Abstract
Human amniotic epithelial cells (HAEs) have a low immunogenic profile and possess potent immunosuppressive properties. HAEs also have several characteristics similar to stem cells, and they are discarded after parturition. Thus, they could potentially be used in cell therapy with fewer ethical problems. HAEs have a short life, so our aim is to establish and characterize immortalized human amniotic epithelial cells (iHAEs). HAEs were introduced with viral oncogenes E6/E7 and with human telomerase reverse transcriptase (hTERT) to create iHAEs. These iHAEs have proliferated around 200 population doublings (PDs) for at least 12 months. High expression of stem cell markers (Oct 3/4, Nanog, Sox2, Klf4) and epithelial markers (CK5, CK18) were detected by immunohistochemistry and reverse transcription polymerase chain reaction (RT-PCR). These iHAEs were expanded in ultra-low-attachment dishes to form spheroids similarly to epithelial stem/precursor cells. High expression of mesenchymal (CD44, CD73, CD90, CD105) and somatic (CD24, CD29, CD271, Nestin) stem cell markers was detected by flow cytometry. The iHAEs showed adipogenic, osteogenic, neuronal, and cardiac differentiation abilities. In conclusion, the immortalization of HAEs with the characteristics of stem cells has been established, allowing these iHAEs to become useful for cell therapy and regenerative medicine.
Introduction
During recent years, human mesenchymal stem cells (hMSCs) have become one of the most promising tools in regenerative medicine. The applicability of these cells for allogeneic transplantation and stem cell–based therapies could further be boosted by standardized collection, quality control, and careful selection of functional and safe cell banking products. However, to provide sufficient stem cell numbers for cell banking and cell-based therapies, their limited replicative potential has to be overcome. In this regard, ectopic expression of human telomerase reverse transcriptase (hTERT) has proven valuable. Besides prolongation of the cellular life span, improvement of growth characteristics, stabilization of the karyotype, and maintenance of the original cellular phenotype (Egusa et al., 2007; Park et al., 2003; Stadler et al., 2008; Takeda et al., 2004; Wai, 2004), hTERT has also been demonstrated to retain or even improve differentiation potential (Jacobs et al., 1999; Kiyono et al., 1998; Lessard and Sauvageau, 2003; Tamagawa et al., 2004; Zhang et al., 2006).
The amnion is a fetal-origin tissue deriving from the inner cell mass (ICM) in the blastocyst and is composed of a single layer of epithelial cells (human amniotic epithelial cells, HAEs) on a thicker basement membrane and collagen spongy layer containing mesenchymal cells (human amniotic mesenchymal cells, HAMs). At days 8–9 after fertilization, the ICM differentiates into two layers, epiblast and hypoblast. From the epiblast, small cells that later constitute the amniotic epithelium appear between the trophoblast and the embryonic disc. The epiblast gives rise to the amnion as well as to all of the germ layers of the embryo (Miki and Strom, 2006; Miki et al., 2005). Thus, HAE cells maintain the plasticity of pregastrulation embryo cells and supposedly have the potential to differentiate into various tissues.
Several studies have shown that HAE cells are a heterologous population positive for stem cell markers, and they display multilineage differentiation potential, differentiating into cells of the endoderm (liver, lung epithelium), mesoderm (bone, fat), and ectoderm (neural cells) (Manuelpillai et al., 2010; Miki et al., 2010; Murphy et al., 2010; Parolini et al., 2008; Toda et al., 2007; Tsutsumi et al., 2001). They have a low immunogenic profile and possess potent immunosuppressive properties, because they do not express major histocompatibility complex (MHC) class II and mildly express MHC class I (Adinolfi et al., 1982; Akle et al., 1981; Lekhanont et al., 2009; Miki et al., 2010; Sakuragawa et al., 1995; Tohyama et al., 1997; Wolbank et al., 2007). Under certain conditions, HAEs also have been reported to differentiate to mature neural cells that synthesize and release neurotransmitters, including acetylcholine, norepinephrine, and dopamine (Sakuragawa et al., 1997; Venkatachalam et al., 2009). HAEs also can be obtained without creating legal or ethical problems and without invasive procedures because they are discarded after parturition (Lekhanont et al., 2009; Wolbank et al., 2007). These observations suggest that cells derived from the fetal side of the placenta may retain a multipotent phenotype long after they differentiate from the epiblast.
These properties are a potentially useful and noncontroversial source of cells for transplantation and regenerative medicine. However, HAE cells, which are usually isolated from fresh amniotic membrane, undergo growth limitation and stop growing after 4–5 passages. These cells are difficult to culture in vitro because of the environment and complexity of cell populations. HAE cells reach senescence because of DNA damage or shortened telomeres, implying that it would be difficult to obtain sufficient quantities of stable cells for cell transplantation therapy (Wai, 2004).
To resolve these problems, we attempted to establish several strains of HAE cells without a life span limitation by introducing retrovirus-carrying hTERT and human papilloma virus type 16 (HPV16) E6/E7 genes (Takeda et al., 2004; Terai et al., 2005). Both Rb/p16INK4a inactivation with E7 and telomerase activation with E6 are required to extend the life span of human epithelial cells (Kiyono et al., 1998). This method was highly efficient in extending the life span of HAE cells.
In the present study, we established iHAE cells and investigated their proliferative ability, differentiation capabilities, and pluripotential markers.
Material and Methods
Isolation of fresh HAE cells and culture
Amniotic membrane was peeled mechanically from the chorion of a placenta obtained with informed consent from a patient undergoing cesarean section. As previously described (Toda et al., 2007), fresh HAE cells (fHAE) were isolated by sequential 0.2% trypsin digestion and cultured in Dulbecco's modified Eagle medium (DMEM) Nutrient Mixture F12 HAM (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS), 1% antibiotic-antimycotic solution (GIBCO BRL, Grand Island, NY, USA), and 5 ng/mL epidermal growth factor (EGF; R&D Systems, Vienna, Austria) at 37°C, 5% CO2, and 95% air humidity to a subconfluent state. Cultured fHAE cells were harvested when they became confluent, and then were seeded into a culture flask to make HAE first generation (HAE p1). The population doubling level (PDL) at each passage was calculated using the following formula: [log (cell number at passage)−log (cell number of seeding)]/log2 (Miki et al., 2010). The population doubling time was calculated from the slope of the linear regression curve of cell number versus time of treatment over a 72-h period. On the basis of the formula, the cell population doubling time (TD)=t[log2/(logNt−logN0)], [N0 and Nt representing the cell number at an initial time point and at time t (hours) of proliferation, respectively], was calculated. The study and the use of amnion membrane were approved by the Research Ethics Committee of the University of Toyama.
Infection of retrovirus constructs and establishment of cell line
HAE cells were stably introduced with HPV16 E6/E7 and hTERT genes by a retrovirus infection, as described previously (Jacobs et al., 1999; Takeda et al., 2004). The CSII-CMV-hTERT was co-infected with the CSII-EF-16E6E7 to create iHAE A cells, and the CSII-CMV-cdk4R24C-PGK-hTERT was co-infected with the CSII-EF-16E6E7 to create iHAE B cells.
Construction of the destination vectors pDEST-CLXSN and pDEST-CMSCVpuro and the expression vectors pCMSCVpuro-16E7 and pCLXSNhTERT were performed. The HPV16 E6E7 segment was cloned into the destination vectors. Briefly, after cloning the segments of HPV16 E6E7 (16E6E7) and the splice of donor site-mutant version of HPV16 E6 (16E6SD) (Sudo et al., 2007) into pDONR201 (Invitrogen), these segments were recombined into retroviral vectors by LR reaction (Invitrogen) to generate pCMSCVpuro-16E6E7. Construction of the destination vector pDEST-CL-SI-MSCVpuro (designated as pSI-CMSCVpuroDEST previously), for retroviral expression of shRNA, pCL-SIMSCVpuro-p53-shRNA (designated as pSI-CMSCVpuro-p53Ri previously), and the entry vector pENTR-H1R-stuffer were as described previously (Terai et al., 2005). To generate p16shRNA expression vectors pSI-CMSCVpuro-H1R-p16shRNA1, 6 and 8, 5′-AAC GCA CCG AAT AGT TAC G-3′, 5′-GGA CGA AGT TTG CAGGGG A-3′, and 5′-GCC CAA CGC ACC GAA TAG TTA CGGTC-3′ were chosen, respectively, as the targeted sequences. Production of recombinant retroviruses was as described earlier (Kiyono et al., 1998). Infected cells were selected in the presence of 0.5 μg/mL puromycin or 50–200 μg/mL G418. For combinations of retroviral infections, cells were first transduced with p16INK4a shRNA, and then with hTERT.
Immunofluorescence
Cells were harvested and fixed in −20°C acetone for 10 min, and then air dried for subsequent immunocytochemical reactions. After blocking with BLOCK ACE (Dainippon Pharmaceutical, Osaka, Japan) for 30 min, cells were incubated in the following primary antibodies diluted 1:200 in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and Triton-×100 for 24 h at 4°C: Oct 3/4 (rabbit, H-134), Nanog (goat, N17), Klf4 (rabbit, H-180), Nestin (rabbit, H-85), c-Myc (mouse, Santa Cruz Biotechnology, CA, USA), Sox2 (mouse, R&D Systems, MN, USA), vimentin (mouse, clone V9, Dako, Glostrup, Denmark), cytokeratin 5 (CK5; mouse, AF 138, Covance, USA), cytokeratin 18 (CK18; mouse, Progen, Heidelberg, Germany), microtubule-associated protein2 (MAP2; mouse, Abcam, Japan), glial fibrillary acidic protein (GFAP; mouse, Progen Biotech., Heidenberg, Germany), β tubulin (Tuj1; mouse, R&D Systems, Inc., Japan), Nkx2.5 (goat, N-19, Santa Cruz), connexin43 (rabbit, Cell Signaling Technology, Boston, MA, USA), myosin light chain-2v (MLC-2v; rabbit, Proteintech, Chicago, IL, USA), α-myosin heavy chain (α-MHC; mouse, clone 3-48, Abcam, Cambrige, UK), and cardiac troponin T (cTnT; mouse, clone 1F11, Abcam).
Cells were incubated with biotinylated secondary antibody (Nichirei, Tokyo, Japan) for 1 h at room temperature, followed by incubation with fluorescein isothiocyanate (FITC) or R-phycoerythrin (RPE)-conjugated streptavidin (DakoCytomation, Glostrup, Denmark) for 30 min at room temperature. Nuclear staining was performed with Hoechst 33342 (Dojindo Laboratories, Kumamoto, Japan). All samples were visualized using fluorescent microscopy (Leica DM/RBE, Wetzlar, Germany), and figures were analyzed with a DP70 digital microscope camera (Olympus, Tokyo, Japan).
Flow cytometry analysis for cell-surface marker
Isolated fHAE cells, HAE p1 cells, and iHAE cells were harvested and washed in 0.5% BSA/0.01 M PBS solution. After 4% paraformaldehyde (PFA) fixation, cells were resuspended in 0.5% BSA/PBS solution and incubated for 1 h at room temperature with the following FITC-conjugated primary antibodies (Beckman Coulter, CA, USA): CD14, CD29, CD34, CD44, CD49f, HLA-DR, CD105, CD271, Nestin, phycoerythrin (PE)-conjugated CD24, CD45, CD133, BCRP (ABCG2), SSEA-4, CD73 (BD Bioscience Pharmingen, CA, USA), and PC 5-conjugated CD90 (Beckman Coulter). Negative control was performed with mouse immunoglobulin G (IgG) 1-isotyped antibodies conjugated to FITC, PE, or PC5 (Beckman Coulter, France). Samples were analyzed on a BD FACSCanto™ II System (BD Biosciences). A total of 3×104events were acquired in a histogram figure with BD FACSDiva™ Software (BD Biosciences). Data were further analyzed with Cell Quest software (BD Biosciences) and WinMDI software v. 2.9 (The Scripps Research Institute).
Reverse transcription polymerase chain reaction
Total RNA was extracted from 5×106 of each of fHAE, HAE p1, HAE p2, and iHAE cells using TRIzol RNA extraction buffer (Nippon Gene, Tokyo, Japan). cDNA was synthesized from 1 μg of total RNA using an Omniscript RT Kit (QIAGEN, Tokyo, Japan). cDNA was subjected to the polymerase chain reaction (PCR) using a Taq PCR Core Kit (QIAGEN, Tokyo, Japan). DNase I digestion of RNA was performed on purified RNA using a One-Step DNase I kit (QIAGEN, Inc., CA, USA). PCR was performed on cDNA under the specific conditions for following genes: OCT 3/4, NANOG, KLF4, SOX2, PPARγ2, alkaline phosphatase (ALP), osteopontin (OPN), and G3PDH primer sequences. The optimal annealing temperatures and cycles were as described in Table 1. For neuronal differentiation markers such as GFAP and MAP2, the primer sequences and optimal annealing temperatures and cycles were as described previously (Habich et al., 2006) and the TUJ1 primer was designated as: forward, 5′-CTC AGG GGC CTT TGG ACA TC-3′, reverse, 5′-CAG GCA GTC GCA GTT TTC AC-3′, 25 cycles of 95°C for 30 sec, 60°C for 30 sec, 72°C for 30 sec. For the cardiac differentiation markers α-MHC, GATA 4, cTNT, and NKX2.5, the primer sequences and optimal annealing temperatures and cycles were as described previously (Zhao et al., 2005). After the reaction, PCR products were size-fractionated by 2% agarose gel electrophoresis and stained with ethidium bromide. Digital images were captured by LAS-3000 (Fujifilm, Tokyo, Japan) and Multi-Gauge v3.0 software (Fujifilm, Tokyo, Japan).
Table 1.
Primers and Conditions Used in RT-PCR Gene Expression
| Gene | Sequence (5’–3’) | Cycles | Product Size (bp) | Anneal. Temp. °C |
|---|---|---|---|---|
| OCT 3/4 | ||||
| Sense | GAA GCT GGA GAA GGA GAA GCT G | 35 | 244 | 60 |
| Antisense | CAA GGG CCG CAG CTT ACA CAT GTT C | |||
| NANOG | ||||
| Sense | CAG AAG GCC TCA GCA CCT AC | 35 | 216 | 56 |
| Antisense | CTG TTC CAG GCC TGA TTG TT | |||
| SOX2 | ||||
| Sense | AGT CTC CAA GCG ACG AAA AA | 35 | 410 | 56 |
| Antisense | GGA AAG TTG GGA TCG AAC AA | |||
| KLF4 | ||||
| Sense | GTT TTG AGG AAG TGC TGA G | 35 | 332 | 55 |
| Antisense | CAG TCA CAG TGG TAA GGT TT | |||
| PPARγ2 | ||||
| Sense | GCT GTT ATG GGT GAA ACT CTG | 35 | 1161 | 58 |
| Antisense | TCG CAG GCT CTT TAG AAA CTC | |||
| ALP | ||||
| Sense | GAC ATC GCC TAC CAG CTC AT | 35 | 307 | 58 |
| Antisense | TCA CGT TGT TCC TGT TCA GC | |||
| OPN | ||||
| Sense | GCG TAA ACC CTG ACC CAT C | 35 | 643 | 58 |
| Antisense | TGC TCA TTG CTC TCA TCA TTG | |||
| G3PDH | ||||
| Sense | CAA GAA GGT GGT GAA GCA GG | 35 | 411 | 57 |
| Antisense | ATG GTA CAT GAC AAG GTG CG | |||
Sphere formation
fHAE, HAE p2, and iHAE cells were cultured at a density of 4×105 cells per well in ultra-low-attachment dishes (Costar, Corning Incorporated, NY, USA) in 24 wells for 2 weeks.
Differentiation-induction experiments
Adipogenic differentiation
Cells were seeded into 8-well Lab-Tek chamber slides (Nalge Nunc International, USA) at 5×103 cells/cm2 in MSCGM™ Mesenchymal Stem Cell Growth Medium (Lonza, Walkersville, MD, USA). Cells were exposed to Adipogenic Induction Medium (AIM, Lonza, Walkersville, MD, USA) followed by 1–3 days of culture in Adipogenic Maintenance Medium (AMM, Lonza) for 3 weeks with the medium changed every third day. Subsequently, cells were fixed with 4% PFA and stained with Oil Red O (Nakarai Tesque, Kyoto, Japan), which was used to visualize fat drops.
Osteogenic differentiation
Cells were seeded into 2-well Lab-Tek chamber slides (Nalge Nunc International, USA) at 5×103 cells/cm2 in the cultured medium, and then treated by the Differentiation BulletKit-Osteogenic (Lonza) followed by 1–3 days of culture in Osteogenic Maintenance Medium (Lonza) for 2–3 weeks with the medium changed every third day. To identify osteogenic differentiation, cells were fixed with 70% ethanol, and stained by Alizarin Red S (Fisher Scientific, Pittsburg, PA). Alkaline phosphatase activity was observed by using the Vector Red Alkaline Phosphatase Substrate Kit I (Vector Labs, Burlingame, CA) as described previously (Egusa et al., 2007; Stadler et al., 2008).
Neuronal differentiation
Cells were seeded into 12-multiwell dishes (Nalge Nunc International, USA) at 5×103 cells/cm2 in NeuroCult® NS-A Basal Medium (Human) (StemCell, Technologies, Inc., Vancouver, BC, CA) containing NeuroCult® NS-A Proliferation Supplements (StemCell, Technologies, Inc.) and 20 ng/mL recombinant human epithelial growth factor (EGF). Twelve-well dishes were coated by Poly-L-Ornithine (BD BioCoat™, BD Biosciences, Canada). Cells were maintained in neuronal induction medium for 3 weeks with the medium changed every third day. Cells were fixed with 4% PFA and stained with MAP2, GFAP, Tuj1, and Nestin antibodies.
Cardiac differentiation
DMEM containing 20% FBS was used as a differentiation medium. Cells were seeded at a density of 1×103 cells/cm2 in the differentiation medium. On the next day, 10 μM 5-azacytidine (5-aza) (Sigma-Aldrich) was added for 24 h, and the differentiation medium was changed two or three times a week for 4 weeks.
Results
Establishment of iHAE cells with an extended life span
To extend the life span of HAE cells, two different types of cells were established by introducing combinations of E6E7 and/or hTERT using a retrovirus infection. HAE cells were isolated from amniotic tissue propagated in vitro until they reached replicative senescence. HAE cells were co-infected with CSII-EF-16E6/E7 and CSII-CMV-hTERT to create iHAE A cells. iHAE B cells were created with CSII-EF-16E6/E7 and CSII-CMV-cdk4R24C-PGK-hTERT. After introduction of the genes, both HAE cell types were immortalized and expanded to at least PDL60 with no signs of growth retardation (Fig. 1A). The two introduced HAE cells were cuboidal-shaped, with epithelial cell-like morphology (Fig. 1B). CK5 and CK18 (cytokeratins), specific markers for epithelial cells, were both expressed in these introduced HAE cells (Fig. 1C). HAE B cells were expandable up to at least 70 PDLs by passage over 50 generations, whereas HAE A cells showed the same growth rate as HAE B cells (Fig. 1A). However in our study, the life span of HAE cells introduced with hTERT alone did not extended beyond 10 passages (data not shown). The doubling times of each iHAE cell population were observed.
FIG. 1.
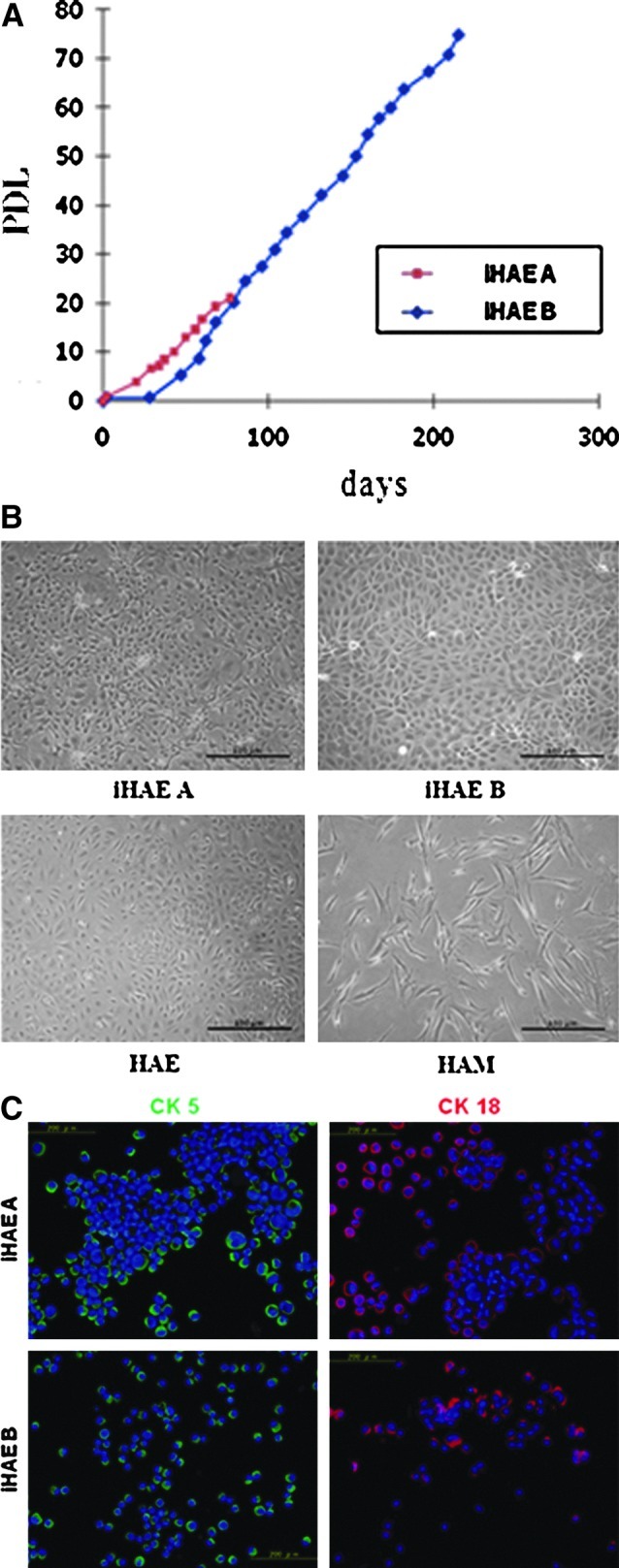
Establishment of immortalized HAE cells. (A) PDL of two introduced HAE cell lines with combination of genes E6/E7 and hTERT. iHAE A (red), iHAE B (blue). (B) Phase-contrast image of the two introduced HAE cells. (Upper left) iHAE A; (upper right) iHAE B; (lower left) fresh isolated HAE; (lower right) fresh isolated HAM cells. Scale bar, 100 μm). (C) Expression of epithelial cell–specific markers Cytokeratin (CK) 5 (green) and CK18 (red) in the two introduced HAE cells. Scale bar, 200 μm.
Analysis of pluripotency of iHAE cells
To investigate the stemness of iHAE cells, RT-PCR, sphere formation assay, and immunocytochemical analysis were performed with cells at passages 35–40. RT-PCR results showed stem cell–specific gene expression, such as OCT 3/4, NANOG, SOX2, and KLF4 in fHAE, HAE p1, HAE p2, and iHAE cells (Fig. 2A). The expression of OCT 3/4 and NANOG genes in iHAE A cells was greater than that in fHAE cells. Other stem cell–specific genes, SOX2 and KLF4, in iHAE A cells showed similar expression to fHAE cells. As in iHAE A cells, iHAE B cells also expressed these stem cell marker genes while SOX2 expression was greater than that in iHAE A cells. When both iHAE cells were cultured in a 24-well ultra-low-attachment dishes, they made spheroids of 1×104 cells/well (Fig. 2B). Numerous spheres of various sizes were observed after 3 or 4 days of floating culture.
FIG. 2.
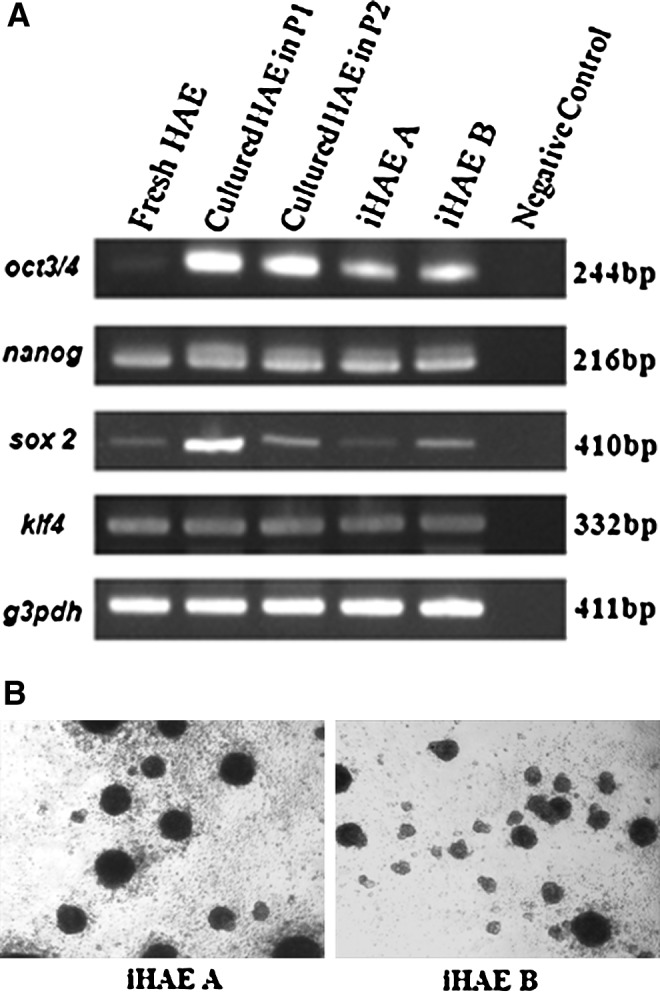
Stem cell marker genes expression and sphere formation. (RT-PCR and sphere formation). (A) RT-PCR of stem cell marker genes OCT 3/4, NANOG, SOX2, and KLF4. Lane 1, freshly isolated HAE cells (fHAE cells); lane 2, HAE cells cultured at passage 1 (HAE cells p1); lane 3, HAE cells cultured at passage 2 (HAE cells p2); lane 4, iHAE A; lane 5, iHAE B; lane 6, negative control. G3PDH mRNA was used as an internal control. (B) iHAE cells made spheroid bodies after they were cultured on ultra-low- attachment dishes for 3 days. Scale bar, 100 μm.
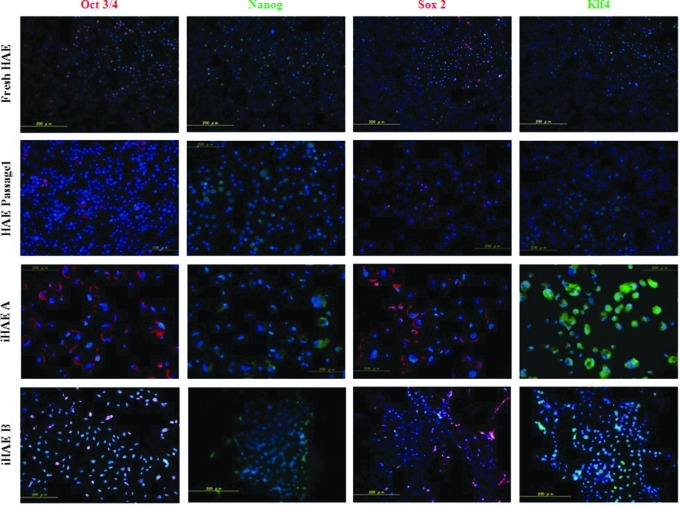
The reactions against stem cell–specific markers Oct 3/4, Sox2, and Klf4 were stronger in iHAE cells. There were scattered positive cells that were stained with anti-Oct 3/4 antibody in the cytoplasm rather than the nucleus and a few cells were positive in the nuclei of fresh HAE and HAE passage 1, whereas all nuclei reacted strongly in iHAE cells. The staining patterns of Klf4 and Sox2 were the same as that of Oct 3/4. Nanog was weakly observed in the perinuclear area (Fig. 3).
FIG. 3.
Expression of stem cell markers Oct 3/4 (red), Nanog (green), Sox2 (red), and Klf4 (green). First row, fHAE cells; second row, HAE p1; third row, iHAE A cells and fourth row, iHAE B cells. All the images are merged with nuclear staining of Hoechst 33342 (blue). Scale bar, 200 μm.
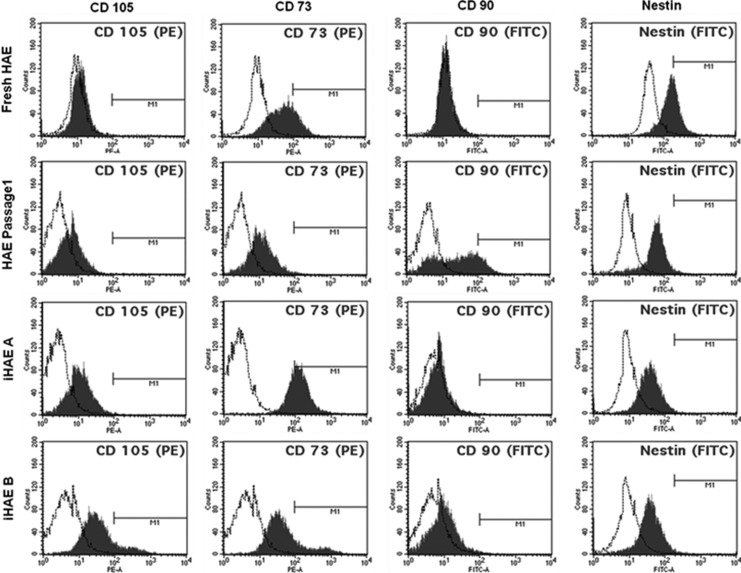
Surface markers analysis of iHAE cells
Surface marker expression of iHAE A and iHAE B was evaluated by flow cytometric analysis. To investigate more characteristics of iHAE cells, hematopoietic (CD14, CD34, CD45, and HLA-DR), mesenchymal (CD44, CD73, CD90, and CD105) and somatic (CD24, CD29, CD49f, CD133, CD271, BCRP, SSEA4, and Nestin) stem cell markers were used, because there are no common markers for epithelial stem cells. The results are summarized in Table 2. Compared to fresh HAE cells, both iHAE cells had higher expression of CD105, CD73, and CD90, whereas Nestin was expressed similarly to fresh HAE cells (Fig. 4). The expression of MSC markers (CD44, CD73, CD90, and CD105) and some somatic stem cell markers (CD24, CD29, CD271, and Nestin) in both iHAE cells was increased compared to fresh isolated HAE cells, even after prolonged in vitro propagation (Table 2). Interestingly, both immortalized HAE cells expressed mesenchymal markers CD44 and CD90, whereas fHAE did not express these at all. The somatic (CD24, CD29, and CD271) stem cell markers showed greater expression in iHAE cells than in fHAE and HAE p1 cells, whereas CD 49f marker was expressed lower in iHAE cells. CD133 and BCRP were expressed at very low levels in iHAE cells. The expression of another stemness marker, SSEA-4, which was positive in about 56.1±11% of fHAE cells, disappeared in both iHAE cells (Table 2).
Table 2.
Surface Markers Expression (Mesenchymal Stem Cell Markers, Hematopoietic Stem Cell Markers and Somatic Stem Cell Markers) by Flow Cytometry Analysis
| Marker | Fresh HAE | HAE Passage 1 | iHAE A | iHAE B |
|---|---|---|---|---|
| Mesenchymal stem cell markers | ||||
| CD105 | 0.1±0 | 4.5±4.25 | 46.6±0.55 | 42.8±0.11 |
| CD90 | 0.0±0 | 19.1±0.02 | 4.1±0.51 | 1.7±0.02 |
| CD73 | 69.2±2.4 | 48.4±1.7 | 95.5±0.07 | 55.6±0.42 |
| CD44 | 0.17±0.0 | 3.4±2.1 | 58.9±0.16 | 21.7±0.09 |
| Haematopoietic stem cell markers | ||||
| CD34 | 0.0±0 | 0.13±0.22 | 0.6±0.0 | 0.13±0.0 |
| CD45 | 0.0±0 | 0.1±0.0 | 0.23±0.0 | 0.1±0.0 |
| HLA-DR | 0.1±0.0 | 0.03±0.0 | 0.03±0.0 | 1.97±0.01 |
| CD14 | 0.0±0 | 0.0±0 | 0.0±0 | 0.1±0.0 |
| Somatic stem cell markers | ||||
| CD24 | 5.5±6.31 | 58.2±3.94 | 100±0.0 | 92.7±0.07 |
| CD29 | 92.0±7.05 | 83.0±1.59 | 100±0.0 | 98.7±0.23 |
| CD49f | 91.4±0.14 | 90.0±5.63 | 67±0.1 | 25.4±0.06 |
| CD133 | 3.1±5.13 | 0.13±0.0 | 0.3±0.0 | 0.33±0.0 |
| CD271 | 55.2±10.79 | 51.9±1.67 | 70.8±0.03 | 60.1±0.01 |
| BCRP | 0.1±0.1 | 0.07±0.0 | 0.0±0 | 0.0±0 |
| SSEA-4 | 56.1±10.91 | 33±2.07 | 0.0±0 | 0.0±0 |
| Nestin | 54.6±10.41 | 43.6±1.45 | 67.1±0.01 | 57.03±0.02 |
Data represent average±standard deviation (SD), n=3.
HAE, human amniotic epithelial cells; iHAE, immortalized human amniotic epithelial cells.
FIG. 4.
Flow cytometric analysis of MSC markers CD105, CD73, CD90, and neural stem cell marker Nestin. First row, freshly isolated HAE cells (fHAE cells); second row, HAE cells cultured at passage 1 (HAE cells p1); third row, iHAE A; fourth row, iHAE B.
Adipogenic, osteogenic, neural, and cardiac differentiation
The multipotency of iHAE cells was assessed by induction using differentiation media, and the results were evaluated by cytochemical staining and RT-PCR assays. Adipogenic differentiation of iHAE cells was estimated by Oil Red O staining. Various sizes of lipids droplets were observed in both iHAE cytoplasm with a cell cluster-like appearance after 3 weeks' induction. However, noninduced cells also showed small lipids droplets in the cytoplasm (Fig. 5A). RT-PCR analysis indicated that PPARγ2 mRNA, the adipogenic differentiation marker, was highly expressed in the induced cells compared to the cells without induction (Fig. 5B).
FIG. 5.
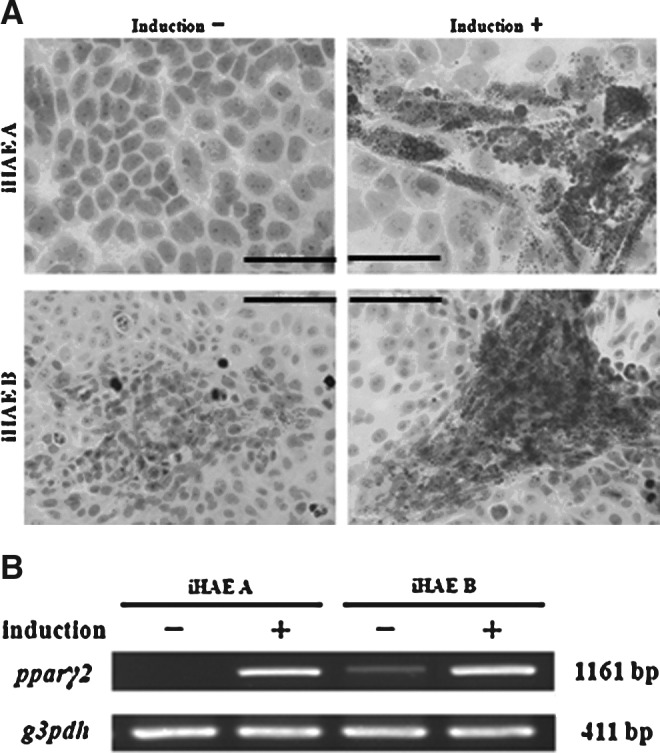
Adipogenic differentiation of the two iHAE cell lines. (A) Oil Red O staining of iHAE A and B cells with or without adipogenic induction. (Upper left) iHAE A cells without induction; (upper right) iHAE A cells with induction; (lower left) iHAE B cells without induction; (lower right) iHAE B cells with induction. Scale bar, 100 μm. (B) RT-PCR of PPARγ2 mRNA (an adipogenic differentiation marker) from iHAE A and iHAE B cells with (+) or without (−) adipogenic differentiation.
Osteogenic differentiation of iHAE cells was detected by staining with Alizarin Red S (Fig. 6A) after 3 weeks of induction. Both iHAE cells showed the presence of mineralization of osteocytes (Alizarin Red–positive cells) with a cluster-like appearance after induction. iHAE B especially was remarkably positively stained. Expression of osteogenic-specific genes ALP and OPN were detected in all cells after differentiation stimulation. Especially, expression of the iHAE B cell line demonstrated OPN mRNA, even without induction (Fig. 6B).
FIG. 6.
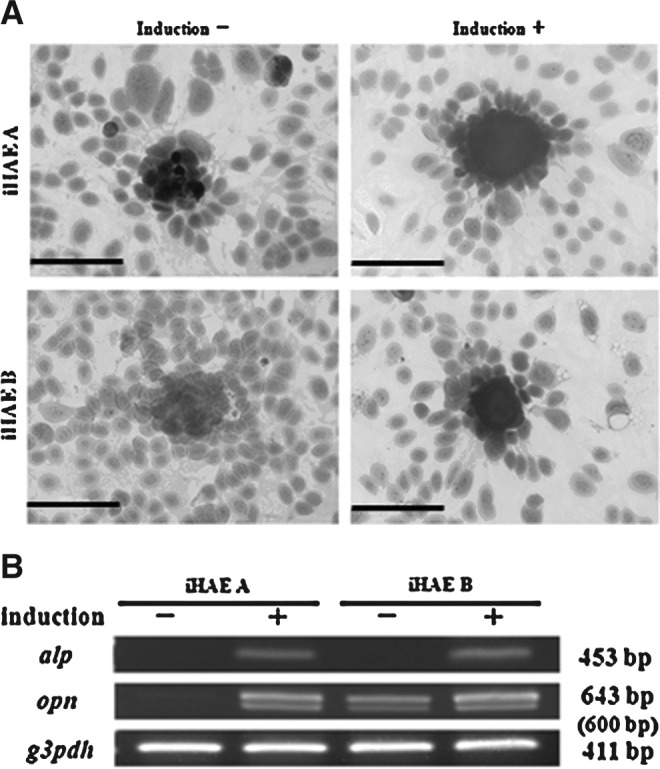
Osteogenic differentiation of the two iHAE cell lines. (A) Alizarin Red S staining of iHAE A and B cells with or without osteogenic induction. (Upper left) iHAE A cells without induction; (upper right) iHAE A cells with induction; (lower left) iHAE B cells without induction; (lower right) iHAE B cells with induction. Scale bar, 200 μm. (B) RT-PCR of osteogenic specific genes (ALP and OPN) from iHAE A and iHAE B cells with (+) or without (−) osteogenic differentiation.
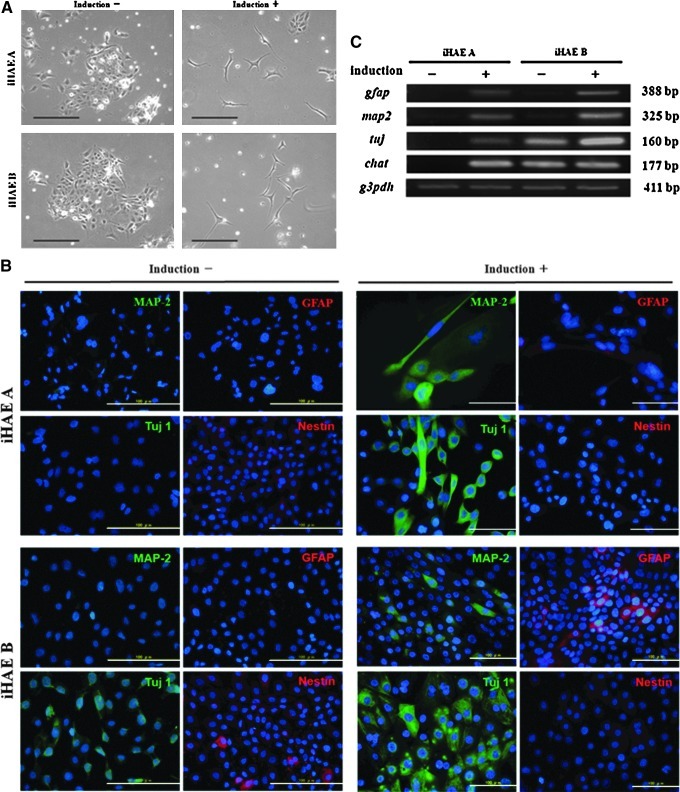
Neuronal differentiation potency was revealed by staining with neuronal-specific markers such as MAP2, GFAP, Tuj1, and Nestin. After induction, MAP2- and Tuj1-positive staining were clearly seen in the cytoplasm with the appearance of microtubules, which are essential for neurogenesis. GFAP was also stained in the cytoplasm with less positive cell numbers than MAP2 or Tuj1. Nestin, as a neural stem cell marker, was weakly stained compared with the other three markers after induction (Fig. 7B). The expression of GFAP, MAP2, TUJ1, and choline acetyltransferase (CHAT) were detected in both iHAE cells by RT-PCR analysis after differentiation stimulation (Fig. 7C). These findings were consistent with the immunofluorescence results.
FIG. 7.
Neuronal differentiation of the two iHAE cell lines. (A) Phase-contrast image of iHAE cells with (+) or without (−) neuronal induction. Scale bar, 100 μm. (B) Immunocytochemistry of neuronal specific markers MAP2 (green), GFAP (red), Tuj1 (green), and Nestin (red) with or without induction of neuronal differentiation. All the images are merged with nuclear staining of Hoechst 33342 (blue). Scale bar, 100 μm. (C) RT-PCR of neuronal-specific genes (MAP2, GFAP, TUJ, and CHAT) from iHAE A and iHAE B cells with (+) or without (−) induction of neuronal differentiation.
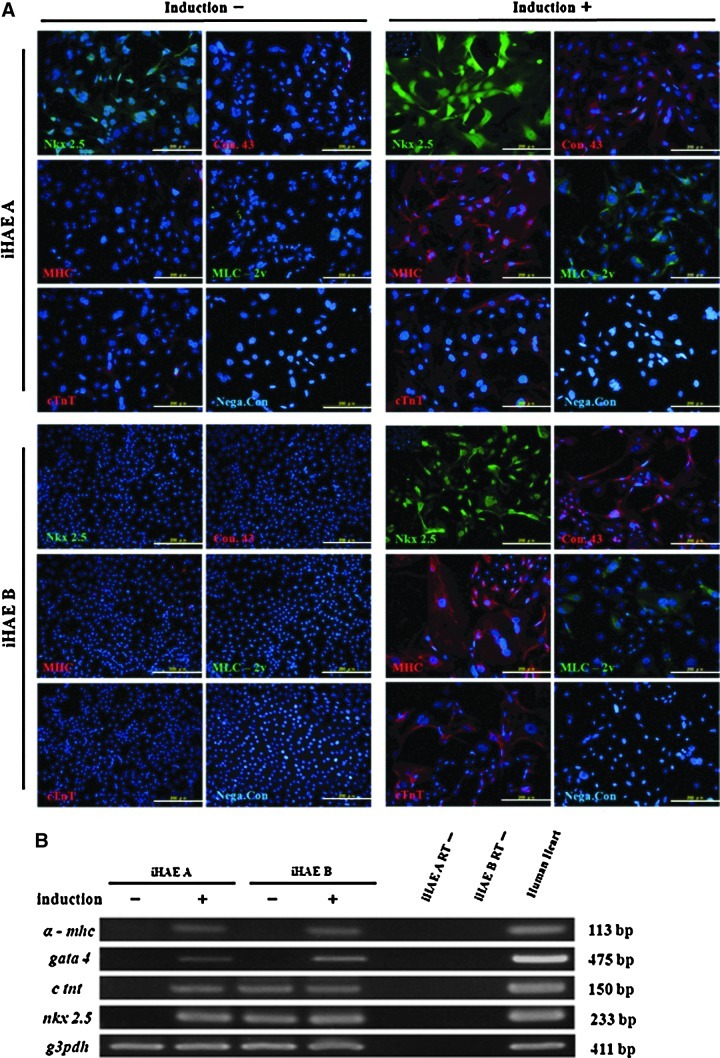
After 4 weeks of cardiac induction, iHAE cells expressed Nkx2.5 protein in nuclei. The expression of connexin 43 was detected in cytoplasm. This protein is present in the gap junction between adjacent cells and makes the possible transmission of the action potential during cardiogenesis. The majority of both iHAE cells were stained with cardiac α-MHC and cTnT in cytoplasm after cardiac induction, whereas cardiac MLC-2v was stained in the perinuclear area (Fig. 8A). As for changes in mRNA, the intensities of these cardiac-specific contracting proteins in the induced iHAE cells were changed more than in the cells without induction (Fig. 8B).
FIG. 8.
Cardiac differentiation of the two iHAE cell lines. (A) Immunocytochemistry of cardiac specific markers Nkx 2.5 (green), Connexin 43 (red), MHC (red), MLC 2v (green), and c TnT (red) with or without induction of cardiac differentiation. All of the images are merged with nuclear staining of Hoechst 33342 (blue). Scale bar, 200 μm. (B) RT-PCR of cardiac specific genes (α-MHC, GATA 4, cTNT, and NKX 2.5) from iHAE A and iHAE B cells with (+) or without (−) induction of cardiac differentiation. Human heart cells were used as a positive control (last lane).
Discussion
In this study, we found that combinations of hTERT with HPV16E6/E7 genes extended the life span of HAE cells, which was difficult with in vitro culture. This result suggested that these immortalized cell lines were successfully established by downregulation of p16INK4a and p53 expression and activation of telomerase (Kiyono et al., 1998; Takeda et al., 2004), although normal somatic cell populations proliferated no more than 50 PDs (Miki et al., 2005; Miki et al., 2010; Sudo et al., 2007). It has been reported that the extension of the life span of human umbilical cord blood-derived mesenchymal stem cells (UCBMSCs) with transfecting hTERT alone did not require inhibition of the p16INK4a/Rb pathway (Terai et al., 2005). This indicates that telomerase activity was not sufficient to immortalize HAE cells, which agrees with inhibition of the p16INK4a/Rb pathway being necessary to immortalize human placenta-derived mesenchymal cells (hPDMCs) (Kiyono et al., 1998; Zhang et al., 2006).
When iHAE cells were transplanted into the testis, liver, and muscle of nude mice at a concentration of 1×106 cells/part, no tumors were seen during the monitoring period of more than 6 months. Even when iHAM cells were transfected with the same oncogenes, carcinogenic effects are not induced in those cells (data not shown).
Surface marker expression of HAE cells was affected by the exogenously expressed E6/E7 and/or hTERT genes. When iHAE cells were compared to freshly isolated HAE cells and cultured HAE cells at p1 or p2, they displayed higher proportions of positivity for CD73, CD105, CD44, CD24, CD29, and CD271 than fHAE and cultured p1 or p2 HAE cells. However, our results suggested that the proportion of MSC markers increased gradually from primary culture, as continuous cultures were performed. Surface markers thought to be absent on HAE cells, including CD 90 (Thy-1), showed positive populations in the iHAE cells. Recently, it was reported that upon cultivation in EGM-2 a subpopulation of CD90-negative human bone marrow MSCs evolved after prolonged culture, probably due to angiogenic growth factors in the medium (Sudo et al., 2007).
In addition to surface markers, iHAE cells expressed molecular markers of pluripotent stem cells, including Oct 3/4, Nanog, Sox2, and Klf4, indicating that HAE cells with an extended life span after long-term culture still maintained “stemness” characteristics (Izumi et al., 2009; Simat et al., 2008). iHAE cells expressed stem cell–specific genes such as OCT 3/4, NANOG, SOX2, and KLF4. But compared to cultured p1 and/or p2 HAE cells, OCT 3/4, and SOX2 expression in iHAE cells were more like those in fHAE cells (Fig. 2A).
In contrast, with adipogenic and osteogenic differentiation, iHAE B cells highly expressed mRNA of PPARγ2 and OPN, even in the noninduced cells, but noninduced iHAE A did not express these two markers. Although iHAE A and iHAE B were introduced with the same genes, such as E6E7 and hTERT, their differentiation potentials were different. Here, cdk4R24C might play an important role. We also found that iHAE B cell can make a cluster-like structure during differentiation induction; this looks like a cell colony or aggregation, and seems to have much more differentiation potency than other two-dimensionally cultured cells. These findings are consistent with the report of Parolini et al. that the surface marker expression of hCMSC was unaffected by the exogenously expressed Bmi-1, E6, E7, and/or hTERT genes (Parolini et al., 2008). It will be necessary to investigate in detail the relationships between the expression of stem cell markers and differentiation ability with immortalization. The iHAE cells can differentiate into many mesodermal lineage-like tissues such as adipose, bone, and neural cells. After induction by 5-aza, iHAE cells expressed some cardiac-specific genes such as NKX 2.5, CONNEXIN 43, GATA 4, cardiac α-MHC, MLC-2v, and c TNT, suggesting that iHAE cells also have the ability to differentiate into cardiomyocyte-like cells.
We also examined the differentiation of iHAE cells to another endodermal tissue, the pancreas, as previously reported. Wei et al. cultured HAE cells for 2–4 weeks in the presence of nicotinamide to induce pancreatic differentiation (Wei et al., 2003). Subsequent transplantation of the insulin-expressing HAE cells corrected the hyperglycemia of streptozotocin (STZ)-induced diabetic mice. In the same setting, HAM cells were ineffective, suggesting that HAE, but not HAM cells, were capable of acquiring a β-cell fate (Bailo et al., 2004; Szukiewicz et al., 2010a; Szukiewicz et al., 2010b). In our study, iHAE cells could spontaneously express some pancreatic-related genes, such as PC1 and PC2 (data not shown), and thus it is possible that iHAE cells could also differentiate into insulin-producing cells, a lineage of endodermal cells.
The recent notion of epithelial-to-mesenchymal transition (EMT) is related to embryonic development and metastasis. Several groups have demonstrated that human pancreatic insulin-producing cells proliferate and undergo EMT in vitro (Cole et al., 2009; Joglekar et al., 2009; Russ et al., 2008; Russ et al., 2009). Another recent report suggested that adult human islets contain a minor fraction of CD90/CD105 double-immunopositive, mesenchymal cells (Carlotti et al., 2010). This concept was also supported by a similar study demonstrating the presence of CD105+/CD73+/CD90+ MSCs in freshly isolated islets (Davani et al., 2007). In our iHAE cells, we also found CD105/CD73/CD90-positive expression, suggesting that EMT occurred in these cells. Although in vivo proliferation involves a mesenchymal intermediate, this remains unclear (Cole et al., 2009). We think that the coming years may realize the possible potential of our iHAE cells for replacement therapy.
In conclusion, the immortalized HAE lines established in this study can be seen as a first step to a proof of principle for their applicability in cell-based therapy approaches. Specifically, their differentiation potential and immunosuppressive effects are of major importance. By introducing genes such as HPV16 E6/E7 and hTERT, we established two HAE cell lines. The immortalized HAE cells retained characteristics of the parental cells with regard to morphology and showed similar or even improved differentiation potential. Collectively, the unique properties of HAE cells, in combination with immortalization by E6E7 and/or hTERT, resulted in an efficient new cell source. These cells may be useful for cell therapy and regenerative medicine research.
Acknowledgments
We would like to thank Mrs. Furuichi and Mr. Kawahara for providing expert technical assistance with this study. This work was supported in part by a special grant from the Ministry of Education, Culture, Sports, Sciences, and Technology of Japan No.K20390430 to C.K., T.Y., M.O., and T.N. Also, it was supported by a grant from Japan Society for the Promotion of Science (JSPS) no. 16390473 to Toshio Nikaido.
Author Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- Adinolfi M. Akle C.A. McColl I. Fensom A.H. Tansley L. Connolly P. Hsi B.L. Faulk W.P. Travers P. Bodmer W.F. Expression of HLA antigens, beta 2-microglobulin and enzymes by human amniotic epithelial cells. Nature. 1982;295:325–327. doi: 10.1038/295325a0. [DOI] [PubMed] [Google Scholar]
- Akle C.A. Adinolfi M. Welsh K.I. Leibowitz S. McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2:1003–1005. doi: 10.1016/s0140-6736(81)91212-5. [DOI] [PubMed] [Google Scholar]
- Bailo M. Soncini M. Vertua E. Signoroni P.B. Sanzone S. Lombardi G. Arienti D. Calamani F. Zatti D. Paul P. Albertini A. Zorzi F. Cavagnini A. Candotti F. Wengler G.S. Parolini O. Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation. 2004;78:1439–1448. doi: 10.1097/01.tp.0000144606.84234.49. [DOI] [PubMed] [Google Scholar]
- Carlotti F. Zaldumbide A. Loomans C.J. van Rossenberg E. Engelse M. de Koning E.J. Hoeben R.C. Isolated human islets contain a distinct population of mesenchymal stem cells. Islets. 2010;2:164–173. doi: 10.4161/isl.2.3.11449. [DOI] [PubMed] [Google Scholar]
- Cole L. Anderson M. Antin P.B. Limesand S.W. One process for pancreatic beta-cell coalescence into islets involves an epithelial-mesenchymal transition. J. Endocrinol. 2009;203:19–31. doi: 10.1677/JOE-09-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davani B. Ikonomou L. Raaka B.M. Geras-Raaka E. Morton R.A. Marcus-Samuels B. Gershengorn M.C. Human islet-derived precursor cells are mesenchymal stromal cells that differentiate and mature to hormone-expressing cells in vivo. Stem Cells. 2007;25:3215–3222. doi: 10.1634/stemcells.2007-0323. [DOI] [PubMed] [Google Scholar]
- Egusa H. Iida K. Kobayashi M. Lin T.Y. Zhu M. Zuk P.A. Wang C.J. Thakor D.K. Hedrick M.H. Nishimura I. Downregulation of extracellular matrix-related gene clusters during osteogenic differentiation of human bone marrow- and adipose tissue-derived stromal cells. Tissue Eng. 2007;13:2589–2600. doi: 10.1089/ten.2007.0080. [DOI] [PubMed] [Google Scholar]
- Habich A. Jurga M. Markiewicz I. Lukomska B. Bany-Laszewicz U. Domanska-Janik K. Early appearance of stem/progenitor cells with neural-like characteristics in human cord blood mononuclear fraction cultured in vitro. Exp. Hematol. 2006;34:914–925. doi: 10.1016/j.exphem.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Izumi M. Pazin B.J. Minervini C.F. Gerlach J. Ross M.A. Stolz D.B. Turner M.E. Thompson R.L. Miki T. Quantitative comparison of stem cell marker-positive cells in fetal and term human amnion. J. Reprod. Immunol. 2009;81:39–43. doi: 10.1016/j.jri.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Jacobs J.J. Kieboom K. Marino S. DePinho R.A. van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- Joglekar M.V. Patil D. Joglekar V.M. Rao G.V. Reddy D.N. Mitnala S. Shouche Y. Hardikar A.A. The miR-30 family microRNAs confer epithelial phenotype to human pancreatic cells. Islets. 2009;1:137–147. doi: 10.4161/isl.1.2.9578. [DOI] [PubMed] [Google Scholar]
- Kiyono T. Foster S.A. Koop J.I. McDougall J.K. Galloway D.A. Klingelhutz A.J. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- Lekhanont K. Choubtum L. Chuck R.S. Sa-ngiampornpanit T. Chuckpaiwong V. Vongthongsri A. A serum- and feeder-free technique of culturing human corneal epithelial stem cells on amniotic membrane. Mol. Vis. 2009;15:1294–1302. [PMC free article] [PubMed] [Google Scholar]
- Lessard J. Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- Manuelpillai U. Tchongue J. Lourensz D. Vaghjiani V. Samuel C.S. Liu A. Williams E.D. Sievert W. Transplantation of human amnion epithelial cells reduces hepatic fibrosis in immunocompetent CCl-treated mice. Cell Transplant. 2010;19:1157–1168. doi: 10.3727/096368910X504496. [DOI] [PubMed] [Google Scholar]
- Miki T. Strom S.C. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2:133–142. doi: 10.1007/s12015-006-0020-0. [DOI] [PubMed] [Google Scholar]
- Miki T. Lehmann T. Cai H. Stolz D.B. Strom S.C. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23:1549–1559. doi: 10.1634/stemcells.2004-0357. [DOI] [PubMed] [Google Scholar]
- Miki T. Marongiu F. Dorko K. Ellis E.C. Strom S.C. Isolation of amniotic epithelial stem cells. Curr. Protoc. Stem Cell Biol. 2010;Chapter 1:3. doi: 10.1002/9780470151808.sc01e03s12. Unit 1E. [DOI] [PubMed] [Google Scholar]
- Murphy S. Rosli S. Acharya R. Mathias L. Lim R. Wallace E. Jenkin G. Amnion epithelial cell isolation and characterization for clinical use. Curr. Protoc. Stem Cell Biol. 2010;Chapter 1:6. doi: 10.1002/9780470151808.sc01e06s13. Unit 1E. [DOI] [PubMed] [Google Scholar]
- Park I.K. Qian D. Kiel M. Becker M.W. Pihalja M. Weissman I.L. Morrison S.J. Clarke M.F. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Parolini O. Alviano F. Bagnara G.P. Bilic G. Buhring H.J. Evangelista M. Hennerbichler S. Liu B. Magatti M. Mao N. Miki T. Marongiu F. Nakajima H. Nikaido T. Portmann-Lanz C.B. Sankar V. Soncini M. Stadler G. Surbek D. Takahashi T.A. Redl H. Sakuragawa N. Wolbank S. Zeisberger S. Zisch A. Strom S.C. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- Russ H.A. Bar Y. Ravassard P. Efrat S. In vitro proliferation of cells derived from adult human beta-cells revealed by cell-lineage tracing. Diabetes. 2008;57:1575–1583. doi: 10.2337/db07-1283. [DOI] [PubMed] [Google Scholar]
- Russ H.A. Ravassard P. Kerr-Conte J. Pattou F. Efrat S. Epithelial-mesenchymal transition in cells expanded in vitro from lineage-traced adult human pancreatic beta cells. PLoS One. 2009;4:e6417. doi: 10.1371/journal.pone.0006417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuragawa N. Misawa H. Ohsugi K. Kakishita K. Ishii T. Thangavel R. Tohyama J. Elwan M. Yokoyama Y. Okuda O. Arai H. Ogino I. Sato K. Evidence for active acetylcholine metabolism in human amniotic epithelial cells: Applicable to intracerebral allografting for neurologic disease. Neurosci. Lett. 1997;232:53–56. doi: 10.1016/s0304-3940(97)00570-3. [DOI] [PubMed] [Google Scholar]
- Sakuragawa N. Tohyama J. Yamamoto H. Immunostaining of human amniotic epithelial cells: possible use as a transgene carrier in gene therapy for inborn errors of metabolism. Cell Transplant. 1995;4:343–346. doi: 10.1177/096368979500400313. [DOI] [PubMed] [Google Scholar]
- Simat S.F. Chua K.H. Abdul Rahman H. Tan A.E. Tan G.C. The stemness gene expression of cultured human amniotic epithelial cells in serial passages. Med. J. Malaysia. 2008;63(Suppl A):53–54. [PubMed] [Google Scholar]
- Stadler G. Hennerbichler S. Lindenmair A. Peterbauer A. Hofer K. van Griensven M. Gabriel C. Redl H. Wolbank S. Phenotypic shift of human amniotic epithelial cells in culture is associated with reduced osteogenic differentiation in vitro. Cytotherapy. 2008;10:743–752. doi: 10.1080/14653240802345804. [DOI] [PubMed] [Google Scholar]
- Sudo K. Kanno M. Miharada K. Ogawa S. Hiroyama T. Saijo K. Nakamura Y. Mesenchymal progenitors able to differentiate into osteogenic, chondrogenic, and/or adipogenic cells in vitro are present in most primary fibroblast-like cell populations. Stem Cells. 2007;25:1610–1617. doi: 10.1634/stemcells.2006-0504. [DOI] [PubMed] [Google Scholar]
- Szukiewicz D. Pyzlak M. Stangret A. Rongies W. Maslinska D. Decrease in expression of histamine H2 receptors by human amniotic epithelial cells during differentiation into pancreatic beta-like cells. Inflamm. Res. 2010a;59(Suppl 2):S205–S207. doi: 10.1007/s00011-009-0131-6. [DOI] [PubMed] [Google Scholar]
- Szukiewicz D. Szewczyk G. Mittal T.K. Rongies W. Maslinski S. Involvement of histamine and histamine H2 receptors in nicotinamide-induced differentiation of human amniotic epithelial cells into insulin-producing cells. Inflamm. Res. 2010b;59(Suppl 2):S209–S211. doi: 10.1007/s00011-009-0132-5. [DOI] [PubMed] [Google Scholar]
- Takeda Y. Mori T. Imabayashi H. Kiyono T. Gojo S. Miyoshi S. Hida N. Ita M. Segawa K. Ogawa S. Sakamoto M. Nakamura S. Umezawa A. Can the life span of human marrow stromal cells be prolonged by bmi-1, E6, E7, and/or telomerase without affecting cardiomyogenic differentiation? J. Gene Med. 2004;6:833–845. doi: 10.1002/jgm.583. [DOI] [PubMed] [Google Scholar]
- Tamagawa T. Ishiwata I. Saito S. Establishment and characterization of a pluripotent stem cell line derived from human amniotic membranes and initiation of germ layers in vitro. Hum. Cell. 2004;17:125–130. doi: 10.1111/j.1749-0774.2004.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Terai M. Uyama T. Sugiki T. Li X.K. Umezawa A. Kiyono T. Immortalization of human fetal cells: The life span of umbilical cord blood-derived cells can be prolonged without manipulating p16INK4a/RB braking pathway. Mol. Biol. Cell. 2005;16:1491–1499. doi: 10.1091/mbc.E04-07-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda A. Okabe M. Yoshida T. and Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J. Pharmacol. Sci. 2007;105:215–228. doi: 10.1254/jphs.cr0070034. [DOI] [PubMed] [Google Scholar]
- Tohyama J. Tsunoda H. Sakuragawa N. Characterization of human amniotic epithelial cells transformed with origin-defective SV40 T-antigen gene. Tohoku J. Exp. Med. 1997;182:75–82. doi: 10.1620/tjem.182.75. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S. Shimazu A. Miyazaki K. Pan H. Koike C. Yoshida E. Takagishi K. Kato Y. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem. Biophys. Res. Commun. 2001;288:413–419. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- Venkatachalam S. Palaniappan T. Jayapal P.K. Neelamegan S. Rajan S.S. Muthiah V.P. Novel neurotrophic factor secreted by amniotic epithelial cells. Biocell. 2009;33:81–89. [PubMed] [Google Scholar]
- Wai L.K. Telomeres, telomerase, and tumorigenesis—a review. MedGenMed. 2004;6:19. [PMC free article] [PubMed] [Google Scholar]
- Wei J.P. Zhang T.S. Kawa S. Aizawa T. Ota M. Akaike T. Kato K. Konishi I. Nikaido T. Human amnion-isolated cells normalize blood glucose in streptozotocin-induced diabetic mice. Cell Transplant. 2003;12:545–552. doi: 10.3727/000000003108747000. [DOI] [PubMed] [Google Scholar]
- Wolbank S. Peterbauer A. Fahrner M. Hennerbichler S. van Griensven M. Stadler G. Redl H. Gabriel C. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: A comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007;13:1173–1183. doi: 10.1089/ten.2006.0313. [DOI] [PubMed] [Google Scholar]
- Zhang X. Soda Y. Takahashi K. Bai Y. Mitsuru A. Igura K. Satoh H. Yamaguchi S. Tani K. Tojo A. Takahashi T.A. Successful immortalization of mesenchymal progenitor cells derived from human placenta and the differentiation abilities of immortalized cells. Biochem. Biophys. Res. Commun. 2006;351:853–859. doi: 10.1016/j.bbrc.2006.10.125. [DOI] [PubMed] [Google Scholar]
- Zhao P. Ise H. Hongo M. Ota M. Konishi I. Nikaido T. Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation. 2005;79:528–535. doi: 10.1097/01.tp.0000149503.92433.39. [DOI] [PubMed] [Google Scholar]