Abstract
The use of tissue transfer flaps has become a common and effective technique for reconstructing or replacing damaged tissue. While the overall failure rate associated with these procedures is relatively low (5-10%), the failure rate of tissue flaps that require additional surgery is significantly higher (40-60%). The reason for this is largely due to the absence of a technique for objectively assessing tissue health after surgery. Here we have investigated spatial frequency domain imaging (SFDI) as a potential tool to do this. By projecting wide-field patterned illumination at multiple wavelengths onto a tissue surface, SFDI is able to quantify absolute concentrations of oxygenated and deoxygenated hemoglobin over a large field of view. We have assessed the sensitivity of SFDI in a swine pedicle flap model by using a controlled vascular occlusion system that reduced blood flow by 25%, 50%, 75%, or 100% of the baseline values in either the vein or artery. SFDI was able to detect significant changes for oxygenated hemoglobin, deoxygenated hemoglobin, or tissue oxygen saturation in partial arterial occlusions of at least 50% and partial venous occlusions of at least 25%. This shows SFDI is sensitive enough to quantify changes in the tissue hemoglobin state during partial occlusions and thus has the potential to be a powerful tool for the early prediction of tissue flap failure.
OCIS codes: (110.4234) Multispectral and hyperspectral imaging; (170.3660) Light propagation in tissues; (170.3880) Medical optics and biotechnology; (170.6510) Spectroscopy, tissue diagnostics
1. Introduction
The use of tissue transfer flaps has become a common and effective technique for reconstructing or replacing damaged tissue, typically in cases of cancer resection or trauma [1]. Tissue flap transfers are a method of moving tissue from a donor location to a recipient location [2,3], and have the potential to suffer from complications from either partial or complete occlusions of the vasculature. While the overall failure rates associated with this procedure are relatively low (5-10%) [2–7], the failure rates of tissue flaps that suffer from these complications and require additional surgery are significantly higher (40-60%) [2,3,7]. Not surprisingly, it has been shown that the chances of salvaging a tissue flap are directly related to the length of time needed to diagnose a problem [2,3]. Many tissue flap studies have focused on ischemic arterial failure, despite the fact that venous failures are more common and can lead to more severe damage [8,9]. Even fewer studies have focused on partial venous occlusions, which may only have subtle signs of congestion in its early stages before developing into a severe thrombosis that can be extremely difficult to treat [10]. The resulting tissue damage can result in the loss of part, or all, of the tissue transfer flap, which in turn can result in increased morbidity to the patient. The ability to detect these vascular compromised flaps when they experience a partial occlusion, be it arterial or venous or both, allows for earlier intervention by the clinician, and thereby improves salvage rates in compromised flaps [3,6].
Currently, clinicians rely primarily on features such as flap size, color, location, and refill rate in order to make treatment decisions [6,10,11]. However, a variety of modalities have been investigated as tools to provide more quantitative assessments of tissue flap health. Near-infrared spectroscopy (NIRS) has been used to differentiate changes in oxygenated hemoglobin (ctO2Hb) and deoxygenated hemoglobin (ctHHb) as a means to detect and even distinguish arterial and venous occlusion in animals [8,12,13] and has shown promising results clinically [7,14]. Laser Doppler based techniques can detect partial occlusions in animals where NIRS has only been able to detect full occlusions [15], however results in the clinic have not been successful [1]. Doppler ultrasound has successfully been used to monitor the recovery of tissue flaps post operatively [16,17]. A limitation shared by these techniques is that they are only capable of monitoring very small regions of tissue at a time making it difficult to thoroughly investigate a large tissue flap. The use of indocyanine green (ICG) and subsequent NIR fluorescence imaging is a technique capable of generating a quantifiable image of tissue perfusion [18]. While this seems to be effective in the operating room, the need to inject ICG means it is unlikely to be practical as a post-surgical surveillance device. Thermal imaging does not require contrast agents and is sometimes used to locate perforators before or during surgery [19], but has not been shown to be effective for assessing tissue flap health.
Spatial frequency domain imaging (SFDI) is a non-contact imaging technique capable of quantifying changes in ctO2Hb and ctHHb by projecting patterned illumination at multiple wavelengths onto a tissue surface. It is capable of generating these images with a large field of view and fast acquisition times without any contrast agents. This makes it ideally suited to monitor tissue flaps in the operating room or post operatively. While some have used SFDI to study complete vascular occlusions [8,20], no one has assessed the sensitivity of this technique to detect partial occlusions, which would allow for earlier intervention in the case of flap failure. In this study we test the ability of spatial frequency domain imaging to detect partial occlusions by monitoring swine pedicle flaps as blood flow to the flap is carefully controlled.
2. Methodology
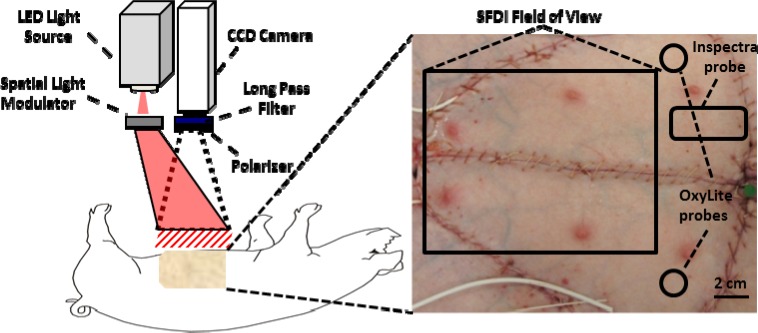
The setup for the SFDI instrumentation has been described previously [21], but a brief description is provided below. The SFDI instrument consists of three fundamental components: a light source, spatial light modulator and camera. For these studies, a prototype clinic-compatible system (v100, Modulated Imaging Inc., Irvine, CA) was used to measure tissue oxygenated hemoglobin, deoxygenated hemoglobin, and tissue oxygen saturation (stO2). The imaging head is contained in a compact cubic enclosure (~1 ft3) and is light weight (~12 lbs) enough to be mounted on an articulating arm attached to a portable cart. The field of view of the camera is 13.5 cm x 10.5 cm. Based on previous work [22], a light source consisting of modules with LED’s centered at 658, 730, and 850 nm were implemented in the system to optimize chromophore quantification. A digital light projector based on a Digital Micromirror Device (DMD Discovery 1100, Texas Instruments Inc., Dallas, TX) was used to project sinusoidal patterns of distinct spatial frequencies onto the tissue flap. The sinusoidal pattern was projected at 3 phases, 0, 120, and 240 degrees. The detection arm consisted of two cameras: a dedicated near-infrared (NIR) camera to collect images of the spatially projected pattern and a color camera. White light was projected after each series of patterns to capture a color photograph of the region of interest, creating a record of clinical appearance so that it could be compared to the SFDI data. Data was collected approximately every 30s, although the time to collect a series of images at each wavelength, frequency, and phase was approximately only 12s. The projector and NIR detection arms were fitted with cross-polarizers to eliminate specular reflection. All system hardware was controlled by custom C# software (Modulated Imaging Inc., Irvine, CA). A schematic of the instrumentation is shown in Fig. 1 below.
Fig. 1.
Diagram of SFDI imaging system and photograph of a typical swine pedicle flap preparation with approximate location of instrumentation.
Custom software was written in MATLAB (R2011b, Mathworks, Natick MA) to analyze the collected images as described previously [21]. For each spatial frequency, the demodulation of the three phases was used to determine the AC component of the reflected image. For two spatial frequencies (0 and 0.2 mm−1), these values were used to estimate the absorption and reduced scattering coefficient properties based on a scaled Monte Carlo generated lookup table at each wavelength [23]. After a surface profilometry calibration measurement of a sample with known optical properties was used to correct for errors resulting from surface curvature [24], chromophore concentration maps of ctO2Hb and ctHHb were generated using absorption maps. Total hemoglobin (ctTHb) maps were generated by summing ctO2Hb and ctHHb. Tissue oxygen saturation (stO2) was then calculated by dividing ctO2Hb by ctTHb.
All experiments were performed in accordance with the University of California, Irvine Institutional Animal Care Use Committee protocol #2006-2693. Yorkshire pigs (n = 8, 30-50 kg) were used in this experiment. The animals were initially anesthetized with an intramuscular injection of ketamine (20 mg/kg) and xylazine (2 mg/kg). Following the onset of sedation, pentobarbital (10 mg/kg) was intravenously applied and the animals were intubated and mechanically ventilated with oxygen (100%) and isoflurane (1-1.5%). Vital signs were continuously monitored and kept constant by adjusting anesthesia levels. A heating blanket was used to maintain a constant body temperature (36-38°C). Two 12 cm x 7 cm bilateral pedicles flaps were created by isolating the flaps from all of the surrounding connective skin and tissue. All of the vasculature between the femoral artery and vein and the flap was ligated and severed except for the branches that led to the deep inferior epigastric artery and vein. This was done so that there would only be one path for blood flow into and out of the flap as is the case in free flap procedures. In half of the animals (n = 4), an appropriately sized occlusion balloon cuff (Docxs Biomedical, Ukiah, CA) was sutured around the isolated artery that had a typical diameter of 3 mm. A programmable syringe pump (NE-1000, New Era, Farmingdale, NY) was used to inject saline into the occlusion balloon cuff causing it to inflate and reduce blood flow. An ultrasound probe (TS-420, Transonic System,, Ithaca, NY) was also attached to the artery to monitor blood flow and provide feedback to the syringe pump so that it could effectively control blood flow to the flap and accurately reduce blood flow to a specific value [10]. The time to reach a desired blood flow level was typically 4 minutes or less. This setup was repeated in both flaps and one side was chosen randomly as the control. The other half of the animals (n = 4) had the same procedure done, but a different sized occlusion cuff and probe were used on the isolated vein that had a typical diameter of 6 mm. Both flaps were then sutured back to their original area. An example of a typical flap preparation is shown in Fig. 1.
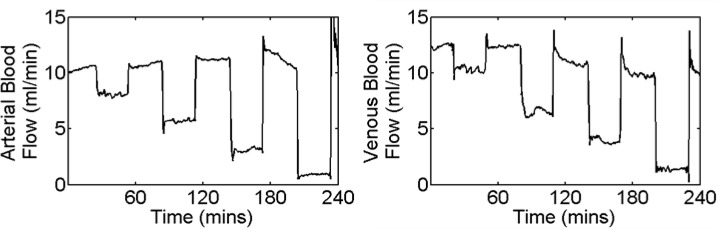
After suturing the tissue flaps back into place and waiting approximately 60 minutes to allow stabilization, the ultrasound probe was used to record the blood flow in either the vein or the artery of both flaps. After establishing the baseline blood flow, the programmable pump was set to adjust the occlusion balloon cuff to reduce blood flow by 25% of the baseline value. After 30 minutes, the cuff was deflated and the tissue flap was allowed to recover for 30 minutes. This process was repeated for reductions of 50%, 75%, and 100% of the baseline values. An example of the blood flow changes, as reported by the TS-420, seen during a typical arterial and venous experiment are shown in Fig. 2 .
Fig. 2.
Time course of typical blood flow changes during a series of partial occlusions.
Both flaps were monitored with the SFDI system as well as with an implanted optical fluorescent probe that was placed outside the field of view of the SFDI system. The latter was used as a means to verify expected changes in absolute regional tissue oxygen tension (Oxylite 2000,Oxford Optronix, Oxfordshire, United Kingdom). Additionally, the occluded flap alone was monitored with a tissue oxygen saturation monitor (Inspectra StO2 monitor, Hutchinson Technology, Hutchinson MN), which provided an additional point of reference to verify SFDI data.
In order to assess if there was a statistically significant change in each SFDI derived parameter at each partial occlusion level, a paired student’s t-test was used to compare the relative change in each parameter for the experimental and control flap. A homogenous region of interest (ROI) near the center of the tissue flap, approximately 2cm x 2cm, was chosen in the experimental and control flaps, so that the SFDI derived parameters could be averaged across the animals in the arterial and venous occlusion groups. A p value less than 0.05 was considered significant for this study.
3. Results
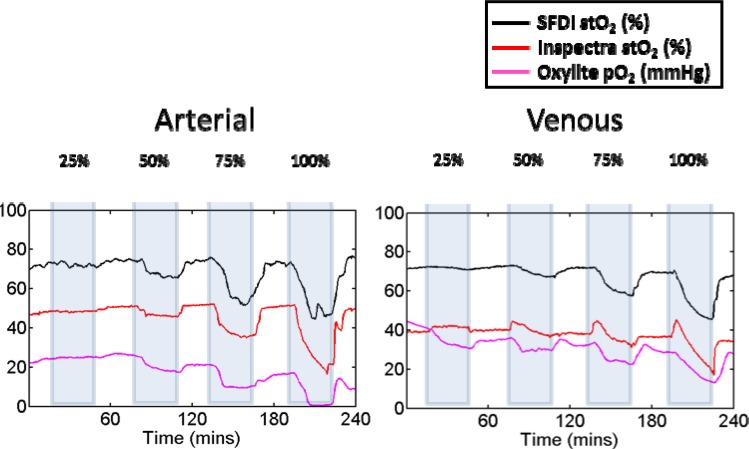
The average arterial baseline blood flow in all the animals was 11.6 ml/min, while the average venous blood flow was 15.4 ml/min. The difference in flow between the artery and vein was not statistically significant. The average tissue oxygen saturation values on the occlusion flap were qualitatively compared against the average Oxylite 2000 and Inspectra measurements to verify the effectiveness of the partial occlusions. The average oxygen tension values across animals from the optical fluorescent probe (Oxylite 2000) and the average tissue oxygen saturation values from the SFDI and Inspectra systems are shown in Fig. 3 below. The blue boxes mark the time that the feedback occlusion system was turned on at a given occlusion level. There was typically a delay of several minutes for the feedback system to adjust blood flow levels to the appropriate level.
Fig. 3.
Time course of average stO2 (%) measured with SFDI compared with tissue oxygen tension (mmHg) measured with Oxylite system and stO2 measured with the Inspectra system in the arterial and venous occlusion flaps.
Similar ROIs in the control and occlusion flaps were used to average several SFDI parameters across all animals in the arterial and venous groups. Table 1 below shows the relative changes in each SFDI parameter for the occlusion flap at different occlusion levels. The values are averaged over a ten minute period ten minutes after the initial onset of each occlusion level and compared against an averaged ten minute baseline taken before any occlusions. Statistical significance was calculated as described above by comparing with relative changes seen in the control group (data not shown).
Table 1. Relative change of SFDI parameters in occlusion flap.
| SFDI parameter | 25% |
50% |
75% |
100% |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arterial | Venous | Arterial | Venous | Arterial | Venous | Arterial | Venous | ||||
| ctO2Hb (%) | 99 | 118 | 91* | 131* | 69* | 129* | 62* | 124 | |||
| ctHHb (%) | 105 | 123* | 126* | 162* | 163* | 237* | 165* | 431* | |||
| ctTHb (%) | 100 | 119* | 100 | 140* | 93 | 158* | 88 | 204* | |||
| stO2 (%) | 98 | 99 | 91* | 94* | 72* | 83* | 65* | 65* | |||
*p-value less than 0.05
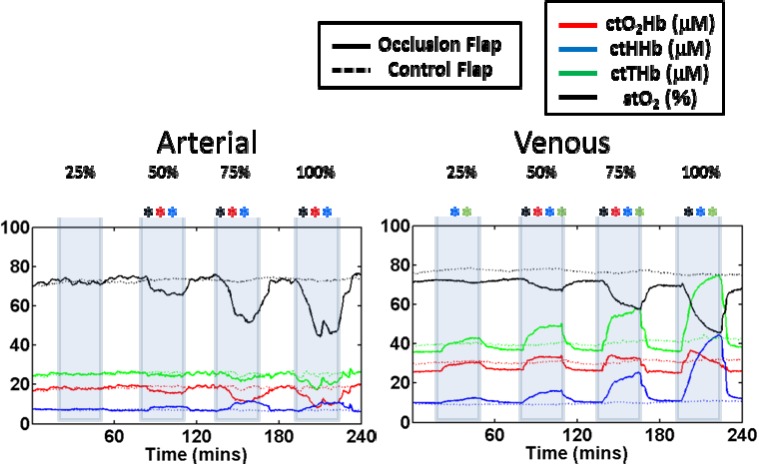
For the arterial occlusions at 25%, none of the parameters were significantly different from their controls. At 50%, stO2, ctO2Hb, and ctHHb were significantly different. At 75%, stO2, ctO2Hb, and ctHHb were again significantly different. At 100% occlusion, the same three parameters were significantly different. For the venous occlusions at 25%, ctHHb and ctTHb were significantly different from its controls. At 50%, stO2, ctO2Hb, ctHHb, and ctTHb were all significantly different. At 75%, stO2, ctO2Hb, ctHHb, and ctTHb were again significantly different. At 100% occlusion, only stO2, ctHHb, and ctTHb were significant different. While stO2 always decreased and ctHHb always increased regardless of the type of occlusion seen, ctO2Hb increased during venous occlusions and decreased during arterial occlusions. The time course of the absolute values for the SFDI derived parameters are shown in Fig. 4 below.
Fig. 4.
Time course of average oxygenated hemoglobin, (ctO2Hb), deoxygenated hemoglobin (ctHHb), total hemoglobin (ctTHb), and oxygen saturation (stO2). Parameters with significant changes at a given occlusion level are shown with an asterisk.
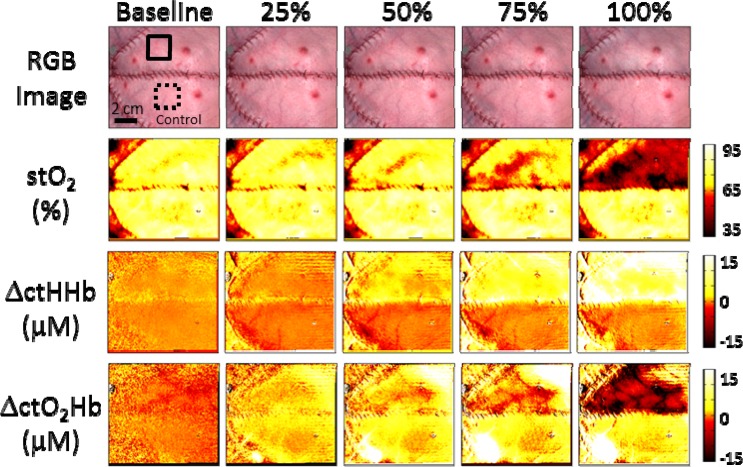
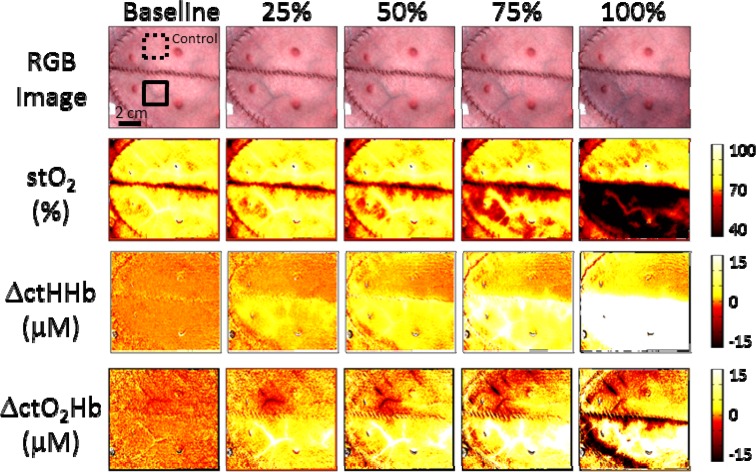
To highlight the imaging capability of SFDI, Fig. 5 (Media 1 (3.9MB, AVI) ) and Fig. 6 (Media 2 (4MB, AVI) ) show image maps of each parameter at different time points Each time point corresponds to the middle of a given occlusion interval, approximately 30 minutes from the start of the occlusion. The baseline values were taken before any occlusions were done. Changes in ctHHb and ctO2Hb were calculated from a reference image taken shortly before the baseline images. An example of typical regions of interest selected for the control and occlusion flaps are shown with dashed and solid lines respectively.
Fig. 5.
Images of tissue oxygen saturation, absolute changes in oxygenated and deoxygenated hemoglobin, and a color image of the flaps are shown at different time points corresponding to a different arterial occlusion level (Media 1 (3.9MB, AVI) ).
Fig. 6.
Images of tissue oxygen saturation, absolute changes in oxygenated and deoxygenated hemoglobin, and a color image of the flaps are shown at different time points corresponding to a different venous occlusion level (Media 2 (4MB, AVI) ).
4. Discussion
While generally successful, tissue flap transfers have the potential to fail if vascular occlusions are not detected in a timely manner. The current standard of care is to closely monitor the tissue flaps in the first 48-72 hours after surgery through intermittent visual clinical inspection [3,10]. A device that could constantly monitor tissue flaps and provide quantitative assessments, would lead to earlier interventions that directly translate into reduced flap failures. Many studies focus on the ability to detect complete occlusions. However, this does not capture the clinical reality in most cases where partial occlusions are the first warning sign. In the case of complete occlusion, the tissue flap may be completely compromised. The ability to detect partial occlusions would be a useful tool for early prevention of flap failure.
In our experiment the measured flow values in the artery and vein were similar to values seen by other groups following a similar protocol and using the same flow measurement device [10]. The fact that flow was slightly higher in the vein is most likely because the measurements on the isolated vein had to be taken further distal at a location for which the vein was generally larger than the artery. Still, the difference in flow between artery and vein was not statistically significant and could simply be attributed to variations among animals.
The expected response for a tissue flap undergoing complete arterial occlusion is an eventual increase in ctHHb and decrease in ctO2Hb and stO2 as the ctO2Hb is consumed and fresh blood cannot deliver more to the flap [25]. While changes in the color images were only visible at 100% occlusion, we were able to see significant changes in all of these parameters at 50% occlusion. The ability to see changes during partial occlusions, presumably before they can become complete occlusions, highlights the sensitivity of the system and its potential for early detection of tissue flap failure. Other studies using similar imaging modalities have shown an inability to detect significant changes in these parameters during complete arterial occlusion in rodents [8]. This highlights an advantage of using a porcine tissue flap model that more closely resemble human flaps [20], specifically in terms of thickness. SFDI measurements are integrated over a volume of tissue. In rodents, the thin nature of the flaps results in the flap being a small partial volume compared to the overall measurement volume. Thus, the changes are occurring in a smaller tissue volume compared to the pig model and the sensitivity to signal changes is much lower in rodents compared to pigs.
The expected response for a tissue flap undergoing complete venous occlusion is an initial increase in ctO2Hb until the pressure build up in the tissue flap prevents incoming blood flow and ctO2Hb begins to decrease. Additionally we would expect ctHHb to increase and stO2 to decrease over time [25]. Unlike the partial arterial occlusions, significant parameter changes could be seen as early as the 25% occlusion where an increase in ctHHb could be detected. Again visible changes in the color images could not be seen until 100% occlusion. At 50%, 75%, and 100% occlusion, changes in all parameters became statistically significant except for ctO2Hb. At 100% occlusion, while the initial change in ctO2Hb was a significant increase, after approximately 10 minutes, it slowly began to decrease towards baseline levels. We suspect that if the occlusion time was longer than 30 minutes, we would have eventually seen a significant decrease in ctO2Hb. Similar studies have seen an almost immediate decrease in ctO2Hb during a complete venous occlusion [8], but this discrepancy can also be explained by the different animal models. If the flap being studied is relatively small, than it is likely that the complete venous occlusion would cause the tissue flap to fill up rapidly and the increase in ctO2Hb would be brief, before it started to decrease.
A significant advantage of monitoring flaps with an imaging system that has a large field of view is the ability to observe several regions of interest simultaneously. During the 100% occlusion experiments, the hemodynamic parameter changes were eventually seen throughout most of the tissue flap. However, during the partial occlusions the parameter changes were more localized, and the system was still able to quickly identify changes. Further analysis is needed to study the spatial changes associated with partial occlusions, but this highlights another benefit of having a system with a large field of view and good spatial resolution to effectively monitor tissue flaps. While these experiments have focused on monitoring hemoglobin changes in tissue flaps, with appropriate modifications, a future system could be used to examine other important chromophores related to tissue flap health. Fat necrosis, another form of flap failure, could be detected with an imaging system capable of monitoring lipid concentrations.
5. Conclusion
The ability to easily quantify tissue flap health would greatly reduce tissue flap failure by allowing clinicians earlier access to failing flaps. SFDI is an ideal tool to monitor these tissue flaps because of its large field of view, fast acquisition times, ease of use, and ability to quantify multiple parameters directly related to tissue health. We have shown that SFDI is sensitive enough to measure chromophore changes in pedicle flaps that are undergoing only partial occlusions. This further highlights its potential to be a powerful modality for assessing tissue flaps and preventing their failure.
Acknowledgments
The authors would like to thank Earl Steward and Roger Geertsema for their help and advice regarding the animals used in the study. We gratefully acknowledge support from the Beckman Foundation and the NIH, including NIBIB P41EB015890 (A Biomedical Technology Resource) NIGMS R42GM077713. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIBIB or NIH.
References and links
- 1.Smit J. M., Werker P. M. N., Liss A. G., Enajat M., de Bock G. H., Audolfsson T., Acosta R., “Introduction of the implantable Doppler system did not lead to an increased salvage rate of compromised flaps: a multivariate analysis,” Plast. Reconstr. Surg. 125(6), 1710–1717 (2010). 10.1097/PRS.0b013e3181d0ace8 [DOI] [PubMed] [Google Scholar]
- 2.Chen K.-T., Mardini S., Chuang D. C.-C., Lin C.-H., Cheng M.-H., Lin Y.-T., Huang W.-C., Tsao C.-K., Wei F.-C., “Timing of presentation of the first signs of vascular compromise dictates the salvage outcome of free flap transfers,” Plast. Reconstr. Surg. 120(1), 187–195 (2007). 10.1097/01.prs.0000264077.07779.50 [DOI] [PubMed] [Google Scholar]
- 3.Kroll S. S., Schusterman M. A., Reece G. P., Miller M. J., Evans G. R., Robb G. L., Baldwin B. J., “Timing of pedicle thrombosis and flap loss after free-tissue transfer,” Plast. Reconstr. Surg. 98(7), 1230–1233 (1996). 10.1097/00006534-199612000-00017 [DOI] [PubMed] [Google Scholar]
- 4.Chubb D., Rozen W. M., Whitaker I. S., Acosta R., Grinsell D., Ashton M. W., “The efficacy of clinical assessment in the postoperative monitoring of free flaps: a review of 1140 consecutive cases,” Plast. Reconstr. Surg. 125(4), 1157–1166 (2010). 10.1097/PRS.0b013e3181d0ac95 [DOI] [PubMed] [Google Scholar]
- 5.Pohlenz P., Blessmann M., Blake F., Li L., Schmelzle R., Heiland M., “Outcome and complications of 540 microvascular free flaps: the Hamburg experience,” Clin. Oral Investig. 11(1), 89–92 (2007). 10.1007/s00784-006-0073-0 [DOI] [PubMed] [Google Scholar]
- 6.Bui D. T., Cordeiro P. G., Hu Q.-Y., Disa J. J., Pusic A., Mehrara B. J., “Free flap reexploration: indications, treatment, and outcomes in 1193 free flaps,” Plast. Reconstr. Surg. 119(7), 2092–2100 (2007). 10.1097/01.prs.0000260598.24376.e1 [DOI] [PubMed] [Google Scholar]
- 7.Steele M. H., “Three-year experience using near infrared spectroscopy tissue oximetry monitoring of free tissue transfers,” Ann. Plast. Surg. 66(5), 540–545 (2011). 10.1097/SAP.0b013e31820909f9 [DOI] [PubMed] [Google Scholar]
- 8.Pharaon M. R., Scholz T., Bogdanoff S., Cuccia D., Durkin A. J., Hoyt D. B., Evans G. R. D., “Early detection of complete vascular occlusion in a pedicle flap model using quantitative [corrected] spectral imaging,” Plast. Reconstr. Surg. 126(6), 1924–1935 (2010). 10.1097/PRS.0b013e3181f447ac [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hjortdal V. E., Hansen E. S., Hauge E., “Myocutaneous flap ischemia: flow dynamics following venous and arterial obstruction,” Plast. Reconstr. Surg. 89(6), 1083–1091 (1992). 10.1097/00006534-199206000-00014 [DOI] [PubMed] [Google Scholar]
- 10.Russell J. A., Conforti M. L., Connor N. P., Hartig G. K., “Cutaneous tissue flap viability following partial venous obstruction,” Plast. Reconstr. Surg. 117(7), 2259–2266, discussion 2267–2268 (2006). 10.1097/01.prs.0000225472.57337.2e [DOI] [PubMed] [Google Scholar]
- 11.Chubb D., Whitaker I. S., Rozen W. M., Ashton M. W., “Continued observations in the postoperative monitoring of free flaps: preliminary experiences with Masimo Radical-7 transcutaneous plethysmography and pulse oximetry,” Plast. Reconstr. Surg. 129(1), 222e–223e (2012). 10.1097/PRS.0b013e3182365db4 [DOI] [PubMed] [Google Scholar]
- 12.Thorniley M. S., Sinclair J. S., Barnett N. J., Shurey C. B., Green C. J., “The use of near-infrared spectroscopy for assessing flap viability during reconstructive surgery,” Br. J. Plast. Surg. 51(3), 218–226 (1998). 10.1054/bjps.1997.0145 [DOI] [PubMed] [Google Scholar]
- 13.Yafi A., Vetter T. S., Scholz T., Patel S., Saager R. B., Cuccia D. J., Evans G. R., Durkin A. J., “Postoperative quantitative assessment of reconstructive tissue status in a cutaneous flap model using spatial frequency domain imaging,” Plast. Reconstr. Surg. 127(1), 117–130 (2011). 10.1097/PRS.0b013e3181f959cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitaker I. S., Pratt G. F., Rozen W. M., Cairns S. A., Barrett M. D., Hiew L. Y., Cooper M. A., Leaper D. J., “Near infrared spectroscopy for monitoring flap viability following breast reconstruction,” J. Reconstr. Microsurg. 28(03), 149–154 (2012). 10.1055/s-0031-1296030 [DOI] [PubMed] [Google Scholar]
- 15.Gimbel M. L., Rollins M. D., Fukaya E., Hopf H. W., “Monitoring partial and full venous outflow compromise in a rabbit skin flap model,” Plast. Reconstr. Surg. 124(3), 796–803 (2009). 10.1097/PRS.0b013e3181b03768 [DOI] [PubMed] [Google Scholar]
- 16.Lorenzetti F., Ahovuo J., Suominen S., Salmi A., Asko-Seljavaara S., “Colour Doppler ultrasound evaluation of haemodynamic changes in free tram flaps and their donor sites,” Scand. J. Plast. Reconstr. Surg. Hand Surg. 36(4), 202–206 (2002). 10.1080/02844310260259851 [DOI] [PubMed] [Google Scholar]
- 17.Vakharia K. T., Henstrom D., Lindsay R., Cunnane M. B., Cheney M., Hadlock T., “Color Doppler ultrasound: effective monitoring of the buried free flap in facial reanimation,” Otolaryngol. Head Neck Surg. 146(3), 372–376 (2012). 10.1177/0194599811427377 [DOI] [PubMed] [Google Scholar]
- 18.Matsui A., Lee B. T., Winer J. H., Laurence R. G., Frangioni J. V., “Quantitative assessment of perfusion and vascular compromise in perforator flaps using a near-infrared fluorescence-guided imaging system,” Plast. Reconstr. Surg. 124(2), 451–460 (2009). 10.1097/PRS.0b013e3181adcf7d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chubb D., Rozen W. M., Whitaker I. S., Ashton M. W., “Images in plastic surgery: digital thermographic photography (“thermal imaging”) for preoperative perforator mapping,” Ann. Plast. Surg. 66(4), 324–325 (2011). 10.1097/SAP.0b013e31820bcc5e [DOI] [PubMed] [Google Scholar]
- 20.Gioux S., Mazhar A., Lee B. T., Lin S. J., Tobias A. M., Cuccia D. J., Stockdale A., Oketokoun R., Ashitate Y., Kelly E., Weinmann M., Durr N. J., Moffitt L. A., Durkin A. J., Tromberg B. J., Frangioni J. V., “First-in-human pilot study of a spatial frequency domain oxygenation imaging system,” J. Biomed. Opt. 16(8), 086015 (2011). 10.1117/1.3614566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuccia D. J., Bevilacqua F., Durkin A. J., Ayers F. R., Tromberg B. J., “Quantitation and mapping of tissue optical properties using modulated imaging,” J. Biomed. Opt. 14(2), 024012 (2009). 10.1117/1.3088140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazhar A., Dell S., Cuccia D. J., Gioux S., Durkin A. J., Frangioni J. V., Tromberg B. J., “Wavelength optimization for rapid chromophore mapping using spatial frequency domain imaging,” J. Biomed. Opt. 15(6), 061716 (2010). 10.1117/1.3523373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erickson T. A., Mazhar A., Cuccia D., Durkin A. J., Tunnell J. W., “Lookup-table method for imaging optical properties with structured illumination beyond the diffusion theory regime,” J. Biomed. Opt. 15(3), 036013 (2010). 10.1117/1.3431728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gioux S., Mazhar A., Cuccia D. J., Durkin A. J., Tromberg B. J., Frangioni J. V., “Three-dimensional surface profile intensity correction for spatially modulated imaging,” J. Biomed. Opt. 14(3), 034045 (2009). 10.1117/1.3156840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin M. S., Thorniley M. S., Doré C. J., Green C. J., “Near infra-red spectroscopy: a non-invasive monitor of perfusion and oxygenation within the microcirculation of limbs and flaps,” Br. J. Plast. Surg. 48(1), 14–22 (1995). 10.1016/0007-1226(95)90024-1 [DOI] [PubMed] [Google Scholar]