Abstract
Five-year survival rate for lung cancer is limited to 10% to 15%. Therefore, the identification of novel therapeutic prognostic factors is an urgent requirement. The aim of this study is thus to highlight specific biomarkers in chemoresistant non-small cell lung cancer cell lines. Therefore, we checked—in the control condition as well as after short-term pharmacological treatment with either docetaxel or gemcitabine—the expression of genes such as tumor suppressor genes (CDKN2A, DAPK, FHIT, GSTP1, MGMT, RARβ2, RASSF1A, and TIMP3), genes associated with drug resistance (BRCA1, COX2, ERCC1, IGFBP3, RRM1, and TUBB3), and stemness-related genes (CD133, OCT4, and SLUG) in two cellular models of squamous carcinoma (CAEP) and adenocarcinoma (RAL) of the lung originally established. Their promoter methylation profile was also evaluated. Drug-related genes were upregulated. Cisplatin resistance matched with high levels of BRCA1 and ERCC1 in both cell lines; docetaxel sensitivity of CAEP cells was associated to levels of TUBB3 lower than RAL cells. Although CAEP cells were more sensitive to gemcitabine, both cell lines showed high levels of RRM1. Stemness-related genes were downregulated in the control condition but became upregulated in docetaxel-resistant cells, indicating the selection of a population with stemness features. We did not find an unequivocal correspondence between gene expression and respective DNA promoter methylation status, suggesting the involvement of additional mechanisms of gene expression regulation. These results highlight specific biomarkers consistent with the different responses of the two cell lines to standard pharmacological treatments and indicate specific molecular traits for their chemoresistance.
Introduction
Non-small cell lung cancer (NSCLC) represents a common cause of tumor death in industrialized countries where, despite a significant improvement in diagnostics, surgery, and chemotherapy, the overall 5-year survival rate is in fact limited to about 10% to 15% [1,2]. The recommended treatment for these tumors is surgery, whereas radiotherapy and chemotherapy are used for the treatment of unresectable or locally advanced tumors and as palliative therapies for metastatic tumors [3]. In tumors without EGFR mutations, platinum compounds, taxanes and gemcitabine—alone or in combination—are the traditional drugs used in standard chemotherapy, even though tumor resistance to these treatments is common [4].
Chemoresistance can arise from different mechanisms, such as reduced drug uptake, increased drug efflux, drug detoxification, DNA repair, or defective apoptosis, depending on the specific drug target. In this respect, several studies highlight relationships among specific gene expression, therapeutic response and tumor progression, and major classes of drugs used in the treatment of NSCLC.
In particular, resistance to platinum compounds is mainly the consequence of the overexpression of DNA repair genes, such as ERCC1 and BRCA1 [5–7]. During taxane treatment of NSCLC, BRCA1 expression represents a marker of drug sensitivity [8,9]. However, a high expression level of TUBB3, encoding for a member of the β-tubulin protein family, is associated with taxane resistance in different tumors, including NSCLCs [5–7]. Conversely, high levels of FHIT expression were shown together with significant apoptosis in in vitro experiments [10]. NSCLC therapy may also include the use of gemcitabine, whose intracellular active forms interfere with DNA synthesis through competition with deoxycytidine and inhibition of ribonucleotide reductase. Therefore, high levels of expression of the subunit 1 of ribonucleotide reductase (RRM1) are associated with increased gemcitabine resistance in NSCLC cell lines [11].
Promoter methylation is an epigenetic event usually associated with gene silencing, implicated in the development and differentiation and frequently deregulated in cancer [12,13]. Several studies have identified a pattern of promoter methylation and a corresponding gene expression profile consistent with tumor progression and response to drug treatment either in NSCLC patients or in cell lines. Several tumor suppressor genes (TSGs) display a hypermethylated promoter in lung cancer, including CDKN2A, RASSF1A, RARβ2, MGMT, GSTP1, DAPK, TIMP3, and FHIT [14–17]. Aberrant methylation of RASSF1A, DAPK, CDKN2A, and FHIT promoters was associated with shorter overall survival [18–21].
Evaluating gene expression and promoter methylation of specific genes significant for monitoring NSCLC progression might thus identify specific biomarkers that could predict disease recurrence and help in keeping watch over therapeutic response.
In the present work, to explore this issue, we used an in vitro model based on two NSCLC cell lines, representative of either a squamous carcinoma (CAEP) or an adenocarcinoma (RAL) of the lung, established and studied at the Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori at Meldola [22–25]. In these cells, we evaluated the expression of genes of interest in lung cancer disease, such as a set of TSGs (CDKN2A, DAPK, FHIT, GSTP1, MGMT, RARβ2, RASSF1A, and TIMP3) found to be implicated in lung cancer progression and therapeutic response [19,26–32], and a set of genes associated with specific drug resistance (BRCA1, COX2, ERCC1, IGFBP3, RRM1, and TUBB3) [3–5,33–36]. In addition, a set of genes expressed in cells with stemness features (CD133, OCT4, and SLUG) [37–43] was also monitored, as increasing evidence points at small cancer cell populations, defined as cancer stem cells, sustaining NSCLC resistance to antitumor agents [39,44,45] and accounting for tumor recurrence [46].
To investigate the involvement of our gene panel in the process of resistance to chemotherapy, we carried out this analysis in the basal control condition as well as after short-term pharmacological treatment with standard chemotherapeutic drugs.
Promoter methylation was also studied to evaluate how the expression profile of these genes might be consistent with this epigenetic modification.
Materials and Methods
Cell Culture
The study was performed on two EGFR wild-type NSCLC cell lines, CAEP and RAL, derived from a squamous carcinoma and an adenocarcinoma of the lung, respectively, established and characterized in our laboratory [22–25]. Cell lines were grown in Dulbecco's modified Eagle's medium/HAM F12 (1:1), supplemented with 10% FBS, 2 mM l-glutamine (PAA, Pashing, Austria), and insulin (10 mg/ml; Sigma-Aldrich, Milan, Italy) in a humidified atmosphere of 95% air and 5% CO2 at 37°C. Cells in the exponential growth phase were used for all the experiments.
Drugs and Treatment
Cisplatin (Ebewe Pharma-Sandoz, Origgio, Italy), docetaxel (Sanofiaventis SpA, Milan, Italy), and gemcitabine (Eli Lilly Italia SpA, Sesto Fiorentino, Italy) were aliquoted, stored at -80°C, and freshly diluted in culture medium before each experiment.
For in vitro chemosensitivity assay, drugs were tested at scalar dilutions of 1:1, 1:10, and 1:100 of plasma peak concentrations (10 µM for cisplatin, 2 µM for docetaxel, and 40 µM for gemcitabine). To ensure that exposure times were compatible with the half-life of the drugs administered in a clinical setting [47–50], we analyzed the effects after 6, 1, and 3 hours of exposure, respectively, followed by 72 hours of culture in drug-free medium.
For gene expression and DNA methylation analysis, cells were treated at either 50% inhibiting concentration (IC50) or plasma peak concentration dosage when IC50 was not reached. These values were 0.09 µM and 2 µM docetaxel and 1.98 µM and 40 µM gemcitabine, for, respectively, CAEP and RAL.
In Vitro Chemosensitivity Assay
Cell viability was evaluated with the sulforodamine B (SRB) assay according to Skehan et al. [51]. Experiments were run in octoplets, and each experiment was repeated twice. Dose-response curves were created by Excel Software and the IC50 values were determined from the plots.
Gene Expression Analysis
The analysis of gene expression was performed as reported by Arienti et al. [52] using a real-time reverse transcription-polymerase chain reaction (PCR) method. The amount of mRNA of each gene was normalized to the endogenous references β2-microglobulin using Gene Expression Macro Software (version 1.1; Bio-Rad Laboratories S.r.l., Segrate (MI), Italy). Commercial control RNA, derived from a normal lung, was used as calibrator (MPV total RNA; Stratagene, Agilent Technologies Italia SpA, Cernusco, Italy). Primers for mRNA amplification were designed using Beacon Designer Software (version 4; PREMIER Biosoft International, Palo Alto, CA); sequences and annealing temperatures are available upon request.
DNA Methylation Analysis
Promoter methylation was analyzed by a standard methylation-specific PCR (MSP) protocol from DNA extracted in phenol-chloroform. Briefly, 1 µg of purified DNA was subjected to bisulfite treatment (EZ DNA Methylation Kit; Zymo Research, Irvine, CA). The methylation status of the gene promoter was determined by MSP [53] and confirmed in two independent experiments. Total volume for MSP reaction was 15 µl, with 4 mM MgCl2, 0.5 mM each dNTP, 0.2 µM each primer, 0.5 U of @Taq Hot Start Thermostable DNA polymerase (EuroClone, Pero, Italy), and 1 µl of bisulfite-treated DNA. Methylation of OCT4 and CD133 gene promoters was tested by bisulfite sequencing, cloning PCR products in pGEM-T Easy Vector System (Promega, Milan, Italy). Sequencing of PCR products was performed using the Big Dye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) using the Applied Biosystems 3130 Avant Genetic Sequencer. Primer sequences and annealing temperatures are available upon request.
Statistical Analysis
Gene expression of CAEP and RAL cell lines in the basal control condition was statistically compared with two-tailed t test using GraphPad Prism (version 4.0; GraphPad Software, San Diego, CA). Data for basal control condition versus drug treatment expression were statistically analyzed using one-way analysis of variance with Tukey's post test (GraphPad Prism, version 4.0; GraphPad Software). Differences in gene expression were considered significant for P < .05.
Results
Pharmacological Treatment
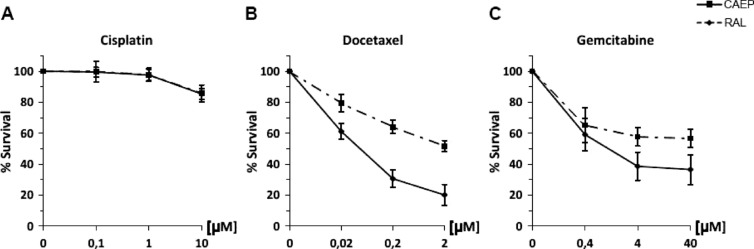
Both the investigated cell lines showed high resistance to cisplatin treatment, even after treatment with the plasma peak concentration (10 µM; Figure 1A).
Figure 1.
Cell survival of CAEP and RAL cell lines after the treatments with (A) cisplatin, (B) docetaxel, and (C) gemcitabine.
The squamous carcinoma cells (CAEP) showed less resistance to docetaxel and gemcitabine than the adenocarcinoma cells (RAL). In fact, the exposure of CAEP cells to 2 µM (the plasma peak concentration) docetaxel induced 80% cell death with an IC50 value of 0.09 µM (Figure 1B). When the CAEP cells were treated with gemcitabine, an IC50 of 1.98 µM was observed with an overall survival of about 36% at the highest dose tested (Figure 1C). Conversely, after either docetaxel or gemcitabine exposure, IC50 values were never reached even at the highest concentrations tested, for the adenocarcinoma cells (RAL; Figure 1, B and C).
Gene Expression Profile
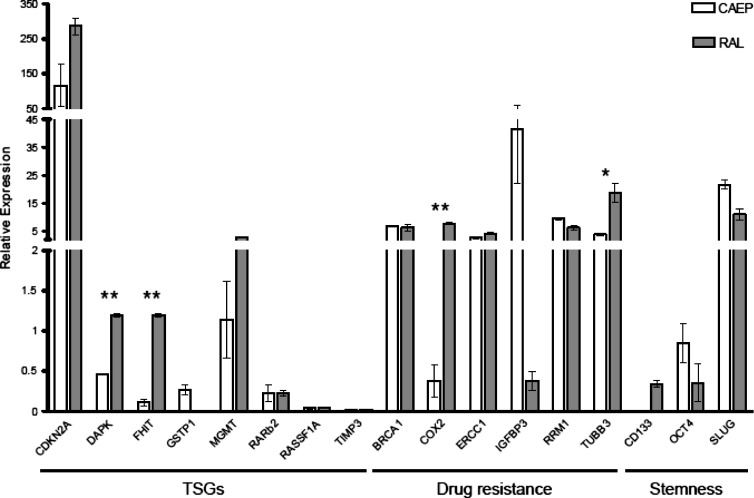
Among the investigated TSGs, only the CDKN2A mRNA was highly upregulated in both lung cancer cell lines with respect to normal lung tissue. MGMT transcript levels were upregulated (five-fold) in RAL but not in CAEP cells. Although similar to normal lung tissue levels, DAPK and FHIT expression in RAL cells was significantly higher (respectively about 3-fold and 11-fold; P < .01) than in CAEP cells. No differences between CAEP and RAL were scored for GSTP1, RARβ2, RASSF1A, and TIMP3, although in both cell lines they were downregulated with respect to the normal lung tissue (Figure 2).
Figure 2.
Gene expression profile of CAEP and RAL cell lines in the basal control condition. Expression data were normalized against normal lung tissue. Statistically significant differences are indicated with asterisks, *P < .05; **P < .01.
As expected, genes associated with specific drug resistance (BRCA1, ERCC1, RRM1, and TUBB3) were significantly upregulated in both cell lines with respect to the normal lung tissue. A significant difference in mRNA levels of RAL versus CAEP cells was scored for COX2 (P < .01) and TUBB3 (P < .05), respectively about 20-fold and 5-fold increases in RAL cells. The reverse was observed for IGFBP3: It was highly upregulated (about 100-fold) in CAEP versus RAL cells (Figure 2).
In both cell lines, CD133 and OCT4 gene products, associated with stemness features, were downregulated, whereas SLUG gene product was overexpressed with respect to normal lung tissue (Figure 2).
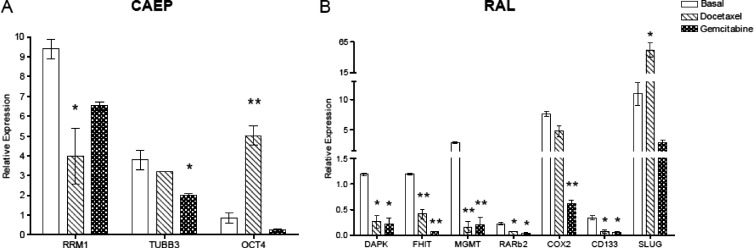
Upon treatment with docetaxel and gemcitabine, the expression of the investigated genes showed specific differences with respect to the basal control condition (Figure 3). Docetaxel treatment in CAEP cells did not modify the expression of TSGs; however, the RRM1 transcript level was significantly decreased (42%) with respect to the basal control condition (P < .05) and OCT4 expression in treated cells increased about six-fold (P < .01; Figure 3A). RAL cells treated with docetaxel, in contrast, showed a significantly decreased expression of the TSGs DAPK, FHIT, MGMT, and RARβ2 (about 22%, 35%, 5%, and 30%, respectively) compared to the basal control condition (P < .05; Figure 3B). CD133 expression was significantly decreased (20%; P < .05), whereas SLUG mRNA level increased about five-fold (P < .05; Figure 3B).
Figure 3.
Genes differentially expressed with respect to the basal control condition in (A) CAEP and (B) RAL cell lines after the treatment with docetaxel and gemcitabine. Statistically significant differences are indicated with asterisks, *P < .05; **P < .01.
The only change in the expression level of the investigated genes induced by gemcitabine in CAEP cells occurred at the TUBB3 gene, which was downregulated (52%) with respect to the basal control condition (P < .05; Figure 3A). In RAL cells, however, several effects were seen, i.e., gemcitabine treatment induced decreased expression of the TSGs DAPK, FHIT, MGMT, and RARβ2 (about 18%, 6%, 7%, and 16%, respectively), compared to the basal control condition (P < .05; Figure 3B); only COX2 was downregulated (8%, P < .01) among genes associated with specific drug resistance; and finally, the mRNA level of the CD133 gene was decreased (16%) with respect to the basal control condition (P < .01; Figure 3B).
Promoter Methylation Analysis
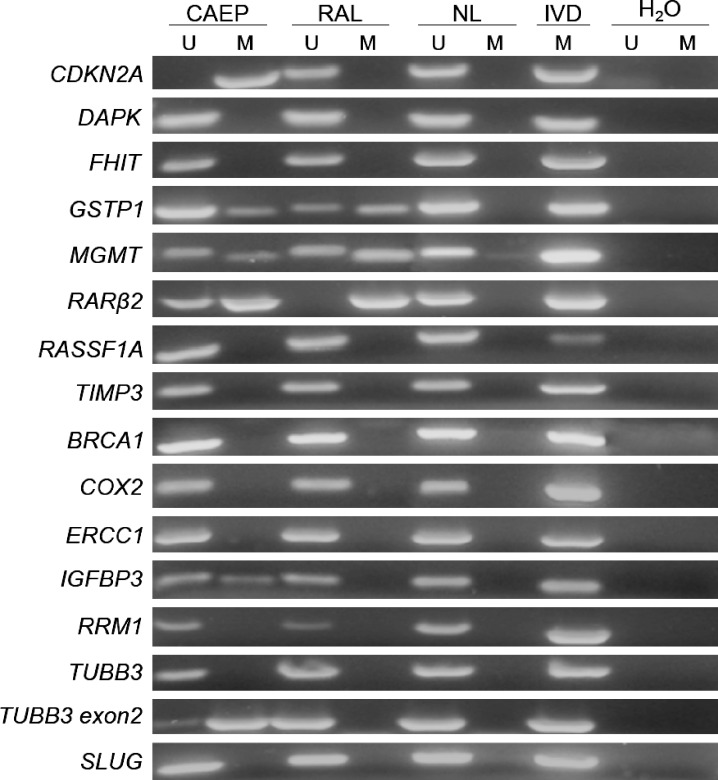
TSG promoter was unmethylated for DAPK, RASSF1A, and TIMP3 and partially or completely methylated for GSTP1, MGMT, and RARβ2, in both cell lines. CDKN2A promoter was methylated in CAEP but unmethylated in RAL cells. Genes associated with specific drug resistance (BRCA1, COX2, ERCC1, RRM1, TUBB3) were unmethylated in both cell lines, except for a region of TUBB3 located at 5′ of exon 2 that was partially methylated in CAEP. The promoter of IGFBP3 showed partial methylation in CAEP and no methylation in RAL. When genes associated with stemness features were considered, CD133 and OCT4 were methylated in both cell lines, whereas SLUG was unmethylated (Figures 4 and 5 and Table 1).
Figure 4.
MSP results for CAEP and RAL cell lines in basal control condition. U, unmethylation-specific reaction; M, methylation-specific reaction; NL, normal lymphocyte DNA; IVD, in vitro methylated DNA; H2O, PCR water control.
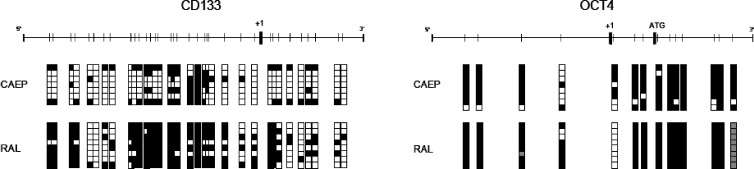
Figure 5.
Bisulfite sequencing results for CD133 and OCT4 genes in CAEP and RAL cell lines in the basal control condition. Vertical bar, CpG site; black square, methylated clone; white square, unmethylated clone; gray square, sequence not defined.
Table 1.
DNA Promoter Methylation Status and Gene Expression in CAEP (Squamous Carcinoma) and RAL (Adenocarcinoma) Cell Lines.
| Set | Gene Name | CAEP | RAL | ||
| Methylation | Expression | Methylation | Expression | ||
| TSGs | CDKN2A | M | +++ | U | +++ |
| DAPK | U | + | U | ++ | |
| FHIT | U | + | U | ++ | |
| GSTP1 | U/M | + | U/M | - | |
| MGMT | U/M | ++ | U/M | ++ | |
| RARβ2 | U/M | + | M | + | |
| RASSF1A | U | - | U | - | |
| TIMP3 | U | - | U | - | |
| Drug resistance | BRCA1 | U | +++ | U | ++ |
| COX2 | U | + | U | ++ | |
| ERCC1 | U | ++ | U | ++ | |
| IGFBP3 | U/M | +++ | U | + | |
| RRM1 | U | +++ | U | +++ | |
| TUBB3 | U | ++ | U | +++ | |
| Stemness | CD133 | M | - | M | + |
| OCT4 | M | + | M | + | |
| SLUG | U | +++ | U | +++ | |
U, unmethylated; M, completely methylated; U/M, partially methylated; +++, highly expressed (>5); ++, medium expressed (>1); +, low expressed (<1); ., not expressed.
Treatment with docetaxel and gemcitabine did not change the above-described methylation profile of our lung cancer cell lines (data not shown).
Discussion
Specific gene expression profiles may be useful for monitoring tumor progression, predicting therapeutic response, and assessing risk of relapse. With the objective of gathering information for clinical purposes, in the present work, we used two NSCLC cell lines, representative of a squamous carcinoma (CAEP) and of an adenocarcinoma (RAL), to explore their expression profiles in the basal control condition and after acute treatment with standard lung cancer chemotherapeutic drugs. As was expected considering the neoplastic nature of the cells, both cell lines showed a low expression profile of the TSGs GSTP1, RASSF1A, RARβ2, and TIMP3; DAPK and FHIT were downregulated in only CAEP cells [18,54–57]. Furthermore, genes related to drug resistance were also more widely expressed in both cell lines than in normal lung, as one would predict from the poor efficacy of the standard chemotherapy in the treatment of NSCLCs. Both our cell lines showed remarkable in vitro resistance to cisplatin, consistent with high basal levels of BRCA1 and ERCC1 mRNAs, as supported by other in vitro and in vivo studies [6,8,58].
However, CAEP and RAL cells differed in their responses to the other drugs investigated, consistent with specific patterns of gene expression. In particular, CAEP cells were less resistant to docetaxel; additionally, in accordance with published data [6–8], they displayed a lower expression level of TUBB3. CAEP cells also showed more sensitivity to gemcitabine, although a plateau in the in vitro chemosensitivity assay was observed in both cell lines. The resistance of both cell lines to plasma peak concentrations of the drug could be explained by the observed up-regulation of RRM1, a target gene of gemcitabine action [11,59].
The difference in sensitivity to docetaxel and gemcitabine of CAEP versus RAL is likely because of different levels of expression of other gene products such as COX2, an important antiapoptotic factor involved in lung carcinogenesis [60] whose expression level was found to be associated with chemoresistance to several agents [4]. Indeed, we found up-regulation of COX2 gene expression in the adenocarcinoma (RAL) cells, consistent with their greater resistance to all the drugs tested.
The recent findings of small cancer cell populations with stem cell features, i.e., cancer stem cells, in cancers resistant to antitumor agents [46] was the reason for investigating the level of expression of stem-related genes, such as OCT4, CD133, and SLUG [39,43–45] in our cell lines. Indeed, the population surviving docetaxel treatment showed OCT4 and SLUG up-regulation, respectively, in CAEP and RAL cells. This finding highlights the survival of a cell population with stemness features, expected to be more aggressive and chemoresistant according to the literature [37–39,45]. The presence of a resistant component with self-renewing capacity in NSCLC cells is in accordance with the limited survival observed in patients treated with docetaxel as a second-line therapy [61,62].
Because epigenetic mechanisms of gene expression regulation are gaining increasing attention in cancer pathophysiology, we evaluated the methylation status of the promoter of the studied genes. Although promoter methylation is usually associated with gene silencing, we did not find unequivocal evidence of DNA methylation and corresponding low mRNA levels for our entire gene panel. The hypermethylated status of GSTP1, RARβ2, CD133, and OCT4 promoters correlated with the gene silencing in both cell lines (Table 1). Loss of expression of GSTP1 and RARβ2 associated with the hypermethylated status of their promoter regions was also shown by Chen et al. [15], Kerr et al. [27], and Virmani et al. [55]; similarly, the lack of methylation on the promoter region of genes associated with specific drug resistance (BRCA1, COX2, ERCC1, RRM1, and TUBB3) was in agreement with the detection of their transcripts (Table 1). In addition, SLUG was expressed and its promoter was unmethylated, as expected, in both cell lines (Table 1). Despite CDKN2A (in CAEP cells) and MGMT gene promoter methylation, as also shown by other authors [15,17,30], these genes were highly expressed in both cell lines (Table 1). The absence of DNA methylation in the promoter of the other genes of interest implies that different mechanism of regulation of gene expression are involved. Genetic alterations, such as allelic loss of chromosome arms, could be also implicated; the 3p arm—harboring RASSF1A (3p24), RARβ2 (3p21.3), and FHIT (3p14.2)—is indeed frequently lost in NSCLC [63,64]. The hypermethylated status of CD133 and OCT4 promoters is consistent with the differentiated phenotype of our NSCLC cell lines [65,66] and is furthermore correlated with their down-regulation in the basal control condition.
Finally, the treatment with docetaxel and gemcitabine did not cause variations in the promoter methylation status of either cell line. A possible explanation could be the short term of drug treatment, which may not have been sufficient to determine a variation in the methylation profile. Further investigation, which also takes the chromatin structure and histone modifications into account, might better describe the mechanism involved in the modulation of gene expression.
The results reported in this work highlight specific biomarkers consistent with the different responses of the two cell lines to standard pharmacological treatment and indicate a molecular trait of their chemoresistance. CAEP and RAL cell lines may, therefore, be considered validated models for testing novel anticancer compounds. These cell lines may, in addition, represent a simple model for evaluating how cell populations with stem cell features impact cancer resistance to antitumor agents.
Acknowledgments
We thank Kristina Mayberry for the careful critical editing of the manuscript.
Footnotes
All authors have contributed significantly and they agree with the content of the manuscript. None of the authors has any conflict of interest.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Danesi R, de Braud F, Fogli S, de Pas TM, Di Paolo A, Curigliano G, Del Tacca M. Pharmacogenetics of anticancer drug sensitivity in non-small cell lung cancer. Pharmacol Rev. 2003;55:57–103. doi: 10.1124/pr.55.1.4. [DOI] [PubMed] [Google Scholar]
- 4.Stewart DJ. Tumor and host factors that may limit efficacy of chemotherapy in non-small cell and small cell lung cancer. Crit Rev Oncol Hematol. 2010;75:173–234. doi: 10.1016/j.critrevonc.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol. 2007;63:12–31. doi: 10.1016/j.critrevonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Danesi R, Pasqualetti G, Giovannetti E, Crea F, Altavilla G, Del Tacca M, Rosell R. Pharmacogenomics in non-small-cell lung cancer chemotherapy. Adv Drug Deliv Rev. 2009;61:408–417. doi: 10.1016/j.addr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Toschi L, Cappuzzo F. Impact of biomarkers on non-small cell lung cancer treatment. Target Oncol. 2010;5:5–17. doi: 10.1007/s11523-010-0132-y. [DOI] [PubMed] [Google Scholar]
- 8.Rosell R, Skrzypski M, Jassem E, Taron M, Bartolucci R, Sanchez JJ, Mendez P, Chaib I, Perez-Roca L, Szymanowska A, et al. BRCA1: a novel prognostic factor in resected non-small-cell lung cancer. PLoS One. 2007;2:e1129. doi: 10.1371/journal.pone.0001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Wei J, Qian X, Yin H, Zhao Y, Yu L, Wang T, Liu B. ERCC1 and BRCA1 mRNA expression levels in metastatic malignant effusions is associated with chemosensitivity to cisplatin and/or docetaxel. BMC Cancer. 2008;8:97. doi: 10.1186/1471-2407-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim CH, Yoo JS, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. FHIT protein enhances paclitaxel-induced apoptosis in lung cancer cells. Int J Cancer. 2006;118:1692–1698. doi: 10.1002/ijc.21573. [DOI] [PubMed] [Google Scholar]
- 11.Davidson JD, Ma L, Flagella M, Geeganage S, Gelbert LM, Slapak CA. An increase in the expression of ribonucleotide reductase large subunit 1 is associated with gemcitabine resistance in non-small cell lung cancer cell lines. Cancer Res. 2004;64:3761–3766. doi: 10.1158/0008-5472.CAN-03-3363. [DOI] [PubMed] [Google Scholar]
- 12.Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 13.Berdasco M, Esteller M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell. 2010;19:698–711. doi: 10.1016/j.devcel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Castro M, Grau L, Puerta P, Gimenez L, Venditti J, Quadrelli S, Sánchez-Carbayo M. Multiplexed methylation profiles of tumor suppressor genes and clinical outcome in lung cancer. J Transl Med. 2010;8:86. doi: 10.1186/1479-5876-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Yin N, Yin B, Lu Q. DNA methylation in thoracic neoplasms. Cancer Lett. 2011;301:7–16. doi: 10.1016/j.canlet.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M, Yoshino I. Aberrant methylation in non-small cell lung cancer. Surg Today. 2010;40:602–607. doi: 10.1007/s00595-009-4094-6. [DOI] [PubMed] [Google Scholar]
- 17.Heller G, Zielinski CC, Zöchbauer-Müller S. Lung cancer: from single-gene methylation to methylome profiling. Cancer Metastasis Rev. 2010;29:95–107. doi: 10.1007/s10555-010-9203-x. [DOI] [PubMed] [Google Scholar]
- 18.Burbee DG, Forgacs E, Zöchbauer-Müller S, Shivakumar L, Fong K, Gao B, Randle D, Kondo M, Virmani A, Bader S, et al. Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression. J Natl Cancer Inst. 2001;93:691–699. doi: 10.1093/jnci/93.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang M, Xu W, Wang Q, Xiao W, Xu R. Potential of DNMT and its epigenetic regulation for lung cancer therapy. Curr Genomics. 2009;10:336–352. doi: 10.2174/138920209788920994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toyooka S, Suzuki M, Maruyama R, Toyooka KO, Tsukuda K, Fukuyama Y, Iizasa T, Aoe M, Date H, Fujisawa T, et al. The relationship between aberrant methylation and survival in non-small-cell lung cancers. Br J Cancer. 2004;91:771–774. doi: 10.1038/sj.bjc.6602013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JS, Kim JW, Han J, Shim YM, Park J, Kim DH. Cohypermethylation of p16 and FHIT promoters as a prognostic factor of recurrence in surgically resected stage I non-small cell lung cancer. Cancer Res. 2006;66:4049–4054. doi: 10.1158/0008-5472.CAN-05-3813. [DOI] [PubMed] [Google Scholar]
- 22.Gasperi-Campani A, Roncuzzi L, Ricotti L, Lenzi L, Gruppioni R, Sensi A, Zini N, Zoli W, Amadori D. Molecular and biological features of two new human squamous and adenocarcinoma of the lung cell lines. Cancer Genet Cytogenet. 1998;107:11–20. doi: 10.1016/s0165-4608(98)00076-4. [DOI] [PubMed] [Google Scholar]
- 23.Zoli W, Ricotti L, Dal Susino M, Barzanti F, Frassineti GL, Folli S, Tesei A, Bacci F, Amadori D. Docetaxel and gemcitabine activity in NSCLC cell lines and in primary cultures from human lung cancer. Br J Cancer. 1999;81:609–615. doi: 10.1038/sj.bjc.6690737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosetti M, Zoli W, Tesei A, Ulivi P, Fabbri F, Vannini I, Brigliadori G, Granato AM, Amadori D, Silvestrini R. Iressa strengthens the cytotoxic effect of docetaxel in NSCLC models that harbor specific molecular characteristics. J Cell Physiol. 2007;212:710–716. doi: 10.1002/jcp.21067. [DOI] [PubMed] [Google Scholar]
- 25.Tesei A, Brigliadori G, Carloni S, Fabbri F, Ulivi P, Arienti C, Sparatore A, Del Soldato P, Pasini A, Amadori D, et al. Organosulfur derivatives of the HDAC inhibitor valproic acid sensitize human lung cancer cell lines to apoptosis and to cisplatin cytotoxicity. J Cell Physiol. 2012;227:3389–3396. doi: 10.1002/jcp.24039. [DOI] [PubMed] [Google Scholar]
- 26.Zöchbauer-Müller S, Minna JD, Gazdar AF. Aberrant DNA methylation in lung cancer: biological and clinical implications. Oncologist. 2002;7:451–457. doi: 10.1634/theoncologist.7-5-451. [DOI] [PubMed] [Google Scholar]
- 27.Kerr KM, Galler JS, Hagen JA, Laird PW, Laird-Offringa IA. The role of DNA methylation in the development and progression of lung adenocarcinoma. Dis Markers. 2007;23:5–30. doi: 10.1155/2007/985474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsou JA, Hagen JA, Carpenter CL, Laird-Offringa IA. DNA methylation analysis: a powerful new tool for lung cancer diagnosis. Oncogene. 2002;21:5450–5461. doi: 10.1038/sj.onc.1205605. [DOI] [PubMed] [Google Scholar]
- 29.Digel W, Lubbert M. DNA methylation disturbances as novel therapeutic target in lung cancer: preclinical and clinical results. Crit Rev Oncol Hematol. 2005;55:1–11. doi: 10.1016/j.critrevonc.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Belinsky SA. Gene-promoter hypermethylation as a biomarker in lung cancer. Nat Rev Cancer. 2004;4:707–717. doi: 10.1038/nrc1432. [DOI] [PubMed] [Google Scholar]
- 31.Mao L. Molecular abnormalities in lung carcinogenesis and their potential clinical implications. Lung Cancer. 2001;34:S27–S34. doi: 10.1016/s0169-5002(01)00341-5. [DOI] [PubMed] [Google Scholar]
- 32.Endoh H, Yatabe Y, Shimizu S, Tajima K, Kuwano H, Takahashi T, Mitsudomi T. RASSF1A gene inactivation in non-small cell lung cancer and its clinical implication. Int J Cancer. 2003;106:45–51. doi: 10.1002/ijc.11184. [DOI] [PubMed] [Google Scholar]
- 33.Adams VR, Harvey RD. Histological and genetic markers for non-small-cell lung cancer: customizing treatment based on individual tumor biology. Am J Health Syst Pharm. 2010;67:S3–S9. doi: 10.2146/ajhp090456. [DOI] [PubMed] [Google Scholar]
- 34.Ibanez de Caceres I, Cortes-Sempere M, Moratilla C, Machado-Pinilla R, Rodriguez-Fanjul V, Manguán-García C, Cejas P, López-Ríos F, Paz-Ares L, de CastroCarpeño J, et al. IGFBP-3 hypermethylation-derived deficiency mediates cisplatin resistance in non-small-cell lung cancer. Oncogene. 2010;29:1681–1690. doi: 10.1038/onc.2009.454. [DOI] [PubMed] [Google Scholar]
- 35.Rosell R, Vergnenegre A, Liu B, Cobo M, Massuti B, Wei J, Molina MA, Costa C, Queralt C, Taron M. Biomarkers in lung oncology. Pulm Pharmacol Ther. 2010;23:508–514. doi: 10.1016/j.pupt.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Kang CH, Jang BG, Kim DW, Chung DH, Kim YT, Jheon S, Sung SW, Kim JH. The prognostic significance of ERCC1, BRCA1, XRCC1, and βIII-tubulin expression in patients with non-small cell lung cancer treated by platinum- and taxane-based neoadjuvant chemotherapy and surgical resection. Lung Cancer. 2010;68:478–483. doi: 10.1016/j.lungcan.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Peacock CD, Watkins DN. Cancer stem cells and the ontogeny of lung cancer. J Clin Oncol. 2008;26:2883–2889. doi: 10.1200/JCO.2007.15.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pine SR, Marshall B, Varticovski L. Lung cancer stem cells. Dis Markers. 2008;24:257–266. doi: 10.1155/2008/396281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS One. 2008;3:e3077. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan JP, Minna JD, Shay JW. Evidence for self-renewing lung cancer stem cells and their implications in tumor initiation, progression, and targeted therapy. Cancer Metastasis Rev. 2010;29:61–72. doi: 10.1007/s10555-010-9216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci USA. 2009;106:16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salnikov AV, Gladkich J, Moldenhauer G, Volm M, Mattern J, Herr I. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int J Cancer. 2010;126:950–958. doi: 10.1002/ijc.24822. [DOI] [PubMed] [Google Scholar]
- 43.Tesei A, Zoli W, Arienti C, Storci G, Granato AM, Pasquinelli G, Valente S, Orrico C, Rosetti M, Vannini I, et al. Isolation of stem/progenitor cells from normal lung tissue of adult humans. Cell Prolif. 2009;42:298–308. doi: 10.1111/j.1365-2184.2009.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 45.Chen YC, Hsu HS, Chen YW, Tsai TH, How CK, Wang CY, Hung SC, Chang YL, Tsai ML, Lee YY, et al. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS One. 2008;3:e2637. doi: 10.1371/journal.pone.0002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dick JE. Looking ahead in cancer stem cell research. Nat Biotechnol. 2009;27:44–46. doi: 10.1038/nbt0109-44. [DOI] [PubMed] [Google Scholar]
- 47.Baker SD, Zhao M, Lee CK, Verweij J, Zabelina Y, Brahmer JR, Wolff AC, Sparreboom A, Carducci MA. Comparative pharmacokinetics of weekly and every-three-weeks docetaxel. Clin Cancer Res. 2004;10:1976–1983. doi: 10.1158/1078-0432.ccr-0842-03. [DOI] [PubMed] [Google Scholar]
- 48.Souid AK, Dubowy RL, Blaney SM, Hershon L, Sullivan J, McLeod WD, Bernstein ML. Phase I clinical and pharmacologic study of weekly cisplatin and irinotecan combined with amifostine for refractory solid tumors. Clin Cancer Res. 2003;9:703–710. [PubMed] [Google Scholar]
- 49.Jacobs SS, Fox E, Dennie C, Morgan LB, McCully CL, Balis FM. Plasma and cerebrospinal fluid pharmacokinetics of intravenous oxaliplatin, cisplatin, and carboplatin in nonhuman primates. Clin Cancer Res. 2005;11:1669–1674. doi: 10.1158/1078-0432.CCR-04-1807. [DOI] [PubMed] [Google Scholar]
- 50.Mavroudis D, Pavlakou G, Blazoyiannakis G, Veslemes M, Apostolopoulou F, Kouroussis C, Kakolyris S, Agelaki S, Androulakis N, Vardakis N, et al. Sequential administration of cisplatin-etoposide followed by topotecan in patients with extensive stage small cell lung cancer. A multicenter phase II study. Lung Cancer. 2003);39:71–76. doi: 10.1016/s0169-5002(02)00307-0. [DOI] [PubMed] [Google Scholar]
- 51.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 52.Arienti C, Tesei A, Verdecchia GM, Framarini M, Virzi S, Grassi A, Scarpi E, Turci L, Silvestrini R, Amadori D, et al. Peritoneal carcinomatosis from ovarian cancer: chemosensitivity test and tissue markers as predictors of response to chemotherapy. J Transl Med. 2011;9:94. doi: 10.1186/1479-5876-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geradts J, Fong KM, Zimmerman PV, Minna JD. Loss of Fhit expression in non-small-cell lung cancer: correlation with molecular genetic abnormalities and clinicopathological features. Br J Cancer. 2000;82:1191–1197. doi: 10.1054/bjoc.1999.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Virmani AK, Rathi A, Zöchbauer-Müller S, Sacchi N, Fukuyama Y, Bryant D, Heda S, Fong KM, Thunnissen F, Minna JD, et al. Promoter methylation and silencing of the retinoic acid receptor-β gene in lung carcinomas. J Natl Cancer Inst. 2000;92:1303–1307. doi: 10.1093/jnci/92.16.1303. [DOI] [PubMed] [Google Scholar]
- 56.Tomizawa Y, Iijima H, Nomoto T, Iwasaki Y, Otani Y, Tsuchiya S, Saito R, Dobashi K, Nakajima T, Mori M. Clinicopathological significance of aberrant methylation of RARβ2 at 3p24, RASSF1A at 3p21.3, and FHIT at 3p14.2 in patients with non-small cell lung cancer. Lung Cancer. 2004;46:305–312. doi: 10.1016/j.lungcan.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Dammann R, Strunnikova M, Schagdarsurengin U, Rastetter M, Papritz M, Hattenhorst UE, Hofmann HS, Silber RE, Burdach S, Hansen G. CpG island methylation and expression of tumour-associated genes in lung carcinoma. Eur J Cancer. 2005;41:1223–1236. doi: 10.1016/j.ejca.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 58.Fujii T, Toyooka S, Ichimura K, Fujiwara Y, Hotta K, Soh J, Suehisa H, Kobayashi N, Aoe M, Yoshino T, et al. ERCC1 protein expression predicts the response of cisplatin-based neoadjuvant chemotherapy in non-small-cell lung cancer. Lung Cancer. 2008;59:377–384. doi: 10.1016/j.lungcan.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 59.Gong W, Zhang X, Wu J, Chen L, Li L, Sun J, Lv Y, Wei X, Du Y, Jin H. RRM1 expression and clinical outcome of gemcitabine-containing chemotherapy for advanced non-small-cell lung cancer: a meta-analysis. Lung Cancer. 2012;75:374–380. doi: 10.1016/j.lungcan.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Dubinett SM, Sharma S, Huang M, Dohadwala M, Pold M, Mao JT. Cyclooxygenase-2 in lung cancer. Prog Exp Tumor Res. 2003;37:138–162. doi: 10.1159/000071371. [DOI] [PubMed] [Google Scholar]
- 61.Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 62.Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 63.Mountzios G, Dimopoulos MA, Soria JC, Sanoudou D, Papadimitriou CA. Histopathologic and genetic alterations as predictors of response to treatment and survival in lung cancer: a review of published data. Crit Rev Oncol Hematol. 2010;75:94–109. doi: 10.1016/j.critrevonc.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Toloza EM, Roth JA, Swisher SG. Molecular events in bronchogenic carcinoma and their implications for therapy. Semin Surg Oncol. 2000;18:91–99. doi: 10.1002/(sici)1098-2388(200003)18:2<91::aid-ssu2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 65.Fisher CL, Fisher AG. Chromatin states in pluripotent, differentiated, and reprogrammed cells. Curr Opin Genet Dev. 2011;21:140–146. doi: 10.1016/j.gde.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 66.Schuettengruber B, Cavalli G. Recruitment of polycomb group complexes and their role in the dynamic regulation of cell fate choice. Development. 2009;136:3531–3542. doi: 10.1242/dev.033902. [DOI] [PubMed] [Google Scholar]