Abstract
The model of Drosophila female meiosis I was recently revised by the discovery that chromosome congression precedes metaphase I arrest. Use of the prior framework to interpret data from meiotic mutants led to the conclusion that chromosome segregation errors (nondisjunction, NDJ) occurred when nonexchange chromosomes moved out on the spindle in a maloriented configuration and became trapped there at metaphase arrest. The discovery that congression returns nonexchange chromosomes to the metaphase plate invalidates this interpretation and raises the question of what events actually do lead to NDJ. To address this, we have assayed an allelic series of ald (mps1) meiotic mutants that complete congression at wild-type rates, but have widely varying NDJ rates in an otherwise isogenic background, as well as a nod mutant background that primarily undergoes loss of chromosome 4. Using genetic assays to measure NDJ rates, and FISH assays to measure chromosome malorientation rates in metaphase-arrested oocytes, shows that these two rates are highly correlated across ald mutants, suggesting that malorientation during congression commits these chromosomes to eventually nondisjoin. Likewise, the rate of chromosome loss observed in nod is similar to the rate at which these chromosomes fail to associate with the main chromosome mass. Together these results provide a proximal mechanism for how these meiotic mutants cause NDJ and chromosome loss and improve our understanding of how prometaphase chromosome congression relates to anaphase chromosome segregation.
Keywords: congression, Drosophila, meiosis, nondisjunction, segregation
THE first meiotic division establishes the proper separation of homologous chromosome pairs, with sister centromeres segregating to the same spindle pole while homologous centromeres go to opposite poles. This is in contrast to both mitosis and the second meiotic division, where it is the sister centromeres that segregate to opposite poles. This implies there must be mechanisms that ensure accurate segregation of homologs, and mutants in loci required for those mechanisms have been identified on the basis of chromosome segregation errors that result in aneuploid progeny (Lake and Hawley 2012). While meiotic crossing over is normally sufficient to ensure homologous chromosomes properly coorient in meiosis I, there are instances where recombination is not necessary. Chromosomes in female Drosophila melanogaster can be nonexchange (either spontaneously, as occurs to the X in 6–10% of meioses, or by heterozygosity for multiply-rearranged balancer chromosomes that block exchange), yet are still able to properly segregate from their homologs through the distributive segregation system (Hawley et al. 1993). Humans are also likely to contain a pathway analogous to distributive segregation; a recent study found that chromosome 21 did not recombine in 20% of human meioses (Oliver et al. 2008), yet the rate of segregation errors in women under 35 is only 1 in 2500 births (Lamb et al. 2005), implying that most nonexchange chromosomes are still able to segregate properly. As ∼90% of human aneuploidies arise in female meiosis I (Hassold and Hunt 2001), this makes the Drosophila system a useful model for studying how aneuploidy occurs.
The first confocal study of distributive chromosome segregation in female meiosis was published 20 years ago (Theurkauf and Hawley 1992). This study proposed an “anti-congression” model for nonexchange chromosome segregation, where once the tubulin spindle forms during prometaphase, the nonexchange chromosomes move unidirectionally toward opposite spindle poles, and metaphase arrest is reached with nonexchange chromosomes out on opposite spindle arms, separated from the metaphase plate. This explained nondisjunction (NDJ), as if two homologs were to move out onto the same arm of the spindle, there would be no connection between the homologs and, lacking any way to detect and repair a malorientation, these chromosomes would be “trapped” facing the same pole and committed to nondisjoin. Further support for this model came from subsequent studies that found that chromosomes could be observed on the same arm of the meiotic spindle at rates very similar to their segregation rates (Theurkauf and Hawley 1992; Harris et al. 2003; Gilliland et al. 2005; Xiang and Hawley 2006).
Recently, however, it was shown that the chromosomes-out configuration is actually an intermediate stage of prometaphase. Females that are aged for several days as virgins accumulate mature metaphase-arrested oocytes, and cytological examination revealed that their chromosomes were found in a single, compact mass (Gilliland et al. 2009). This indicates that the nonexchange chromosomes in Drosophila females must undergo congression prior to metaphase arrest and rejoin the exchange chromosomes at the metaphase plate. The apparent trapping of nonexchange chromosomes on the same arm of the spindle could be explained as a consequence of a rejoin-and-reorient cycle, which is mediated by nonexchange homologs remaining physically connected by previously undetected DNA threads that tether homologs together (Hughes et al. 2009, 2011). Nonexchange chromosomes can therefore detect their homologs and have a mechanism to correct malorientations prior to congression and metaphase arrest.
This change to the model of meiotic progression requires a reevaluation of prior studies of meiotic mutants. Is NDJ a consequence of defects in congression, and if not, what events actually do result in NDJ? What is the true metaphase arrest configuration in meiotic mutants? How does congression account for maloriented chromosomes being found on the spindle at rates similar to their NDJ rates? To address these questions, we have examined how congression and metaphase arrest are related to nondisjunctional segregation events. Our hypothesis is that NDJ is caused when oocytes complete congression without properly coorienting homologous chromosomes. This will result in a metaphase I chromosome mass with both homologs oriented toward the same spindle pole (Figure 1). Those chromosomes would then segregate to the facing pole at anaphase I, producing equal numbers of nullo and diplo NDJ progeny depending on which pole becomes the egg pronucleus. This predicts that the rate of genetic NDJ should be equal to the rate of cytological malorientation. To test this hypothesis, we have assayed an allelic series of ald meiotic mutants, which provides a wide range of NDJ rates within a common genetic background. The ald locus was first identified in a screen for mutants causing NDJ in female meiosis (O’Tousa 1982) and encodes the fly homolog of the widely conserved spindle assembly checkpoint (SAC) gene mps1 (Fischer et al. 2004; Gilliland et al. 2005). Mutations in ald primarily cause NDJ in nonexchange chromosomes, but can also cause chiasmate chromosomes to nondisjoin as well, without significant chromosome loss. Live imaging of ald mutants reveals there is loss of sister chromatid cohesion early in prometaphase (Gilliland et al. 2005, 2007), but we show here that even highly compromised ald genotypes remain fully competent to congress their chromosomes to a single mass by metaphase arrest. Therefore, ald is a good candidate for studying how NDJ can occur in the presence of congression, and this allelic series provides widely varying genetic NDJ rates with minimal differences in the genetic background.
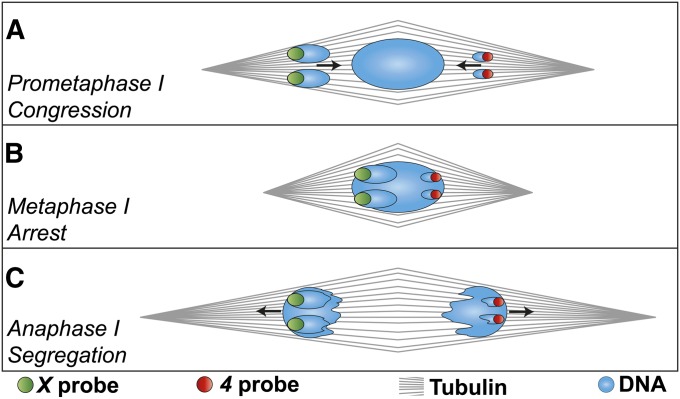
Figure 1 .
Model for nondisjunctional segregation with proper congression. A heterologous segregation event is drawn here; black arrows indicate chromosome movements. (A) If chromosomes are out on the spindle in a maloriented configuration, and complete congression without correcting the defect, then (B) the metaphase-arrested chromosome mass would contain all the chromosomes, yet will have both homologs pointed toward the same spindle pole. (C) When sister chromatid cohesion along chromosome arms is dissolved at anaphase, the maloriented chromosomes would proceed to opposite poles. If the left pole becomes the pronucleus, the oocyte will be diplo-X nullo-4, while the other pole would produce nullo-X diplo-4.
Each ald allele was assayed genetically by estimation of X and 4 NDJ rates in experimental crosses and cytologically by measuring the rates of X and 4 malorientation in metaphase-arrested oocytes using chromosome-specific fluorescent in situ hybridization (FISH). We show that these rates of genetically measured NDJ and cytologically measured malorientation are highly correlated. This implies that ald mutations must cause meiotic NDJ in mid-to-late prometaphase and prior to the completion of congression. To show that this hypothesis can be extended to other meiotic mutants, we also performed a similar cytological assay with nod, a kinesin-like protein that primarily causes nonexchange chromosome loss, with exchange chromosomes being largely unaffected (Zhang and Hawley 1990; Zhang et al. 1990). These genetic data were supported by the original cytological study of nod oocytes, which found that nonexchange chromosomes were frequently ejected from the meiotic spindle (Theurkauf and Hawley 1992). As that study did not use oocytes with known staging, we examined metaphase arrested nod oocytes from females with exchange X chromosomes (which have very high rates of 4 loss and a much lower rate of X loss, limited to those 6–10% of meioses with spontaneously nonexchange X chromosomes), and generated a sample size that allows estimation of segregation class frequencies. Similar to Theurkauf and Hawley’s result, we found chromosome 4’s frequently, and X chromosomes infrequently, isolated in the ooplasm at metaphase arrest, as well as a low rate of oocytes with two homologs at the same pole. Consistent with our hypothesis, these cytological data predict segregation rates that closely match those observed genetically, suggesting that the disposition of these chromosomes by metaphase arrest leads to their eventual segregation pattern.
Materials and Methods
Fly stocks and crosses
The ald1, aldA15, Df(3R)AN6, and noda alleles are described elsewhere (Zhang and Hawley 1990; Gilliland et al. 2005, 2007; Hawley and Gilliland 2006). The ald allelic series was generated in a previous study by excision of aldP{GS:13084} (Gilliland et al. 2005); some of these alleles have been molecularly characterized elsewhere (Gilliland et al. 2007). The NDJ rates of 24 excision lines were tested in a preliminary experiment, to identify eight lines with a range of NDJ rates (Supporting Information, Table S1). Virgin females from these eight lines were crossed in bottles to FM7, y w B; Df(3R)AN6/TM3, Sb; pol males, which carries a deficiency that deletes the ald locus (Gilliland et al. 2007), and FM7, y w B/y w; aldexcision/Df(3R)AN6; pol hemizygous virgin experimental females were collected. Nondisjunction assays were done by crossing individual experimental females to YSX·YL, In(1)EN, v f B/0; C(4)RM, ci eyR/Ø males in vials. Adults were allowed to lay eggs for 5 days, brooded once to new vials for 5 additional days, and then discarded. Experimental progeny were counted up through day 18 (d18) after vials were set up. The target sample size for each line was at least 2000 progeny; two lines (aldexcision-4 and aldexcision-15) did not reach this threshold so an additional set of crosses was done for each. Finally, X and 4 nondisjunctional progeny were scored per standard methods (Zitron and Hawley 1989). No significant differences were found in mean NDJ between broods 1 and 2 for any line, even before correcting for multiple tests (data not shown) so broods were pooled for data analysis.
The noda chromosome is maintained in y w noda/y+Y; pol males crossed to C(1)DX, y f/y+Y; pol females. To produce homozygotes, males were crossed to FM7w, y w B; pol females, heterozygous virgin females were backcrossed to y w noda/y+Y; pol males, and noda homozygous virgin experimental females were collected. For the nod heterozygous control, y w; pol virgin females were crossed to y w noda/y+Y; pol males, and experimental female heterozygous progeny were collected. Experimental females were aged for assays as above.
Rate and confidence interval estimations
NDJ assays:
NDJ rates were calculated using Cooper’s method (Cooper 1948), with only the number of X NDJ progeny doubled due to sperm-induced inviability, as X NDJ oocytes can only produce viable progeny with one of the two male sex chromosomes, while all normal progeny are expected to be viable. Confidence intervals for both X and 4 rates were calculated using the hierarchical Poisson method (Zeng et al. 2010).
Cytological assays:
The 95% confidence intervals were estimated independently for X and 4 rates using the normal approximation to the binomial, , where is the sample mean and N is the total number of oocytes. For the line with zero malorientations, the 95% confidence interval used was (Hanley and Lippman-Hand 1983).
Calculations and data plots were done in Microsoft Excel and R (http://www.r-project.org/).
Cytological preparations
Metaphase-arrested oocytes were enriched by aging experimental females for 4–5 days posteclosion (dpe) in vials with yeast paste and no males prior to dissection, which causes females to retain unfertilized eggs and results in >90% of wild-type oocytes being at metaphase arrest (Gilliland et al. 2009). While only oocytes with the chromosomes in a single mass were scored for metaphase arrest in ald genotypes, the chromosome loss in nod meant that the same criteria could not be used. Metaphase arrest was therefore determined by the presence of fully mature dorsal appendages, a standard that cannot be used with immunolocalization.
A protocol for combined immuno-FISH was developed based on a method originally used in mitosis (Ferree and Barbash 2009); this new protocol was necessary as removal of the chorion (to allow antibody penetration) appears to increase the stringency of formamide washes, resulting in complete loss of FISH signal after only a few washes. Ovaries were hand dissected in 1× Robb’s media + 1% BSA (Sullivan et al. 2000), then fixed for 4 min in 1 ml of prewarmed 39° fixative, a 1:1 mix of 16% EM grade paraformaldehyde and 2× fixative buffer (William’s hypotonic oocyte preservation and stabilization solution: 100 mM sodium cacodylate, 100 mM sucrose, 40 mM potassium acetate, and 10 mM EGTA) combined just before use. Ovaries were rinsed four times in PBST (PBS + 0.1% Triton X-100) for 15 min, and then dechorionated by rolling between sandblasted glass slides, and briefly washed three times in PBST. Oocytes were blocked for 1 hr in PBST-NGS (PBST + 5% normal goat serum), then new PBST-NGS plus primary antibody (Serotec MCA786 rat antitubulin, 1:250) was added and hybridized at 4° overnight. Oocytes were washed briefly three times in PBST followed by once in PBST for 15 min. Oocytes were blocked for 1 hr as before, then hybridized to secondary antibody (Invitrogen goat antirat IgG with Alexa Fluor 647 conjugate, 1:250) overnight as before. Oocytes were washed briefly three times in PBST, and then postfixed in PBST plus 4% paraformaldehyde for 30 min. After postfixation, ovaries were briefly washed three times in PBST, then three times in 2× SSCT (0.3 M sodium chloride, 0.03 M sodium citrate, 0.1% Tween-20) for 10 min each. The ovarioles were then ramped into formamide by successive 10-min washes in 2× SSCT containing 20, 40, and 50% formamide, followed by incubation in 50% formamide at 37° for 2 hr. The buffer was thoroughly aspirated and 40 µl of hybridization solution was added (36 µl of 1.1× hybridization buffer (Sullivan et al. 2000) plus 4 µl of probe mix (4 µl H2O containing 25 ng X 359-bp satellite probe (TTT-TCC-AAA-TTT-CGG-TCA-TCA-AAT-AAT-CAT) and 50 ng 4 probe (AATAT)6 (Dernburg et al. 1996) denatured for 3 min at 92°, and incubated at 32° overnight. Hybridization solution was removed, and then the sample was ramped out of formamide by 10-min washes in 2× SSCT + 40% formamide, 2× SSCT + 20% formamide, and then 2× SSCT. Oocytes were incubated with 0.5 ml 2× SSCT plus DAPI for 10 min, washed twice in 2× SSCT for 10 min, and finally mounted on slides in Slowfade Gold mounting medium (Invitrogen). FISH without immunostaining was done with the same X and 4 probes as above in addition to the 2L–3L probe (AATAACATAG)3 using standard protocols (Dernburg et al. 1996), while congression rate assays without FISH used a DAPI-only fixation protocol (Gilliland et al. 2009). Probes with fluorophore conjugates were either synthesized by Integrated DNA Technologies (www.idtdna.com) or were a generous gift from the Hawley lab. To avoid double counting oocytes, an image of each microscope slide was taken on a dissecting microscope camera and used as a map to guide confocal image collection. Cytological images were collected on a Leica TCS SPE II confocal microscope using LAS AF software (www.leica.com), and deconvolved using Huygens Essential (www.svi.nl).
Results
We first determined the effect of ald mutations on chromosome congression. Previous work indicates a number of steps must occur during wild-type congression, including the nonexchange chromosomes rejoining the exchange chromosomes at the metaphase plate, chromosome compaction and loss of chromosome individualization, and shortening of the meiotic spindle (Gilliland et al. 2009). These processes are all correlated with aspects of oocyte maturation, such as the growth of the dorsal appendages; the present study is focused on the first process, whether chromosomes form a single mass by metaphase arrest. As live imaging of ald mutants observed the loss of sister chromatid cohesion early in prometaphase (Gilliland et al. 2007), it was possible that these chromosomes may be entering early anaphase. We therefore aged virgin FM7/X; ald1/Df females for 4 days, fixed their ovaries, and examined the chromosomes of mature oocytes. While homologous segregation in this genotype is quite compromised, with ∼39% X and ∼28% 4 NDJ (Gilliland et al. 2005), we found that 47/50 oocytes (94%) had congressed their chromosomes to a single mass, while the three remaining oocytes appeared to still be in prometaphase. This rate is similar to that of wild-type FM7/X females, where 149/164 (91%) of oocytes from similarly aged females were found to have chromosomes in a single mass (Gilliland et al. 2009). Similarly high rates of successful congression were also observed in homozygotes of another strong ald allele, aldA15 (W. D. Gilliland, unpublished observations), as well as in the cytological assays described below. These results indicate that reducing the level of ald function does not compromise the ability of oocytes to carry out congression, although as all of these alleles still retain some level of ald function (Gilliland et al. 2007), we cannot comment on congression in a completely null background.
To determine how nondisjunction could still occur in the presence of normal congression, we then assayed an allelic series of ald mutations that were generated by P element excision. These alleles exhibit widely varying NDJ rates, while minimizing genetic background differences, as all genotypes are derived from the same source chromosome. Each allele was crossed to a stock carrying the X chromosome balancer FM7 as well as a small deficiency that removes the ald locus, resulting in experimental females with nonexchange X chromosomes that were hemizygous for the ald excision alleles. Virgin experimental females from each excision line were mated to males from a tester stock that allows the recovery and identification of progeny that are nondisjunctional for both X and 4 chromosomes (Zitron and Hawley 1989). These progeny-count data (Table S2) were used to calculate NDJ rates for each line (Table 1). This shows that the lines used vary from wild-type levels (0.7% X and 0.6% 4 NDJ for aldexcision-25) to almost random segregation (39.7% X and 28.6% 4 for aldexcision-23). To measure chromosome coorientation rates at metaphase arrest, virgin experimental females from each line were also aged for 4 days posteclosion with yeast and no males, to enrich the proportion of ovaries at metaphase arrest. Ovaries were fixed, and oocytes were labeled using chromosome-specific heterochromatin FISH (Dernburg et al. 1996) to reveal homologous coorientation in the metaphase-arrested chromosome mass. As spindle orientation can be reliably inferred from chromosome shape in wild-type oocytes, we initially attempted to use FISH labeling of the exchange chromosomes 2 and 3 to indicate the directions of the spindle poles. While this worked for the precise excision allele aldexcision-25, in more strongly compromised genotypes it proved difficult to confidently determine spindle orientation by this method. Therefore we used a combined immuno-FISH protocol to label the spindle with antitubulin antibodies and the X and 4 chromosomes with FISH. This allowed us to identify oocytes that exhibited proper X and 4 coorientation (Figure 2A), 4-only malorientation (Figure 2B), X-only malorientation (Figure 2C), and X and 4 double malorientation in either heterologous (Figure 2D) or nonheterologous (Figure 2E) configurations. At least 200 oocytes from each excision line were scored, and the numbers of oocytes in each segregation pattern (Table S3) were used to calculate the rates of malorientation for each chromosome (Table 2). These results were comparable to the genetic data, with a range of 0 to 35.5% X malorientation and a range of 0 to 31.3% 4 malorientation.
Table 1 . Genetic nondisjunction rates.
| Excision | X NDJ rate | 4 NDJ rate | Adjusted N |
|---|---|---|---|
| 1 | 10.9 ± 1.72 | 8.3 ± 1.50 | 2690 |
| 4 | 10.9 ± 1.35 | 8.8 ± 1.22 | 4376 |
| 14 | 10.9 ± 1.85 | 10.5 ± 1.82 | 2319 |
| 15 | 8.2 ± 1.12 | 6.4 ± 0.99 | 4843 |
| 23 | 39.8 ± 2.91 | 28.6 ± 2.55 | 2901 |
| 25 | 0.7 ± 0.47 | 0.6 ± 0.42 | 2475 |
| 26 | 13.8 ± 2.07 | 10.4 ± 1.81 | 2309 |
| 30 | 10.4 ± 1.75 | 8.3 ± 1.57 | 2469 |
The progeny counts in Table S2 were used to estimate the genetic rates of X and 4 nondisjunction (NDJ) for each line, using Cooper’s method for calculating means and the Heirarchical–Poisson method for confidence intervals (Zeng et al. 2010).
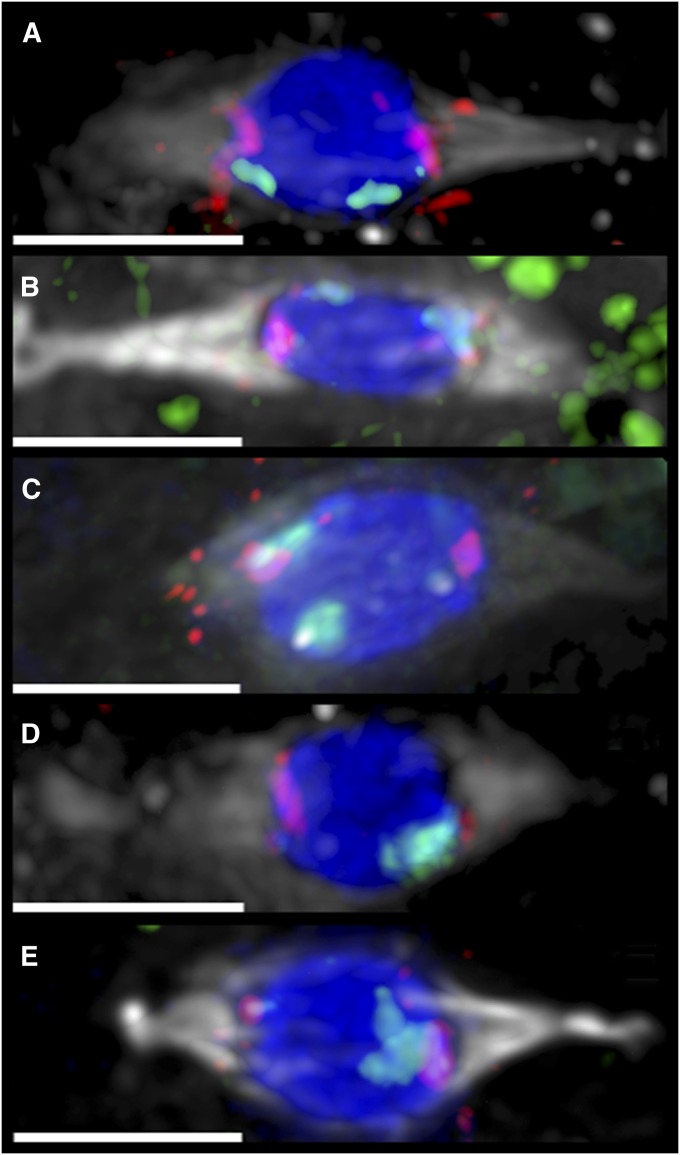
Figure 2 .
Immuno-FISH of metaphase arrested oocytes. Oocytes from experimental females from the indicated lines are shown; each was FM7/y w; aldexcision/Df(3R)AN6; pol. For each image, the tubulin spindle (antibody) is shown in gray, DNA (DAPI) in blue, the 4 (FISH probe) in red, and the X (FISH probe) in green. All bars, 4 µm. Small and variable foci of both probes can be found on other chromosomes (Hughes et al. 2009) so the size of the block of probe signal is needed to determine chromosome locations. (A) An aldexcision-14 oocyte showing proper coorientation of both X and 4. FM7 is facing the right pole. (B) An aldexcision-1 oocyte showing proper X coorientation and both 4 chromosomes oriented to the left pole. The left X mass is the distal FM7 signal, with its centromeric spot on top of the combined 4 signal. (C) An aldexcision-4 oocyte showing proper coorientation of both 4 chromosomes with both X chromosomes oriented to the left pole. (D) An aldexcision-23 oocyte showing heterologous segregation, with both 4 chromosomes oriented to the left pole and both X chromosomes oriented to the right. (E) An aldexcision-15 oocyte showing nonheterologous segregation, with all four X and 4 homologs facing the right pole.
Table 2 . Cytological malorientation rates.
| Excision | X malorientation rate | 4 malorientation rate | N |
|---|---|---|---|
| 1 | 15.2 ± 4.9 | 12.3 ± 4.5 | 204 |
| 4 | 14.7 ± 4.7 | 8.7 ± 3.7 | 218 |
| 14 | 12.6 ± 4.3 | 9.5 ± 3.8 | 231 |
| 15 | 9.8 ± 4.1 | 7.8 ± 3.7 | 204 |
| 23 | 35.5 ± 6.4 | 31.3 ± 6.2 | 214 |
| 25 | 0 + 1.5 | 0 + 1.5 | 201 |
| 26 | 18.0 ± 4.8 | 14.0 ± 4.3 | 250 |
| 30 | 11.7 ± 4.4 | 5.8 ± 3.2 | 206 |
The coorientation counts in Table S3 were used to estimate the malorientation rates of X and 4 for each line, with confidence intervals indicated (see Materials and Methods). As viability is not an issue at this stage, these two rates are treated as independent binomial processes.
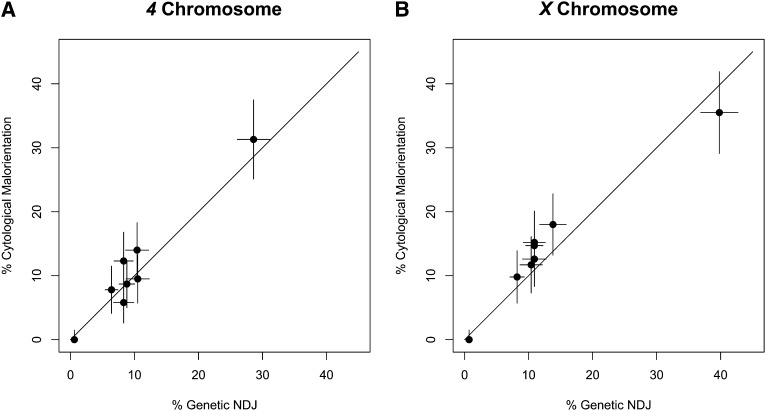
We then compared the rates of genetic NDJ and cytological malorientation for each chromosome separately. For both the 4 and X (Figure 3) the rates of genetic nondisjunction and cytological malorientation were almost completely correlated (r = 0.970 for X and r = 0.972 for 4, Pearson correlation coefficient) and not far from the prediction that the two measurements should be equal (diagonal lines). This supports the hypothesis that nondisjunction without chromosome loss arises when the metaphase-arrested chromosome mass forms with one or more homologs maloriented and suggests that those metaphase-arrested oocytes with both homologs facing the same pole are committed to subsequently nondisjoin at anaphase I.
Figure 3 .
Comparison of NDJ and malorientation rates. The rates and 95% confidence intervals in Tables 1 and 2 are plotted for (A) 4 chromosome and (B) X chromosome. The two rates are almost perfectly correlated for both chromosomes (4: r = 0.972, X: r = 0.970, Pearson correlation coefficient) and close to equal (diagonal line).
To see if this hypothesis can be applied to other loci, we also assayed nod mutant females. Nod is a kinesin-like protein required for distributive segregation (Zhang et al. 1990) that crosslinks nonexchange chromosomes to the meiotic spindle (Cui et al. 2005). Genetically, mutant females lacking nod primarily undergo chromosome loss during meiosis I, which results in an excess of nullo over diplo progeny (Carpenter 1973). Homozygous y w noda; pol females exhibit high rates of NDJ for the obligately achiasmate 4 chromosome, while only the 6–10% of meioses with spontaneously achiasmate X chromosomes nondisjoin. When assayed genetically as before, we found this genotype has 78.4% 4 and 2.2% X NDJ, with 95.1% of chromosome 4 errors being nullo-4 (Table S4). Our hypothesis that congression sets up nondisjunctional segregations predicts that in this genotype, most X chromosomes should be congressed and properly cooriented with the autosomes, while the 4 would fail to remain associated with the main mass. When examined by FISH, metaphase-arrested oocytes from aged virgin females were indeed found to frequently have 4 chromosomes that were well separated from the autosomes, while the X chromosomes usually remained associated with the autosomes (Figure 4). We scored 200 oocytes for how the X and 4 chromosomes were associated with the autosomal mass (Table S5). Rate estimation from these figures is less straightforward than in the NDJ-only case, due to the possibility of getting euploid oocytes from abnormal metaphase figures; if we assume that every chromosome that is separated from the chiasmate autosomes will be lost, and that both spindle poles are equally likely to become the egg pronucleus, then the cytological data predict rates of 87.8% 4 and 3.0% X NDJ, with 98.0% of chromosome 4 errors being nullo-4. Similar to ald, these results are close to the genetic data from this genotype.
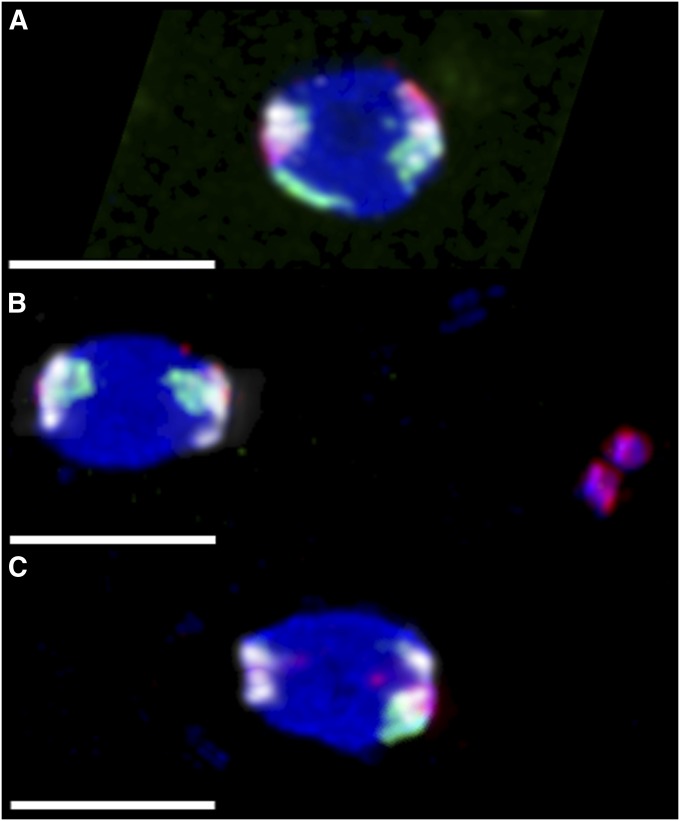
Figure 4 .
Chromosome loss in nod mutant oocytes. Experimental virgin females were aged 4 dpe with yeast and no males, and then FISH labeled with probes that predominantly hybridized to the X (green), 4 (red), and 2L–3L (white). As chiasmate chromosomes do not undergo meiotic errors in nod, the two pairs of white 2L–3L foci were used to infer the direction of the meiotic spindles, which are positioned horizontally. Three configurations are shown here, out of 20 possible configurations, not all of which were actually observed (Table S5). All bars, 4 µm. (A) An oocyte from a heterozygous control y w/y w noda; pol female, showing biorientation of both X and 4. This oocyte would always produce a euploid oocyte; 200/200 control oocytes scored showed this proper biorientation. (B) An oocyte from a homozygous y w noda; pol female, showing the two X homologs correctly bioriented, while the two 4 homologs are together off of the spindle to the right. This X⇔X oocyte would always produce normal-X, nullo-4 progeny. (C) An oocyte from a homozygous y w noda; pol female, showing the left pole with no X or 4, and single X and 4 chromosomes associated with the right pole; the other X and 4 homologs were visible elsewhere in the ooplasm (not shown). If the left spindle pole became the egg pronucleus, this Ø⇔X4 oocyte would produce a nullo-X, nullo-4 progeny, while the right pole would result in euploid progeny.
Discussion
Our ald results are in good agreement with the prediction that the rates of chromosome malorientation at metaphase arrest should be equal to the rates of genetic NDJ and support the model that nondisjunctional segregations are established by events during chromosome congression in these mutants. These results also provide a straightforward explanation for how NDJ can still occur in the presence of normal congression, by causing the congressed chromosome mass to be assembled with the homologs maloriented. These oocytes may have successfully gotten their chromosomes into a single DNA mass, but they have not achieved a configuration that will result in proper chromosome segregation; a rather apt analogy for this situation would be a skydiver’s parachute that was packed incorrectly. This finding suggests that one of the functions of the elaborate movements of nonexchange chromosome during Drosophila female meiosis is to ensure that the metaphase-arrested chromosome mass establishes this configuration properly. While we have not yet achieved our stated goal of completely determining the causes of meiotic nondisjunction, we have demonstrated a proximal mechanism that restricts both the possible types of defect induced by these mutant alleles and the time frame when they are induced. Live imaging of intermediate prometaphase and the process of congression will probably be the most direct way to further characterize the exact defects that these mutations cause. Likewise, our results for nod confirm that the nonexchange chromosomes that are ejected from the main meiotic spindle are mostly unable to reassociate with it by metaphase arrest, and that those chromosomes that fail to do so are eventually lost. Our data show that the original cytological study of nod mutant defects (Theurkauf and Hawley 1992) was not adversely affected by the use of unstaged oocytes. Furthermore, our larger data set allows us to predict NDJ rates, and identify the small number of meiosis that lead to diplo progeny, suggesting these arise from the recapture of both homologs by the same pole of the spindle.
Several previous studies had reported that the frequencies of maloriented chromosomes observed out on the spindle were also similar to the nondisjunction frequencies in genetic data (Theurkauf and Hawley 1992; Harris et al. 2003; Gilliland et al. 2005; Xiang and Hawley 2006). While this observation was taken to confirm the prior model of metaphase arrest, now it is known that this chromosomes-out configuration is an intermediate stage of prometaphase. In the present study, we show that chromosome malorientation rates at metaphase arrest are likewise equal to the genetic nondisjunction rates. This equality of the malorientation rates in both prometaphase and metaphase-arrested oocytes allows us to infer how congression probably proceeds in these genotypes. As each oocyte was at a particular time point in meiosis when it was fixed, the abundance of fixed oocytes in any configuration should be proportional to the length of time that oocytes dwell in that configuration during oogenesis. Live imaging of wild-type oocytes shows that congression is a slow process, with nonexchange chromosomes contracting without changing positions relative to other chromosomes over at least several hours (Gilliland et al. 2009). Therefore, the agreement of cytological rates at these two time points suggests that malorientation in mutant oocytes is established prior to congression, and then stabilized for the extended period that chromosomes need to complete congression.
Our data allow us to draw several further conclusions. First, our data provide direct validation of Cooper’s method of doubling the number of X NDJ progeny observed when estimating nondisjunction rates. This doubling is necessary because while all normal oocytes are expected to be viable with any sperm genotype, X nondisjunctional oocytes will produce viable progeny for only one of the two types of sperm. Therefore for every X NDJ progeny observed, another is expected to have died unseen. For the 4, both normal and NDJ progeny are inviable with one of the two sperm genotypes, and therefore no proportional correction is necessary. Viability is not an issue for the cytological data, which should reflect the meiotic segregation rate. We found that across all ald alleles, the frequency of X maloriented oocytes (14.9%) was 2.14 times the uncorrected frequency of X nondisjunctional progeny (6.9%), quite consistent with Cooper’s method. The same comparison cannot be made directly for chromosome 4 rates, as half the X–4 doubles would still die due to X chromosome content in the sperm, but if the number of X–4 double NDJ progeny is doubled, then the frequency of 4 maloriented oocytes (11.3% across all alleles) is only 1.12 times the frequency of 4 NDJ progeny (10.1%), again supporting Cooper’s methodology. In addition to validating the historical inference, this finding also allows us to be confident that viability differences between the normal and nondisjunctional progeny classes are changing the genetic estimate of NDJ rates by at most a few percent, at least for these mutants.
Second, X and 4 NDJ in ald mutants are known to be nonindependent, with a higher rate of X and 4 double nondisjunctional progeny than predicted by chance, and an excess of heterologous (XX⇔44) over nonheterologous (XX44⇔Ø) segregations (O’Tousa 1982). We see a similar lack of independence and excess of heterologous segregations in both our genetic and cytological data, although we note that nonheterologous doubles are rather more abundant in the cytological data. One possible reason for this higher abundance could be that some oocytes in that category have actually oriented the XX44 chromosomes away from one (or both) chiasmate autosomes. While NDJ of chiasmate autosomes in ald mutants is clearly possible, as it allowed for the recovery of new ald alleles in a germline clone screen (Page et al. 2007), in the NDJ assay used here all such progeny would die and therefore could not be observed genetically.
Third, our nod data also indicate that this mutant likely causes abnormal 4 behavior in nearly 100% of meioses, including those that produce oocytes with a normal chromosome complement. Even though ∼12% of meiotic spindles are predicted to produce normal X4 oocytes, most of those (20.5/24.5) are from spindles where the other 4 was off the spindle. We can therefore conclude that most of the euploid progeny of nod females were likely from abnormal meioses where the only 4 that remained on the spindle formed the pronucleus. Likewise, the number of oocytes with both 4 homologs facing the same pole (7) is greater than the number of oocytes with proper 4 coorientation (4). If nod causes the two 4 homologs to associate with the spindle independently (a reasonable assumption if the DNA tethers are breaking), then these two classes are expected to be equal. Therefore, it is possible that even the 2% of oocytes with normal 4 biorientation achieved this configuration by chance and not through normal segregation.
Fourth, our data reveal some of the mechanistic differences that lead to NDJ vs. chromosome loss. The observation that ald mutations primarily cause NDJ without significant loss is consistent with the observation that all metaphase-arrested chromosomes are found in a single mass at wild-type rates in ald females (the exceptions being oocytes still in prometaphase, as indicated by their incompletely formed dorsal appendages). Previous studies of ald found a loss of sister chromatid cohesion in both fixed and live images (Gilliland et al. 2005, 2007). While this was interpreted as resulting in a premature entry into anaphase, based on the current work it is clear that these mutants must subsequently complete congression to a single DNA mass, indicating that loss of cohesion is not sufficient to induce entry into meiotic anaphase. One possible mechanism that could explain both the NDJ as well as the excess of heterologous segregations would be if the loss of sister chromatid cohesion leads to too many chromosomes entering the distributive system. This would require multiple chromosomes to rely on their heterochromatin tethers to achieve coorientation (Hughes et al. 2009). If the tether connecting the X homologs were to become entangled with the tether connecting the 4 homologs, then tension could be established by orienting XX and 44 toward opposite poles, resulting in a heterologous double NDJ event in a manner directly analogous to two interlocked chiasmate bivalents each monooriented to opposite poles (Nicklas 1974). In contrast, nod mutations primarily cause chromosome loss, but can still produce some diplo progeny. Live imaging in a nod background revealed that the nonexchange chromosomes moved rapidly back and forth across the meiotic spindle, followed by an abrupt change in behavior where a chromosome was suddenly ejected from the spindle (Hughes et al. 2009), although subsequent live imaging has indicated that ejection often happens much earlier in prometaphase, without this prolonged back-and-forth movement (K. A. Collins and R. S. Hawley, personal communication). One interpretation is that the lack of plateward force provided by Nod destabilizes these chromosomes, but the chromosomes are restricted to the spindle until the DNA tether breaks. By metaphase arrest, chromosomes on and off the spindle appear to have become more compact (as in wild type) but those chromosomes that fail to rejoin the chiasmate chromosome mass may become lost. The present study cannot rule out the possibility that these nonexchange chromosomes would later rejoin the main mass (Figure 4), although we note that the nod cytological data has more nullo-4 and fewer diplo-4 malorientations than the genetic data, a pattern that is consistent with these chromosomes rejoining the main mass over time. This question could be addressed in a time series experiment, where progressively older females were fixed and the proportions of nullo-4 oocytes estimated.
Finally, in addition to informing how nondisjunction arises during meiosis I, our data suggest a number of future questions to address. First, if nonexchange chromosomes are able to congress while in a maloriented configuration, what mechanism is allowing them to do so? With both homologous kinetochores monooriented toward the same pole, something must generate the opposing force that moves the chromosomes toward the metaphase plate to rejoin the main chromosome mass. Second, what is the role of the SAC in reaching metaphase I arrest? The present results suggest the SAC is dispensable for achieving metaphase arrest in female meiosis, although we have not examined a complete null background due to viability issues. This is concordant with other evidence that the SAC is dispensable in flies from a study on mad2 knockout flies, which had no NDJ in female meiosis (Buffin et al. 2007). Third, if chromosomes are unable to repair a maloriented configuration, it suggests that Ald/Mps1 may play a more direct role in establishing proper chromosome coorientation than just delaying cell-cycle progression to allow time for reorientations to occur. One experiment that may start to answer these questions would be an aging series in ald mutant females, comparing the proportion of oocytes that reach metaphase arrest in progressively older virgin females. If the main role of the Ald is delaying metaphase arrest to allow time for reorientation, then these mutants would be predicted to reach metaphase arrest faster than in wild type. Conversely, if Ald protein is directly involved in establishing reorientations, then cell cycle progression in the mutants should be delayed.
Supplementary Material
Acknowledgments
The present work would not have been possible without the encouragement and support of Scott Hawley and his lab, and his generosity is gratefully acknowledged. We also thank the anonymous reviewers for their helpful comments and suggestions. This work was supported by the National Institutes of Health (NIGMS R15 award 1R15GM099054-01 to W.D.G.) and the DePaul College of Liberal Arts and Sciences (Undergraduate Research Council and Faculty Summer Research Grant awards to W.D.G., an Undergraduate Research Assistant Program award to W.P., and a teaching assistantship to S.G.).
Footnotes
Communicating editor: M. Colaiácovo
Literature Cited
- Buffin E., Emre D., Karess R., 2007. Flies without a spindle checkpoint. Nat. Cell Biol. 9: 565–572 [DOI] [PubMed] [Google Scholar]
- Carpenter A. T., 1973. A meiotic mutant defective in distributive disjunction in Drosophila melanogaster. Genetics 73: 393–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K. W., 1948. A new theory of secondary non-disjunction in female Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 34: 179–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W., Sproul L. R., Gustafson S. M., Matthies H. J., Gilbert S. P., et al. , 2005. Drosophila Nod protein binds preferentially to the plus ends of microtubules and promotes microtubule polymerization in vitro. Mol. Biol. Cell 16: 5400–5409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg A. F., Sedat J. W., Hawley R. S., 1996. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86: 135–146 [DOI] [PubMed] [Google Scholar]
- Ferree P. M., Barbash D. A., 2009. Species-specific heterochromatin prevents mitotic chromosome segregation to cause hybrid lethality in Drosophila. PLoS Biol. 7: e1000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M., Heeger S., Hacker U., Lehner C., 2004. The mitotic arrest in response to hypoxia and of polar bodies during early embryogenesis requires Mps1. Curr. Biol. 14: 2019–2024 [DOI] [PubMed] [Google Scholar]
- Gilliland W. D., Wayson S. M., Hawley R. S., 2005. The meiotic defects of mutants in the Drosophila mps1 gene reveal a critical role of Mps1 in the segregation of achiasmate homologs. Curr. Biol. 15: 672–677 [DOI] [PubMed] [Google Scholar]
- Gilliland W. D., Hughes S. E., Cotitta J. L., Takeo S., Xiang Y., et al. , 2007. The multiple roles of mps1 in Drosophila female meiosis. PLoS Genet. 3: e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland W. D., Hughes S. F., Vietti D. R., Hawley R. S., 2009. Congression of achiasmate chromosomes to the metaphase plate in Drosophila melanogaster oocytes. Dev. Biol. 325: 122–128 [DOI] [PubMed] [Google Scholar]
- Hanley J. A., Lippman-Hand A., 1983. If nothing goes wrong, is everything all right? Interpreting zero numerators. JAMA 249: 1743–1745 [PubMed] [Google Scholar]
- Harris D., Orme C., Kramer J., Namba L., Champion M., et al. , 2003. A deficiency screen of the major autosomes identifies a gene (matrimony) that is haplo-insufficient for achiasmate segregation in Drosophila oocytes. Genetics 165: 637–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold T., Hunt P., 2001. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2: 280–291 [DOI] [PubMed] [Google Scholar]
- Hawley R., Irick H., Haddox D., Whitley M., Arbel T., et al. , 1993. There are two mechanisms of achiasmate segregation in Drosophila females, one of which requires heterochromatic homology. Dev. Genet. 13: 440–467 [DOI] [PubMed] [Google Scholar]
- Hawley R. S., Gilliland W. D., 2006. Sometimes the result is not the answer: the truths and the lies that come from using the complementation test. Genetics 174: 5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. E., Gilliland W. D., Cotitta J. L., Takeo S., Collins K. A., et al. , 2009. Heterochromatic threads connect oscillating chromosomes during prometaphase I in Drosophila oocytes. PLoS Genet. 5: e1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. E., Beeler J. S., Seat A., Slaughter B. D., Unruh J. R., et al. , 2011. Gamma-tubulin is required for bipolar spindle assembly and for proper kinetochore microtubule attachments during prometaphase I in Drosophila oocytes. PLoS Genet. 7: e1002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake C. M., Hawley R. S., 2012. The molecular control of meiotic chromosomal behavior: events in early meiotic prophase in Drosophila oocytes. Annu. Rev. Physiol. 74: 425–451 [DOI] [PubMed] [Google Scholar]
- Lamb N. E., Sherman S. L., Hassold T. J., 2005. Effect of meiotic recombination on the production of aneuploid gametes in humans. Cytogenet. Genome Res. 111: 250–255 [DOI] [PubMed] [Google Scholar]
- Nicklas R. B., 1974. Chromosome segregation mechanisms. Genetics 78: 205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver T. R., Feingold E., Yu K., Cheung V., Tinker S., et al. , 2008. New insights into human nondisjunction of chromosome 21 in oocytes. PLoS Genet. 4: e1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Tousa J., 1982. Meiotic chromosome behavior influenced by mutation: altered disjunction in Drosophila melanogaster females. Genetics 102: 503–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. L., Nielsen R. J., Teeter K., Lake C. M., Ong S., et al. , 2007. A germline clone screen for meiotic mutants in Drosophila melanogaster. Fly (Austin) 1: 172–181 [DOI] [PubMed] [Google Scholar]
- Sullivan W., Ashburner M., Hawley R. S., 2000. Drosophila Protocols, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Theurkauf W. E., Hawley R. S., 1992. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol. 116: 1167–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Hawley R. S., 2006. The mechanism of secondary nondisjunction in Drosophila melanogaster females. Genetics 174: 67–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Li H., Schweppe N. M., Hawley R. S., Gilliland W. D., 2010. Statistical Analysis of Nondisjunction Assays in Drosophila. Genetics 186: 505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Hawley R. S., 1990. The genetic analysis of distributive segregation in Drosophila melanogaster. II. Further genetic analysis of the nod locus. Genetics 125: 115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Knowles B. A., Goldstein L. S., Hawley R. S., 1990. A kinesin-like protein required for distributive chromosome segregation in Drosophila. Cell 62: 1053–1062 [DOI] [PubMed] [Google Scholar]
- Zitron A. E., Hawley R. S., 1989. The genetic analysis of distributive segregation in Drosophila melanogaster. I. Isolation and characterization of Aberrant X segregation (Axs), a mutation defective in chromosome partner choice. Genetics 122: 801–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.