Abstract
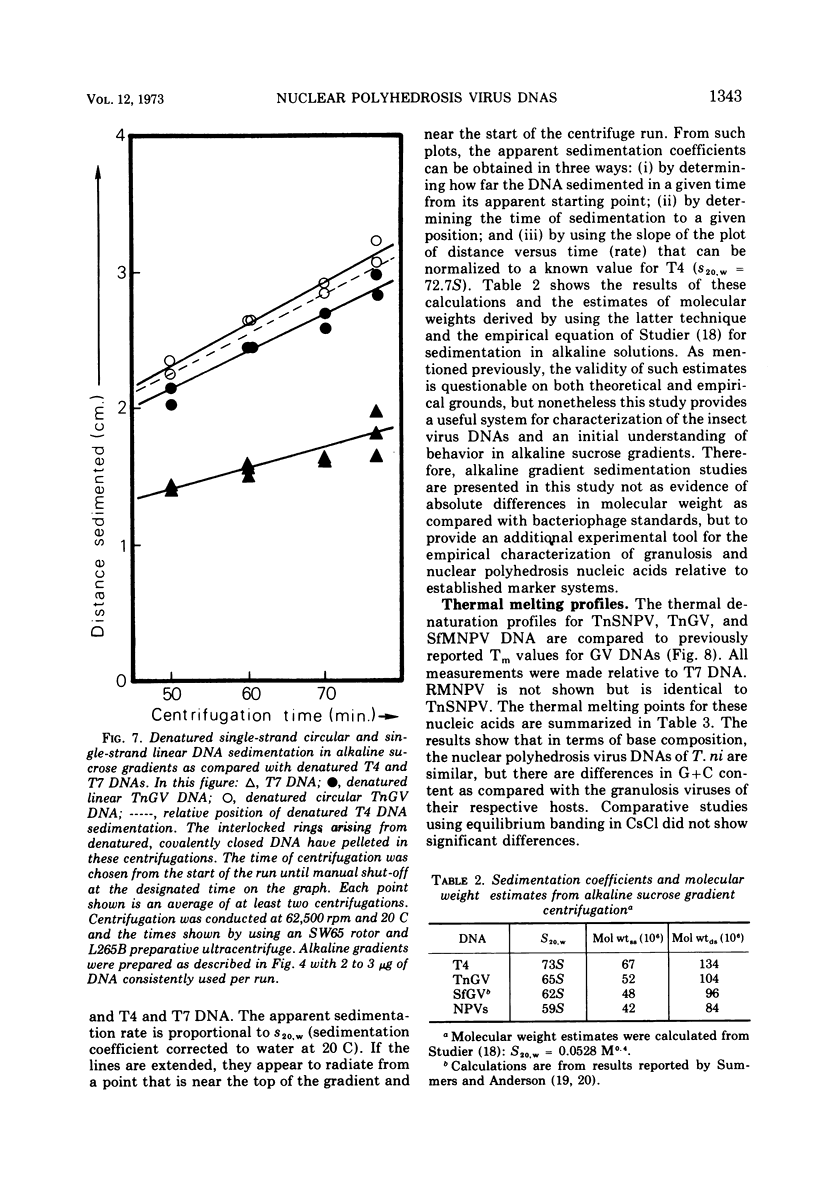
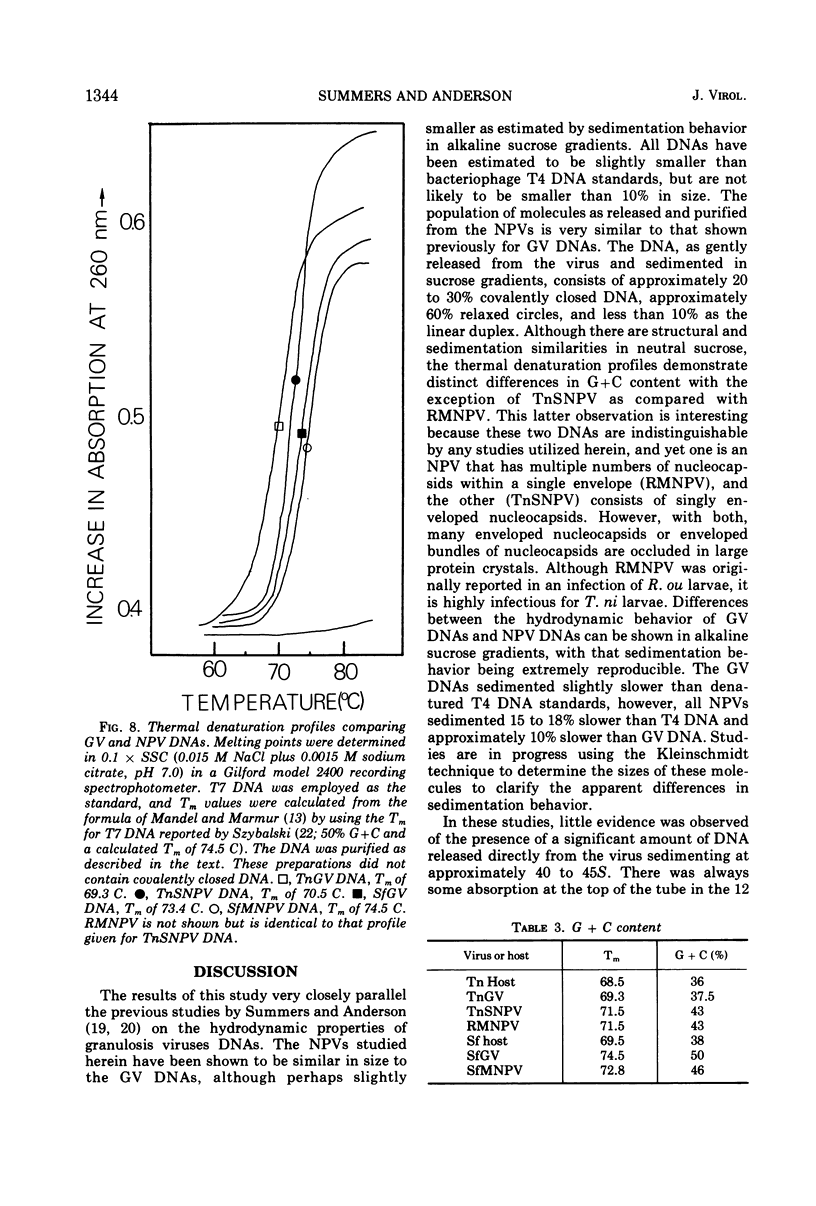
The nuclear polyhedrosis virus DNAs characterized and compared in this study consist of the singly-enveloped nucleocapsids (SNPV) of Trichoplusia ni and the bundles of nucleocapsids common to a single envelope (MNPV) from Spodoptera frugiperda and Rachiplusia ou. The SNPV and MNPV DNAs are very similar in hydrodynamic properties and molecular weights. In addition, the NPV DNAs are similar in size to those extracted from the granulosis viruses that infect T. ni and S. frugiperda. As isolated from purified virus or directly from occluded virus, the nuclear polyhedrosis virus DNAs consist of a mixture of about 20 to 30% double-stranded covalently closed molecules and approximately 60% relaxed circles, with less than 10% in linear duplex form. The molecular weights of all nuclear polyhedrosis virus DNAs as compared in this study are slightly smaller than those of T4 bacteriophage DNA and perhaps slightly smaller than those of the granulosis virus DNAs. The best estimates of these molecular weights by neutral sucrose sedimentation for the nuclear polyhedrosis viruses range from 90 to 100 × 106 relative to a size of 108 × 106 for T4 DNA. The base compositions of the nuclear polyhedrosis viruses that infect T. ni and S. frugiperda are compared with the respective insect host DNAs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode V. C., MacHattie L. A. Electron microscopy of superhelical circular lambda DNA. J Mol Biol. 1968 Mar 28;32(3):673–679. doi: 10.1016/0022-2836(68)90350-1. [DOI] [PubMed] [Google Scholar]
- Böttger M., Kuhn W. Sedimentation analysis of conformation changes of circular PM 2 DNA in relation to the ionic strength. Biochim Biophys Acta. 1971 Dec 30;254(3):407–411. doi: 10.1016/0005-2787(71)90872-0. [DOI] [PubMed] [Google Scholar]
- Faulkner P., Henderson J. F. Serial passage of a nuclear polyhedrosis disease virus of the cabbage looper (Trichoplusia ni) in a continuous tissue culture cell line. Virology. 1972 Dec;50(3):920–924. doi: 10.1016/0042-6822(72)90448-5. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Studies on Escherichia coli sex factors. 3. Covalently closed F'Lac DNA molecules. J Mol Biol. 1968 May 28;34(1):31–38. doi: 10.1016/0022-2836(68)90232-5. [DOI] [PubMed] [Google Scholar]
- Gafford L. G., Randall C. C. The high molecular weight of the fowlpox virus genome. J Mol Biol. 1967 Jun 14;26(2):303–310. doi: 10.1016/0022-2836(67)90299-9. [DOI] [PubMed] [Google Scholar]
- Laird C. D., McCarthy B. J. Magnitude of interspecific nucleotide sequence variability in Drosophila. Genetics. 1968 Oct;60(2):303–322. doi: 10.1093/genetics/60.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod M. G., Lehmann A. R. Artefacts arising from the sedimentation of high molecular weight DNA on sucrose gradients. Biochim Biophys Acta. 1971 Oct;247(3):369–372. doi: 10.1016/0005-2787(71)90021-9. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Shvedchikova N. G., Tarasevich L. M. Electron microscope investigation of granulosis viruses of Dendrolimus sibiricus and Agrotis segetum. J Invertebr Pathol. 1971 Jul;18(1):25–32. doi: 10.1016/0022-2011(91)90004-a. [DOI] [PubMed] [Google Scholar]
- Summers M. D., Anderson D. L. Characterization of deoxyribonucleic acid isolated from the granulosis viruses of the cabbage looper, Trichoplusia ni and the fall armyworm, Spodoptera frugiperda. Virology. 1972 Nov;50(2):459–471. doi: 10.1016/0042-6822(72)90397-2. [DOI] [PubMed] [Google Scholar]
- Summers M. D., Anderson D. L. Granulosis virus deoxyribonucleic acid: a closed, double-stranded molecule. J Virol. 1972 Apr;9(4):710–713. doi: 10.1128/jvi.9.4.710-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upholt W. B., Gray H. B., Jr, Vinograd J. Sedimentation velocity behavior of closed circular SV40 DNA as a function of superhelix density, ionic strength, counterion and temperature. J Mol Biol. 1971 Nov 28;62(1):21–38. doi: 10.1016/0022-2836(71)90128-8. [DOI] [PubMed] [Google Scholar]
- Zherebtsova E. N., Strokovskaya L. I., Gudz-Gorban A. P. Subviral infectivity in nuclear polyhedrosis of the great wax moth (Galleria mellonella L.). Acta Virol. 1972 Sep;16(5):427–431. [PubMed] [Google Scholar]




