Abstract
Purpose.
Retinal hemorrhages occur in a variety of sight-threatening conditions including ocular trauma, high altitude retinopathy, and chronic diseases such as diabetic and hypertensive retinopathies. The goal of this study is to investigate the effects of blood in the vitreous on retinal vascular function in rats.
Methods.
Intravitreal injections of autologous blood, plasma kallikrein (PK), bradykinin, and collagenase were performed in Sprague-Dawley and Long-Evans rats. Retinal vascular permeability was measured using vitreous fluorophotometry and Evans blue dye permeation. Leukostasis was measured by fluorescein isothiocyanate–coupled concanavalin A lectin and acridine orange labeling. Retinal hemorrhage was examined on retinal flatmounts. Primary cultures of bovine retinal pericytes were cultured in the presence of 25 nM PK for 24 hours. The pericyte-conditioned medium was collected and the collagen proteome was analyzed by tandem mass spectrometry.
Results.
Intravitreal injection of autologous blood induced retinal vascular permeability and retinal leukostasis, and these responses were ameliorated by PK inhibition. Intravitreal injections of exogenous PK induced retinal vascular permeability, leukostasis, and retinal hemorrhage. Proteomic analyses showed that PK increased collagen degradation in pericyte-conditioned medium and purified type IV collagen. Intravitreal injection of collagenase mimicked PK's effect on retinal hemorrhage.
Conclusions.
Intraocular hemorrhage increases retinal vascular permeability and leukostasis, and these responses are mediated, in part, via PK. Intravitreal injections of either PK or collagenase, but not bradykinin, induce retinal hemorrhage in rats. PK exerts collagenase-like activity that may contribute to blood–retinal barrier dysfunction.
Intravitreal bleeding increases retinal vascular permeability and leukostasis, and these responses are mediated via plasma kallikrein (PK). Intravitreal injections of PK induce retinal hemorrhage in rats. PK exerts collagenase-like activity that may contribute to blood–retinal barrier dysfunction.
Introduction
Retinal hemorrhage is a hallmark of ocular trauma and sight-threatening retinopathies, including diabetic and hypertensive retinopathies. In infants, retinal hemorrhages have long been linked with child abuse caused by the shaken baby/shaking-impact syndrome.1,2 Retinal hemorrhage also commonly occurs during exposure to high altitudes.3 Unless severe, these transient intraocular hemorrhages in otherwise healthy eyes usually resolve spontaneously without long-term effects on vision. However, retinal and vitreous hemorrhages also commonly occur in certain chronic diseases, such as diabetic retinopathy. The incidence and severity of retinal hemorrhage often increase with retinopathy disease progression. Recent proteomic analyses of vitreous fluid obtained from patients with advanced diabetic retinopathy have revealed abundant quantities of intracellular red blood cell proteins, including hemoglobin and carbonic anhydrase-1 (CA-1),4 suggesting that intraocular bleeding markedly alters the vitreous proteome. In diabetic retinopathy, intraretinal hemorrhages can occur at all stages of the disease, and this bleeding has been attributed to vascular dysfunction and rupture.5 In addition, preretinal and vitreous hemorrhage can occur from fragile newly formed vessels generated during proliferative diabetic retinopathy. Retinal and vitreous hemorrhage can lead to blurred vision, spots, lines, or streaks in the field of vision. The mechanisms contributing to these recurrent retinal hemorrhages in diabetic retinopathy and the potential effects of intraocular blood on the retina are not fully understood.
Previously, we have reported that extracellular erythrocyte CA-1 in the vitreous activates plasma kallikrein (PK) and thereby increases retinal vascular permeability (RVP).4 Plasma prekallikrein (PPK) is one of the most abundant protease zymogens in blood, and undergoes activation to PK by factor XII (FXII) following interactions with negatively charged surfaces,6 activated platelets,7 mast cells,8 and misfolded proteins.9 The reciprocal conversion of FXII to factor XIIa by PK initiates the intrinsic coagulation pathway via the activation of factor XI. PK mostly circulates (∼75%) as a complex with high-molecular-weight kininogen (HK) and induces the release of bradykinin (BK), which activates the G-protein coupled BK 2 (B2) receptor. Subsequent cleavage of bradykinin by carboxypeptidases generates des-Arg9-BK, which activates the BK 1 (B1) receptor. Activation of B1 and B2 receptors exerts an array of effects on inflammation, vasodilatation, and an increase in vessel permeability.10,11
Our group and others have previously shown that intravitreal injection of BK or PK increased RVP and bradykinin's effect was inhibited by the B2 receptor antagonist Hoe-140.11,12 We have also shown that treatment with a selective PK inhibitor, ASP-440, reduced RVP in rats with hypertension or streptozotocin-induced diabetes.12,13 While these studies have revealed a role of the kallikrein-kinin system in RVP, the effects of this system in retinal hemorrhage are not yet available. Contact system activation of PPK/FXII leads to FXI activation and the intrinsic coagulation pathway, which may contribute to hemostasis. Indeed, PK-deficient mice display reduced thrombosis.14,15 However, in the congenital PPK deficiency, there is a peculiar discrepancy between a severe in vitro defect and bleeding diathesis.14,16 Recently, we have shown that PK can interfere with collagen-induced platelet activation leading to intracerebral hematoma expansion in rodent models.17 These reports have revealed that PK can exert effects on thrombosis and hemostasis at multiple levels. In the current study, we investigated the effects of autologous blood and PK in the vitreous on retinal vascular functions and hemorrhage.
Methods
RVP Measurement by Evans Blue Dye Permeation
Male Sprague-Dawley (SD) rats (250 g) were obtained from Taconic Farms. Experiments were performed in accordance with guidelines from the Association for Research in Vision and Ophthalmology and with approval from the Animal Care and Use Committee of the Joslin Diabetes Center. RVP was measured by the Evans blue dye permeation technique.12 Under anesthesia, a 31-G needle was inserted into the vitreal cavity of the rats through the sclera at 2 mm below the limbus and the needle tip positioned above the optic nerve by direct observation. The vitreous of rat eyes were injected using a Hamilton syringe with either 10 μL of autologous blood or a mixture of autologous blood with 5 μM 1-benzyl-1H-pyrazole-4-carboxylic acid 4-carbamimidoyl-benzylamide (BPCCB; Creagen Biosciences, Woburn, MA). We have previously shown that 10 μL intravitreal injection volume in rats does not significantly affect retinal blood flow.18 The escape of injected fluid from the injection site during needle withdrawal was estimated at less than 10% of the injected volume. Twenty-four hours later, rats were infused with Evans blue dye (45 mg/kg, Sigma-Aldrich, St. Louis, MO) through a 1-mm polyvinyl catheter (Braintree Scientific, Braintree, MA) which was inserted into the right jugular vein. The dye was allowed to circulate for 2 hours prior to sacrifice. Following tissue fixation, the eyes were enucleated. The retina from each eye was extracted with dimethylformamide and the resulting supernatant was used to determine Evans blue tissue content. Results are expressed as μL plasma per gram of dry retina tissue per hour.
RVP Measurement by Fluorescein Leakage
Under anesthesia, eyes were dilated and a focused image of the retina was obtained using a Rodenstock scanning laser ophthalmoscope (SLO). Video fluorescein angiography (VFA) was performed under fixed gain and power settings. Video sequences were digitized with 8 bits of grayscale by a capture board (Matrox Orion AGP; Matrox Electronic Systems Ltd., Dorval, Quebec, Canada) at a rate of 30 frames/second. Each image represents an average of three consecutive TIFF frames to improve signal to noise levels. Rats were placed in a three-axis holder for positioning of the eye and the optic nerve head was centered in the field of view. Focusing was achieved by the addition of a +20-diopter (D) contact lens and additional instrument correction of +3 to +7 D. Focus was centered on the retinal nerve fiber layer and primary retinal vessels before VFA were recorded. Baseline video sequences of retinal fluorescein transit (excitation 488 nm, emission 520 nm, band pass 515–550 nm) were performed by injection of 5 μL sodium fluorescein through an indwelling jugular catheter. Intravitreal injection of activated contact system (100 nM purified PPK, FXII, and HK from human plasma, American Diagnostics, Deerfield Beach, FL) or BSS was performed. After 10 minutes, a secondary infusion of 300 mL/kg 10% sodium fluorescein was given systemically via a jugular vein catheter. Postintravitreal injection images were obtained to observe fluorescein leakage by the retinal vasculature.
To quantify acute RVP, rat eyes were dilated and received intravitreal injections of purified PK (500 ng, final concentration in the vitreous is 34 nM); bradykinin (1 μM, Calbiotech, Spring Valley, CA); or PBS alone. Fifteen minutes after the injection, 300 mL/kg 10% sodium fluorescein was infused via a jugular vein catheter. At 15 minutes after dye infusion, RVP was measured by quantifying vitreous fluorescein levels by vitreous fluorophotometry (VFP), as described previously.4 We have previously shown that intravitreal injection of saline, human serum albumin, and human C1-INH does not significantly increase RVP.4
Retinal Leukostasis Analysis Using Concavalin A
Male Long-Evans (LE) rats (Taconic Farms, Hudson, NY) with initial body weight of 200 g were used for leukostasis measurement because the retinal pigment epithelium improves contrast between fluorescent leukocytes and the choroidal background. BPCCB (40 μg/kg body weight per hour) or its vehicle (5% polyethylene glycol in PBS) was delivered by subcutaneous osmotic pump (ALZET, Cupertino, CA) into rats 48 hours before injection. Twenty-four hours after intravitreal injection of 10 μL autologous blood or PBS, rats were perfused with 30 mL PBS to eliminate erythrocytes and nonadherent leukocytes. Fluorescein-isothiocyanate (FITC)-coupled Concanavalin A (Con A) lectin (20 μg/mL in PBS, 5 mg/kg body weight; Vector Labs, Burlingame, CA) was infused to label adherent leukocytes and vascular endothelial cells. Rats were then perfused with 30 mL 4% paraformaldehyde followed by 25 mL 1% Albumin in PBS.19 Retinas were mounted on a glass slide and imaged using a fluorescence microscope (Olympus FSX100; Olympus, Center Valley, PA). Adherent leukocytes were counted in the upper retinal plane closest to the inner limiting membrane. Images were obtained at a 10× magnification centered at the optic nerve head. A composite image of each retina was created from the 10× images to illustrate a 1.5- × 1.5-mm area of the retina. Adherent leukocytes were counted within the visible vascular areas.
Retinal Leukostasis Analysis Using Acridine Orange
Male LE rats were subjected to intravitreal injections of activated contact system or BSS alone. Twenty-four hours after injection, rats were placed in front of an SLO (Rodenstock, Munich, Germany) with an Argon light source. A fluorescent barrier filter was placed in the optical path and acridine orange (AO, 4 mg/kg body weight; Sigma-Aldrich) was infused at 1.5 mL/minute. Twenty minutes after the AO infusion, the focus of the SLO was centered on the retinal nerve fiber layer and fluorescent scans of the retina were obtained of static leukocytes within the retinal capillaries from nine segments (three optic disc diameters radially from the center) of the retina. Digitized images were analyzed using numerical computing software (MATLAB; MathWorks, Natick, MA).
Retinal Hemorrhage
The vitreous of rat eyes were injected with 10 μL bolus of PK (500 ng); bradykinin (1 μM); collagenase (100 ng, Sigma-Aldrich); or PBS alone in 10 μL final volume. A repeated injection was performed 24 hours after the first injection. Twenty-four hours after the second injection, the rats were perfused with saline via the left ventricle. The retinas were harvested and flatmounted on slides with antifade mounting medium. Mounted retinas were examined with a dissection scope (Zeiss Stemi 2000C; Carl Zeiss Meditec, Munich, Germany) with a digital camera (Olympus QColor3; Olympus, Melville, NY) mounted on the observation port. Retinal images were captured and digitized using imaging software (QCapture Pro 6; QImaging, Surry, BC, Canada). Retinas showing bleeding spots were determined as retinas with the presence of hemorrhages.
Cell Culture and Extraction of the Secreted Proteins
Fresh calf eyes were obtained from a local abattoir. Primary cultures of bovine retina pericytes (BRPC) were isolated by homogenization and a series of filtration steps as described previously.20 BRPC were cultured in Dulbecco's modified Eagle medium (DMEM) with 5.5 mM glucose and 20% FBS. Cells (70% confluent) from passages 2 through 5 were incubated in serum-free DMEM-F12 media containing 0.1% BSA for 16 hours and then cultured in fresh serum-free media, in the presence or absence of 25 nM PK, for 24 hours. The pericyte-conditioned medium was collected, centrifuged at 20,800g for 30 minutes, and filtered through a 0.22-μm filter. Sodium deoxycholate was added to 0.02% final concentration. The mixture was incubated on ice for 30 minutes and precipitated in 15% trichloroacetic acid. The samples were kept at 4°C for 1 hour and then spun at 20,800g for 15 minutes. The pellets were resuspended in 1% SDS, 75 mM Tris/HCl (pH 8.0). The protein concentration was determined by Bradford reagent (Bio-Rad Laboratories, Hercules, CA).
Identification of Protein by LC-MS/MS
Precipitated proteins from pericyte-conditioned medium were separated by 10% SDS-PAGE. The SDS-PAGE gel was stained with Coomassie Brilliant Blue (Bio-Rad Laboratories). Each lane from the SDS-PAGE gel for each sample was divided into 40 slices. Gel slices were individually digested with trypsin (Promega, Madison, WI). In-gel tryptic digests from the entire lane were analyzed by tandem mass spectrometry (MS/MS) using an LTQ linear ion trap mass spectrometer (Thermo Fisher, Waltham, MA). Assignment of MS/MS data was performed using open source software (X!Tandem, version 2006.09.15; The Global Proteome Machine Organization, provided in the public domain by http://www.thegpm.org) search against the International Protein Index (IPI) rat sequence database (IPI_RAT_v3.44, http://www.ebi.ac.uk). An in-house program based on PHP-MySQL-Apache platform was used to perform proteomic computational analyses. The protein spectral count for each protein from multiple slices was used to generate grayscale digital images to display the distribution of peptide matches for each protein within the gel lane.21 This visualization technique enables the comparison of protein molecular weight, intensity, and distribution differences among individual samples.
Analyses of Proteolysis In Vitro
For the PK-induced collagen proteolysis study, 20 μg collagen IV (Sigma-Aldrich) from human placenta was incubated with 200 nM PK in 60 μL reaction buffer, in the absence or presence of 20 μM BPCCB at 37°C for 2 hours, 6 hours, and 24 hours, respectively. The mixture was then subjected to SDS-PAGE and the degradation of collagen IV was detected by Coomassie blue staining.
Statistical Analysis
All data are presented as means ± SEM. Statistical analysis was performed by one-way ANOVA followed by Bonferroni's multiple comparisons test or Students' t-test as appropriate. Fisher exact test was used for counting data analysis. Statistically significant differences between groups were defined as P < 0.05 and are indicated in the legends to the figures.
Results
Autologous Blood in the Vitreous Induces RVP
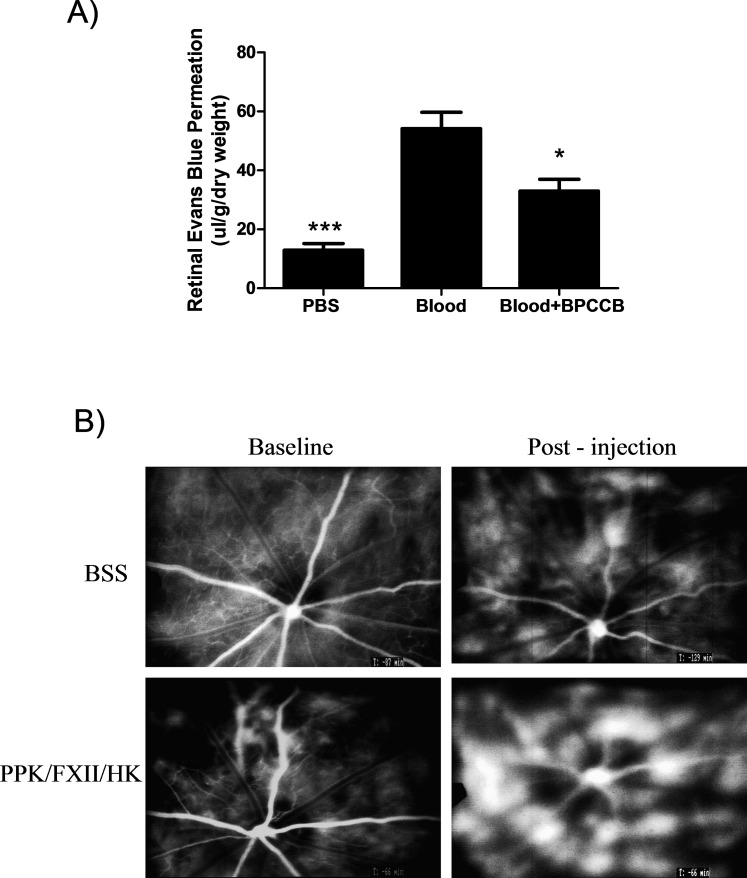
We evaluated the effect of intravitreal injection of autologous blood on RVP in rats using Evans blue dye permeation. RVP in eyes 24 hours after intravitreal injection of blood was 4-fold greater than RVP in control eyes receiving a PBS-injection (PBS: 12.91 ± 2.24 versus blood: 54.19 ± 5.54, P < 0.001; Fig. 1A). Since we have previously shown that PK induces RVP in rats,13 we investigated the role of PK in autologous blood-induced RVP. We show that coinjection of PK inhibitor, BPCCB, with autologous blood significantly inhibited RVP (blood: 54.19 ± 5.54 versus blood + BPCCB: 32.96 ± 4.02, P < 0.05; Fig. 1A), suggesting that PK contributes to hemorrhage-induced RVP. Fluorescein angiography of rat retina showed that intravitreal injection of contact system components, PPK/FXII/HK, increased acute RVP compared with saline injected control (Fig. 1B).
Figure 1. .
Effect of autologous blood on retinal vascular permeability. (A) Retinal Evans blue-albumin permeation was measured 2 hours after intravitreal injection of 10 μL autologous blood (n = 18); 10 μL autologous blood mixed with 5 μM BPCCB (n = 12); or PBS (n = 6) into rat eyes. Blood increased retinal Evans blue permeation by 4-fold compared with PBS injection; the effect of blood on dye permeation was inhibited by BPCCB by 40%. *P < 0.05; ***P < 0.001 versus blood group. (B) Fluorescein angiography of rat retina at baseline and 30 minutes after intravitreal injection of BSS vehicle or activated contact system (PPK/FXII/HK).
Autologous Blood in the Vitreous Induces Leukostasis
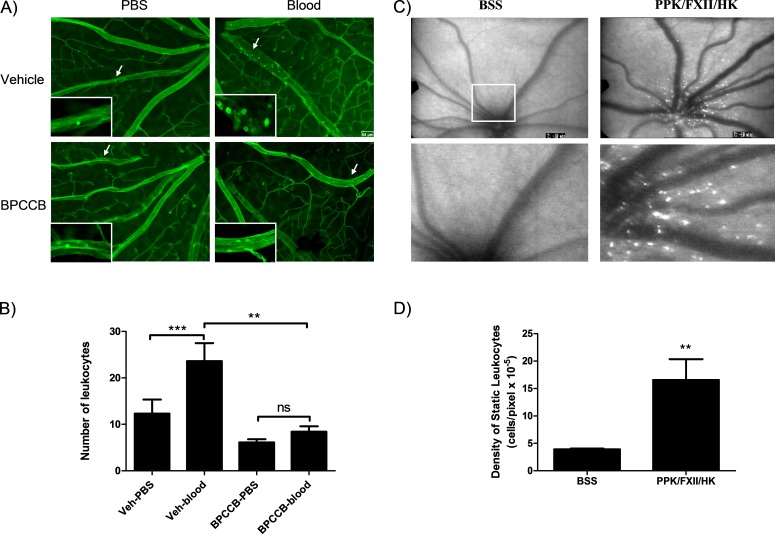
To evaluate the effect of retinal hemorrhage on inflammation, retinal adherent leukocytes were labeled with FITC-Con A lectin in LE rats (Fig. 2A). Total number of adherent leukocytes in the retinal wall was significantly increased in autologous blood-injected rat eyes compared with PBS-injected eyes (Fig. 2B). Three-day systemic administration of PK inhibitor BPCCB significantly decreased retinal leukostasis induced by autologous blood injection. AO fluorescein angiography of SD rat retina showed that intravitreal injection of PPK/FXII/HK increased static leukostasis in the retina 4-fold compared with eyes receiving BSS injections (Figs. 2C, 2D). We observed that Con A mainly detected adherent leukocytes in veins, whereas AO detected static leukocytes in capillaries.
Figure 2. .
Effect of autologous blood on retinal leukostasis. (A) Representative images of adherent leukocytes in retinal vessels of a vehicle-treated rat, a BPCCB treated rat subjected to PBS or 10 μL autologous blood intravitreal injection. The majority of the adherent leucocytes were observed in the veins of the Veh-blood group. Scale bar is 64 μm. (B) The number of adherent leukocytes was 4-fold greater in eyes injected with blood than PBS alone in vehicle group (n = 8). BPCCB treatment reduced the number of blood-induced adherent leukocytes (n = 7) and had no effect on PBS injection. **P < 0.01; ***P < 0.001; ns, no significance. (C) Representative retinal images obtained by SLO from eye following intravitreal injection of either BSS or PPK/FXII/HK. Fluorescent acridine orange bound static leukocytes are observed in a greater number within the vascular retina of contact system treated eyes (top row) compared with BSS injected eyes. Magnified section from each retina is shown in the bottom row of images. (D) Quantification of static leukocytes per mm2. The number of leukocytes was normalized by the total measured area within a 40-degree field of view centered at the optic nerve head. The number of static leukocytes was 4.2 times greater (**P < 0.01) in eyes treated with contact system (n = 5) versus BSS (n = 6) alone.
PK Induces Retinal Hemorrhage in Rats
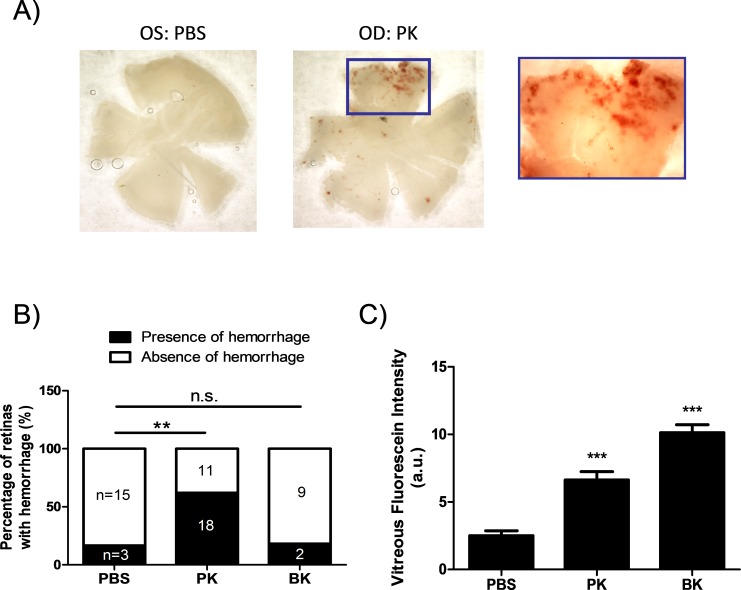
To test the effect of PK on retinal hemorrhage, rats were subjected to either 500 ng/10 μL PK or same volume of PBS injections into the vitreous. Injections were performed twice in 24-hour intervals. Twenty-four hours after the second injection, retina flatmounts were examined under microscope. We observed hemorrhage spots on the retinas from eyes injected with PK (Fig. 3A). As shown in Figure 3B, 18 out of 29 eyes injected with PK had hemorrhage spots, whereas only 3 out of 18 PBS injected eyes had hemorrhage spots (P < 0.01 by Fisher exact test). The majority of hemorrhages appeared in the peripheral retina away from the injection site. No hemorrhages were observed in the eyes subjected to a single injection of PBS (data not shown). However, a low incidence of retinal hemorrhage was detected in eyes subjected to two consecutive vehicle injections.
Figure 3. .
Effect of PK on retinal hemorrhage in rats. (A) Representative images of the retinal hemorrhage 24 hours after the second intravitreal injection of PK into the right eyes (OD) or PBS injection into the left eyes (OS) from rats. (B) The percentage of the number of eyes with or without hemorrhages to the total number of eyes subjected to double intravitreal injection of PK (n = 29); BK (n = 11); or PBS (n = 18) from rats. **P < 0.01 by Fisher exact test for counting data analysis. (C) Effect of exogenous PK and BK on acute RVP in rats. Both PK and BK-induced leakage were greater (***P < 0.001) than the PBS-injected eyes. a.u., arbitrary unit.
Since PK induces the release of bradykinin from HK upon activation, we next examined whether the observed retinal hemorrhages were caused by bradykinin-mediated RVP. Consistent with our previous report,13 intravitreal injection of either PK or bradykinin significantly increased acute RVP compared with PBS injection in rats measured by VFP (PBS: 2.51 ± 0.35 versus PK: 6.64 ± 0.61 or BK: 10.14 ± 0.59; P < 0.001; Fig. 3C). However, only 2 out of 11 eyes showed retinal bleeding after double-intravitreal injection of BK, which was a frequency similar to the observation following saline injections (Fig. 3B). These results suggested that BK-mediated RVP did not contribute to retinal hemorrhage in rats.
PK Induces Proteolysis of Collagen in Pericyte Culture Medium
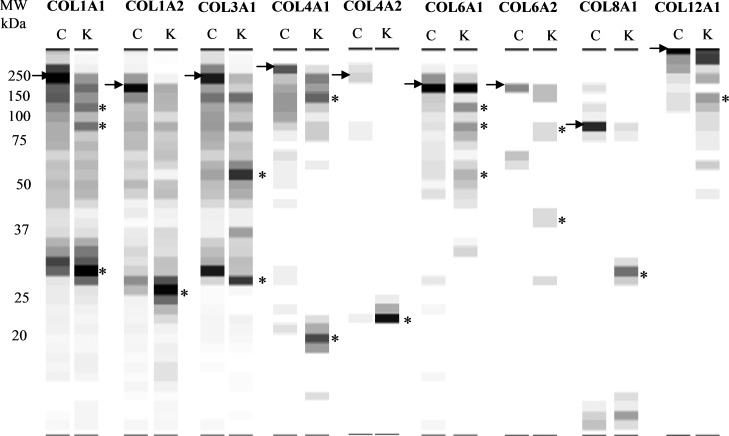
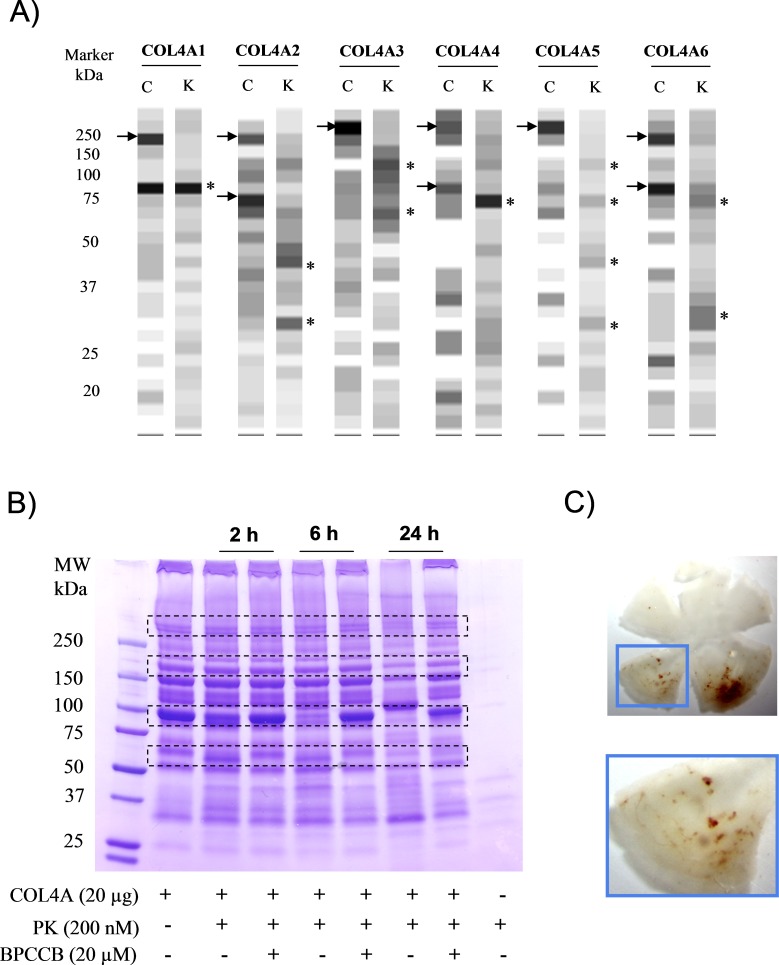
One potential mechanism underlying the effect of PK on retinal hemorrhage could be mediated by bradykinin-independent effects on components of the blood–retinal barrier (BRB). The BRB plays an important role in the maintenance of homeostasis of the microenvironment in the retina. Both astrocyte and pericyte contribute to the integrity of the BRB. We have previously shown that PK induces proteolysis of basement membrane and extracellular matrix proteins such as collagens, laminin β1 and γ1, nidogen 1 and 2, and fibronectin in brain astrocyte culture media.22 Here we performed a proteomic study to characterize the effects of PK on secretome of pericytes. We identified the appearance of low molecular weight fragments between 20 and 40 kDa of collagens in conditioned media from pericytes exposed to 25 nM PK for 24 hours, compared with that from untreated cells. In particular, we found that PK treatment was associated with an increase in low molecular weight fragments of collagen alpha-1(I) (COL1A1); collagen alpha-2(I) (COL1A2); collagen alpha-1(III) (COL3A1); collagen alpha-1(IV) (COL4A1); collagen alpha-2(IV) (COL4A2); collagen alpha-1(VI) (COL6A1); collagen alpha-2(VI) (COL6A2); collagen alpha-1(VIII) (COL8A1); collagen alpha-1(XI) (COL11A1); and collagen alpha-2(XII) (COL12A2; Fig. 4). The appearance of distinct bands of collagen fragments suggests the presence of sites on collagen fibrils that are susceptible to cleavage by PK. The generation of distinct bands of collagen fragments (indicated with *) and the absence of cleavage of other proteins, such as fibronectin 1 isoform 4, preproprotein isoform 2 (data not shown), suggests that collagen contains a limited number of sites that are sensitive to PK cleavage.
Figure 4. .
Effect of PK on collagen proteolysis in pericyte-conditioned media. Extracted spectral counting of collagen displayed as a grayscale digital image from pericyte conditioned media (24 hours) in the absence or presence of 25 nM PK. The spectral counting from each slice was converted to 256 grayscale intensities. The black bands correspond to positions in the gel with the highest numbers of spectral/peptide matches and the white indicates areas where peptides were not detected. C, control; K, PK. Arrow indicates the high molecular weight full length collagen; star indicates collagen fragments.
PK Induces Proteolysis of Purified Collagen In Vitro
Collagen IV is a major constituent of the basement membrane. To determine whether collagen IV is a direct substrate for PK, we incubated PK with purified human COL4A and utilized mass spectrometry to evaluate its cleavage. We found that PK generates fragments from human COL4A (Fig. 5A), which was similar to the generation of rat collagen fragments observed in pericyte-conditioned media (Fig. 4). The differences in the pattern of collagen fragments in rat and human COL4A exposed to PK could be due to sequence differences or modifications of collagen in these species. The PK-induced proteolysis of COL4A was also shown by Coomassie blue staining and changes were blocked by coincubation with the PK inhibitor BPCCB (Fig. 5B). The time course of PK-induced COL4A proteolysis revealed the appearance of collagen chains that were differentially sensitive to cleavage at 2 hours, 6 hours, and 24 hours (Fig. 5B). These findings suggest that PK can exert a direct role in the proteolysis of collagen. To further investigate whether collagenase activity could cause retinal hemorrhage, we injected bacteria collagenase into rat vitreous and observed retinal hemorrhages that were similar in appearance to but more prevalent than PK-injected eyes (Fig. 5C). The discrepancy of the hemorrhage severity could be explained by the finding that PK only has limited collagenase-like activity compared with collagenase per se (data not shown).
Figure 5. .
Effect of PK on collagen proteolysis in vitro. (A) Extracted spectral counting of collagen from purified COL4A cleaved by PK displayed as grayscale digital image. Arrow indicates the high molecular weight full length collagen; star indicates collagen fragments after cleavage. (B) Coomassie blue staining of the cleavage of COL4A by PK at 2, 6, and 24 hours in the absence or presence of BPCCB. Bands highlighted with dotted frame indicate the major changes of the COL4A after PK treatment. The figure is representative of three independent experiments. (C) Representative images of the retinal hemorrhage at 24 hours after the second intravitreal injection of collagenase into rat eyes. The figure is representative of images from nine rats.
Discussion
Retinal hemorrhage is a hallmark of both diabetic and hypertensive retinopathies, as well as a number of other ocular disorders. While increased frequency and severity of retinal hemorrhages are often associated with advanced stages of these retinopathies, the potential effects of blood inside the BRB are not fully understood. In this report, we show that intravitreal injection of autologous blood in rats increases RVP and induces leukostasis in the retina. These findings suggest that blood in the vitreous may contribute to BRB dysfunction and inflammatory processes that are associated with certain ocular injuries and retinopathies.
The retinal and cerebral microvasculatures share many morphological and physiological properties. For example, the retina is an extension of the diencephalon embryologically and both organs share a similar pattern of vascularization during development; both vascular networks have similar vascular regulatory processes.23 Moreover, both the retinal and cerebral endothelium have highly restrictive tight junctions, which separate neuronal tissue from blood. While the effects of hemorrhage on the retina have received relatively little attention, the effects of hemorrhage in the brain have been studied extensively. Hemorrhage in the brain triggers a cascade of events, including acute increases in cerebrovascular permeability, inflammation, and both vasogenic and cytotoxic edema.24,25 In addition, hemorrhage in the brain can lead to dysfunction, activation, and death of glial and neuronal cells.26 The adverse effects of hemorrhage in the brain have been attributed to a plethora of factors, including components of the kallikrein, complement, and coagulation cascades, as well as inflammatory cytokines and iron.27,28 Our group and others have identified many of these factors in the vitreous fluid from people with advanced diabetic retinopathy.4 Since retinal hemorrhages occur repeatedly in diabetic retinopathy, it is possible that components of blood released to the neuroretinal interstitial fluid and vitreous could contribute to retinal vascular dysfunction. Indeed, we have previously shown that injection of isolated components of blood (i.e., CA-1 and PK) into the vitreous could increase RVP and retinal thickening.4,13 The current report shows that intravitreal injection of autologous blood into the vitreous similarly induces RVP and also leukostasis. The effects of blood on RVP and leukostasis were reduced by a PK inhibitor and mimicked by injection of PPK/FXII/HK. These findings suggest that PK is a significant contributing factor to the vascular permeability and proinflammatory effects of blood in the vitreous on retinal dysfunction.
The studies on RVP and hemorrhage were performed on SD rats, which have been primarily used in our previous studies.4,12,13 However, since SD rats are not pigmented, strong background fluorescence from the choroid can obscure adherent leukocytes labeled with AO. In order to increase contrast and decrease background fluorescence, pigmented LE rats were chosen for leukostasis studies. We assessed PK-induced retinal leukostasis using two different methods, including AO labeling of static leukocytes in vivo and Con A labeling of adherent leukocytes in perfused retina ex vivo. We show that intravitreal injection of PPK/FXII/HK increases the number of static leukocytes that are detected at 20 minutes postintravenous infusion of AO. Although leukocyte adherence and rolling are observed in the first few minutes post AO injection, at 20 minutes, the AO-labeled static leukocytes are primarily detected in microvascular regions adjacent to primary retinal vessels, and may include cells that have undergone extravasation.29 Since blood injection into the vitreous obscured visualization of the retina in vivo by SLO imaging, the effects of blood on retinal leukostasis were measured using Con A staining. We show that intravitreal injection of autologous blood increased leukocytes in veins and venules, and this response was decreased by PK inhibition. In contrast to in vivo labeling used in the AO method, the Con A labeling method is performed ex vivo and involves extensive pre- and postperfusion with saline. We observed Con A–labeled leukocytes in retinal veins and venules, whereas AO-labeled cells were observed mainly in areas adjacent to primary vessels, which are similar findings using these labeling methods to previous studies of leukostasis in diabetic rats.29,30 The penetration of intravitreal PK across the inner limiting membrane into the retina is unknown. Since PK was injected with its substrate HK, it is likely that bradykinin was generated and may contribute to the effects of the intravitreal KKS on retinal leukostasis. Bradykinin receptors are expressed on endothelial cells, neurons, glia, and circulating mononuclear cells; therefore, the mechanisms that contribute to KKS-induced retinal vascular leukostasis will require additional studies. Results from AO and Con A studies suggest that leukostasis occurs on primary and secondary veins and within the microvessels.
The estimated vitreous volume of adult rat is approximately 60 μL; therefore, the expected molar concentration of PPK in 10 μL autologous blood injected into each eye is 22 to 30 nM. The expected molar concentrations of 500 ng PK injected into each eye is approximately 34 nM, which is comparable with 10 μL blood. We have found that PK levels in diabetic macular edema patients is approximately 100 nM (data not shown), suggesting that the amounts of blood and PK we injected into rat vitreous are within a physiological relevant range. Following injection, outflow toward the anterior chamber would likely facilitate anterior diffusion of PK (Fig. 3A) and collagenase (Fig. 5C) toward the periphery of the retina, which is consistent with the localization of retinal hemorrhage.
In previous studies, we found that a single injection of PK alone into the vitreous induced both acute RVP and focal areas of sustained RVP at 24 hours postinjection.13 Since PK is normally bound to HK in plasma, in this study, we injected eyes with the combination of PPK, HK, and FXII to examine the effect of PK in the presence of HK. Comparison with our previous reports12,13 revealed that PK in the absence or presence of HK exerts similar effects on increasing RVP. In this study, we also observed retinal hemorrhages in eyes receiving two intravitreal injections of PK, whereas hemorrhages were infrequently observed in eyes receiving either saline or bradykinin. Penn et al.31 reported that multiple dry-needle punctures exert an additive effect to decrease retinal neovascularization in a rodent model of oxygen-induced retinopathy. We have previously reported that a single intravitreal injection of saline in adult rats produced a small but consistent increase in RVP.4 The appearance of retinal hemorrhage in eyes receiving multiple saline injections, which was not observed in eyes receiving a single saline injection, suggests that consecutive intravitreal injections can exert cumulative effects on BRB dysfunction in adult animals. The absence of an effect of bradykinin on hemorrhage suggests that PK may exert bradykinin-independent effects on the retina. Indeed, recent reports have identified a number of novel PK substrates; for example, PK can function as a plasminogen activator, which could contribute to plasmin-mediated fibrinolysis, activation of matrix metalloproteinases (MMPs), and adipocyte differentiation.32–34 To search for PK substrates from retinal cells, we exposed culture bovine retinal pericytes to PK for 24 hours and used proteomics to characterize proteins in the conditioned medium. This analysis revealed increased levels of low molecular weight fragments of collagen chain in medium exposed to PK compared with the control. These findings are consistent with a previous study that has shown that PK increased collagen fragments in the secretome from rat brain astrocytes.22 Since PK has been previously reported to influence plasminogen and MMP activities, the effect of PK on collagen proteolysis could be due to both direct and indirect effects. To examine the potential direct effect of PK on collagen, we incubated purified PK with purified human type IV collagen and used mass spectrometry to analyze individual type IV collagen chains. Although direct comparisons of PK-mediated type IV collagen cleavage fragments obtained from bovine cells and from human placenta is limited, these studies showed that multiple COL4A chains are cleaved by PK and these effects of PK on COL4A cleavage were blocked by a selective PK inhibitor. These findings suggest that PK has a previously unrecognized direct collagenase-like activity. Intracerebral bacterial collagenase injection into the brain is an established experimental model of intracerebral hemorrhage in rodents,35 and we show that intravitreal injection of collagenase induced retinal hemorrhage. These results suggest that the collagenase-like activity of PK may contribute to its effects on retinal hemorrhage, which requires disruption of the vascular basement membrane.
Basement membrane is a specialized form of extracellular matrix comprised of an interwoven mixture of type IV collagen, laminins, nidogen, fibronectin, and sulfated proteoglycans and regulated by MMPs and plasmin. The basement membrane has many functions, including maintenance of capillary vessel morphology, cell adhesion, and prevention of plasma protein leakage from capillary vessels. The integrity of BRB is maintained by the presence of an intact basement membrane that provides structural support to the endothelial cell wall, pericytes, and astrocytes. Disruption of the BRB can cause increased vascular permeability, vasogenic edema, and tissue damage.36 The functional integrity of the blood-brain barrier (BBB) is regulated by pericytes during development and in adulthood.37,38 Pericytes also contribute to basement membrane formation by synthesizing type IV collagen, glycosaminoglycans, and laminin39 and play an important role in the maintenance of the basement membrane at the BBB.40 In this current experiment, we found that pericytes also secrete collagens and the majority of these proteins are cleaved by PK—in particular, COL4, which is the major structural protein in extracellular matrix in all types of vessels.41
The predominant components of basement membrane are intertwined meshworks of polymeric laminin and type IV collagen, which are the main components, comprising 50% of all basement membrane proteins.42 The type IV procollagens form specific heterotrimer molecules, and their proper incorporation into the extracellular matrix is important for basement membrane stability. A number of studies have demonstrated that type IV collagen was degraded in experimental intracerebral stroke models.43,44 In addition, mutation of COL4A1 may cause a spectrum of cerebrovascular phenotypes and persons with COL4A1 mutations may be predisposed to hemorrhage.45–47 Although it has been shown that degradation and breakdown of basal lamina by proteases such as MMPs contributes to intracerebral hemorrhage severity,43,48 our in vitro experiments demonstrated that PK can directly mediate the proteolysis of collagen. These results reveal that PK has collagenase-like activity that might induce hemorrhage under certain pathological conditions.
In summary, this report shows that intravitreal blood increases both RVP and leukostasis in rats and these effects are ameliorated by PK inhibition. In addition, we show that PK has collagenase-like activity that may contribute to basement membrane damage and retinal hemorrhage. Thus, PK may contribute to chronic intraocular hemorrhage in certain retinopathies, such as diabetic retinopathy. These findings suggest that PK could be a therapeutic target for the treatment of retinopathies with recurrent retinal hemorrhage.
Footnotes
Supported in part by the US National Institutes of Health Grants EY19029 and DK36836, and Juvenile Diabetes Research Foundation (JDRF) Grant 17-2011-251.
Disclosure: J. Liu, None; A.C. Clermont, None; B.-B. Gao, None; E.P. Feener, KalVista Pharmaceuticals Ltd. (C), P
References
- 1. Levin AV. Retinal hemorrhages: advances in understanding. Pediatr Clin North Am. 2009; 56: 333–344 [DOI] [PubMed] [Google Scholar]
- 2. Levin AV. Retinal hemorrhage in abusive head trauma. Pediatrics. 2010; 126: 961–970 [DOI] [PubMed] [Google Scholar]
- 3. Barthelmes D, Bosch MM, Merz TM, et al. Delayed appearance of high altitude retinal hemorrhages. PLoS One. 2011; 6: e11532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gao BB, Clermont A, Rook S, et al. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med. 2007; 13: 181–188 [DOI] [PubMed] [Google Scholar]
- 5. Frank RN. Diabetic retinopathy. N Engl J Med. 2004; 350: 48–58 [DOI] [PubMed] [Google Scholar]
- 6. Schmaier AH, McCrae KR. The plasma kallikrein-kinin system: its evolution from contact activation. J Thromb Haemost. 2007; 5: 2323–2329 [DOI] [PubMed] [Google Scholar]
- 7. Muller F, Mutch NJ, Schenk WA, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009; 139: 1143–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oschatz C, Maas C, Lecher B, et al. Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity. 2011; 34: 258–268 [DOI] [PubMed] [Google Scholar]
- 9. Maas C, Govers-Riemslag JW, Bouma B, et al. Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008; 118: 3208–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sainz IM, Pixley RA, Colman RW. Fifty years of research on the plasma kallikrein-kinin system: from protein structure and function to cell biology and in-vivo pathophysiology. Thromb Haemost. 2007; 98: 77–83 [PubMed] [Google Scholar]
- 11. Abdouh M, Talbot S, Couture R, Hassessian HM. Retinal plasma extravasation in streptozotocin-diabetic rats mediated by kinin B(1) and B(2) receptors. Br J Pharmacol. 2008; 154: 136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phipps JA, Clermont AC, Sinha S, Chilcote TJ, Bursell SE, Feener EP. Plasma kallikrein mediates angiotensin II type 1 receptor-stimulated retinal vascular permeability. Hypertension. 2009; 53: 175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clermont A, Chilcote TJ, Kita T, et al. Plasma kallikrein mediates retinal vascular dysfunction and induces retinal thickening in diabetic rats. Diabetes. 2011; 60: 1590–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bird JE, Smith PL, Wang X, et al. Effects of plasma kallikrein deficiency on haemostasis and thrombosis in mice: murine ortholog of the Fletcher trait. Thromb Haemost. 2012; 107: 1141–1150 [DOI] [PubMed] [Google Scholar]
- 15. Revenko AS, Gao D, Crosby JR, et al. Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood. 2011; 118: 5302–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Acar K, Yagci M, Sucak GT, Haznedar R. Isolated prolonged activated partial thromboplastin time in an asymptomatic patient: Fletcher factor deficiency. Thromb Res. 2006; 118: 765–766 [DOI] [PubMed] [Google Scholar]
- 17. Liu J, Gao BB, Clermont AC, et al. Hyperglycemia-induced cerebral hematoma expansion is mediated by plasma kallikrein. Nat Med. 2011; 17: 206–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horio N, Clermont AC, Abiko A, et al. Angiotensin AT(1) receptor antagonism normalizes retinal blood flow and acetylcholine-induced vasodilatation in normotensive diabetic rats. Diabetologia. 2004; 47: 113–123 [DOI] [PubMed] [Google Scholar]
- 19. Pouliot M, Talbot S, Sénécal J, Dotigny F, Vaucher E, Couture R. Ocular application of the kinin B(1) receptor antagonist LF22-0542 inhibits retinal inflammation and oxidative stress in streptozotocin-diabetic rats. PLoS One. 2012; 7: e33864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nayak RC, Berman AB, George KL, Eisenbarth GS, King GL. A monoclonal antibody (3G5)-defined ganglioside antigen is expressed on the cell surface of microvascular pericytes. J Exp Med. 1988; 167: 1003–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao BB, Stuart L, Feener EP. Label-free quantitative analysis of one-dimensional PAGE LC/MS/MS proteome: application on angiotensin II-stimulated smooth muscle cells secretome. Mol Cell Proteomics. 2008; 7: 2399–2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu J, Gao BB, Feener EP. Proteomic identification of novel plasma kallikrein substrates in the astrocyte secretome. Transl Stroke Res. 2010; 1: 276–286 [DOI] [PubMed] [Google Scholar]
- 23. Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005; 206: 319–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J, Doré S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007; 27: 894–908 [DOI] [PubMed] [Google Scholar]
- 25. Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol. 2012; 11: 101–118 [DOI] [PubMed] [Google Scholar]
- 26. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001; 344: 1450–1460 [DOI] [PubMed] [Google Scholar]
- 27. Keep RF, Xiang J, Ennis SR, et al. Blood-brain barrier function in intracerebral hemorrhage. Acta Neurochir Suppl. 2008; 105: 73–77 [DOI] [PubMed] [Google Scholar]
- 28. Wadas TM. Emerging inflammatory biomarkers with acute stroke. Crit Care Nurs Clin North Am. 2009; 21: 493–505 [DOI] [PubMed] [Google Scholar]
- 29. Noda K, Nakao S, Zandi S, Engelstädter V, Mashima Y, Hafezi-Moghadam A. Vascular adhesion protein-1 regulates leukocyte transmigration rate in the retina during diabetes. Exp Eye Res. 2009; 89: 774–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miyahara S, Kiryu J, Yamashiro K, et al. Simvastatin inhibits leukocyte accumulation and vascular permeability in the retinas of rats with streptozotocin-induced diabetes. Am J Pathol. 2004; 164: 1697–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Penn JS, McCollum GW, Barnett JM, Werdich XQ, Koepke KA, Rajaratnam VS. Angiostatic effect of penetrating ocular injury: role of pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2006; 47: 405–414 [DOI] [PubMed] [Google Scholar]
- 32. Selvarajan S, Lund LR, Takeuchi T, Craik CS, Werb Z. A plasma kallikrein-dependent plasminogen cascade required for adipocyte differentiation. Nat Cell Biol. 2001; 3: 267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lund LR, Green KA, Stoop AA, et al. Plasminogen activation independent of uPA and tPA maintains wound healing in gene-deficient mice. EMBO J. 2006; 25: 2686–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saunders WB, Bayless KJ, Davis GE. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci. 2005; 118: 2325–2340 [DOI] [PubMed] [Google Scholar]
- 35. MacLellan CL, Silasi G, Auriat AM, Colbourne F. Rodent models of intracerebral hemorrhage. Stroke. 2010; 41: S95–S98 [DOI] [PubMed] [Google Scholar]
- 36. Kaur C, Foulds WS, Ling EA. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog Retin Eye Res. 2008; 27: 622–647 [DOI] [PubMed] [Google Scholar]
- 37. Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010; 468: 562–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Armulik A, Genové G, Mäe M, et al. Pericytes regulate the blood-brain barrier. Nature. 2010; 468: 557–561 [DOI] [PubMed] [Google Scholar]
- 39. Fisher M. Pericyte signaling in the neurovascular unit. Stroke. 2009; 40 (suppl 3): S13–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kose N, Asashima T, Muta M, et al. Altered expression of basement membrane-related molecules in rat brain pericyte, endothelial, and astrocyte cell lines after transforming growth factor-beta1 treatment. Drug Metab Pharmacokinet. 2007; 22: 255–266 [DOI] [PubMed] [Google Scholar]
- 41. Bou-Gharios G, Ponticos M, Rajkumar V, Abraham D. Extra-cellular matrix in vascular networks. Cell Prolif. 2004; 37: 207–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003; 3: 422–433 [DOI] [PubMed] [Google Scholar]
- 43. Rosell A, Cuadrado E, Ortega-Aznar A, Hernández-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008; 39: 1121–1126 [DOI] [PubMed] [Google Scholar]
- 44. Scholler K, Trinkl A, Klopotowski M, et al. Characterization of microvascular basal lamina damage and blood-brain barrier dysfunction following subarachnoid hemorrhage in rats. Brain Res. 2007; 1142: 237–246 [DOI] [PubMed] [Google Scholar]
- 45. Gould DB, Phalan FC, Breedveld GJ, et al. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005; 308: 1167–1171 [DOI] [PubMed] [Google Scholar]
- 46. Gould DB, Phalan FC, van Mil SE, et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006; 354: 1489–1496 [DOI] [PubMed] [Google Scholar]
- 47. Vahed K, Kubis N, Boukobza M, et al. COL4A1 mutation in a patient with sporadic, recurrent intracerebral hemorrhage. Stroke. 2007; 38: 1461–1464 [DOI] [PubMed] [Google Scholar]
- 48. Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002; 39: 279–291 [DOI] [PubMed] [Google Scholar]