Abstract
In a previous study, we reported that the short-term treatment with celecoxib, a non-steroidal anti-inflammatory drug (NSAID) attenuates the activation of brain structures related to nociception and does not interfere with orthodontic incisor separation in rats. The conclusion was that celecoxib could possibly be prescribed for pain in orthodontic patients. However, we did not analyze the effects of this drug in periodontium. The aim of this follow-up study was to analyze effects of celecoxib treatment on recruitment and activation of osteoclasts and alveolar bone resorption after inserting an activated orthodontic appliance between the incisors in our rat model. Twenty rats (400–420 g) were pretreated through oral gavage with celecoxib (50 mg/kg) or vehicle (carboxymethyl-cellulose 0.4%). After 30 min, they received an activated (30 g) orthodontic appliance, set not to cause any palate disjunction. In sham animals, the appliance was immediately removed after introduction. All animals received ground food and, every 12 h, celecoxib or vehicle. After 48 h, they were anesthetized and transcardiacally perfused through the aorta with 4% formaldehyde. Subsequently, maxillae were removed, post-fixed and processed for histomorphometry or immunohistochemical analyses. As expected, incisor distalization induced an inflammatory response with certain histological changes, including an increase in the number of active osteoclasts at the compression side in group treated with vehicle (appliance:32.2±2.49 vs sham: 4.8±1.79, P<0.05) and celecoxib (appliance: 31.0±1.45 vs sham: 4.6±1.82, P<0.05). The treatment with celecoxib did not modify substantially the histological alterations and the number of active osteoclasts after activation of orthodontic appliance. Moreover, we did not see any difference between the groups with respect to percentage of bone resorption area. Taken together with our previous results we conclude that short-term treatment with celecoxib can indeed be a therapeutic alternative for pain relieve during orthodontic procedures.
Key words: orthodontic treatment, analgesic, inflammation, bone resorption, osteoclasts.
Introduction
Orthodontic procedures may cause unpleasant and even painful sensations, and these are probably the most important factors for aversion in patients and discontinuation of the treatment. As a solution, orthodontists commonly use painkillers such as acetaminophen, a non-steroidal anti-inflammatory drug (NSAIDs), which is also the most frequently prescribed analgesic to patients.1–4 The reason for this being the drug of choice is that it does not interfere with the tooth movement that is intended and necessary in any such orthodontic treatment. Acetaminophen only slightly inhibits prostaglandin formation and does not interfere in the bone resorption process. Its analgesic action seems to be a central nervous system effect associated with a minor local anti-inflammatory effect.5,6 Notwithstanding, like other non-selective NSAIDs, its use may imply in adverse effects, with hepatic toxicity and drug hypersensibility being the most common.7–10 Celecoxib is a possible candidate to be prescribed to orthodontic patients when acetais counter-indicated. It acts selectively on cyclooxygenase type 2, which results in less adverse effects than those produced by non-selective NSAIDs.8,9,11–15 Despite this apparent advantage, the effects of celecoxib on tooth movement are still unclear, especially since experimental studies performed until now report contradictory results.14,16–19
To address this question, we started to use a rat model designed to reveal nociception pathways when applying an orthodontic force between the incisors, and, in a previous study, we could show that a short-term treatment (48 h) with celecoxib reduced neuronal activation in brain structures related to nociception, while not interfering with tooth movement.20 Given this, we concluded that in the short-term application protocol, this drug worked as an effective analgesic, without apparently compromising the kinetics of orthodontic movement. An important aspect however, possible effects on local bone resorption, was not analyzed. The aim of this follow-up study, thus, was to analyze whether or not celecoxib treatment affects the recruitment and activation of osteoclasts and alveolar bone resorption in rats submitted to orthodontic force.
Materials and Methods
Animals
Twenty male Wistar rats, weighing 400 g each, were kept in Plexiglas cages in a temperature-controlled room (24±1°C) with a 12:12 hours L:D cycle. They had free access to water and food. The experiments were reviewed and approved by the Ethics Committee on the Use of Experimental Animals of the University of São Paulo, Campus of Ribeirão Preto (protocol number: 06.1.1045.53.6).
Experimental protocol
The animals were first anesthetized by an intramuscular injection of ketamine (Vetaset, 100 mg/kg) and xylazine (Dopaser, 14 mg/kg). They then received an activated orthodontic appliance set on the superior incisors, submitting each tooth to a force of 30 g. This appliance was either left in place for 48 h (appliance group, n=10), or was immediately removed after insertion (sham group, n=10). Thirty minutes before, and 12, 24 and 36 h after inserting the appliance, a 1 mL solution containing celecoxib (CEL - Celebra, Pfizer, New York, NY, USA) in a dose of 50 mg/kg, or 0.4% carboxymethylcellulose (CMC - vehicle) were administered in subsets of both groups by oral gavage. After 48 h, the animals were transcardially perfused with 100 mL of phosphate-buffered saline (PBS 0.01M, pH 7.4), followed by 400 mL of 4% formaldehyde in 0.1 M phosphate buffer. The maxillae were removed postfixed by 24 h, and placed in an EDTA solution (0.5M) containing TRIS 0,2M (pH7.4) for approximately 28 days.
Orthodontic appliance
A fixed orthodontic appliance designed to induce tipping tooth movement and constructed in our laboratory was used.21–23 It consists of a torsion spring made of 0.016 inch stainless steel wire that had each edge welded to two stainless steel rings (adapted orthodontic bands) of 0.004×0.06 inches. These were cut open in the middle so that they could be fixed to the right and left incisors. After activating the spring, tension was measured with a dynamometer (Zeusan) and the appliance inserted with the torsion spring adapted to the rats' mouth palate in a way that the orthodontic band could be cemented to the incisor with zinc oxyphosphate cement.
Tissue preparation
The segments of the maxilla containing the root and crown of the incisors were submitted to a demineralization procedure, followed by dehydration in an ascending ethanol series (50% for 30 min, 70% and 95% for 2 h each). Subsequently, they were immersed and left overnight in equal parts of alcohol and xylene, diaphanized three times in xylene, with solutions being changed every 2 h, and finally embedded in paraffin. Coronal sections through the maxilla including the upper incisors region were cut at 6 µm thickness on a microtome and further processed for hematoxylin-eosin staining or tartrate-resistant acid phosphatase (TRAP) immunohistochemistry.
Immunohistochemistry for tartrate-resistant acid phosphatase detection
After deparaffinization with xylene, selected sections were rehydrated in a descending ethanol series, followed by washing with PBS (0.1M, pH 7.4) and incubation for 1 h with PBS containing 3% hydrogen peroxide to inactivate endogenous peroxidase activity. After several rinses in PBS, the sections were placed in 3% bovine albumin serum for 1 h and then incubated with the primary goat anti-TRAP (Santa Cruz Biotechnology, Santa Cruz, CA, USA), diluted 1:100 in PBS containing 0.3% Triton X-100, for 24 h in a humid chamber at 4ºC. After several washes in PBS, the sections were incubated at room temperature with biotinylated anti-goat IgG (Dako Laboratories, Carpinteria, CA, USA) for 1 h. Subsequently, they were washed in PBS and placed for 1 h in Streptavidin-HRP complex (Universal Dako Labeled -HR, Streptavidin-Biotin Kit®, Dako Laboratories). Peroxidase activity was revealed by 3, 3-diamino benzidine tetrachloride (DAB chromogen Kit, Dako Laboratories). After rinses in PBS the sections were counter-staining with Harris-Haematoxylin. Finally, they were immersed in an ascending ethanol series, xylene-cleared, and coverslipped with Entellan. As negative control some sections were submitted to the same procedure but without the anti-TRAP serum.
Analysis
For each selected section, five microscopic fields of the distal periodontium were captured by a light microscope connected to a digital camera. The Image J, public domain software (http://rsb.info.nih.gov/ij) was used to calculate percentage bone resorption. Osteoclasts were counted using this same software and in each of five histological sections showing the alveolar bone surface (compression side) adjacent to the entire root. Cells were considered osteoclasts if they were multinucleated, TRAP positive, and located on or close to bone surfaces. All data were analyzed by using the non-parametric Kruskal-Wallis test followed by Dunn's multiple comparison test. All calculations were performed with the software GraphPad Prism 4 (GraphPad Software Inc.) and the accepted significance level was P<0.05.
Results
In both groups, treated with vehicle or celecoxib, it was possible to observe a histological difference between the mesial (tension) and distal (compression) area of the periodontal ligament within 48 h after insertion and fixation of the orthodontic appliance. The tension area showed disruption of collagen fibers and the periodontal space was filled with an extravascular fluid. In the compression area we could see neutrophils that transmigrate through the endothelium to the space among several collagen fibers of the periodontal ligament. The activation of orthodontic appliance increased the number of active osteoclasts at the compression side in group treated with vehicle (appliance: 32.2±2.49 vs sham: 4.8±1.79, P<0.05) and celecoxib (appliance:31.0±1.45 vs sham: 4.6±1.82, P<0.05). The bone resorption percentage at the compression side only slightly increased but not significantly in group treated with vehicle (appliance: 6.21±0.06% vs 4.18±0.05%, P>0.05) and celecoxib sham (5.55±0.06% vs sham: 2.98±0.04%, P>0.05). Moreover, after the activation of orthodontic appliance we could not see any difference between the groups with respect to the active osteoclasts recruitment (celecoxib: 31.0±1.45 vs vehicle: 32.2±2.49, P>0.05) and percentage of bone resorption area (celecoxib: 5.55±0.06% vs vehicle: 6.21±0.06%, P>0.05). Data are plotted in the Table 1. Representative photomicrographics are showed in the Figures 1 and 2.
Table 1. Bone resorption and osteoclast number in the periodontium of sham animals (appliance removed) and those that received the appliance for 48 h and were treated with either vehicle (carboxymethyl cellulose) or celecoxib.
| Sham | Appliance | |||
|---|---|---|---|---|
| Vehicle | Celecoxib | Vehicle | Celecoxib | |
| Bone resorption (%) | 4.18±0.05 | 2.98±0.04 | 6.21±0.06 | 5.55±0.06 |
| Osteoclast number | 4.8±1.79 | 4.6±1.82 | 32.2±2.49* | 31.0±1.45* |
Values represent mean + SEM (for 5 animals/group);
P<0.05 in relation to sham.
Figure 1.
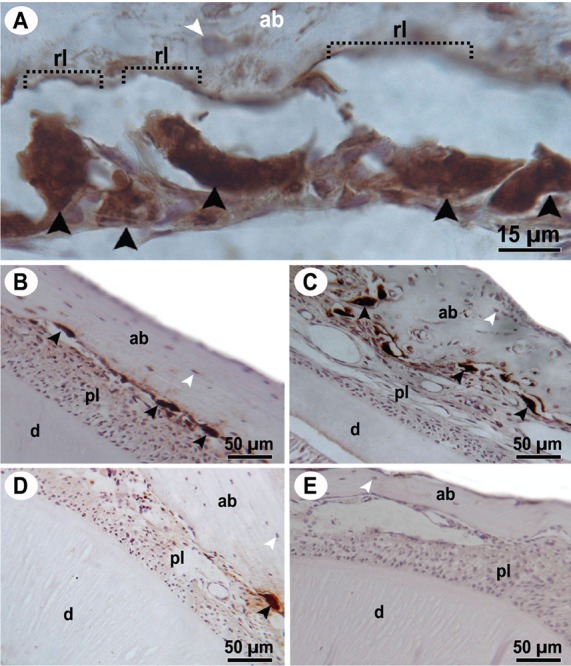
Photomicrographies of periodontium showing immunolabeling for the tartrate-resistant acid phosphatase. Osteocyte are seen as weakly stained cells (white arrowheads) and osteoclasts are large multinuclear cells with intense brown staining in the cytoplasm (black arrowheads). Most of these cells showed intense resorption activity once they were adjusted to the resorption lacuna (rl) in the alveolar bone (ab), as visible in panel A. Panels B and C are from the periodontium of animals that received the appliance, and D and E are from the sham group (appliance removed). Periodontium of animals treated with celecoxib (B and D) and with vehicle (C and E). ab, alveolar bone; d, dentine; pl, periodontal ligament, rl, resorption lacuna.
Figure 2.
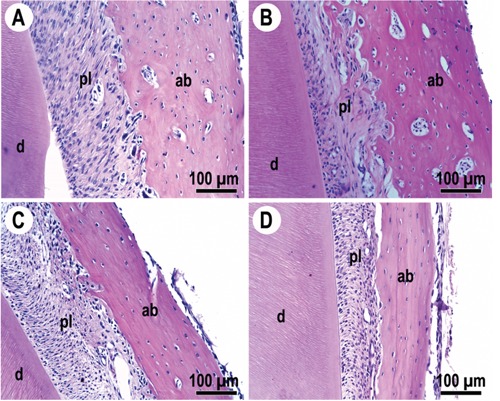
Photomicrographs of the periodontium (H&E stain) of sham animals (A and C) or those that received the appliance (B and D). Respective vehicle-treated animals are shown in A and B, and those receiving celecoxib in C and D. ab, alveolar bone; d, dentine; pl, periodontal ligament.
Discussion
In our rat model, a short-term celecoxib treatment did not alter the recruitment and activation o osteoclasts and bone resorption area at the compression side after the application of orthodontic force. Hence, this treatment does not affect the tooth movement as already reported by our previous work.20 Our results are in accordance with those of previous investigators that also used experimental tooth movement to study the effect of coxib therapies.14,16 Though in distinction to these, drug treatment in our study was applied via oral 30 min before and then four times every 12 h after the application of the orthodontic force. This treatment schedule was designed to mimic the typical clinical procedure for cele-coxib prescription, following recommendations in orthodontia on the preemptive or pre-operative administration of analgesics to decrease postoperative pain.24–28
Mechanical forces applied to a tooth affect its relationship with the alveolar bone, creating pressure and tension zones, and eventually elicits a local inflammatory reaction which allows the tooth to migrate inside the alveolar bone.21,22,29–35 In fact, we could see a local inflammatory reaction in the compression periodontal ligament following insertion of the orthodontic appliance. This included local alterations in vascularity, as well as cellular and extracellular matrix reorganization and a periodontal space narrowing. Even though the molecular alterations occuring in the periodontal ligament, especially in the initial phases following mechanical stress, are not yet clear, it is established that neurotransmitters, cytokines, growth factors, colony-stimulating factors, and metabolites of arachidonic acid are involved.31 We herein chose to analyze the periodontium 48 h after the application of the orthodontic appliance because this is the period when pain is frequently reported in clinical routine.3,36–40 Furthermore, this period represents the initial phase of dental movement, when pressure stimulates periodontal receptors and elicits inflammatory reactions accompanied by the production of hyperalgesic substances.41 Prostaglandins, which are among such hyperalgesic compounds, are thought to affect bone remodelation, while themselves being affected by NSAIDs.34,42 Bone remodeling, thus, requires the inflammatory process to cause the desired tooth movement in an orthodontic treatment, but could in turn be affected by NSAID treatment.
Osteoclasts play an important role in the remodeling bone process.43 It was reported that these cells migrate from the medullar cavity to the periodontal ligament approximately within 48 h after installation of an orthodontic apparatus,44 which is in accordance with the present study. The osteoclast is a monocyte-derived cell whose development is dependent on factors produced by activated osteoblasts in response to inflammatory or immunological signaling.45,46 The cytoplasm membrane of an inactive osteoclast is straight and faces to the bone, but without adhering to its surface. Such cells differentiate into active osteoclasts when stimulated by certain factors and then begin to express a proton pump in the folded edge of their cell membrane facing the bone surface gap.47 The osteoclasts can be identified through histochemistry or immunohistochemistry methods by detection of the tartrate-resistant acid phosphatase (TRAP), an isoenzyme of the group of acid phosphatases, which is highly expressed by activated osteoclasts.46 Using immunohistochemistry we observed an increase in the number of active osteoclasts in the distal periodontal ligament following the orthodontic force application, there was no significant increase in the bone resorption area. This could be explained by the fact that in our analysis we targeted an early period of events that preceded the actual bone resorption process. Altogether, this and previous work showed that celexocib did not affect the recruitment and activation of osteoclasts and incisor separation but attenuated the activation of brain structures related to nociception following experimental tooth movement. Consi dering that orthodontic patients usually will not use NSAIDs for a prolonged period of time, because pain rarely lasts more than 48 h, we believe that celecoxib may be a safe alternative medication for orthodontic patients.
Acknowledgements:
the authors would like to thank the financial support of FAPESP. Eduardo P.C. Filho was recipient of a CNPq scholarship.
References
- 1.Arias OR, Marquez-Orozco MC. Aspirin, acetaminophen, and ibuprofen: their effects on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2006;130:364–70. doi: 10.1016/j.ajodo.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Kehoe MJ, Cohen SM, Zarrinnia K, Cowan A. The effect of acetaminophen, ibuprofen, and misoprostol on prostaglandin E2 synthesis and the degree and rate of orthodontic tooth movement. Angle Orthod. 1996;66:339–49. doi: 10.1043/0003-3219(1996)066<0339:TEOAIA>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan V. Orthodontic pain: from causes to management--a review. Eur J Orthod. 2007;29:170–9. doi: 10.1093/ejo/cjl081. [DOI] [PubMed] [Google Scholar]
- 4.Farzanegan F, Zebarjad SM, Alizadeh S, Ahrari F. Pain reduction after initial arch-wire placement in orthodontic patients: a randomized clinical trial. Am J Orthod Dentofacial Orthop. 2012;141:169–73. doi: 10.1016/j.ajodo.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi M, Panerai AE. The dose-related effects of paracetamol on hyperalgesia and nociception in the rat. Br J Pharmacol. 1996;117:130–2. doi: 10.1111/j.1476-5381.1996.tb15164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickering G, Loriot MA, Libert F, Eschalier A, Beaune P, Dubray C. Analgesic effect of acetaminophen in humans: first evidence of a central serotonergic mechanism. Clin Pharmacol Ther. 2006;79:371–8. doi: 10.1016/j.clpt.2005.12.307. [DOI] [PubMed] [Google Scholar]
- 7.Anker AL, Smilkstein MJ. Acetaminophen. Concepts and controversies. Emerg Med Clin North Am. 1994;12:335–49. [PubMed] [Google Scholar]
- 8.Lichtenstein DR, Syngal S, Wolfe MM. Nonsteroidal antiinflammatory drugs and the gastrointestinal tract. The double-edged sword. Arthritis Rheum. 1995;38:5–18. doi: 10.1002/art.1780380103. [DOI] [PubMed] [Google Scholar]
- 9.Singh G, Rosen Ramey D. NSAID induced gastrointestinal complications: the ARAMIS perspective--1997. Arthritis, Rheumatism, and Aging Medical Information System. J Rheumatol Suppl. 1998;51:8–16. [PubMed] [Google Scholar]
- 10.Peterson GM. Selecting nonprescription analgesics. Am J Ther. 2005;12:67–79. doi: 10.1097/00045391-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Walker JB, Buring SM. NSAID impairment of orthodontic tooth movement. Ann Pharmacother. 2001;35:113–5. doi: 10.1345/aph.10185. [DOI] [PubMed] [Google Scholar]
- 12.Sooriakumaran P. COX-2 inhibitors and the heart: are all coxibs the same? Postgrad Med J. 2006;82:242–5. doi: 10.1136/pgmj.2005.042234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore PA, Hersh EV. Celecoxib and rofecoxib. The role of COX-2 inhibitors in dental practice. J Am Dent Assoc. 2001;132:451–6. doi: 10.14219/jada.archive.2001.0207. [DOI] [PubMed] [Google Scholar]
- 14.de Carlos F, Cobo J, Perillan C, Garcia MA, Arguelles J, Vijande M, et al. Orthodontic tooth movement after different coxib therapies. Eur J Orthod. 2007;29:596–9. doi: 10.1093/ejo/cjm072. [DOI] [PubMed] [Google Scholar]
- 15.Garavito RM. The cyclooxygenase-2 structure: new drugs for an old target? Nat Struct Biol. 1996;3:897–901. doi: 10.1038/nsb1196-897. [DOI] [PubMed] [Google Scholar]
- 16.Jerome J, Brunson T, Takeoka G, Foster C, Moon HB, Grageda E, et al. Celebrex offers a small protection from root resorption associated with orthodontic movement. J Calif Dent Assoc. 2005;33:951–9. [PubMed] [Google Scholar]
- 17.Hauber Gameiro G, Nouer DF, Pereira Neto JS, Siqueira VC, Andrade ED, Duarte Novaes P, et al. Effects of short- and long-term celecoxib on orthodontic tooth movement. Angle Orthod. 2008;78:860–5. doi: 10.2319/100207-474.1. [DOI] [PubMed] [Google Scholar]
- 18.Gameiro GH, Nouer DF, Pereira-Neto JS, de Araujo Magnani MB, de Andrade ED, Novaes PD, et al. Histological analysis of orthodontic root resorption in rats treated with the cyclooxygenase-2 (COX-2) inhibitor celecoxib. Orthod Craniofac Res. 2008;11:156–61. doi: 10.1111/j.1601-6343.2008.00424.x. [DOI] [PubMed] [Google Scholar]
- 19.Hammad SM, El-Hawary YM, El-Hawary AK. The use of different analgesics in orthodontic tooth movements. Angle Orthod. 2012;82:820–6. doi: 10.2319/110911-691.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stabile AC, Stuani MB, Leite-Panissi CR, Rocha MJ. Effects of short-term acetaminophen and celecoxib treatment on orthodontic tooth movement and neuronal activation in rat. Brain Res Bull. 2009;79:396–401. doi: 10.1016/j.brainresbull.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Engstrom C, Granstrom G, Thilander B. Effect of orthodontic force on periodontal tissue metabolism. A histologic and biochemical study in normal and hypocalcemic young rats. Am J Orthod Dentofacial Orthop. 1988;93:486–95. doi: 10.1016/0889-5406(88)90077-7. [DOI] [PubMed] [Google Scholar]
- 22.King GJ, Thiems S. Chemical mediation of bone resorption induced by tooth movement in the rat. Arch Oral Biol. 1979;24:811–5. doi: 10.1016/0003-9969(79)90043-8. [DOI] [PubMed] [Google Scholar]
- 23.Magdalena CM, Navarro VP, Park DM, Stuani MB, Rocha MJ. C-fos expression in rat brain nuclei following incisor tooth movement. J Dent Res. 2004;83:50–4. doi: 10.1177/154405910408300110. [DOI] [PubMed] [Google Scholar]
- 24.Minor V, Marris CK, McGorray SP, Yezierski R, Fillingim R, Logan H, et al. Effects of preoperative ibuprofen on pain after separator placement. Am J Orthod Dentofacial Orthop. 2009;136:510–7. doi: 10.1016/j.ajodo.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Bradley RL, Ellis PE, Thomas P, Bellis H, Ireland AJ, Sandy JR. A randomized clinical trial comparing the efficacy of ibuprofen and paracetamol in the control of orthodontic pain. Am J Orthod Dentofacial Orthop. 2007;132:511–7. doi: 10.1016/j.ajodo.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Bird SE, Williams K, Kula K. Preoperative acetaminophen vs ibuprofen for control of pain after orthodontic separator placement. Am J Orthod Dentofacial Orthop. 2007;132:504–10. doi: 10.1016/j.ajodo.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Polat O, Karaman AI, Durmus E. Effects of preoperative ibuprofen and naproxen sodium on orthodontic pain. Angle Orthod. 2005;75:791–6. doi: 10.1043/0003-3219(2005)75[791:EOPIAN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Xiaoting L, Yin T, Yangxi C. Interventions for pain during fixed orthodontic appliance therapy. A systematic review. Angle Orthod. 2010;80:925–32. doi: 10.2319/010410-10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furstman L, Bernick S. Clinical considerations of the periodontium. Am J Orthod. 1972;61:138–55. doi: 10.1016/0002-9416(72)90092-9. [DOI] [PubMed] [Google Scholar]
- 30.Grieve WG, 3rd, Johnson GK, Moore RN, Reinhardt RA, DuBois LM. Prostaglandin E (PGE) and interleukin-1 beta (IL-1 beta) levels in gingival crevicular fluid during human orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1994;105:369–74. doi: 10.1016/s0889-5406(94)70131-8. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129:469 e1–32. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Saito M, Saito S, Ngan PW, Shanfeld J, Davidovitch Z. Interleukin 1 beta and prostaglandin E are involved in the response of periodontal cells to mechanical stress in vivo and in vitro. Am J Orthod Dentofacial Orthop. 1991;99:226–40. doi: 10.1016/0889-5406(91)70005-H. [DOI] [PubMed] [Google Scholar]
- 33.Sandy JR, Farndale RW, Meikle MC. Recent advances in understanding mechanically induced bone remodeling and their relevance to orthodontic theory and practice. Am J Orthod Dentofacial Orthop. 1993;103:212–22. doi: 10.1016/0889-5406(93)70002-6. [DOI] [PubMed] [Google Scholar]
- 34.Valiathan A, Siddhartha D. Prostaglandins and enhanced orthodontic tooth movement: In search of the silver bullet. Current Sci. 2006;90:311–3. [Google Scholar]
- 35.Waldo CM, Rothblatt JM. Histologic response to tooth movement in the laboratory rat; procedure and preliminary observations. J Dent Res. 1954;33:481–6. doi: 10.1177/00220345540330040701. [DOI] [PubMed] [Google Scholar]
- 36.Brown DF, Moerenhout RG. The pain experience and psychological adjustment to orthodontic treatment of preadolescents, adolescents, and adults. Am J Orthod Dentofacial Orthop. 1991;100:349–56. doi: 10.1016/0889-5406(91)70073-6. [DOI] [PubMed] [Google Scholar]
- 37.Jones ML, Chan C. Pain in the early stages of orthodontic treatment. J Clin Orthod. 1992;26:311–3. [PubMed] [Google Scholar]
- 38.Ngan P, Kess B, Wilson S. Perception of discomfort by patients undergoing orthodontic treatment. Am J Orthod Dentofacial Orthop. 1989;96:47–53. doi: 10.1016/0889-5406(89)90228-x. [DOI] [PubMed] [Google Scholar]
- 39.Erdinc AM, Dincer B. Perception of pain during orthodontic treatment with fixed appliances. Eur J Orthod. 2004;26:79–85. doi: 10.1093/ejo/26.1.79. [DOI] [PubMed] [Google Scholar]
- 40.Lew KK. Attitudes and perceptions of adults towards orthodontic treatment in an Asian community. Community Dent Oral Epidemiol. 1993;21:31–5. doi: 10.1111/j.1600-0528.1993.tb00715.x. [DOI] [PubMed] [Google Scholar]
- 41.King GJ, Keeling SD, McCoy EA, Ward TH. Measuring dental drift and orthodontic tooth movement in response to various initial forces in adult rats. Am J Orthod Dentofacial Orthop. 1991;99:456–65. doi: 10.1016/S0889-5406(05)81579-3. [DOI] [PubMed] [Google Scholar]
- 42.Krishnan V, Davidovitch Z. The effect of drugs on orthodontic tooth movement. Orthod Craniofac Res. 2006;9:163–71. doi: 10.1111/j.1601-6343.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- 43.Nicolin V, Dal Piaz F, Nori SL, Narducci P, De Tommasi N. Inhibition of bone resorption by Tanshinone VI isolated from Salvia miltiorrhiza Bunge. Eur J Histochem. 2010;54:e21–e21. doi: 10.4081/ejh.2010.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie R, Kuijpers-Jagtman AM, Maltha JC. Osteoclast differentiation and recruitment during early stages of experimental tooth movement in rats. Eur J Oral Sci. 2009;117:43–50. doi: 10.1111/j.1600-0722.2008.00588.x. [DOI] [PubMed] [Google Scholar]
- 45.Narducci P, Bortul R, Bareggi R, Nicolin V. Clathrin-dependent endocytosis of membrane-bound RANKL in differentiated osteoclasts. Eur J Histochem. 2010;54:e6–e6. doi: 10.4081/ejh.2010.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galvao MJ, Santos A, Ribeiro MD, Ferreira A, Nolasco F. Optimization of the tartrate-resistant acid phosphatase detection by histochemical method. Eur J Histochem. 2011;55:e1–e1. doi: 10.4081/ejh.2011.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farina C, Gagliardi S. Selective inhibition of osteoclast vacuolar H(+)-ATPase. Curr Pharm Des. 2002;8:2033–48. doi: 10.2174/1381612023393369. [DOI] [PubMed] [Google Scholar]


