Abstract
Ubiquitin-specific protease 22 (USP22), a novel ubiquitin hydrolase, has been implicated in oncogenesis and cancer progression in various types of human cancer. However, the clinical significance of USP22 expression in non-small cell lung cancer (NSCLC) has not been determined. In the present study, USP22 messenger RNA (mRNA) and protein levels were analyzed by quantitative real-time polymerase chain reaction (PCR) and western blot analysis in 30 cases of NSCLC and in corresponding non-tumor tissue samples. Furthermore, immunohistochemistry was performed to detect USP22 protein expression in 86 primary tumor tissues derived from clinically annotated NSCLC cases at stage I-II. In our analysis we found that both USP22 mRNA and protein levels in NSCLC tissues were significantly higher than those in corresponding non-tumor tissues and that there was a significant correlation between the expression of USP22 mRNA and protein (P=0.000, κ=0.732). In addition, a high-level of USP22 expression was observed in 53.3% (39 out of 86) cases and it was correlated with large tumor size (P=0.029) and lymph node metastasis (P=0.026). Patients with tumors displaying a high-level of USP22 expression showed significantly shorter survival (P=0.006, log-rank test). Importantly, multivariate analysis showed that high USP22 protein expression was an independent prognostic factor for NSCLC patients (P=0.003). In sum, our data suggest that USP22 plays an important role in NSCLC progression at the early stage, and that overexpression of USP22 in tumor tissues could be used as a potential prognostic marker for patients with early clinical stage of NSCLC.
Key words: Ubiquitin-specific protease 22, non-small cell lung cancer, prognosis.
Introduction
Lung cancer is the most frequently diagnosed cancer and the leading cause of cancer-related mortality worldwide, accounting for an estimated 1.6 million new cases and 1.4 million deaths in 2008.1 Non-small cell lung cancer (NSCLC) comprises approximately 80–85% of all lung cancers, with squamous cell carcinoma (SCC) and adenocarcinoma (AC) representing the majority of them.2 Currently, complete surgical resection remains the optimal treatment option for early-stage NSCLC, but even in stage I, approximately one third of patients will relapse after the initial surgery and die of metastatic recurrence within five years.3,4 According to many randomized clinical trials, adjuvant chemotherapy is now considered the unequivocal standard treatment for resected early-stage NSCLC patients, with an estimated 4–15% survival advantage at 5 years.5–9 This survival advantage clearly indicates that only a proportion of patients benefit from adjuvant chemotherapy, while others may receive potentially toxic chemotherapy unnecessarily. Thus, it is imperative to identify novel prognostic biomarkers that can precisely predict survival in patients with early-stage NSCLC. Such advances would be helpful to stratify patients with resected NSCLC and select high-risk patients who should receive aggressive adjuvant chemotherapy.
Ubiquitin-specific protease 22 (USP22) is a novel ubiquitin hydrolase that catalyzes the deubiquitination of both histones H2A and H2B, thereby acting as a co-activator. USP22 was initially identified by Glinsky et al. in a microarray-based study comparing gene expression profiles in metastatic lesions and primary tumors of prostate cancer.10 Subsequent sequence analysis revealed that USP22 is a dedicated subunit of the human Spt-Ada-Gcn5 acetyltransferase (SAGA) co-activator complex and that it functions as an activator for nuclear receptor-mediated transactivation.11 Furthermore, USP22 has been shown to regulate proliferation and oncogenic transformation through the modulation of the transcription factors BMI, c-myc, p38 mitogen-activated protein kinase (MAPK) and others.12–15 USP22 is moderately expressed in a variety of normal human tissues, such as heart and skeletal muscle, but weakly expressed in lung and liver.16 Recently, overexpression of USP22 has been reported in several epithelial cancer types and demonstrated to be associated with poor survival.17–20 Zhang and colleagues have demonstrated that ectopic overexpression of USP22 promotes cell proliferation and that suppression of USP22 expression by small hairpin RNA induces cell cycle arrest in human lung cancer cells.21 To our knowledge, however, no reports have been published on the relationship between USP22 expression and clinicopathological features and prognosis of lung cancer patients.
In the present study, we detected and compared USP22 expression in primary cancer tissues and adjacent normal tissues derived from early-stage NSCLC patients. Furthermore, we also sought to determine whether there is a correlation between USP22 expression and clinicopathological parameters of NSCLC patients and their survival.
Materials and Methods
Patients and tissue specimens
Between January 2006 and December 2006, a total of 86 primary lung cancer samples and 30 matched normal lung tissues were collected from early-stage NSCLC patients who underwent complete resection at the Department of Thoracic Surgery in the Third Affiliated Hospital of Harbin Medical University (Harbin, China). None of these patients received preoperative chemotherapy or radiotherapy, and all of them were treated with routine chemotherapy after the operation. The study population consisted of 57 men and 29 women (mean age: 58.8 years; age range: 38–80 years). The histological type and grade of cell differentiation were evaluated using hematoxylin-eosin stained sections according to the criteria of the World Health Organization (WHO).22 Pathological staging was determined by the latest tumor-node-metastasis (TNM) classification system.23 Detailed information about demography, clinical manifestation and histopathology was collected retrospectively for all patients. Follow-up lasted until December 2011, with a median follow-up period of 51.9 months for living patients (range: 26–67 months). Informed consent was obtained from all patients prior to the surgical operations. This study was reviewed and approved by the Ethics Committee of Harbin Medical University (Harbin, China). Surgically excised tumors and matched non-cancerous tissues used for quantitative real-time polymerase chain reaction (PCR) and Western blot analysis were immediately immersed in liquid nitrogen and stored at −80°C until needed.
RNA isolation, complementary DNA synthesis and quantitative real-time polymerase chain reaction
Total RNA was isolated from frozen tissue samples using the RNA simple total-RNA kit (Tiangen Biotech, Beijing, China) according to the manufacturer's instructions. Reverse transcription was performed with 1of total RNA from each sample using the TIANScript RT kit (Tiangen Biotech). Quantitative real-time RT-PCR was carried out using SYBR Green (Tiangen Biotech) on an Exicycler™ 96 real-time quantitative thermal block (Bioneer Corporation, Daejeon, Republic of South Korea). The PCR primer sequences were designed according to the human USP22 and β-actin gene sequences reported in GenBank and were chemically synthesized as follows:
USP22: forward, 5′-ATATATCTCGAGTTATGGTGTCCCGGCCAGAGC-3′ and reverse, 5′-TGTGTGGAATTCTCGTATTCCAGGAACTGTTTG-3′;
βactin: forward, 5′-ACGTTGACATCCGTAAAGAC-3′ and reverse, 5′-GAAGGTGGACAGTGAGGC-3′
A melting curve was generated at the end of every run to ensure product uniformity. β-actin served as the constitutive control. PCR reactions of each sample were conducted in triplicate. Data were analyzed through the comparative threshold cycle (CT) method.24 The relative USP22 mRNA expression was calculated by the 2−▵CTmethod (▵CT=CT of USP22 - CT of β-actin). The fold change of USP22 expression in each tissue was defined as the ratio of relative USP22 mRNA expression in tumor tissue to that in corresponding normal tissues.
Immunohistochemical staining
Formalin-fixed and paraffin-embedded specimens were cut into 5µm thick sections and mounted on glass slides. After dewaxing in xylene and rehydrating stepwise in ethanol, the sections were subjected to heat-induced antigen retrieval. Subsequently, endogenous peroxidase activity and non-specific protein binding were blocked with 3% hydrogen peroxide and 10% normal goat serum, respectively. The sections were then incubated at 4°C overnight with a rabbit anti-USP22 polyclonal antibody (dilution 1:50; Abcam plc., Cambridge, MA, USA). After being thoroughly washed with 0.01 mol/L phosphate-buffered saline (PBS) solution, the corresponding secondary antibody was applied and incubated at room temperature for 30 min. Immunolabeled sections were visualized by using 3,3′-diaminobenzidine (DAB), then counterstained with hematoxylin, dehydrated, and mounted. Sections were stained in parallel without primary antibody to provide a negative control.
Evaluation of immunohistochemical staining
Two pathologists independently evaluated all the sections in a blind manner. In the occasional case of discrepancy, the sections were re-evaluated until consensus was obtained. The scoring approach used in the assessment of immunostaining was in accordance with a relatively simple and reproducible protocol. Scores representing the percentage of tumor cells stained positive were as follows: 0, 0%; 1, 1%∼10%; 2, 11%∼50%; and 3, >50%. Intensity was estimated in comparison to the control and scored as follows: 0, negative staining; 1, weak staining; 2, moderate staining; and 3, strong staining. The sum of the intensity and extent score was used as the final staining score. According to the final scores, the tumor tissues were then divided into two groups: low-level USP22 expression (with a score ≤2) and high-level USP22 expression (with a score ≥3).
Western blot analysis
Total proteins were isolated from frozen lung cancer tissues using radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Haimen, China), and protein concentrations were quantified using the Bradford method. Equal amounts of protein lysates were resolved on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel, electrotransferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA), and blocked with 5% skimmed milk at room temperature for 2 h. Membranes were immunoblotted overnight at 4°C with anti-USP22 polyclonal antibody (dilution 1:1000; Abcam plc.) or anti-β-actin (dilution 1:5000; Sigma-Aldrich, St. Louis, MO, USA), followed by their respective horseradish peroxidase-conjugated secondary antibodies. After extensive washing, the band detection was revealed by an ECL plus chemiluminescence kit (Millipore). β-actin was used as an internal reference for relative quantification. Densitometric analysis of the immunoblots was done using the Gel pro 3.0 software.
Statistical analysis
The χ2-test was used for comparison between categorical variables and Student's t-test for parametric continuous variables. The Kappa test was used to assess the coincidence of USP22 mRNA and protein expression in NSCLC tissues. Survival curves were plotted by the Kaplan-Meier method and compared using the log-rank test. Survival data were evaluated using univariate and multivariate Cox regression analysis. All statistical analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) for Microsoft Windows. Probability values less than 0.05 were considered to be statistically significant.
Results
Ubiquitin-specific protease 22 is frequently overexpressed in primary non-small cell lung cancer tissues
We first quantitatively examined the expression of USP22 mRNA levels in 30 pairs of primary NSCLC tissues and their corresponding non-tumor samples by using quantitative real-time PCR. Our results showed that 14 out of 30 patients (46.7%) showed a higher expression level of USP22 mRNA in NSCLC specimens than in non-cancerous tissue specimens (at least 2-fold change). In addition, the relative expression of USP22 mRNA in the NSCLC specimens was significantly higher than that in the corresponding normal tissues (0.000207±0.000292 vs 0.000301±0.000428, P=0.043, paired Student's t-test; Figure 1 A).
Figure 1.
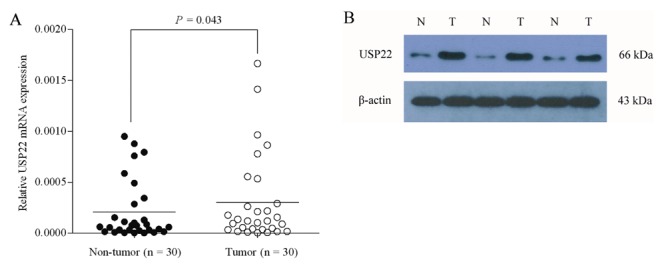
USP22 mRNA and protein expression primary lung cancer and paired adjacent normal tissues examined by quantitative real-time PCR (A) and western blot analysis (B), respectively. In (A) data were normalized to expression of β-actin. Bars represent the means of USP22 relative expression in cancer tissues and normal tissues. In (B) representative blots of three independent experiments are shown, and the protein size is expressed in kDa. In both (A) and (B) T represents tumor specimens, while N represents non-tumor specimens.
To investigate whether the difference in USP22 expression between tumor and non-neoplastic samples also occurs at the protein-expression level, we detected the expression of USP22 protein in the 30 paired samples by Western blot analysis. As expected, changes in 26 paired tissues (26/30, 86.7%) observed by Western blot analysis were in accordance with the findings in the quantitative real-time RT-PCR study, including up-regulated in 12 tumor tissues and down-regulated in 14 non-cancerous tissues. Representative blots are shown in Figure 1 B. The Kappa test revealed a significant correlation between the expression of USP22 mRNA and protein (P=0.000, =0.732; Table 1). Taken together, not only do these observations indicate that overexpression of USP22 plays an important role in the development of lung cancer, but they also suggest that USP22 gene expression may cause the differing levels of USP22 protein expression in NSCLC specimens.
Table 1. Coincidence of ubiquitin-specific protease 22 messenger RNA and protein expression in non-small cell lung cancer tissues.
| USP22 mRNA | P value | κ | |||
|---|---|---|---|---|---|
| High | Low or unchanged | ||||
| USP22 protein | High | 12 | 2 | 0.000 | 0.732 |
| Low or unchanged | 2 | 14 | |||
USP22, ubiquitin-specific protease 22; mRNA, messenger RNA.
Correlation of ubiquitin-specific protease 22 expression with clinicopathological parameters
To further investigate whether USP22 protein up-regulation is linked to the clinico-pathological parameters of NSCLC patients, 86 paraffin-embedded, archived NSCLC tissues were examined by immunohistochemical staining with an antibody against human USP22. As shown in Figure 2, the immunostaining of USP22 was mostly found in the cytoplasm of tumor cells in NSCLC tissues. In addition, USP22 staining was observed in primary NSCLC tissues, and the intensity of staining varied from tumor to tumor and/or from one area to another within the same tumor. According to the USP22 immunoreactive intensity, 47 (54.7%) patients were classified as low USP22 group and 39 (45.3%) patients were classified as high USP22 group. We then evaluated the association between USP22 expression and the clinicopathological data of the patients. As summarized in Table 2, we observed that the expression of USP22 protein was significantly associated with large tumor size (P=0.029) and lymph node metastasis (P=0.026). Nevertheless, there were no significant correlation between USP22 protein expression and other clinicopathological factors including age, gender, smoking history, histological type, tumor differentiation and TNM stage.
Figure 2.
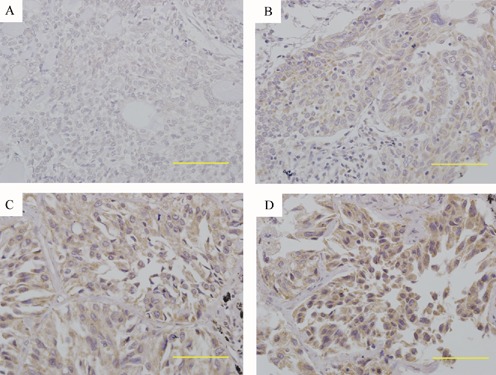
Immunohistochemical staining of USP22 protein in NSCLC tissue samples. Tissue sections were immunohistochemically stained with an anti-USP22 antibody and scored as 0 (A), 1+ (B), 2+ (C) and 3+ (D). Positive USP22 immunostaining was mainly localized in the cytoplasm of tumor cells. Scale bars=50 µm. Original magnifications=400×.
Table 2. Association between ubiquitin-specific protease 22 expression and various clinicopathological factors of patients with non-small cell lung cancer.
| Variables | Categories | Cases | USP22 protein expression | P value | |
|---|---|---|---|---|---|
| (n=86) | Low (n=47) | High (n=39) | |||
| Age | <60 | 44 | 27 (61.4%) | 17 (38.6%) | NS |
| ≥60 | 42 | 20 (47.6%) | 22 (52.4%) | ||
| Gender | Male | 57 | 31 (54.4%) | 26 (45.6%) | NS |
| Female | 29 | 16 (55.2%) | 13 (44.8%) | ||
| Smoking history | Non-smoker | 29 | 15 (51.7%) | 14 (48.3%) | NS |
| Smoker | 57 | 32 (56.1%) | 25 (43.9%) | ||
| Histological type | Scc | 31 | 18 (58.1%) | 13 (41.9%) | NS |
| Ac | 49 | 25 (51.0%) | 24 (49.0%) | ||
| Others | 6 | 4 (66.7%) | 2 (33.3%) | ||
| Tumor differentiation | Well | 6 | 1 (16.7%) | 5 (83.3%) | NS |
| Moderate | 47 | 24 (51.1%) | 23 (48.9%) | ||
| Poor | 33 | 22 (66.7%) | 11 (33.3%) | ||
| TNM stage | I | 55 | 32 (58.2%) | 23 (41.8%) | NS |
| II | 31 | 15 (48.4%) | 16 (51.6%) | ||
| Tumor size | ≤3 cm | 42 | 28 (66.7%) | 14 (33.3%) | 0.029 |
| >3 cm | 44 | 19 (43.2%) | 25 (56.8%) | ||
| Lymph node metastasis | Absent | 57 | 36 (63.2%) | 21 (36.8%) | 0.026 |
| Present | 29 | 11 (37.9%) | 18 (62.1%) | ||
USP22, ubiquitin-specific protease 22; NS, not significant; SCC, squamous cell carcinomas; AC, adenocarcinomas; TNM, tumor-nodes-metastasis.
Association of ubiquitin-specific protease 22 expression with prognosis of patients with non-small cell lung cancer
After surgery, all patients were followed-up for overall survival, and the mean duration of the follow-up period for 35 survivors was 51.9 months (range: 26–67 months). The Kaplan-Meier method was used to further analyze the association of USP22 expression with prognosis of NSCLC patients. We found that the survival of patients with high USP22 protein expression was significantly shorter than that of patients with low USP22 protein expression (P=0.006; Figure 3).
Figure 3.
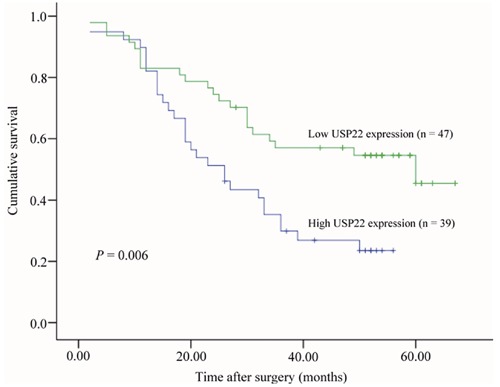
Kaplan-Meier analysis of overall survival rate of NSCLC patients with high USP22 expression (n=39) and low USP22 expression (n=47), respectively. The overall survival rate between the two groups showed significantly different (P=0.006, log-rank test).
To evaluate the possibility of USP22 used as an independent risk factor for poor prognosis, conventional clinicopathological factors and USP22 protein levels were assessed by Cox's univariate and multivariate hazard regression model (Table 3). Univariate analysis indicated that histological type, tumor differentiation, TNM stage, tumor size, and USP22 protein expression were significantly associated with overall survival of NSCLC patients (P=0.001, 0.014, 0.007, 0.011 and 0.008, respectively). By multivariate analysis, we showed that histological type, tumor differentiation, tumor size, and USP22 protein expression were independent prognostic factors for overall survival of NSCLC patients (P=0.000, 0.013, 0.016 and 0.003, respectively).
Table 3. Univariate and multivariate analysis of prognostic factors in 86 patients with non-small cell lung cancer.
| Variables | Categories | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95% CI) | P value | ||
| Age | <60/≥60 | 1.02 (0.98–1.06) | 0.333 | 1.00 (0.97–1.04) | 0.874 |
| Gender | Male/female | 1.11 (0.63–1.98) | 0.716 | 0.95 (0.45–2.01) | 0.891 |
| Smoking history | Non-smoker/smoker | 1.04 (0.58–1.84) | 0.904 | 1.39 (0.68–2.83) | 0.370 |
| Histological type | SCC/AC/others | 2.21 (1.42–3.44) | 0.001 | 2.81 (1.62–4.85) | 0.000 |
| Tumor differentiation | Well/moderate/poor | 1.84 (1.13–2.98) | 0.014 | 2.10 (1.17–3.75) | 0.013 |
| TNM stage | I/II | 2.15 (1.24–3.73) | 0.007 | 1.16 (0.61–2.20) | 0.644 |
| Tumor size | ≤3 cm/>3 cm | 2.07 (1.18–3.62) | 0.011 | 2.41 (1.18–4.93) | 0.016 |
| Lymph node metastasis | Absent/present | 1.03 (0.58–1.83) | 0.920 | 1.18 (0.61–2.28) | 0.628 |
| USP22 protein expression | Low/high | 2.16 (1.23–3.80) | 0.008 | 2.91 (1.42–5.97) | 0.003 |
HR, hazard ratio; CI, confidence interval; SCC, squamous cell carcinomas; AC, adenocarcinomas; TNM, tumor-nodes-metastasis; USP22, ubiquitin-specific protease 22.
Discussion
In the present study, we reported for the first time that the relative level of USP22 mRNA expression in primary NSCLC tissues is significantly higher than that in corresponding non-tumor lung tissues. High-level USP22 protein expression was also observed in 39 out of 86 early-stage NSCLC patients, suggesting that elevated expression of USP22 may contribute to the development and progression of NSCLC. By analyzing the correlation between clinico-pathological factors of patients and USP22 protein expression, we showed that high level of USP22 protein was significantly associated with large tumor size and lymph node metastasis, but not with other factors of early-stage NSCLC patients, including age, gender, smoking history, histological type, tumor differentiation and TNM stage. Furthermore, statistical analysis also indicated that patients with higher USP22 expression had a shorter overall survival time, whereas patients with lower USP22 expression had a better survival. Taken together, our results suggest that overexpression of USP22 is associated with poorer prognosis and is an independent prognostic factor for survival in patients with early-stage NSCLC.
Several previous reports have documented that USP22 is frequently overexpressed in several epithelial cancer types, such as colorectal cancer,17,18 breast cancer19 and gastric cancer.20 These observations suggest that USP22 may have important implications in carcinogenesis. To the best of our knowledge, however, the expression of USP22 in NSCLC tissues and its correlation to clinicopathological features have not yet been determined. To address these issues, we first detected its mRNA and protein levels in 30 paired NSCLC tissues by quantitative real-time PCR and western blot analysis, respectively. Furthermore, we assessed the USP22 protein in 86 NSCLC tumor specimens by immunohistochemistry and analyzed its correlation to clinicopathological features. The results showed that both USP22 mRNA and protein levels were significantly higher in tumor tissues than in corresponding non-tumor tissues, which are reminiscent of previous reports in other malignancies.17–20 Our findings also suggest that up-regulation of USP22 may be an important event in the development and progression of NSCLC. In addition, USP22 positively correlates with large tumor size and lymph node metastasis in these NSCLC patients. These findings agree with the fact that a number of studies have indicated a possible link between USP22 expression and malignant behavior of cancer cells. For example, Liu et al. have demonstrated that USP22 acts as an oncogene in colorectal cancer by the activation of the BMI-1-mediated INK4a/ARF pathway and the Akt pathway.15 In addition, it is also reported that aberrant expression of USP22 is associated with liver metastasis in colorectal cancer.18 Our results may also support this notion as we showed that overexpression of USP22 occurred in 45% of the NSCLC tissues, and USP22 protein expression was significantly correlated with large tumor size and lymph node metastasis of NSCLC patients. Our data suggest that USP22 is up-regulated in NSCLC tissues and positively participates in NSCLC progression.
In this study, univariate and multivariate analyses indicated that USP22 expression was recognized as an independent prognostic factor for the outcome of patients with early-stage NSCLC. Not only do our findings suggest a potentially promising use of USP22 as a valuable prognostic indicator, but they also imply a possible link between the biological function of USP22 and the pathogenesis of NSCLC. This could lead to the development of a novel anti-lung cancer strategy. Nevertheless, further studies are needed to elucidate the molecular mechanisms by which USP22 participates in the development and progression of lung cancer and to address whether USP22 could be used as a target for novel therapeutic approaches.
Nevertheless, this study has several limitations. First, the relatively small number of analyzed patients may reduce the power to detect statistical associations and significantly affects survival analyses. Second, beyond cause of mortality, data on cancer recurrences were not available in these cohorts. Third, all patients did not receive the same chemotherapy regimen both in term of schedule and in term of associated drug. Thus, our findings need to be further confirmed in a large number of patients with a uniform treatment protocol.
In conclusion, the data from the current study have demonstrated that USP22 is overexpressed in NSCLC and correlated with large tumor size and lymph node metastasis as well as unfavorable prognosis of NSCLC patients with early clinical stage. Our study revealed that overexpression of USP22 is a potential prognostic predictor in early-stage NSCLC patients and may provide a valuable tool in selecting patients for adjuvant treatment.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. Ca-Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. New Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung JJ, Hsu WH, Hsieh CC, Huang BS, Huang MH, Liu JS, et al. Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence. Thorax. 2009;64:192–6. doi: 10.1136/thx.2007.094912. [DOI] [PubMed] [Google Scholar]
- 4.Hung JJ, Jeng WJ, Hsu WH, Wu KJ, Chou TY, Hsieh CC, et al. Prognostic factors of postrecurrence survival in completely resected stage I non-small cell lung cancer with distant metastasis. Thorax. 2010;65:241–5. doi: 10.1136/thx.2008.110825. [DOI] [PubMed] [Google Scholar]
- 5.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. New Engl J Med. 2004;350:351–60. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 6.Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al. Vinorelbine plus cisplatin vs observation in resected non-small-cell lung cancer. New Engl J Med. 2005;352:2589–97. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 7.Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzales-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–27. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 8.Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–9. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 9.Strauss GM, Herndon JE, II, Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the cancer and leukemia group b, radiation therapy oncology group, and north central cancer treatment group study groups. J Clin Oncol. 2008;26:5043–51. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glinsky GV, Berezovska O, Glinskii AB. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest. 2005;115:1503–21. doi: 10.1172/JCI23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–12. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 12.Lee KK, Florens L, Swanson SK, Washburn MP, Workman JL. The deubiquitylation activity of Ubp8 is dependent upon Sgf11 and its association with the SAGA complex. Mol Cell Biol. 2005;25:1173–82. doi: 10.1128/MCB.25.3.1173-1182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berezovska OP, Glinskii AB, Yang Z, Li XM, Hoffman RM, Glinsky GV. Essential role for activation of the Polycomb group (PcG) protein chromatin silencing pathway in metastatic prostate cancer. Cell Cycle. 2006;5:1886–901. doi: 10.4161/cc.5.16.3222. [DOI] [PubMed] [Google Scholar]
- 14.Zhang XY, Pfeiffer HK, Thorne AW, McMahon SB. USP22, an hSAGA subunit and potential cancer stem cell marker, reverses the polycomb-catalyzed ubiquitylation of histone H2A. Cell Cycle. 2008;7:1522–4. doi: 10.4161/cc.7.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu YL, Jiang SX, Yang YM, Xu H, Liu JL, Wang XS. USP22 acts as an oncogene by the activation of BMI-1-mediated INK4a/ ARF pathway and Akt pathway. Cell Biochem Biophys. 2012;62:229–35. doi: 10.1007/s12013-011-9287-0. [DOI] [PubMed] [Google Scholar]
- 16.Lee HJ, Kim MS, Shin JM, Park TJ, Chung HM, Baek KH. The expression patterns of deubiquitinating enzymes, USP22 and Usp22. Gene Expr Patterns. 2006;6:277–84. doi: 10.1016/j.modgep.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Liu YL, Yang YM, Xu H, Dong XS. Increased expression of ubiquitin-specific protease 22 can promote cancer progression and predict therapy failure in human colorectal cancer. J Gastroenterol Hepatol. 2010;25:1800–5. doi: 10.1111/j.1440-1746.2010.06352.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu YL, Yang YM, Xu H, Dong XS. Aberrant expression of USP22 is associated with liver metastasis and poor prognosis of colorectal cancer. J Surg Oncol. 2011;103:283–9. doi: 10.1002/jso.21802. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Yao L, Zhang X, Ji H, Wang L, Sun S, et al. Elevated expression of USP22 in correlation with poor prognosis in patients with invasive breast cancer. J Cancer Res Clin. 2011;137:1245–53. doi: 10.1007/s00432-011-0998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang DD, Cui BB, Sun LY, Zheng HQ, Huang Q, Tong JX, et al. The co-expression of USP22 and BMI-1 may promote cancer progression and predict therapy failure in gastric carcinoma. Cell Biochem Biophys. 2011;61:703–10. doi: 10.1007/s12013-011-9229-x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang XY, Varthi M, Sykes SM, Phillips C, Warzecha C, Zhu W, et al. The putative cancer stem cell marker USP22 is a subunit of the human SAGA complex required for activated transcription and cell-cycle progression. Mol Cell. 2008;29:102–11. doi: 10.1016/j.molcel.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18:1059–68. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- 23.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–7. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]


