Abstract
Reduced reproduction is associated with increased fat storage and prolonged lifespan in multiple organisms, but the underlying regulatory mechanisms remain poorly understood. Recent studies in several species provide evidence that reproduction, fat metabolism, and longevity are directly coupled. For instance, germline removal in the nematode Caenorhabditis elegans promotes longevity in part by modulating lipid metabolism through effects on fatty acid desaturation, lipolysis, and autophagy. Here, we review these recent studies and discuss the mechanisms by which reproduction modulates fat metabolism and lifespan. Elucidating the relationship between these processes could contribute to our understanding of age-related diseases, including metabolic disorders.
Keywords: Reproduction, Fat Metabolism, Resource Allocation, Somatic Maintenance, Aging, Insulin Signaling, Steroid Signaling, Autophagy, Metabolism
Introduction
Reproduction is an energetically costly process that has profound effects on the metabolism of fat, the major form of energy storage in animals (Bronson, 1989). During reproduction, animals mobilize their fat reserves, whereas reduced or abolished reproduction can increase lipid storage and lead to weight gain in many species (Corona et al., 2009; Judd et al., 2011; McElroy and Wade, 1987). This inverse relationship between reproduction and fat storage seems to reflect an inevitable energetic trade-off. In this so-called "cost of reproduction" scenario, depletion of energy reserves to support reproduction is thought to compromise the organism’s ability to support somatic maintenance and survival (Kirkwood, 1977; Williams, 1966). Hence, lipids apportioned to reproduction, for instance, would be unavailable for other life-sustaining processes to support somatic maintenance and survival (Shanley and Kirkwood, 2000). Indeed, in most animals, reproduction trades off with maintenance and survival so that individuals with reduced or curtailed reproduction survive better and live longer than those with higher reproductive effort, and vice versa (Bell and Koufopanou, 1986; Partridge et al., 2005). Interestingly, in a variety of organisms, increased lifespan is associated with reduced reproduction but markedly increased lipid storage and thus improved survival under starvation conditions (Gems et al., 1998; Judd et al., 2011; Rion and Kawecki, 2007; Tatar et al., 2001). Despite these observations, the mechanisms connecting reproduction, fat metabolism, and lifespan remain poorly understood.
Recent evidence suggests that the three processes might be causally linked through a reproductive–endocrine signaling axis. In the nematode C. elegans and the fruit fly Drosophila melanogaster, for example, ablation of the germline increases lifespan (Flatt et al., 2008; Hsin and Kenyon, 1999) and significantly alters lipid metabolism (O'Rourke et al., 2009; Parisi et al., 2010). Moreover, studies in C. elegans have begun to uncover the molecular mechanisms by which signals from the reproductive system regulate lipid metabolism and lifespan (Goudeau et al., 2011; Lapierre et al., 2011; McCormick et al., 2011; Wang et al., 2008).
Here, we review these recent findings on the complex interplay between reproduction, fat metabolism, and aging. We first summarize seminal observations and recent findings that suggest that the processes are physiologically linked in many organisms (Figure 1). We then discuss recent insights into the molecular mechanisms that may underlie these connections (Figure 2). Finally, we highlight the implications of these findings for our understanding of aging and age-related diseases, including metabolic disorders, and summarize some of the future challenges (Box 1).
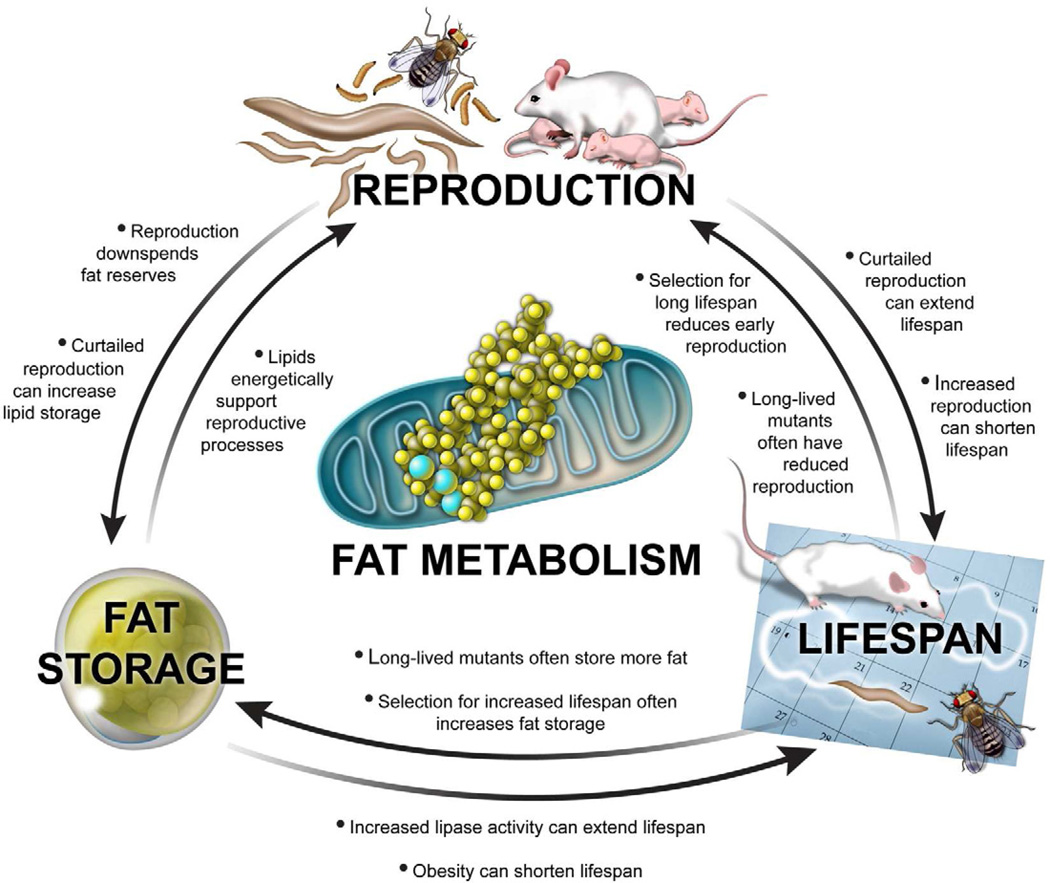
Figure 1. Reproduction, Fat Metabolism, and Lifespan are Intimately Interconnected.
Although the mechanistic cause-and-effect relationships are not yet clear, multiple lines of experimental evidence point to tight links between reproduction, fat metabolism, and aging (see bullet points in figure). For example, observations in worms, insects, and rodents indicate that reproduction (top center) can directly affect fat storage and lifespan. Moreover, increased lifespan (bottom right) is often negatively correlated with reproduction and positively correlated with increased fat storage, whereas fat metabolism (bottom left) influences the energetic cost of reproduction and may directly modulate lifespan.
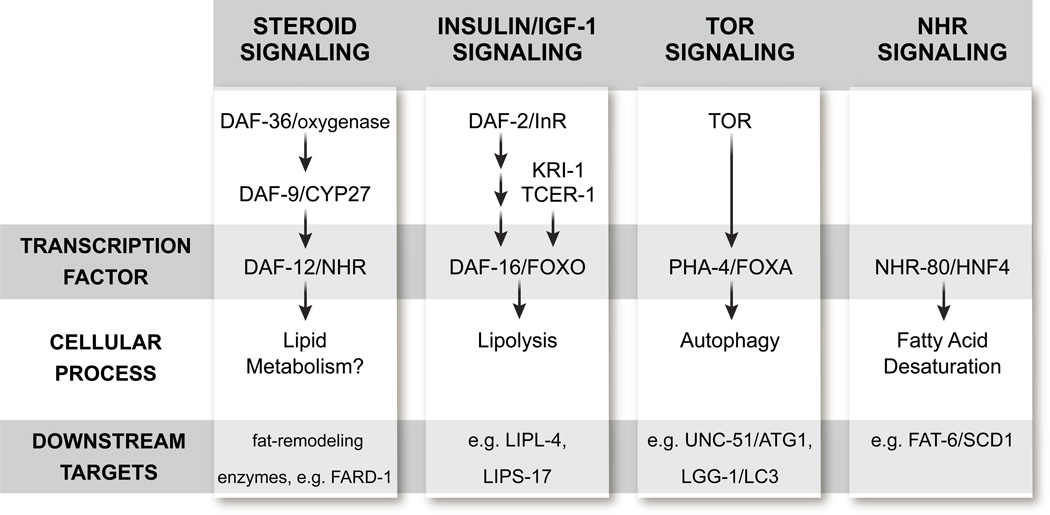
Figure 2. Model of Germline Signaling and Its Impact on Fat Metabolism.
Ablation of the germline increases lifespan in C. elegans and D. melanogaster and profoundly alters fat metabolism. To date, our understanding of the underlying molecular mechanisms are mainly based on findings in C. elegans; however, Drosophila homologs are indicated where possible. For functional parallels between steroid signaling in C. elegans and D. melanogaster seeGalikova et al. (2011). Each of the four longevity-promoting transcription factors (DAF-12/EcR, DAF-16/FOXO, PHA-4/FOXA, and NHR-80/HNF4) either target fat remodeling enzymes or activate cellular processes that can affect fat metabolism. Lifespan extension through removal of the germline therefore provides insights into the mechanisms that link reproduction, fat metabolism, and lifespan.
Box 1. Outstanding Questions and Futures Perspectives.
The links between fat metabolism, reproduction and lifespan are only starting to be uncovered. The table summarizes some of the key questions that are likely to significantly improve our understanding of these links at the molecular level, and highlights current technical challenges such as engineering germline-less mice.
| Conceptual Questions |
|
| Technical Questions |
|
Reproduction, Fat Metabolism, and Lifespan are Interconnected
Animal physiologists have long known that reproduction affects the storage and mobilization of fat, and similarly, evolutionary biologists have made numerous observations suggesting that reproduction influences organismal lifespan and aging. It is thus tempting to speculate that fat might be a causal, physiologic link coupling reproduction and lifespan. Indeed, several lines of evidence indicate that reproduction, resistance to starvation stress, and lifespan might be energetically linked through lipid metabolism (Figure 1).
Effects of the reproductive system on fat metabolism
Numerous studies in mice, rats, cats, monkeys, and other mammals have reported that gonadectomy leads to weight gain and increased fat mass (e.g., Crane, 1991; Fettman et al., 1997; McElroy and Wade, 1987; Pallier et al., 1980; Salmeri et al., 1991; Stotsenburg, 1913, and references therein). In humans and other mammals, hypogonadism, a deficiency in gonadal hormone production and function (reviewed in Wilson and Roehrborn, 1999), is often associated with accumulation of excess fat (obesity), insulin resistance (inability of cells to properly respond to insulin), and metabolic syndrome (diabetes and other metabolic disorders) (Corona et al., 2009). The link between hypogonadism and excess fat seems to be bidirectional: while it is clear that visceral obesity can cause hypogonadism, gonadectomy experiments in animals and other data suggest that hypogonadism can also cause fat accumulation and insulin resistance (Corona et al., 2009).
The connection between impaired or reduced reproduction and excess fat storage is not restricted to mammals. In a variety of insects, including blow flies, bugs, migratory locusts, and grasshoppers, ovariectomy causes hypertrophy of the fat body, the insect equivalent of mammalian adipose and liver tissue (Judd et al., 2011; Socha et al., 1991; Strong, 1967; Thomsen and Hamburger, 1955). Such fat-body hypertrophy is also seen in two female-sterile mutants of D. melanogaster, called mama1 (Doane, 1961) and Rbp9 (Butterworth and Bodenstein, 1968). Remarkably, implantation of wild-type ovaries restores the fat-body tissue to its normal size in both mutants. Consistent with these findings, transgenic ablation of the germline by overexpression of bag of marbles (bam), a protein involved in germline differentiation and maintenance, increases fat storage and starvation resistance in adult female flies (M. Galikova, HA and TF, unpublished data). Similarly, germline-less C. elegans glp-1 mutants, which carry a mutation in the GLP-1/Notch receptor (Arantes-Oliveira et al., 2002), have greatly increased fat stores (McCormick et al., 2011; O'Rourke et al., 2009; MH, and HA, unpublished data).
Effects of the reproductive system on lifespan
Surgical or genetic ablation of the reproductive system significantly extends lifespan in many animals (e.g., Drori and Folman, 1976; Flatt, 2011; Flatt et al., 2008; Hatle et al., 2008; Hsin and Kenyon, 1999; Leroi, 2001; Maynard Smith, 1958; Partridge et al., 2005). Ovariectomized grasshoppers, for example, live significantly longer than sham-operated control animals (Drewry et al., 2011; Judd et al., 2011), and gonadectomy of Pacific salmon also increases their lifespan (Robertson, 1961). Similarly, castration of male rats extends their lifespan by a short but significant period (Drori and Folman, 1976), and castrated men have been reported to live significantly longer than fertile men (Hamilton and Mestler, 1969; Min et al., 2012).
The effect of interventions that abolish reproduction is particularly dramatic in C. elegans and Drosophila, where targeted ablation of germline stem cells significantly extends lifespan (Arantes-Oliveira et al., 2002; Flatt et al., 2008; Hsin and Kenyon, 1999; Maynard Smith, 1958). Notably, this effect is abrogated in C. elegans when the somatic gonad is also removed (Hsin and Kenyon, 1999), suggesting that lifespan extension is not simply a result of sterility but is regulated by opposing signals produced by the germline and the somatic gonad.
Together, these findings suggest that gonadal signals not only affect fat metabolism in a number of species but also modulate their rate of aging. Although these signals have not yet been unambiguously identified, steroid hormones are promising candidates (Galikova et al., 2011; Gerisch et al., 2007; Tatar et al., 2003). Thus, given that reproduction profoundly affects both fat metabolism and lifespan, it is plausible to hypothesize that the three physiological functions might be causally interconnected.
The tripartite interconnection between reduced reproduction, increased fat storage, and improved adult survival (see Figure 1) is perhaps most clearly seen in experiments with C. elegans and Drosophila, although similar observations have been made in other animals (Reznick, 1985; Bell and Koufopanou, 1986). For example, several independent studies have shown that wild-type flies artificially selected for increased lifespan also exhibit impaired early fecundity, and enhanced starvation resistance due to increased fat storage (Chippindale et al., 1993; Djawdan et al., 1998; Djawdan et al., 1996; Service et al., 1985; Zwaan et al., 1995). Similarly, many long-lived single-gene mutants of C. elegans and Drosophila (e.g., mutations in the insulin/IGF-1 and TOR (Target of Rapamycin) signaling pathways) exhibit reduced fecundity, increased lipid storage, and improved starvation stress resistance (Broughton et al., 2005; Gems et al., 1998; Tatar et al., 2001; Tissenbaum and Ruvkun, 1998). Conversely, artificial selection for increased starvation resistance in Drosophila is often correlated with increased lipid storage, reduced fecundity, and prolonged lifespan (reviewed in Hoffmann and Harshman, 1999; Rion and Kawecki, 2007). In mice, most but not all long-lived models exhibit increased fat levels (Wolf, 2010). While enhanced fat storage in response to lifespan-promoting mutations may be necessary to support somatic maintenance (Liao et al., 2011), excessive fat is also an important risk factor for metabolic syndrome, diabetes, and cardiovascular disease, which can significantly curtail lifespan (Das et al., 2004). Thus, the role of fat in long-lived mouse models remains unclear.
Although the association between reproduction, lipid storage, and lifespan is not always observed, suggesting that these processes can be dissociated (Flatt, 2011; Force et al., 1995; Gems et al., 1998; Hoffmann and Harshman, 1999; Rion and Kawecki, 2007; Vermeulen et al., 2006), it is clear that such an association—be it correlative or causal—very often exists. In the following sections, we discuss our current understanding of the molecular underpinnings of the links between the three processes.
Molecular Connections between Reproduction, Fat Metabolism, and Lifespan
The most direct molecular evidence for a causal connection between reproduction, fat metabolism, and lifespan comes from recent studies in C. elegans showing that germline removal not only extends lifespan but also profoundly affects fat metabolism (Goudeau et al., 2011; Lapierre et al., 2011; Wang et al., 2008). Moreover, several fat remodeling enzymes were found to be necessary, and sometimes sufficient, to promote longevity through removal of the germline (Goudeau et al., 2011; Lapierre et al., 2011; O'Rourke et al., 2009; Wang et al., 2008; Xie, 2008). Much progress has been made in identifying genes involved in both fat metabolism and lifespan determination that are activated by germline ablation in C. elegans (Berman and Kenyon, 2006; Gerisch et al., 2007; Ghazi et al., 2009; Goudeau et al., 2011; Hsin and Kenyon, 1999; Lapierre et al., 2011; McCormick et al., 2011; Yamawaki et al., 2008; Yamawaki et al., 2010). Together with work in other systems, including Drosophila, these studies provide a first glimpse of the molecular mechanisms (Figure 2) that might form the regulatory framework linking reproduction, fat metabolism, and lifespan.
Insulin/IGF-1 and steroid signaling pathways: common regulators of reproduction, fat metabolism, and lifespan
The first genes found to affect lifespan and metabolism in germline-less animals were identified in C. elegans and are examples of genes that function through a process we refer to as germline signaling. The impact of germline ablation on lifespan was first shown to be mediated by two dauer formation (daf) genes: daf-16, a FOXO transcription factor in the insulin/IGF-1 signaling pathway and daf-12, a nuclear hormone receptor that functions in steroid hormone signaling (Hsin and Kenyon, 1999). These pathways are described in detail below.
Insulin/IGF-1 signaling
Insulin/IGF-1 signaling is well known to affect both lifespan and fat metabolism in fertile animals (Kenyon et al., 1993; Perez and Van Gilst, 2008; Wolf, 2010). The positive impact of reduced insulin/IGF-1 signaling on lifespan and fat storage has been observed in worms, flies, and mice, suggesting an evolutionarily conserved association (Clancy et al., 2001; Holzenberger et al., 2003; Kenyon et al., 1993; Wolf, 2010; Wang et al., 2008); however, the role of insulin/IGF-1 signaling in germline-ablated animals has only recently been uncovered. In C. elegans, several components of the insulin/IGF-1 signaling pathway, such as daf-16/FOXO and daf-18/PTEN, are required for lifespan extension upon germline loss (Hsin and Kenyon, 1999; Larsen et al., 1995). Despite this connection, it should be noted that germline-mediated lifespan extension is influenced by many factors that are not components of the insulin/IGF-1 signaling pathway. For instance, genes such as kri-1, an ankyrin-repeat protein orthologous to the human gene KRIT1, and tcer-1, a transcription elongation factor related to TCERG1, promote lifespan extension in germline-less animals by interacting with daf-16/FOXO but are not involved in canonical insulin signaling (Ghazi et al., 2009). Other observations support the existence of a noncanonical insulin/IGF-1 signaling pathway in germline-less C. elegans. For example, many daf-16/FOXO target genes are non-overlapping in insulin/IGF-1 signaling-deficient versus germline-less mutants (McCormick et al., 2011; Murphy, 2006). Furthermore, kri-1 mediates DAF-16/FOXO translocation into intestinal nuclei upon germline ablation but not under conditions of reduced insulin signaling in daf-2 (insulin/IGF-1–like receptor, InR) mutants (Berman and Kenyon, 2006). Similarly, intestinal DAF-16/FOXO is activated (Lin et al., 2001; Libina et al., 2003) cell non-autonomously by microRNAs such as mir-71 that are produced in the neurons of germline-less animals, but not of daf-2/InR mutants (Boulias and Horvitz, 2012). Some of these regulatory connections may be evolutionarily conserved because long-lived germline-ablated Drosophila also exhibit altered insulin/IGF-1 signaling (Flatt et al., 2008).
Intriguingly, three independent studies have found that DAF-16/FOXO activates fat-processing enzymes in response to germline ablation in worms (Goudeau et al., 2011; McCormick et al., 2011; Wang et al., 2008). Indeed, genes involved in fat metabolism are overrepresented among daf-16/FOXO targets in germline-less animals (McCormick et al., 2011). In this study, daf-16/FOXO was found to regulate the transcription of genes involved in fat catabolism (e.g., lipases) and anabolism (e.g., diacylglyceride transferases, desaturases). As discussed below, at least two of these daf-16/FOXO targets, the lipases lipl-4 and lips-17, have been directly linked to the long lifespan of germline-less animals (Wang et al., 2008; McCormick et al., 2011). Thus, alterations in fat mobilization may represent one mechanism by which longevity and fat content are linked, perhaps as a means to optimize sustained energy consumption. Finally, daf-16/FOXO also regulates many genes involved in steroid hormone metabolism (e.g., steroid hormone dehydrogenases, cytochrome P450s), suggesting the possibility of crosstalk between the insulin and steroid hormone signaling pathways. The production of hydrophobic signaling molecules such as hormones would be an efficient way to trigger systemic responses. Thus, it is possible that changes in fat metabolism occurring in response to altered reproductive status may induce hormonal signaling that in turn elicits lifespan extension.
Steroid signaling
The second pathway known to be relevant for lifespan extension in response to germline loss, at least in C. elegans, is a steroid hormone pathway that includes daf-12, a nuclear hormone receptor (NHR) related to the vitamin D, pregnane X, and liver X receptors. This pathway also includes daf-36, a Rieske-like oxygenase, daf-9, a cytochrome P450 similar to CYP27A1, and dhs-16, a 3-hydroxysteroid dehydrogenase, all of which are involved in the production of dafachronic acids, the bile acid-like steroid hormone ligands of DAF-12/NHR (Hsin and Kenyon, 1999; Jia et al., 2002; Rottiers et al., 2006; Motola et al., 2006; Wollam et al., 2012). Of note, other genes involved in steroid signaling have not been reported to be required for germline-mediated lifespan extension in C. elegans. The longevity-promoting effects of daf-9/CYP27 and daf-12/NHR are specific to germline signaling because loss-of-function mutations in daf-9/CYP27 extend the lifespan of fertile animals (Jia et al., 2002), and some daf-12/NHR alleles increase the lifespan of males and mutants that exhibit reduced insulin/IGF-1 signaling (Gems et al., 1998; Hochbaum et al., 2011). The detailed mechanisms by which these genes govern germline-mediated longevity remain to be clarified. Interestingly, a recent study has suggested that dafachronic acid might be produced in the somatic gonad (Yamawaki et al., 2010), which could explain the requirement for these tissues in lifespan extension upon germline ablation (Hsin and Kenyon, 1999). The somatic gonad requirement is alleviated in animals carrying reduction-of-function alleles of daf-2/InR that result in markedly reduced insulin/IGF-1 signaling (Hsin and Kenyon, 1999; Yamawaki et al., 2008). One potential explanation for this observation is that low insulin/IGF-1 signaling might induce ectopic production of longevity-promoting steroid hormones in tissues other than the somatic gonad.
The impact of steroid signaling on fat metabolism has been demonstrated most clearly in mammals. Gonadectomy in mammals (mice, rats, and humans) causes a significant redistribution of fat to the periabdominal area and a gross increase in fat mass (Colombel and Charbonnel, 1997; Hausberger and Hausberger, 1966; McElroy and Wade, 1987; O'Rourke et al., 2009). Conversely, steroid hormone treatment restores normal fat metabolism in mice with reproductive deficits (Bjorntorp, 1997; Wohlers and Spangenburg, 2010). Worms also exhibit increased fat storage upon germline ablation (O'Rourke et al., 2009), although the impact of steroid hormones on fat storage has not yet been directly assessed. In fact, microarray studies show that fat remodeling enzymes are not overrepresented among DAF-12/NHR targets (McCormick et al., 2011). However, at least one DAF-12/NHR target, predicted to be the fatty acyl reductase fard-1, is essential for lifespan extension in germline-depleted animals (McCormick et al., 2011).
Although our limited understanding of the role of steroid signaling in fat metabolism makes it difficult to identify parallels among the molecularly tractable models, some noteworthy similarities do exist. For example, DAF-12/NHR interacts with another nuclear hormone receptor, NHR-80, to promote fatty acid desaturation in response to germline ablation (Goudeau et al., 2011). Fatty acid desaturation is also upregulated in ovariectomized mice and requires the presence of the nuclear receptors LXR and PPARα (Chu et al., 2006; Paquette et al., 2008). Thus, it will be interesting to explore the functional parallels between PPARα/LXR and NHR-80/DAF- 12.
Steroid hormones also affect fat metabolism in mammals through the estrogen receptor (ERα). Indeed, the importance of this receptor in regulating fat metabolism is clearly demonstrated by ERα deficient mice, which are prone to obesity (Heine et al., 2000). Moreover, it was recently shown that hypothalamic neurons are important sites of action for the regulation of fat metabolism by the ERα (Xu et al., 2011). The Drosophila ER homolog, dERR, has been found to regulate energy and carbohydrate metabolism (Tennessen et al., 2011), whereas the role of nhr-14—the closest worm ER homolog—remains unknown (Mimoto et al., 2007). Hence, it is not yet clear whether the relationship between estrogen signaling, fat metabolism, and reproduction can be easily modeled in simpler genetic systems.
Several reports suggest that insulin/IGF-1 and steroid hormone/NHR signaling interact to promote lifespan extension of germline-ablated animals. For instance, DAF-16/FOXO translocation into intestinal nuclei following germline removal is impaired in the absence of daf-12/NHR (Berman and Kenyon, 2006), suggesting that DAF-12/NHR signaling promotes longevity, at least in part, by promoting insulin/IGF-1 signaling. Collectively, the available evidence suggests that the insulin/IGF and steroid hormone signaling pathways play pivotal roles in modulating the effects of germline ablation on lifespan extension and lipid metabolism in C. elegans. The precise role played by each pathway in regulating fat metabolism in nematodes clearly requires further investigation, as does their contribution to lifespan and fat metabolism in other model systems. Preliminary work in Drosophila suggests that the effect of germline ablation on lifespan and fat metabolism might be conserved between worms and flies (Flatt et al., 2008; Galikova et al., 2011; M. Galikova, HA, and TF, unpublished data). Moreover, insulin/IGF-1 and steroid hormone (ecdysone) signaling also appear to regulate fat metabolism in Drosophila and other insects (DiAngelo and Birnbaum, 2009), and it is noteworthy that these hormonal signaling pathways also control germline stem cell proliferation and maintenance (Ables and Drummond-Barbosa, 2010; LaFever and Drummond-Barbosa, 2005). The greatest challenge, however, will be to determine if a mammalian model with curtailed reproduction has increased lifespan (see Box 1 for Outstanding Questions and Future Perspectives; also see, for example, Drori and Folman, 1976).
Role of lipolysis in germline-mediated longevity
The first identified target gene of the DAF-16/FOXO transcription factor in germline-ablated animals was the predicted triacylglycerol lipase and cholesterol esterase LIPL-4 (Wang et al., 2008). lipl-4 is induced in germline-less glp-1 mutants in a daf-16/FOXO-dependent fashion. Interestingly, the induction of lipl-4 requires kri- 1/KRIT but is independent of daf-12/NHR, suggesting the existence of a steroid hormone signaling-independent pathway for lipl-4 expression induced by germline ablation. In turn, inactivation of lipl-4 shortens the long lifespan of germline-less glp-1/Notch animals (Wang et al., 2008), supporting a crucial role for LIPL-4 activity in lifespan extension in these animals. Consistent with this notion, overexpression of LIPL-4 is sufficient to extend lifespan when expressed in wild-type animals from a ubiquitous promoter (Lapierre et al., 2011) or an intestinal promoter (Wang et al., 2008). The latter finding provided one of the first direct links between lipid metabolism and longevity.
LIPL-4 has lipase activity in vitro (Lapierre et al., 2011) and is most prominently expressed in the gut and hypodermal seam cells (Wang et al., 2008; Lapierre et al., 2011); notably, these tissues are fat storage sites in C. elegans (Mak, 2012). The cellular substrate(s) and the subcellular localization of LIPL-4 are not yet known, but it is interesting to note that LIPL-4 has the highest homology to human lysosomal acid lipase (LAL), an enzyme involved in cholesterol ester processing and trafficking from autophagic and endocytic vesicles (Ouimet et al., 2011).
Other lipases are also upregulated in germline-less animals, including lips-17, which is required for germline-less glp-1/Notch mutants to live long (McCormick et al., 2011). Similarly, germline-less Drosophila tudor mutants exhibit increased expression of four triacylglycerol lipases (CG8093, CG6277, CG6271, CG2772) and one lipid storage gene (lipid storage droplet protein 1), suggesting that lipid metabolism might be enhanced in germline-less flies (Parisi et al., 2010). Despite these effects, little difference was observed between the lipid profiles of germline-less and fertile control flies in this study (Parisi et al., 2010). It will be interesting to determine whether lipolysis also plays a role in germline-mediated longevity and reproduction in other organisms, and whether changes in lipolysis and expression of longevity-relevant lipases are observed during normal aging.
Role of autophagy in germline-mediated longevity
The cellular recycling process of autophagy has recently been linked to the longevity of germline-less animals. Autophagy sequesters and degrades cytosolic components and is typically induced under stress conditions such as nutrient deprivation. One type of autophagy called macroautophagy (referred to here as autophagy) has been directly linked to aging (Rubinsztein et al., 2011). Specifically, pharmacological and genetic manipulations that increase lifespan in model organisms, including C. elegans and Drosophila, often stimulate autophagy. Conversely, inhibition of autophagy compromises the longevity-promoting effects of multiple paradigms, suggesting that increased autophagy might function as a potent anti-aging mechanism. Consistent with this notion, germline-less C. elegans show increased rates of autophagy (Lapierre et al., 2011), and the expression of multiple autophagy genes is induced, at least in part, by the FOXA transcription factor PHA-4, another forkhead transcription factor. This suggests that autophagy is likely to be transcriptionally regulated in germline-less animals. Finally, pha-4, as well as multiple genes with autophagy-related functions, are required for germline-less animals to live long (Lapierre et al., 2011). These findings imply that longevity of germline-less animals is regulated by at least two transcription factors, PHA-4/FOXA and DAF- 16/FOXO, through induction of autophagy and metabolic genes, respectively.
The nutrient sensor TOR, a major conserved regulator of autophagy (Kapahi et al., 2010), may serve as a common upstream regulator of lipid metabolism and autophagy in germline-less nematodes. Lapierre et al. found that germline-less animals have lower intrinsic TOR levels (Lapierre et al., 2011), which is consistent with the possibility that reduced TOR levels constitutively induce autophagy in these animals. Moreover, TOR inactivation upregulates lipl-4 expression in a daf-16/FOXO-dependent fashion (Lapierre et al., 2011). Future work examining TOR pathway components will shed light on the dual regulatory role of TOR signaling in germline-less animals.
Multiple types of autophagy have been described that are cargo-specific, including one that mediates lipid breakdown and has been termed “lipophagy.” Evidence for this pathway comes from observations with cultured hepatocytes and mouse liver in which inhibition of autophagy increases triglyceride storage in lipid droplets (Singh et al., 2009). Interestingly, overexpression of LIPL-4 alone is sufficient to induce autophagy in C. elegans, probably via downregulation of TOR (Lapierre et al., 2011), and reciprocally, autophagy activity is required for the lipase activity of LIPL-4. Likewise, autophagy genes are required for LIPL-4–overexpressing animals to live long (Lapierre et al., 2011). Taken together, these data suggest that autophagy is intimately linked to lipid hydrolysis in the adult animal, possibly through a mechanism similar to lipophagy (Singh et al., 2009). Indeed, autophagosomes can be observed close to lipid droplets in germline-less animals (Lapierre et al., 2011). Alternatively, autophagy could be triggered by a substrate produced by LIPL-4. Regardless of the exact mechanism, these findings suggest for the first time that autophagy engages lipid metabolism as a mechanism to extend lifespan in response to germline removal. Future experiments are needed to clarify the nature of this link, and to determine whether it is conserved in higher organisms.
Autophagy is not always associated with increased lipid hydrolysis, because inhibition of autophagy in adipocytes results in decreased lipid accumulation as a result of impaired differentiation of white adipocytes (Singh et al., 2009). Taken together, these observations suggest that autophagy not only mediates breakdown of triacylglycerol droplets (i.e., through the catabolic process of lipophagy) but may also facilitate fat storage. The relationship between autophagy and fat storage needs more thorough investigation, including in germline-less C. elegans.
NHR signaling and fatty acid desaturation in germline-mediated longevity
Germline ablation in C. elegans was recently shown to induce transcription of the nuclear hormone receptor NHR-80. In turn, NHR-80 triggers the induction of fat-6/scd1, a gene encoding a stearoyl coenzyme A desaturase (Goudeau et al., 2011). FAT-6/SCD1 converts saturated C16 or C18 fatty acids into their corresponding monodesaturated forms. This induction is physiologically relevant because it results in increased levels of desaturated fatty acids. Remarkably, obstructing the induction of nhr-80 or fat-6 abolishes lifespan extension through germline ablation (Goudeau et al., 2011). Although the effect of fat-6/scd1 upregulation on fat content has not yet been determined in the worm, SCD1 deficiency in mice protects against lipid accumulation (Kim et al., 2011). These findings suggest that signals from the germline impinge on longevity in C. elegans and on fat content in mice by inhibiting fatty acid desaturation through SCD1.
In mice and rats, ovariectomy causes a strong induction of SCD1 levels and an associated increase in fatty acid desaturation (Jackson et al., 2011; Paquette et al., 2008). Although the mechanism by which this occurs is not yet well understood, the finding indicates that increased fatty acid monodesaturation might be a conserved response to impaired reproduction. The observation that germline ablation in C. elegans and ovariectomy in mammals cause increased fat content (McElroy and Wade, 1987; O'Rourke et al., 2009) is consistent with the observations that the desaturation of stearic acid leads to the production of oleic acid, a prime substrate for triacylglycerol synthesis and that SCD1 activity correlates with overall adiposity (Chu et al., 2006; Mauvoisin et al., 2007). However, whether scd1(-) ovariectomized mice or fat-6(-)/fat-7(-) germline-less worms are resistant to weight gain has not yet been tested.
Studies of the regulation of SCD1 in mammals provide additional interesting insights. SCD1 responds to many stimuli, including hormones and nutrients, and it is striking that many important SCD regulators are known to affect longevity and/or fat content (Mauvoisin et al., 2007). For example, SCD1 activity is strongly affected by the insulin/IGF-1 pathway and by numerous NHRs (Lundholm et al., 2004; Mauvoisin et al., 2007; Paquette et al., 2008; Waters and Ntambi, 1994). Interestingly, the increased lifespan observed in C. elegans daf-2/InR mutants has been partly attributed to the upregulation of fat-7/SCD1 (Murphy et al., 2003), suggesting that fatty acid desaturation may also promote the lifespan of fertile animals. It will be interesting to discover why fatty acid desaturation is required to support extended longevity when the germline is ablated and when insulin signaling is low in other systems such as Drosophila.
SCD1 also appears to be regulated by NHRs in mice and worms. In mice, PPARα and LXR regulate SCD1 (Mauvoisin et al., 2007), and genes encoding for SCD1 homologs in worms are under the control of NHR-49 and NHR-80 (Goudeau et al., 2011). Moreover, although the sequences of NHR-49 and PPARα are clearly divergent, they are both involved in the regulation of fat metabolism and have been proposed to be functional analogs (Van Gilst et al., 2005). Interestingly, nhr-80 and PPARα are upregulated in response to germline ablation in worms and ovariectomy in mice, respectively (Goudeau et al., 2011; Paquette et al., 2008).
Thus, both SCD1 and PPARα are upregulated upon ovariectomy and SCD1 and LXR are downregulated upon treatment with sex steroid hormones in mammals (Lundholm et al., 2004; Paquette et al., 2008). Together, these data suggest that SCD1 activity is regulated by reproduction through nuclear hormone receptors. Notably, fertile nhr-80 mutants and SCD-deficient worms exhibit altered fatty acid desaturation (Brock et al. 2007) but have normal lifespans (Goudeau et al. 2011).
In summary, the induction of SCD1 seems to be an evolutionarily conserved response to reduced or curtailed reproduction. Although SCD1 has been shown in the nematode system to be required for lifespan extension upon germline ablation, its effect on fat content in germline-less animals remains unknown. Ovariectomy in mice also induces SCD1 and increases fat content but fails to extend lifespan. While it is not yet clear whether SCD1 directly affects fat content in ovariectomized mice, one might expect that loss of function of SCD1 would prevent the associated weight gain. Therefore, the data gathered thus far suggest that the role of SCD1 may be conserved across species, but its effects on fat metabolism and lifespan require further study. One key point will be to determine whether SCD1 activity regulates fat metabolism and longevity separately or whether one process is required for the induction of the other.
Summary and Future Perspectives
The work discussed here clearly supports the existence of multiple links between reproduction, lipid metabolism, and aging (Figures 1 and 2), but also raises many intriguing questions to be addressed in future work (see Box 1 for Outstanding Questions and Future Perspectives).
Aging is often accompanied by metabolic disorders that predispose to diabetes, cardiovascular disease, and numerous complications. Such disorders affect lipid turnover and are accompanied by an accumulation of visceral fat as well as decreased insulin sensitivity (Arner et al., 2011). Interestingly, removal of visceral fat in rats improves insulin sensitivity and increases longevity (Barzilai et al., 1998; Gabriely et al., 2002) but wild-type mice, which mobilize fat less efficiently, seem to respond better to dietary restriction to enjoy longer lifespans (Liao et al., 2011). However, long-lived genetic mouse models often have increased fat stores, suggesting that increased fat levels might also have a positive impact on longevity (Wolf, 2010). It seems likely that the absolute quantity of stored fat could influence whether it has a beneficial or detrimental effect on the lifespan of these models. It will therefore be of great interest to investigate whether the fat levels of such animals are correlated with their reproductive profiles.
Because data from genetic models show that the vast majority of longevity-promoting interventions alter fat metabolism, it is legitimate to ask whether specifically targeting fat-processing enzymes will increase longevity. The clearest evidence that this might be possible was reported by Wang et al., who found that overexpression of the lipase LIPL-4 is sufficient to extend the lifespan of wild-type worms (Wang et al., 2008). Similarly, the desaturases FAT-5 and FAT-6 are required for both insulin- and germline-mediated lifespan extension, suggesting that fatty acid desaturation might play an important role in regulating longevity (Goudeau et al., 2011; Murphy et al., 2003). Autophagy is another effector of fat metabolism that might be critical for longevity (Lapierre et al., 2011). Although the mechanism through which autophagy modulates lifespan is not yet clear, the notion that it may do so in part by affecting fat metabolism is appealing (Lapierre et al., 2011; McCormick et al., 2011).
Other aspects of fat metabolism and their roles in aging remain underexplored. For example, understanding whether and how fat composition and localization (Ackerman and Gems, 2012) can modulate lifespan is an exciting challenge for future studies. Likewise, it will be interesting to determine whether modification of fat metabolism could be a strategy for more efficient energy consumption under stressful conditions. It may also contribute to the production of lipids that act as signaling molecules (such as hormones). Finally, it is possible that alteration of fat content and/or composition contributes to lifespan extension by affecting the overall metabolism of animals with impaired reproduction. To address these fascinating but largely unresolved questions, the use of powerful genetic models such as C. elegans and Drosophila will be essential.
Acknowledgments
We thank Leanne Jones, Sean Oldham, Louis Lapierre, Manjunatha Thondamal and Anne O’Rourke for helpful comments on the manuscript, and to Jamie Simon for help with figures. We apologize to those colleagues whose work we could not cite due to space limitations. MH is funded by NIH/NIA (R01 AG038664 and R01 AG039756) and is also an Ellison Medical Foundation New Scholar in Aging (AG-NS-0481-08). TF is supported by grants from the Austrian Science Foundation (FWF) and the Swiss National Science Foundation (PP00P3_133641) and a fellowship from the Wissenschaftskolleg zu Berlin. HA is supported by a grant "Equipe FRM" (DEQ20110421275) from the Fondation pour la Recherche Médicale.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ables ET, Drummond-Barbosa D. The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell. 2010;7:581–592. doi: 10.1016/j.stem.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman D, Gems D. The mystery of C. elegans aging: An emerging role for fat: Distant parallels between C. elegans aging and metabolic syndrome? Bioessays. 2012;34:466–471. doi: 10.1002/bies.201100189. [DOI] [PubMed] [Google Scholar]
- Arner P, Bernard S, Salehpour M, Possnert G, Liebl J, Steier P, Buchholz BA, Eriksson M, Arner E, Hauner, et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature. 2011;478:110–113. doi: 10.1038/nature10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Banerjee S, Hawkins M, Chang CJ, Chen W, Rossetti L. The effect of age-dependent increase in fat mass on peripheral insulin action is saturable. J. Gerontol. A Biol. Med. Sci. 1998;53:141–146. doi: 10.1093/gerona/53a.2.b141. [DOI] [PubMed] [Google Scholar]
- Bell G, Koufopanou V. The cost of reproduction. Oxford Surveys in Evolutionary Biology. 1986;3:83–131. [Google Scholar]
- Berman JR, Kenyon C. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell. 2006;124:1055–1068. doi: 10.1016/j.cell.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. Hormonal control of regional fat distribution. Hum. Reprod. 1997;12:21–25. doi: 10.1093/humrep/12.suppl_1.21. [DOI] [PubMed] [Google Scholar]
- Brock TJ, Browse J, Watts JL. Fatty acid desaturation and the regulation of adiposity in Caenorhabditis elegans. Genetics. 2007;176:865–875. doi: 10.1534/genetics.107.071860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson FH. Mammalian reproductive biology. Chicago: University of Chicago Press; 1989. [Google Scholar]
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. USA. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth FM, Bodenstein D. Adipose tissue of Drosophila melanogaster. 3. The effect of the ovary on cell growth and the storage of lipid and glycogen in the adult tissue. J. Exp. Zool. 1968;167:207–217. doi: 10.1002/jez.1401670209. [DOI] [PubMed] [Google Scholar]
- Chippindale AK, Leroi AM, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life-history evolution. I. Nutrition and the cost of reproduction. J. Evol. Biol. 1993;6:171–193. [Google Scholar]
- Chu K, Miyazaki M, Man WC, Ntambi JM. Stearoyl-coenzyme A desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol. Cell. Biol. 2006;26:6786–6798. doi: 10.1128/MCB.00077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Colombel A, Charbonnel B. Weight gain and cardiovascular risk factors in the post-menopausal women. Hum. Reprod. 1997;12:134–145. doi: 10.1093/humrep/12.suppl_1.134. [DOI] [PubMed] [Google Scholar]
- Corona G, Mannucci E, Forti G, Maggi M. Hypogonadism, ED, metabolic syndrome and obesity: a pathological link supporting cardiovascular diseases. Int. J. Androl. 2009;32:587–598. doi: 10.1111/j.1365-2605.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- Crane SW. Occurrence and management of obesity in companion animals. J. Small Anim. Pract. 1991;32:275–282. [Google Scholar]
- Das M, Gabriely I, Barzilai N. Caloric restriction, body fat and ageing in experimental models. Obes. Rev. 2004;5:13–19. doi: 10.1111/j.1467-789x.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- DiAngelo JR, Birnbaum MJ. Regulation of fat cell mass by insulin in Drosophila melanogaster. Mol. Cell Biol. 2009;29:6341–6352. doi: 10.1128/MCB.00675-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djawdan M, Chippindale AK, Rose MR, Bradley TJ. Metabolic reserves and evolved stress resistance in Drosophila melanogaster. Physiol. Zool. 1998;71:584–594. doi: 10.1086/515963. [DOI] [PubMed] [Google Scholar]
- Djawdan M, Sugiyama TT, Schlaeger LK, Bradley TJ, Rose MR. Metabolic aspects of the trade-off between fecundity and longevity in Drosophila melanogaster. Physiol. Zool. 1996;69:1176–1195. [Google Scholar]
- Doane WW. Developmental physiology of the mutant female sterile(2)adipose of Drosophila melanogaster. III. Corpus allatum-complex and ovarian transplantations. J. Exp. Zool. 1961;146:275–298. doi: 10.1002/jez.1401460307. [DOI] [PubMed] [Google Scholar]
- Drewry MD, Williams JM, Hatle JD. Life-extending dietary restriction and ovariectomy result in similar feeding rates but different physiologic responses in grasshoppers. Exp. Gerontol. 2011;46:781–786. doi: 10.1016/j.exger.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drori D, Folman Y. Environmental effects on longevity in the male rat: exercise, mating, castration and restricted feeding. Exp. Gerontol. 1976;11:25–32. doi: 10.1016/0531-5565(76)90007-3. [DOI] [PubMed] [Google Scholar]
- Fettman MJ, Stanton CA, Banks LL, Hamar DW, Johnson DE, Hegstad RL, Johnston S. Effects of neutering on bodyweight, metabolic rate and glucose tolerance of domestic cats. Res. Vet. Sci. 1997;62:131–136. doi: 10.1016/s0034-5288(97)90134-x. [DOI] [PubMed] [Google Scholar]
- Flatt T. Survival costs of reproduction in Drosophila. Exp. Gerontol. 2011;46:369–375. doi: 10.1016/j.exger.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Flatt T, Min KJ, D'Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones DL, Tatar M. Drosophila germ-line modulation of insulin signaling and lifespan. Proc. Natl. Acad. Sci. USA. 2008;105:6368–6373. doi: 10.1073/pnas.0709128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force AG, Staples T, Soliman S, Arking R. Comparative biochemical and stress analysis of genetically selected Drosophila strains with different longevities. Dev. Genet. 1995;17:340–351. doi: 10.1002/dvg.1020170407. [DOI] [PubMed] [Google Scholar]
- Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, Scherer P, Rossetti L, Barzilai N. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51:2951–2958. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- Galikova M, Klepsatel P, Senti G, Flatt T. Steroid hormone regulation of C. elegans and Drosophila aging and life history. Exp. Gerontol. 2011;46:141–147. doi: 10.1016/j.exger.2010.08.021. [DOI] [PubMed] [Google Scholar]
- Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch B, Rottiers V, Li D, Motola DL, Cummins CL, Lehrach H, Mangelsdorf DJ, Antebi A. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc. Natl. Acad. Sci. USA. 2007;104:5014–5019. doi: 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi A, Henis-Korenblit S, Kenyon C. A Transcription Elongation Factor That Links Signals from the Reproductive System to Lifespan Extension in Caenorhabditis elegans. PLoS Genetics. 2009;5:e1000639. doi: 10.1371/journal.pgen.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudeau J, Bellemin S, Toselli-Mollereau E, Shamalnasab M, Chen Y, Aguilaniu H. Fatty Acid Desaturation Links Germ Cell Loss to Longevity Through NHR-80/HNF4 in C. elegans. PLoS Biol. 2011;9:e1000599. doi: 10.1371/journal.pbio.1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JB, Mestler GE. Mortality and survival: comparison of eunuchs with intact men and women in a mentally retarded population. J. Gerontol. 1969;24:395–411. doi: 10.1093/geronj/24.4.395. [DOI] [PubMed] [Google Scholar]
- Hatle JD, Paterson CS, Jawaid I, Lentz C, Wells SM, Fronstin RB. Protein accumulation underlying lifespan extension via ovariectomy in grasshoppers is consistent with the disposable soma hypothesis but is not due to dietary restriction. Exp. Gerontol. 2008;43:900–908. doi: 10.1016/j.exger.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausberger FX, Hausberger BC. Castration-induced obesity in mice. Body composition, histology of adrenal cortex and islets of Langerhans in castrated mice. Acta Endocrinol. 1966;53:571–583. [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. USA. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochbaum D, Zhang Y, Stuckenholz C, Labhart P, Alexiadis V, Martin R, Knolker HJ, Fisher AL. DAF-12 regulates a connected network of genes to ensure robust developmental decisions. PLoS Genet. 2011;7:e1002179. doi: 10.1371/journal.pgen.1002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Harshman LG. Desiccation and starvation resistance in Drosophila: patterns of variation at the species, population and intrapopulation levels. Heredity. 1999;83:637–643. doi: 10.1046/j.1365-2540.1999.00649.x. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- Jackson KC, Wohlers LM, Valencia AP, Cilenti M, Borengasser SJ, Thyfault JP, Spangenburg EE. Wheel running prevents the accumulation of monounsaturated fatty acids in the liver of ovariectomized mice by attenuating changes in SCD-1 content. Appl. Physiol. Nutr. Metab. 2011;36:798–810. doi: 10.1139/h11-099. [DOI] [PubMed] [Google Scholar]
- Jia K, Albert PS, Riddle DL. DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development. 2002;129:221–231. doi: 10.1242/dev.129.1.221. [DOI] [PubMed] [Google Scholar]
- Judd ET, Wessels FJ, Drewry MD, Grove M, Wright K, Hahn DA, Hatle JD. Ovariectomy in grasshoppers increases somatic storage, but proportional allocation of ingested nutrients to somatic tissues is unchanged. Aging Cell. 2011;6:972–979. doi: 10.1111/j.1474-9726.2011.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrientsensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kim E, Lee JH, Ntambi JM, Hyun CK. Inhibition of stearoyl-CoA desaturase1 activates AMPK and exhibits beneficial lipid metabolic effects in vitro. Eur. J. Pharmacol. 2011;672:38–44. doi: 10.1016/j.ejphar.2011.09.172. [DOI] [PubMed] [Google Scholar]
- Kirkwood T. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309:1071–1073. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- Lapierre LR, Gelino S, Melendez A, Hansen M. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr. Biol. 2011;21:1507–1514. doi: 10.1016/j.cub.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PL, Albert PS, Riddle DL. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics. 1995;139:1567–1583. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroi A. Molecular signals versus the Loi de Balancement. Trends Ecol. Evol. 2001;16:24–29. doi: 10.1016/s0169-5347(00)02032-2. [DOI] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Gelfond JA, Diaz V, Nelson JF. Fat maintenance is a predictor of the murine lifespan response to dietary restriction. Aging Cell. 2011;10:629–639. doi: 10.1111/j.1474-9726.2011.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholm L, Moverare S, Steffensen KR, Nilsson M, Otsuki M, Ohlsson C, Gustafsson JA, Dahlman-Wright K. Gene expression profiling identifies liver X receptor alpha as an estrogen-regulated gene in mouse adipose tissue. J. Mol. Endocrinol. 2004;32:879–892. doi: 10.1677/jme.0.0320879. [DOI] [PubMed] [Google Scholar]
- Mak HY. Lipid droplets as fat storage organelles in Caenorhabditis elegans: Thematic review series: Lipid droplet synthesis and metabolism: from yeast to man. J. Lipid Res. 2012;53:28–33. doi: 10.1194/jlr.R021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvoisin D, Rocque G, Arfa O, Radenne A, Boissier P, Mounier C. Role of the PI3-kinase/mTor pathway in the regulation of the stearoyl CoA desaturase (SCD1) gene expression by insulin in liver. J. Cell. Commun. Signal. 2007;1:113–125. doi: 10.1007/s12079-007-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J. The effects of temperature and of egg-laying on the longevity of Drosophila subobscura. J. Exp. Biol. 1958;35:832–842. I. [Google Scholar]
- McCormick M, Chen K, Ramaswamy P, Kenyon C. New genes that extend Caenorhabditis elegans’ lifespan in response to reproductive signals. Aging Cell. 2011;2:192–202. doi: 10.1111/j.1474-9726.2011.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy JF, Wade GN. Short- and long-term effects of ovariectomy on food intake, body weight, carcass composition, and brown adipose tissue in rats. Physiol. Behav. 1987;39:361–365. doi: 10.1016/0031-9384(87)90235-6. [DOI] [PubMed] [Google Scholar]
- Mimoto A, Fujii M, Usami M, Shimamura M, Hirabayashi N, Kaneko T, Sasagawa N, Ishiura S. Identification of an estrogenic hormone receptor in Caenorhabditis elegans. Biochem. Biophys. Res .Commun. 2007;364:883–888. doi: 10.1016/j.bbrc.2007.10.089. [DOI] [PubMed] [Google Scholar]
- Min KJ, Lee CK, Park HN. The lifespan of Korean eunuchs. Curr Biol. 2012;18:792–793. doi: 10.1016/j.cub.2012.06.036. [DOI] [PubMed] [Google Scholar]
- Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- O'Rourke EJ, Soukas AA, Carr CE, Ruvkun G. C. elegans Major Fats Are Stored in Vesicles Distinct from Lysosome-Related Organelles. Cell Metab. 2009;10:430–435. doi: 10.1016/j.cmet.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13:655–667. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallier E, Aubert R, Lemonnier D. Effect of diet and ovariectomy on adipose tissue cellularity in mice. Reprod. Nutr. Dev. 1980;20:631–636. doi: 10.1051/rnd:19800404. [DOI] [PubMed] [Google Scholar]
- Paquette A, Wang D, Jankowski M, Gutkowska J, Lavoie JM. Effects of ovariectomy on PPAR alpha, SREBP-1c, and SCD-1 gene expression in the rat liver. Menopause. 2008;15:1169–1175. doi: 10.1097/gme.0b013e31817b8159. [DOI] [PubMed] [Google Scholar]
- Parisi MJ, Gupta V, Sturgill D, Warren JT, Jallon JM, Malone JH, Zhang Y, Gilbert LI, Oliver B. Germline-dependent gene expression in distant non-gonadal somatic tissues of Drosophila. BMC Genomics. 2010;11:346. doi: 10.1186/1471-2164-11-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Gems D, Withers DJ. Sex and Death: What Is the Connection? Cell. 2005;120:461. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Perez CL, Van Gilst MR. A 13C isotope labeling strategy reveals the influence of insulin signaling on lipogenesis in C. elegans. Cell Metab. 2008;8:266–274. doi: 10.1016/j.cmet.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Rion S, Kawecki TJ. Evolutionary biology of starvation resistance: what we have learned from Drosophila. J. Evol. Biol. 2007;20:1655–1664. doi: 10.1111/j.1420-9101.2007.01405.x. [DOI] [PubMed] [Google Scholar]
- Robertson OH. Relation of gonadal maturation to length of life in Pacific salmon. Fed. Proc. 1961;20:29–30. [PubMed] [Google Scholar]
- Rottiers V, Motola DL, Gerisch B, Cummins CL, Nishiwaki K, Mangelsdorf DJ, Antebi A. Hormonal control of C. elegans dauer formation and life span by a Rieske-like oxygenase. Dev. Cell. 2006;10:473–482. doi: 10.1016/j.devcel.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Salmeri KR, Bloomberg MS, Scruggs SL, Shille V. Gonadectomy in immature dogs: effects on skeletal, physical, and behavioral development. J. Am. Vet. Med. Assoc. 1991;198:1193–1203. [PubMed] [Google Scholar]
- Service PM, Hutchinson EW, MacKinley MD, Rose MR. Resistance to Environmental Stress in Drosophila melanogaster Selected for Postponed Senescence. Physiol. Zool. 1985;58:380–389. [Google Scholar]
- Shanley DP, Kirkwood TB. Calorie restriction and aging: a life-history analysis. Evolution. 2000;54:740–750. doi: 10.1111/j.0014-3820.2000.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socha R, Šula J, Kodrík D, Gelbič I. Hormonal control of vitellogenin synthesis in Pyrrhocoris apterus (L.) (Heteroptera) J. Insect. Physiol. 1991;37:805–816. [Google Scholar]
- Stotsenburg JM. The effect of spaying and semi-spaying young albino rats (Mus norvegicus albinus) on the growth in body weight and body length. Anat. Rec. 1913;7:183–194. [Google Scholar]
- Strong L. Feeding activity, sexual maturation, hormones, and water balance in the female African migratory locust. J. Insect. Physiol. 1967;13:495–507. doi: 10.1016/0022-1910(66)90088-6. [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Tennessen JM, Baker KD, Lam G, Evans J, Thummel CS. The Drosophila estrogen-related receptor directs a metabolic switch that supports developmental growth. Cell Metab. 2011;13:139–148. doi: 10.1016/j.cmet.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen E, Hamburger K. Oxygen Consumption of Castrated Females of the Blow-Fly, Calliphora erythrocephala. Meig. J. Exp. Biol. 1955;32:692–699. [Google Scholar]
- Tissenbaum HA, Ruvkun G. An insulin-like signaling pathway affects both longevity and reproduction in Caenorhabditis elegans. Genetics. 1998;148:703–717. doi: 10.1093/genetics/148.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen CJ, Van De Zande L, Bijlsma R. Developmental and agespecific effects of selection on divergent virgin life span on fat content and starvation resistance in Drosophila melanogaster. J. Insect. Physiol. 2006;52:910–919. doi: 10.1016/j.jinsphys.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Wang MC, O'Rourke EJ, Ruvkun G. Fat Metabolism Links Germline Stem Cells and Longevity in C. elegans. Science. 2008;322:957–960. doi: 10.1126/science.1162011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters KM, Ntambi JM. Insulin and dietary fructose induce stearoyl-CoA desaturase 1 gene expression of diabetic mice. J. Biol. Chem. 1994;269:27773–27777. [PubMed] [Google Scholar]
- Williams GC. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 1966;100:687–690. [Google Scholar]
- Wilson JD, Roehrborn C. Long-term consequences of castration in men: lessons from the Skoptzy and the eunuchs of the Chinese and Ottoman courts. J. Clin. Endocrinol. Metab. 1999;84:4324–4331. doi: 10.1210/jcem.84.12.6206. [DOI] [PubMed] [Google Scholar]
- Wohlers LM, Spangenburg EE. 17beta-estradiol supplementation attenuates ovariectomy-induced increases in ATGL signaling and reduced perilipin expression in visceral adipose tissue. J. Cell. Biochem. 2010;110:420–427. doi: 10.1002/jcb.22553. [DOI] [PubMed] [Google Scholar]
- Wolf NS, editor. Comparative Biology of Aging. Springer; 2010. [Google Scholar]
- Wollam J, Magner DB, Magomedova L, Rass E, Shen Y, Rottiers V, Habermann B, Cummins CL, Antebi A, editors. A Novel 3-Hydroxysteroid Dehydrogenase That Regulates Reproductive Development and Longevity. PLoS Biol. 2012;10:e1001305. doi: 10.1371/journal.pbio.1001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T. Burn Fat, Live Longer. Science. 2008;322:865–866. doi: 10.1126/science.1166150. [DOI] [PubMed] [Google Scholar]
- Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki TM, Arantes-Oliveira N, Berman JR, Zhang P, Kenyon C. Distinct activities of the germline and somatic reproductive tissues in the regulation of Caenorhabditis elegans' longevity. Genetics. 2008;178:513–526. doi: 10.1534/genetics.107.083253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki TM, Berman JR, Suchanek-Kavipurapu M, McCormick M, Maria Gaglia M, Lee S-J, Kenyon C. The Somatic Reproductive Tissues of C. elegans Promote Longevity through Steroid Hormone Signaling. PLoS Biol. 2010;8:e1000468. doi: 10.1371/journal.pbio.1000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaan B, Bijlsma R, Hoekstra RF. Direct selection on life span in Drosophila melanogaster. Evolution. 1995;49:649–659. doi: 10.1111/j.1558-5646.1995.tb02301.x. [DOI] [PubMed] [Google Scholar]