Abstract
Significance: In heart failure (HF), contractile dysfunction and arrhythmias result from disturbed intracellular Ca handling. Activated stress kinases like cAMP-dependent protein kinase A (PKA), protein kinase C (PKC), and Ca/calmodulin-dependent protein kinase II (CaMKII), which are known to influence many Ca-regulatory proteins, are mechanistically involved. Recent Advances: Beside classical activation pathways, it is becoming increasingly evident that reactive oxygen species (ROS) can directly oxidize these kinases, leading to alternative activation. Since HF is associated with increased ROS generation, ROS-activated serine/threonine kinases may play a crucial role in the disturbance of cellular Ca homeostasis. Many of the previously described ROS effects on ion channels and transporters are possibly mediated by these stress kinases. For instance, ROS have been shown to oxidize and activate CaMKII, thereby increasing Na influx through voltage-gated Na channels, which can lead to intracellular Na accumulation and action potential prolongation. Consequently, Ca entry via activated NCX is favored, which together with ROS-induced dysfunction of the sarcoplasmic reticulum can lead to dramatic intracellular Ca accumulation, diminished contractility, and arrhythmias. Critical Issues: While low amounts of ROS may regulate kinase activity, excessive uncontrolled ROS production may lead to direct redox modification of Ca handling proteins. Therefore, depending on the source and amount of ROS generated, ROS could have very different effects on Ca-handling proteins. Future Directions: The discrimination between fine-tuned ROS signaling and unspecific ROS damage may be crucial for the understanding of heart failure development and important for the investigation of targeted treatment strategies. Antioxid. Redox Signal. 18, 1063–1077.
Introduction
Heart failure (HF) can result from myocardial contractile dysfunction and is associated with increased propensity for arrhythmias. Beside detrimental changes in the extracellular matrix, the vasculature or the connective tissue, severe alterations of the functional core of the heart, the cardiomyocyte, are essentially involved in the development of HF. Excitation–contraction coupling is central to the function of cardiomyocytes (see review (88)).
Excitation is initiated by opening of voltage-gated Na channels. The generated current (INa) is large in amplitude (>10 nA). Due to its short in duration (∼10 ms), the amount of Na ions entering the cell is not sufficient to change intracellular Na concentration greatly. Its large amplitude leads to the fast upstroke of the action potential (AP). Fast Na current inactivation and reduced driving force at positive potentials, together with activation of transient outward rectifying K current (Ito), limits AP amplitude and generates the AP notch. During the AP plateau phase, L-type Ca channels open, resulting in ICa, which maintains AP plateau until delayed rectifying K currents initiate repolarization. Mainly during the AP plateau phase, Ca ions enter the cell via ICa into the dyadic cleft very close to the Ca release channel (ryanodine receptor, RyR2) of the sarcoplasmic reticulum (SR). This relatively small Ca influx results in a Ca-induced Ca release from the SR, which is mainly responsible for the transient increase in cytosolic Ca concentration (Ca transient), resulting in myofilament activation and contraction. For Ca removal, two major pathways are involved: SR Ca ATPase (SERCA2a) and sarcolemmal Na–Ca exchange (NCX1) transfer Ca either into the SR or into the extracellular space, respectively.
There is substantial evidence that disturbed Ca handling is central for contractile dysfunction in HF (17). The mechanisms, however, are incompletely understood but involve activation of stress kinases such as cAMP-dependent protein kinase A (PKA), protein kinase C (PKC), and Ca/calmodulin-dependent protein kinase II (CaMKII) (17). Under pathological stress, excessive and/or protracted phosphorylation of target proteins like the L-type Ca channel, phospholamban, and RyR2 appear to contribute to dysregulation of normal intracellular Ca homeostasis. In addition, expression patterns of Ca regulatory proteins are altered.
SERCA2a expression (and activity), for instance, is reduced, which reduces SR Ca content, Ca transients, and impairs systolic contractile function (17). Increased diastolic RyR2 open probability contributes to reduced SR Ca load and increased diastolic Ca (89). Since intracellular Na and Ca handling are tightly interrelated, changes in Ca handling are accompanied by disturbed Na handling. Accumulation of intracellular Na has been observed in HF (105), mainly due to enhanced Na influx through voltage-gated Na channels (135) and Na/H-exchanger (NHE, 12, 13). Increased intracellular Na enhances Ca entry via reverse mode NCX activity during the AP, while it compromises NCX-mediated Ca export during diastole (9, 11, 18, 38, 104, 141–143). Thus, increased NCX expression as shown in HF (57, 122), together with increased activation upon ROS (52) may partly compensate for decreased SR Ca load by contributing to the systolic Ca transient (18).
However, increased NCX-mediated Ca influx and reduced Ca efflux may also lead to cytosolic Ca accumulation (137).
Intriguingly, HF is also associated with increased oxidative stress defined as excess production of reactive oxygen species (ROS) and/or reduced antioxidative capacity. Mallat and colleagues showed that levels of lipid peroxides and 8-iso-prostaglandin F2α, the major biochemical markers of ROS generation, were elevated in the plasma and pericardial fluid of patients with HF and correlated with disease severity (92). Electron spin resonance (ESR) spectroscopy provided direct evidence for increased ROS production in HF (69).
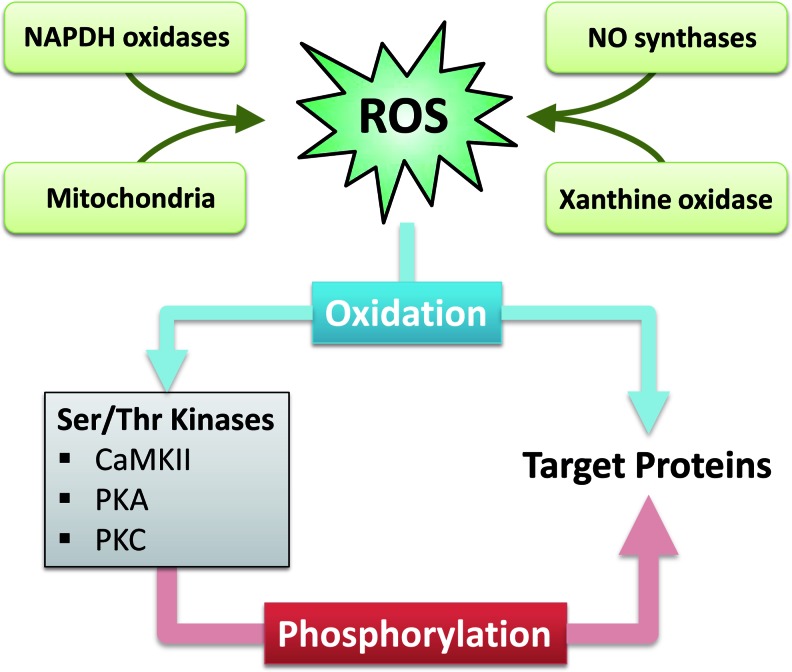
It is known that ROS can impair the function of Ca-regulatory proteins. The mechanisms, however, are incompletely understood but involve direct modification of target proteins (i.e., ion channels and transporters) as well as activation of serine/threonine kinases. The latter may act as second messengers translating the ROS signal into an altered function of ion channels and transporters (Fig. 1). Both these pathways may be involved in the initiation and progression of HF.
FIG. 1.
Sources of ROS in heart failure, and potential pathways for ROS-dependent oxidative regulation of target proteins. Left panel: indirect pathway with activation of serine/threonine kinases by oxidation, which in turn phosphorylate target proteins. Right panel: direct pathway with oxidation of target proteins. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
This review will focus on the redox-regulation of intracellular Ca. However, ROS may also play an important role in prohypertrophic and maybe proapoptotic signaling and thus, structural remodeling. Therefore, the reader is encouraged to see references 4, 5, 130, and 131 for a more complete picture of ROS effects on the heart.
The Redox System of the Heart
The redox system of the heart consists of a delicate balance of ROS-generating proteins and antioxidative capacities.
Sources of ROS and antioxidative capacities of the heart
ROS are generated from several intracellular sources including mitochondria, NADPH oxidase, xanthine oxidase, and uncoupled nitric oxide synthase (Fig. 1).
Physiologically, small amounts of superoxide (O2−) occur upon mitochondrial oxidative phosphorylation but are rapidly inactivated by superoxide dismutase (SOD) into H2O2. O2− has very limited diffusion distance, in the range of only a few nanometers due to its high reactivity. H2O2, on the other hand, is less reactive and can reach the cytoplasm. H2O2 itself is reduced by gluthathione peroxidase, catalase, and the thioredoxin (Trx) system (Trx reductase and Trx peroxidase) into H2O (73, 127, 148). Gluthathione peroxidase requires gluthathione, the major cytosolic redox buffer. The ratio of reduced to oxidized gluthathione (GSH/GSSG) is usually >10 but can become significantly decreased under pathological conditions (102). Gluthathione peroxidase is present in high amounts in mitochondria and cytosol.
Under conditions of enhanced superoxide production, increased amounts of H2O2 may overwhelm the antioxidative capacities and may lead to the formation of highly reactive hydroxyl radicals (OH−) via Haber–Weiss reaction (with a second molecule O2−) or via Fenton reaction. Alternatively, superoxide may lead to the generation of peroxynitrite (ONOO−) by reaction with NO (101).
In HF, it has been shown that increased amounts of superoxide are generated in mitochondria (69, 71, 103). In addition, NADPH oxidase (Nox) 2 and 4 are richly expressed in cardiomyocytes, and myocardial Nox activity has been shown to be increased in human HF (59, 87, 119). Stimuli relevant to the pathophysiology of HF (mechanical stretch, endothelin-1 and angiotensin II) are known to induce Nox activity (2, 82). Interestingly, recent evidence suggests that there may be a connection between Nox activation and mitochondrial ROS production. Nox-dependent ROS production may be amplified by mitochondria in a ROS-induced ROS release manner (32–35). The role of Nox 4, however, is controversially discussed. It was proposed that upregulation and translocation of Nox 4 to mitochondria may augment mitochondrial ROS production and contribute to the progression of heart failure (2, 82). On the other hand, it was shown that genetic deletion of Nox 4 aggravated heart failure development, which was attributed to beneficial effects of Nox 4 on angiogenesis (152).
The third source of ROS, xanthine oxidase, has also been shown to be increased in expression and activity in HF (27). Finally, nitric oxide synthase (NOS) that is exposed to oxidative stress becomes structurally unstable and generates ROS (NOS uncoupling). It was shown that uncoupled NOS contributes to myocardial remodeling upon pressure overload in mice (126). Interestingly, endothelial NOS (eNOS) was shown to co-localize with L-type Ca channels in caveolae, and neuronal NOS (nNOS) was reported to co-localize with RyR2 in the SR (14). This suggests that NOS may play an important role for ROS-dependent Ca handling (25, 154).
Controversy exists with respect to antioxidative capacity in HF. While it was shown in HF following myocardial infarction that antioxidative capacity was reduced (62), others reported increased activity of gluthathione peroxidase in hearts with pacing-induced HF (129). Because of the very short half-life of ROS, there are very different effects on ion channels or ion transporters depending on the source and localization of ROS generation.
Redox-modification of proteins
ROS are known to oxidize sulfhydryl (SH) groups of cysteine residues in proteins, which can lead to the formation of disulfide bonds. The latter affects the tertiary and quaternary structure of proteins, resulting in altered function. Only recently, it was shown that ROS can also oxidize methionine residues, which can also influence structure and function of proteins (42, 74). Many Ca handling proteins have been shown to be subject to ROS-dependent oxidation but the physiological and/or pathophysiological relevance is largely unknown.
Redox Modification of Serine/Threonine Kinases
Since ROS are highly reactive molecules, their intracellular diffusion is very limited. Therefore, ROS generated by endogenous systems can only affect close targets, resulting in very compartmentalized signaling. On the other hand, it has been shown that ROS can have much broader effects on the cardiomyocyte. One explanation for this discrepancy is ROS-dependent activation of serine/threonine kinases. These kinases may translocate, leading to changes in the activity of a broad range of Ca-regulatory proteins by phosphorylation (Fig. 1).
Ca/calmodulin-dependent protein kinase II
In recent years it has become evident that Ca/calmodulin-dependent protein kinase II (CaMKII) is crucial for the regulation of intracellular Ca and excitation–contraction coupling.
CaMKII is a serine/threonine kinase that is robustly expressed in heart tissue. It forms homo-multimeric structures via its association domain (Fig. 2). CaMKII can phosphorylate numerous Ca-regulatory proteins including L-type Ca channel (54, 78), ryanodine receptor (RyR2), phospholamban (PLN, 88). In addition, we have recently shown that CaMKII can phosphorylate cardiac voltage-gated Na channels, which increases Na influx (persistent or late Na current) and prolongs action potential duration (APD, 68, 135).
FIG. 2.
Schematic structure of CaMKII and activation pathways. Upper panel: Schematic depiction of the CaMKII holoenzyme that consists of two stacked hexameric rings (upper right), each ring consisting of six subunits (upper left) which contain the association domain, regulatory domain, and catalytic domain (lower left). Lower panel: Possible ways of CaMKII activation by Ca/CaM that lead to Ca2+ and calmodulin- autonomous activity: by intersubunit autophosphorylation of threonine 286 and/or methionine 281/282 oxidation. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
The typical activation is Ca-dependent (116). Active, Ca-bound calmodulin (Ca-CaM) can bind to the regulatory domain of CaMKII, resulting in a conformational change disturbing the interaction of regulatory (autoinhibitory) domain and catalytic domain, which results in ATP-binding and target protein phosphorylation (Fig. 2). Upon activation, inter-subunit autophosphorylation at threonine 286 or 287 (T286 or T287, dependent on species) occurs, which prevents re-association of catalytic and regulatory domain, even when Ca-CaM has dissociated from the catalytic domain (Fig. 2; 81, 117). Therefore, T286 autophosphorylation confers prolonged and autonomous (Ca-CaM-independent) activity. By integrating the relatively fast transient changes in the intracellular Ca concentration, T286 autophosphorylation serves as the Ca “memory” for CaMKII. Beside changes in the enzyme kinetics of inactivation, T286 autophosphorylation is also required for maximal kinetic activity.
Erickson and colleagues described a novel mechanism of Ca-independent CaMKII activation (42). They showed that ROS can oxidize methionine 281/282 (M281/282) in the regulatory domain resulting in an activation mode very similar to autophosphorylation at T286 (i.e., Ca-CaM-independent activity) (Fig. 2). This type of activation can occur, for instance, upon stimulation of endogenous NADPH oxidase 2 (Nox2) by angiotensin II binding to its receptor on cardiomyocytes. Oxidation at M281/282 is a reversible process and oxidized methionine can be reduced by methionine sulfoxide reductase A (42). The pathophysiological relevance of this oxidized CaMKII has recently been verified for the development of sinus node dysfunction (124) and aldosterone-induced heart injury (58). The role of oxidized CaMKII in the cardiovascular system was reviewed recently in detail (43).
Protein kinase A
Catecholamine hormone-binding to the G-protein coupled β receptor stimulates adenylate cyclase, resulting in increased cyclic adenosine monophosphate (cAMP), which activates cAMP-dependent protein kinase A (PKA, 144). Two major forms of PKA, type I and type II, have been described, both of which are organized as tetramers comprising two catalytic and two regulatory subunits. The regulatory subunit can bind to protein kinase A anchor protein (AKAP), which targets PKA to its substrate proteins. Activation of PKA can occur upon binding of two molecules of cAMP to each regulatory subunit. This favors dissociation of the catalytic and regulatory subunit, which results in substrate phosphorylation. It is known that PKA can phosphorylate several Ca-regulatory proteins, including RyR2, L-type Ca channel, and PLN. In addition, PKA phosphorylation of troponin I regulates myofilament Ca sensitivity (109).
There are two types of regulatory subunits (RI and RII) and accordingly, the enzyme is defined as type I or II, respectively. Type I PKA is localized in the cytosol, whereas type II appears to be primarily targeted to AKAP proteins associated with subcellular compartments. There is a large uncertainty about target specificity of the PKA subtypes. Interestingly, peptide substrate enhanced the activation of PKA type I at low, physiologically relevant concentrations of cAMP through competitive displacement of the regulatory RI subunit. This substrate-induced sensitization is not present in type II PKA (133, 134). This suggests that the activity of PKA type I is determined not only by the cAMP level but also by the availability of substrate.
Additionally, it was recently shown that type I regulatory subunit I is subject to oxidation by ROS (24). The oxidation of cysteines 17 and 38 leads to inter-subunit disulfide bond formation (between two regulatory subunits) and dissociation of the PKA holoenzyme complex. The type I PKA translocation (from cytosol to membrane and myofilaments) and activation results in increased cellular contractility without elevations in cAMP. It was suggested that increased PLN phosphoryation and SERCA2a activation underlies the observed increase in contractility (24). The translocation of oxidized type I PKA seems to be beneficial for activation since it favors substrate binding, which is required for full activation.
To date, however, the relevance of this novel PKA type I activation in cardiomyocytes under physiological and pathophysiologocal conditions, especially in comparison to cAMP-dependent activation, is completely unknown.
Protein kinase C
By molecular cloning, at least 12 isozymes of protein kinase C (PKC) have been identified, which are classified by their activation characteristics. The conventional PKC isozymes (α, βI, βII, and γ) are activated by Ca and diacylglycerol (DAG). In contrast, novel isozymes (δ, ɛ, θ, and η) and atypical isozymes (ζ and λ) are Ca-independent but activated by distinct lipids (39). PKCs consist of N-terminal regulatory (autoinhibitory) and C-terminal catalytic domain. When inactive, the regulatory domain is bound to the catalytic domain (63). The binding of the activating factors (distinct lipids and Ca) results in a conformational change, resulting in release of the autoinhibition and activation of the enzyme. Moreover, activation leads to PKC tranlocation and increased membrane association. Recently, intracellular receptor proteins have been described, which bind activated PKC in the presence of phosphatidylserine and Ca (95). The binding site on PKC is distinct from the substrate binding site, and binding was further increased with the addition of diacylglycerol (95). These binding proteins are called receptors for activated C-kinase (RACKs) and may play a role in activation-induced translocation of PKC.
The effects of PKC activation are complex, especially due to parallel activation of several isozymes, isozyme interdependence, cross-talk, and overlapping isozyme effects. The cellular activity of the individual isozymes depends on their expression levels, subcellular localization, and phosphorylation state (91). All these factors, however, vary dramatically between species and cell types. This may explain why experiments using knock-out of single PKC isozymes did not show consistent results. Despite these confounders, it has been consistently shown that G protein-coupled receptor agonists such as isoproterenol and angiotensin II, but also mechanical stretch, induce cardiac hypertrophy through the activation of PKC (20, 125). Also, in various in vivo models of cardiac hypertrophy, it was shown that PKCα and PKCβ are upregulated, PKCɛ is either upregulated or preferentially activated, and levels of PKCδ,λ or ζ do not change (22, 125, 139). During ischemic preconditioning, on the other hand, it was shown that PKCδ and PKCɛ have opposite roles (29, 30, 40). For more information about the complex role of PKC for cardiac hypertrophy, heart failure, and ischemic preconditioning, we refer to the following reviews (30, 39, 40, 91).
PKC has been shown to regulate excitation–contraction coupling. PKCα can phosphorylate inhibitor 1 at Ser67, resulting in increased protein phosphatase 1 activity, leading to phospholamban dephosphorylation and reduced SERCA2a activity (23). PKCα (and maybe also PKCβ) has been shown to phosphorylate troponin I, troponin T, titin, and myosin binding protein C, which leads to decreased myofilament Ca sensitivity (16, 60, 65, 123, 138). Also, PKCα, βI, βII, and γ have been shown to phosphorylate the α1c subunit of the L-type Ca channel (151).
In addition to the Ca and lipid-induced activation, it was shown that mild oxidative stress can activate PKC (53). At high doses of ROS (5 mmol/L H2O2), both catalytic and regulatory domain are oxidized, leading to irreversible inactivation of the enzyme. Low doses of ROS (50 μmol/L H2O2), on the other hand, selectively oxidize the regulatory domain generating a Ca and phospholipid-independent PKC activation (53). The pathophysiological relevance of this novel redox-dependent PKC activation is largely unknown.
Redox Modification of Ca and Na Handling Proteins
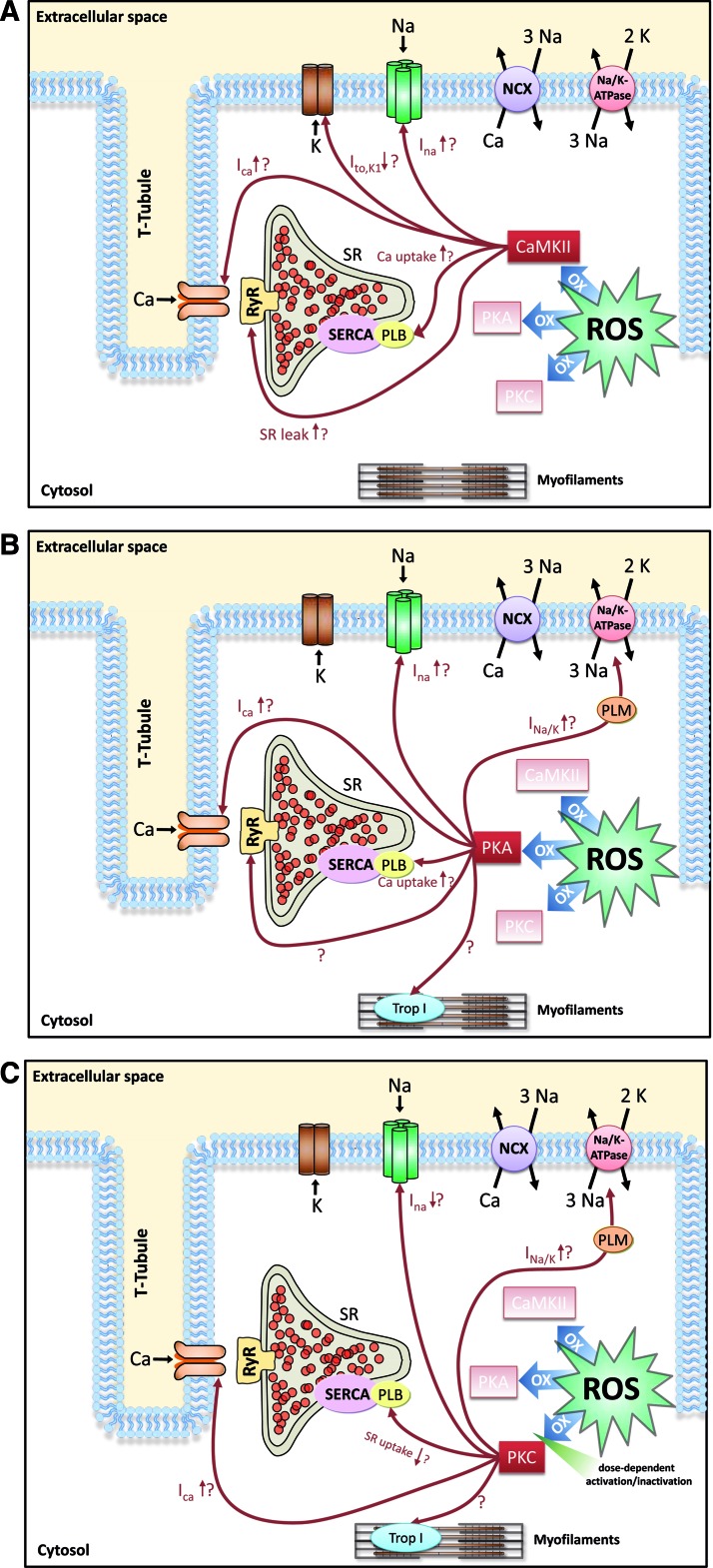
It is well established that accumulation of ROS lead to cytosolic Ca overload (49, 120, 128, 134, 136). ROS-induced accumulation of intracellular Na has also been reported (134). Interestingly, it was shown that in failing cardiomyocytes, cytosolic Na overload greatly enhances mitochondrial ROS production (77). This would result in a positive feedback loop greatly augmenting ROS-induced injury. Since intracellular Na and Ca homeostasis are tightly interrelated, changes in Na handling proteins with consequent changes in intracellular Na can have a profound impact on intracellular Ca and contractility. A synopsis of ROS effects on Na and Ca handling proteins is shown in Figure 3 and Table 1.
FIG. 3.
Overview of known and putative ROS effects by direct oxidation of proteins of the excitation–contraction coupling. CaMKII, Ca/CaM dependent protein kinase II; NCX, Na/Ca-exchanger; OX, activation by oxidation; PKA, proteinkinase A; PKC, proteinkinase C; PLB, phospholamban; RyR, ryanodin receptor; SERCA, SR Ca ATPase; Trop I, troponin I. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Table 1.
Summary of ROS, CaMKII, PKA, PKC and ROS-Mediated Effects on Important Targets in the Cardiomyocyte
| Target effect | ROS | CaMKII | ox-CaMKII | PKA | ox-PKA | PKC | Reference Nos. |
|---|---|---|---|---|---|---|---|
| LTCC ICa | ↓,↑[Ref. 121] | 45,50,51,64,83,121 | |||||
| ↑ | 19,41,54,66,78 | ||||||
| ↑ | 121 | ||||||
| ↑ | 28 | ||||||
| ↑ | 151 | ||||||
| SERCA2a Activity |
↓ | 79,96,115,146 | |||||
| ↑ | 88 | ||||||
| ↑ | 88 | ||||||
| ↓ | 23 | ||||||
| PLB Phosphorylation |
↑ | 88 | |||||
| ↑ | 88 | ||||||
| ↑ | 24 | ||||||
| ↓via PP1-I1 | 23 | ||||||
| RyR2 SR Ca release SR Ca leak |
↑ ↓in low ROS |
1,6,21,45,84,147,155,154 | |||||
| ↑ | 88 | ||||||
| ↑ | 88 | ||||||
| Nav1.5 peak INa late INa | Avail↓, IM↑, late↑ | 15,48,120 | |||||
| Avail↓, IM↑, late↑ | late↑[136] | 68,135,136,10 | |||||
| peak↑ | 56,47 | ||||||
| peak↓[85,107] | 85,107 | ||||||
| Troponin I Phosphorylation |
↑ | 24 | |||||
| ↑ | 102 | ||||||
| ↑ | 102, 138 | ||||||
| Na-K ATPase Na-K pump current |
↓ | 76,80,118,145 | |||||
| ↑via PLM | 37 | ||||||
| ↑via PLM | 37 | ||||||
| NCX INCX | ↑ | 52,75,110,114 | |||||
| ↔[61],↑[72] | 61,72 | ||||||
| ↔[61],↑[72] | 61,72 | ||||||
| Other Targets | MMP-9 expression↑ | 58 | |||||
| Sinus nodal cell death↑ | 124 |
Avail↓, reduced INa availability, enhanced steady-state inactivation; IM, intermediate inactivation; LTCC, L-type Ca channel; NaV1.5, pore-forming subunit of the Na channel encoded by the SCN5A gene; NCX, Na/Ca exchanger; MMP-9, matrix metalloproteinase-9; PLB, phospholamban; PLM, phospholemman; PP1-I1, phosphatase-1 inhibitor-1; RyR2, ryanodine receptor 2.
Voltage-gated Na channels
Cardiac Na channels consist of a pore-forming α-subunit NaV1.5 and a regulatory β-subunit. NaV1.5 contains methionine residues that may be substrate to ROS-dependent oxidation (74). Oxidation of these methionine residues have been shown to impair open-state inactivation (74). ROS also reduce Na channel availability, causing a negative shift in the voltage dependence of inactivation due to enhanced intermediate inactivation and delayed recovery from inactivation. Activation, on the other hand, was unaltered (48).
Interestingly, a novel INa gating mode has been described recently that is also activated by ROS (120). Beside peak INa lasting only for a few ms (ca. 10 ms), there is a late INa component persisting over hundreds of milliseconds (90). Because of its persistent nature, the amount of Na entering the cell via late INa is substantial despite its small amplitude (approximately 1% of peak INa). In fact, it has been suggested that late INa is an important regulator of intracellular Na under pathophysiological conditions (135, 136).
ROS have also been shown to increase intracellular Na, prolong the action potential, and induce EADs, and ROS-enhanced late INa may be involved (15, 48, 120).
In addition to direct ROS-dependent oxidation of NaV1.5, changes in the lipid environment or ROS-induced activation of PKA, PKC, and CaMKII may be involved in the ROS regulation of cardiac Na channels (48, 74, 85, 132, 135, 136).
The α-subunit NaV1.5 is substrate to phosphorylation by PKA, PKC, and CaMKII. CaMKII has been shown to phosphorylate serine 571, serine 516, and threonine 594, which results in a negative shift in the voltage dependence of inactivation due to enhanced intermediate inactivation (10, 68). Although the relevant phosphorylation site remains to be determined, CaMKII has been also been shown to enhance late INa, which leads to accumulation of intracellular Na, AP prolongation and arrhythmias (135, 136). These effects resemble the above described ROS effects on Na channel gating. Moreover, the ROS-induced late INa was not observed in CaMKIIδ knock-out mice, suggesting a crucial role for CaMKII in redox regulation of INa (136).
Beside CaMKII, PKA and PKC have been shown to influence cardiac Na channels. NaV1.5 has two serine residues (serine 526 and 529) in the I-II cytoplasmic linker that can be phosphorylated by PKA. The PKA-dependent phosphorylation of NaV1.5 increases peak INa current density via acceleration of channel trafficking to the plasma membrane (56), but does not change inactivation kinetics (47, 98).
Also PKC can modify INa. Ward and Giles have shown that the H2O2-dependent slowing of INa open state inactivation was blocked in the presence of the PKC inhibitor Bis-indolylmaleimide (140). In heterologeous expression systems (rat and human NaV1.5), however, PKC activation did not change INa inactivation (99, 108) questioning the role of PKC for regulation of INa gating. The most consistent effect of PKC on cardiac Na channels is phosphorylation of serine 1505 in the III–IV linker, which reduces peak INa current density (107).
Although both PKA and PKC have been shown to divergently influence peak INa possibly by changing the number of functional channels in the membrane, they both do not appear to regulate INa gating (i.e., the activation and inactivation process). Therefore, ROS-activated PKC or PKA do not account for the ROS-induced changes in Na channel gating. However, it has been shown that ROS generated from mitochondria (by elevation of cytosolic NADH) may reduce peak Na current density (85) via a PKC-dependent pathway (132).
Sarcolemmal Na/K-ATPase
Physiologically, intracellular Na is heavily controlled by the Na-K-ATPase (NKA). Indeed, computational modeling of rabbit ventricular myocytes exposed to H2O2 revealed that even a 16-fold ROS-induced increase in INa conductance would not result in increased intracellular Na due to dramatic activation of NKA (136). Therefore, a substantial inhibition of NKA activity needs to occur to explain the ROS-induced increase in intracellular Na. Interestingly, ROS have been shown to inhibit NKA function potently, although the underlying mechanism is not known (76, 80, 118, 145). Beside changes in the lipid environment, direct NKA oxidation has been suggested. In this respect it is interesting that the β-subunit of NKA contains a sulfhydryl group that has been shown to be essential for catalytic activity.
Beside direct oxidation, NKA may also be redox-regulated via PKA or PKC. Phospholemman (PLM), which inhibits NKA by reducing its affinity for internal Na, has been shown to be phosphorylated by PKA (serine 68) and PKC (serine 63 and 68). PLM phosphorylation results in dissociation from the catalytic subunit, which activates the pump (37). Since ROS inhibit NKA activity, but ROS-induced activation of PKA or PKC would result in PLM phosphorylation, dissociation and NKA activation, it is unlikely that PKA or PKC are involved in the ROS regulation of NKA.
If a substantial reduction in NKA function is incorporated into the computer model, intracellular Na increases dramatically. Beside enhanced Na influx via INa, enhanced Na-proton-exchanger-dependent Na influx has been suggested, but the pathophysiological relevance in unclear (112).
Intracellular Na is tightly associated with intracellular Ca. The activity of cardiac Na-Ca-exchange (NCX) is a function of membrane potential and trans-sarcolemmal gradients for Na and Ca. Upon prolonged action potential duration and increased intracellular Na, reduced Ca efflux (forward mode) and/or Ca influx (reverse mode) via NCX contributes to intracellular Ca accumulation. Indeed, computational modeling revealed that the Ca entry mode of the NCX was dramatically favored under conditions of increased intracellular Na (136).
Sarcolemmal sodium–calcium exchanger
For cardiac NCX (NCX1), intramolecular disulfide bonds between cysteine residues of different domains have been implicated to be functionally relevant (100). ROS have been shown to activate NCX (52, 75, 110, 114), but the effect was inconsistent with respect to ROS source and ROS level. There is substantial controversy about the role of serine/threonine kinases in the regulation of NCX. No functional change in cardiac NCX has been shown upon application of catalytic subunits of PKA and PKC (61), suggesting that NCX may not be subject to regulation by kinases. On the other hand, it was shown that the intracellular loop of NCX can be phosphorylated by PKA or PKC, which may increase NCX activity and may be responsible for part of the ROS-induced NCX activation (72).
Under conditions of increased intracellular Na, ROS-dependent stimulation of NCX may result in cellular Ca overload. Indeed, it was shown previously that increased expression of NCX, as observed in HF, augmented ROS-induced cellular injury (134), and that pharmacological inhibition of Ca entry via NCX reduced ROS-induced Ca overload and diminishes cellular injury (136).
However, the situation in human HF may be more complex: human failing myocardium was paradoxically more resistant to ROS-induced contractile dysfunction, while human nonfailing was not (86). It was suggested that an increased endogenous activation of mitochondrial KATP channels in human failing myocardium may explain this “stunning paradox” such that failing myocardium may be endogenously preconditioned.
Voltage-gated L-type Ca channel
The pore-forming subunit α1C of the cardiac L-type Ca channel contains more than 10 cysteine residues (94), which can potentially undergo redox modification. It was shown that thiol-oxidizing agents or ROS irreversibly decreased ICa in heterologeous expression systems (HEK293 cells, 46, 64) or cardiomyocytes (50, 51, 83). These results are, however, controversial since others have shown that thiol-oxidizing agents (26) or ROS may increase ICa (121). Part of the discrepancy may be a result of the fact that CaMKII (19, 41, 54, 66), PKA (28), and PKC (151) can activate ICa by phosphorylation, and all three kinases are subject to ROS-dependent oxidation/activation. PKC has been shown to phosphorylate the α1c subunit of the L-type Ca channel at several sites including serine 1928 (150,151). PKA also phosphorylates α1c at serine 1928 (36, 67). However, the key phosphorylation site involved in Ca current modulation is still a matter of debate since phosphorylation of both α1C as well as the auxiliary β subunit (55, 106) can result in increased ICa.
Therefore, ROS can simultaneously induce ICa activation (via serine kinases) and ICa inhibition (via direct cysteine oxidation). The net result depends on the sources and levels of ROS generated. However, since it has been shown that H2O2 causes Ca overload due to Ca release from intracellular stores and Ca entry via NCX but not due to activation of ICa (49, 136), it is likely that the direct redox modification of α1C is the predominant ROS effect.
Cardiac ryanodine receptor
The RyR2 (cardiac isoform) is a large tetrameric complex that contains up to 89 cysteine residues per monomer, of which approximately 21 are free (147).
Thiol-oxiziding agents such as H2O2 activate RyR Ca release after oxidation of more than 7 thiols per subunit (1, 6, 21, 45, 84, 147, 155). Since the RyR2 has multiple sites for regulation by phosophorylation and/or interaction with Ca, Mg, ATP, CaM, or regulatory proteins (FKBP12.6 also called calstabin), oxidation may interfere with these regulators. Indeed, the mechanism of ROS-induced increase in RyR Ca release involves a change in RyR sensitivity to cytosolic Ca and ATP (42, 93), the alteration of the RyR interaction with triadin, which regulates RyR sensitivity to luminal Ca (84), and the disturbance of FKBP12.6 binding (155). For activation to occur, it requires the oxidation of more than 7 thiols per subunit (147). In contrast, the oxidation of less than 5.5 thiols per subunit occurs readily without affecting RyR function (147). Moreover, it has been shown that low ROS levels increase diastolic RyR Ca release (Ca spark frequency), whereas excessive ROS production reduces Ca spark frequency (149). Therefore, the quality of ROS-dependent RyR regulation may be closely related to the amount of oxidized thiols.
Beside direct oxidation of cysteine residues of RyR2, ROS may increase RyR Ca release via activation of serine/threonine kinases (113). It is well known that CaMKII and PKA phosphorylate RyR2 increasing diastolic Ca leak (88). Both kinases can be oxidized (see above) and activated by ROS (24, 42). Therefore, it is conceivable that part of the observed ROS effects on RyR2 are mediated via oxidized CaMKII or oxidized PKA. The relative contribution of each kinase and the pathophysiological relevance, however, is completely unclear.
One consequence of increased diastolic Ca leak is reduced SR Ca content, especially if ROS reduce SR Ca ATPase function as well (153). ROS have been shown to reduce SR Ca content (136, 153). This results in smaller Ca transients and reduced contractility (136). Interestingly, due to rapid activation of Na-Ca-exchange in forward mode (Ca exit mode, net positive charge movement into the cell) diastolic Ca leak is also mainly responsible for delayed afterdepolarizations. The ROS-induced increase in Ca spark frequency is therefore associated with increased propensity for delayed afterdepolarizations and arrhythmias (136), especially if Na-Ca-exchange is enhanced via ROS (52).
Sarcoplasmic reticulum Ca-ATPase
SERCA2a and its regulatory (inhibitory) protein PLN are substrate to redox modification. SERCA2a contains 25 cysteine residues, of which only 1 or 2 are essential for enzyme action (97). Thiol-oxidizing agents or ROS have been shown to inhibit cardiac SERCA function (79, 96, 115, 146). Part of this effect may be a result of direct interference with the ATP binding site (146). In addition, PLN is also substrate to phosphorylation by CaMKII (threonine 17) and PKA (serine 16) resulting in PLN dissociation and activation of SERCA (88). The latter effect, however, appears to be less relevant for ROS-dependent SERCA regulation, since ROS have been unequivocally shown to reduce SERCA function. Possibly PKC-dependent signaling is also involved. In contrast to CaMKII and PKA activating SERCA, PKC has been shown to reduce SERCA activity. Hearts of PKCα-deficient mice are hypercontractile, whereas those of transgenic mice overexpressing PKCα are hypocontractile. The underlying mechanism involves PKCα-dependent phosphorylation of protein phosphatase inhibitor-1 (PPI-1), resulting in dephosphorylation of PLN. The reduced SERCA2a activity leads to reduced SR Ca content and diminished Ca transient amplitude (23).
To summarize the discussed ROS effects on Na and Ca handling proteins, Figure 4 and Table 1 give an overview of the effects possibly mediated by ROS-activated CaMKII (Fig. 4A), ROS-activated PKA (Fig. 4B) or ROS-activated PKC (Fig. 4C).
FIG. 4.
Overview of known and putative ROS effects on Na and Ca handling proteins that may be mediated by ROS-activated CaMKII (A), ROS-activated PKA (B), and ROS-activated PKC (C). (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
ROS-Associated Arrhythmias
Changes in intracellular Na and Ca handling are associated with electrical instability.
It was shown previously that ROS increase AP duration resulting in early afterdepolarizations (EADs) due to reactivation of ICa (120). Since the selective Na channel blocker tetrodotoxin (TTX) could reverse this ROS effect, enhanced late INa was attributed as underlying mechanism of AP prolongation. Indeed, recently this mechanistic link was confirmed showing that redox-activated CaMKII and enhanced late INa are required for AP prolongation and EADs (68, 136). Depending on the sources and levels of ROS, a ROS-induced enhancement of ICa and transient outward K current (Ito) inhibition by oxidation of SH groups may additionally contribute (111).
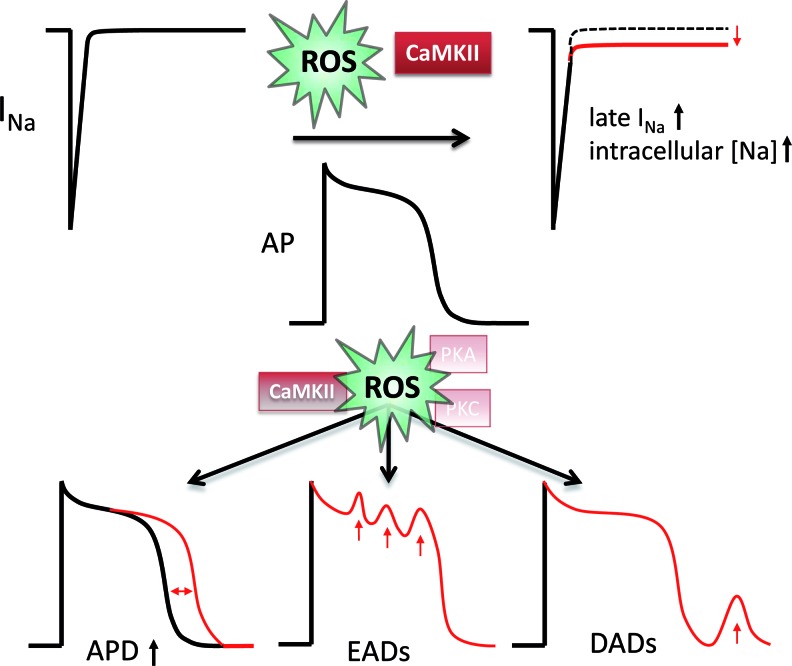
On the other hand, cellular Ca overload and ROS-induced diastolic Ca leak predispose to increased transient inward INCX (Iti), which transports the released diastolic Ca outside the cell, generating a depolarizing current and predisposing to delayed afterdepolarizations (DADs). The propensity for EADs and DADs has been shown to be dramatically increased upon ROS exposition but reduced in CaMKIIδ knockout mice (136). The relevance of CaMKII for life-threatening arrhythmias was confirmed in CaMKII-transgenic mice showing a dramatic increase in the propensity for monomorphic and polymorphic ventricular arrhythmias (135). Therefore, redox-modified CaMKII may be crucially involved in the ROS-induced arrhythmogenesis (Fig. 5).
FIG. 5.
Effects of ROS-activated CaMKII on pathological, proarrhythmic, membrane excitability. ROS-activated CaMKII leads to increased intracellular [Na] by increasing late INa (upper panel). This leads to prolongation of the action potential duration, early after depolarizations (EADs), and delayed afterdepolarizations (DADs, lower panel). The possible contributions of PKA and PKC are still unknown. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Beside dysregulated Na and Ca handling, ROS-effects on mitochondrial ATP production may also increase arrhythmogenesis (3, 7, 8). It was shown that ROS-induced ROS release from mitochondria results in mitochondrial depolarization, during which ATP production is inhibited and sarcolemmal KATP channels are activated, leading to shortened AP duration and slowed conduction. Due to spatiotemporal differences of this mitochondrial membrane depolarization between different regions of the heart, re-entry and life-threatening arrhythmias may develop.
Conclusion and Perspectives
Here we have discussed ROS effects in Na and Ca handling. ROS have been shown to induce activation of late INa that in the face of reduced NKA activity lead to intracellular Na accumulation and action potential prolongation. As a consequence, Ca entry mode of ROS-activated NCX is favored. On the other hand, ROS increase diastolic leak through enhanced RyR2 open probability, which leads together with dysfunctional SERCA to reduced SR Ca load. The dysfunctional SR cannot compensate the Ca entry via NCX, leading to a dramatic increase in intracellular Ca, reduced SR Ca transients, diminished contractility, arrhythmias, and cellular injury (Fig. 6). Interestingly, all these changes are also present in the failing heart, suggesting that ROS may be involved in the development of the disease.
FIG. 6.
Effects of the ROS-mediated increase in intracellular Na on Ca transients and contractility. Increased intracellular Na disables the Ca extruding capacity of the Na/Ca exchanger (NCX) and favors ‘reverse mode’ of the NCX, which increases intracellular and diastolic Ca and contributes to diastolic dysfunction. Increased SR Ca leak leads to reduced SR Ca load and reduced contractility, and also contributes to increased intracellular Ca. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Low amounts of ROS constitute redox signaling, which may have physiological relevance but may also be involved in the processes leading to myocardial remodeling. Part of these ROS signaling effects is mediated via serine/threonine kinases, translating the short living ROS signal into a signal of longer duration. These kinases are known to be involved in the pathophysiology of heart failure. Excessive uncontrolled ROS generation, however, results in profound myocardial damage, including substantial direct and possibly irreversible redox-modification of Ca handling proteins, which contributes to the progression of HF. Depending on the source, localization, and amount of ROS generated, ROS could have very different effects on Ca handling proteins.
Future treatment strategies interfering with ROS signaling in HF have to deal with these issues.
Abbreviations Used
- AKAP
protein kinase A anchor protein
- AP
action potential
- Ca
free ionized calcium
- Ca-CaM
Ca-bound calmodulin
- CaMKII
Ca2+ calmodulin dependent protein kinase II
- cAMP
cyclic adenosine monophosphate
- DAD
delayed after-depolarizations
- DAG
diacylglycerol
- EAD
early after-depolarization
- FKBP12.6
FK506 binding protein 12.6
- GSH/GSSG
ratio of reduced to oxidized gluthathione
- HF
heart failure
- H2O2
hydrogen peroxide
- ICa
Ca2+ influx current via L-type Ca channels
- INa
Na+ influx current via cardiac voltage-gated Na channels
- Iti
Ca2+ activated transient inward current
- Ito
transient outward potassium current
- Na
free ionized sodium
- NADH
nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NaV1.5
pore forming α-subunit of cardiac voltage-gated Na channel
- NCX
Na+/Ca2+ exchanger
- NKA
Na-K-ATPase
- NO
nitric oxide
- NOS
nitric oxide synthase
- Nox
NADPH oxidase
- O2−
superoxide
- OH−
hydroxyl radicals
- ONOO−
peroxynitrite
- PKA
cAMP-dependent protein kinase A
- PKC
protein kinase C
- PLM
phospholemman
- PLN
phospholamban
- ROS
reactive oxygen species
- RyR2
cardiac ryanodine receptor
- SERCA2a
sarco-endoplasmic reticulum Ca2+ pump
- SH
sulfhydryl
- SOD
superoxide dismutase
- SR
sarcoplasmic reticulum Ca2+ storage organelle
- Trx
thioredoxin
- TTX
tetrodotoxin
Acknowledgments
SW was funded by the Forschungsförderprogramm der Universitätsmedizin Göttingen. SW was supported by a travel grant from the Fondation Leducq. AGR is supported by a career development grant from the Fondation Leducq. MEA is funded by NIH Grants R01HL70250, R01HL079031, and R01HL096652. MEA and LSM are funded by a grant (08CVD01) from the Fondation Leducq as part of the 'Alliance for CaMKII Signaling in Heart'. LSM is funded by Deutsche Forschungsgemeinschaft (DFG) Grants MA 1982/4-1, MA 1982/2-2, as well as TPA03 SFB 1002. LSM is also funded by the Fondation Leducq Transatlantic Network of Excellence on 'Redox and Nitrosative Regulation of Cardiac Remodeling: Novel Therapeutic Approaches for Heart Failure.
Author Disclosure Statement
MEA is a named inventor on patents claiming to treat heart failure and arrhythmias by CaMKII inhibition. LSM acknowledges research grants and funding from CVT, GILEAD, and MENARINI/Berlin-Chemie.
References
- 1.Abramson JJ. Salama G. Critical sulfhydryls regulate calcium release from sarcoplasmic reticulum. J Bioenerg Biomembr. 1989;21:283–294. doi: 10.1007/BF00812073. [DOI] [PubMed] [Google Scholar]
- 2.Ago T. Kuroda J. Pain J. Fu C. Li H. Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akar FG. Aon MA. Tomaselli GF. O'Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005;115:3527–3535. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akki A. Zhang M. Murdoch C. Brewer A. Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol. 2009;47:15–22. doi: 10.1016/j.yjmcc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Anilkumar N. Sirker A. Shah AM. Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci. 2009;14:3168–3187. doi: 10.2741/3443. [DOI] [PubMed] [Google Scholar]
- 6.Anzai K. Ogawa K. Kuniyasu A. Ozawa T. Yamamoto H. Nakayama H. Effects of hydroxyl radical and sulfhydryl reagents on the open probability of the purified cardiac ryanodine receptor channel incorporated into planar lipid bilayers. Biochem Biophys Res Comm. 1998;249:938–942. doi: 10.1006/bbrc.1998.9244. [DOI] [PubMed] [Google Scholar]
- 7.Aon MA. Cortassa S. O'Rourke B. The fundamental organization of cardiac mitochondria as a network of coupled oscillators. Biophys J. 2006;91:4317–4327. doi: 10.1529/biophysj.106.087817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aon MA. Cortassa S. Marban E. O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 9.Armoundas AA. Hobai IA. Tomaselli GF. Winslow RL. O'Rourke B. Role of sodium-calcium exchanger in modulating the action potential of ventricular myocytes from normal and failing hearts. Circ Res. 2003;93:46–53. doi: 10.1161/01.RES.0000080932.98903.D8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashpole NM. Herren AW. Ginsburg KS. Brogan JD. Johnson DE. Cummins TR. Bers DM. Hudmon A. Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel NaV1.5 gating by multiple phosphorylation sites. J Biol Chem. 2012;287:19856–19869. doi: 10.1074/jbc.M111.322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baartscheer A. Schumacher CA. Belterman CN. Coronel R. Fiolet JW. [Na+]i and the driving force of the Na+/Ca2+-exchanger in heart failure. Cardiovasc Res. 2003;57:986–995. doi: 10.1016/s0008-6363(02)00848-9. [DOI] [PubMed] [Google Scholar]
- 12.Baartscheer A. Schumacher CA. van Borren MM. Belterman CN. Coronel R. Fiolet JW. Increased Na+/H+-exchange activity is the cause of increased [Na+]i and underlies disturbed calcium handling in the rabbit pressure and volume overload heart failure model. Cardiovasc Res. 2003;57:1015–1024. doi: 10.1016/s0008-6363(02)00809-x. [DOI] [PubMed] [Google Scholar]
- 13.Baartscheer A. Schumacher CA. van Borren MM. Belterman CN. Coronel R. Opthof T. Fiolet JW. Chronic inhibition of Na+/H+-exchanger attenuates cardiac hypertrophy and prevents cellular remodeling in heart failure. Cardiovasc Res. 2005;65:83–92. doi: 10.1016/j.cardiores.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Barouch LA. Harrison RW. Skaf MW. Rosas GO. Cappola TP. Kobeissi ZA. Hobai IA. Lemmon CA. Burnett AL. O'Rourke B. Rodriguez ER. Huang PL. Lima JA. Berkowitz DE. Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 15.Barrington P. Meier C. Weglicki W. Abnormal electrical-activity induced by free-radical generating systems in isolated cardiocytes. J Mol Cell Cardiol. 1988;20:1163–1178. doi: 10.1016/0022-2828(88)90596-2. [DOI] [PubMed] [Google Scholar]
- 16.Belin RJ. Sumandea MP. Allen EJ. Schoenfelt K. Wang H. Solaro RJ. de Tombe PP. Augmented protein kinase C-α-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007;101:195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- 17.Bers DM. Despa S. Bossuyt J. Regulation of Ca2+ and Na+ in normal and failing cardiac myocytes. Ann NY Acad Sci. 2006;1080:165–177. doi: 10.1196/annals.1380.015. [DOI] [PubMed] [Google Scholar]
- 18.Bers DM. Altered cardiac myocyte Ca regulation In heart failure. Physiology (Bethesda) 2006;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 19.Blaich A. Welling A. Fischer S. Wegener JW. Köstner K. Hofmann F. Moosmang S. Facilitation of murine cardiac L-type CaV1.2 channel is modulated by calmodulin kinase II-dependent phosphorylation of S1512 and S1570. Proc Natl Acad Sci USA. 2010;107:10285–10289. doi: 10.1073/pnas.0914287107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogoyevitch MA. Parker PJ. Sugden PH. Characterization of protein kinase C isotype expression in adult rat heart. Protein kinase C-epsilon is a major isotype present, and it is activated by phorbol esters, epinephrine, and endothelin. Circ Res. 1993;72:757–767. doi: 10.1161/01.res.72.4.757. [DOI] [PubMed] [Google Scholar]
- 21.Boraso A. Williams AJ. Modification of the gating of the cardiac sarcoplasmic reticulum Ca2+-release channel by H2O2 and dithiothreitol. Am J Physiol. 1994;267:H1010–1016. doi: 10.1152/ajpheart.1994.267.3.H1010. [DOI] [PubMed] [Google Scholar]
- 22.Bowling N. Walsh RA. Song G. Estridge T. Sandusky GE. Fouts RL. Mintze K. Pickard T. Roden R. Bristow MR. Sabbah HN. Mizrahi JL. Gromo G. King GL. Vlahos CJ. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 23.Braz JC. Gregory K. Pathak A. Zhao W. Sahin B. Klevitsky R. Kimball TF. Lorenz JN. Nairn AC. Liggett SB. Bodi I. Wang S. Schwartz A. Lakatta EG. DePaoli-Roach AA. Robbins J. Hewett TE. Bibb JA. Westfall MV. Kranias EG. Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 24.Brennan J. Bardswell S. Burgoyne J. Fuller W. Schroder E. Wait R. Begum S. Kentish J. Eaton P. Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J Biol Chem. 2006;281:21827–21836. doi: 10.1074/jbc.M603952200. [DOI] [PubMed] [Google Scholar]
- 25.Burkard N. Rokita AG. Kaufmann SG. Hallhuber M. Wu R. Hu K. Hofmann U. Bonz A. Frantz S. Cartwright EJ. Neyses L. Maier LS. Maier SK. Renné T. Schuh K. Ritter O. Conditional neuronal nitric oxide synthase overexpression impairs myocardial contractility. Circ Res. 2007;100:e32–44. doi: 10.1161/01.RES.0000259042.04576.6a. [DOI] [PubMed] [Google Scholar]
- 26.Campbell DL. Stamler JS. Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Dual mechanism regulation by nitric oxide and S-nitrosothiols. J Gen Physiol. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cappola TP. Kass DA. Nelson GS. Berger RD. Rosas GO. Kobeissi ZA. Marban E. Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- 28.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 29.Chen L. Hahn H. Wu G. Chen CH. Liron T. Schechtman D. Cavallaro G. Banci L. Guo Y. Bolli R. Dorn GW., 2nd Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci USA. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Churchill EN. Mochly-Rosen D. The roles of PKCdelta and epsilon isoenzymes in the regulation of myocardial ischaemia/reperfusion injury. Biochem Soc Trans. 2007;35:1040–1042. doi: 10.1042/BST0351040. [DOI] [PubMed] [Google Scholar]
- 31.Coetzee WA. Opie LH. Effects of oxygen free radicals on isolated cardiac myocytes from guinea-pig ventricle: Electrophysiological studies. J Mol Cell Cardiol. 1992;24:651–663. doi: 10.1016/0022-2828(92)91049-b. [DOI] [PubMed] [Google Scholar]
- 32.Dai DF. Rabinovitch P. Mitochondrial oxidative stress mediates induction of autophagy and hypertrophy in angiotensin-II treated mouse hearts. Autophagy. 2011;7:917–918. doi: 10.4161/auto.7.8.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai DF. Chen T. Szeto H. Nieves-Cintrón M. Kutyavin V. Santana LF. Rabinovitch PS. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai DF. Hsieh EJ. Liu Y. Chen T. Beyer RP. Chin MT. MacCoss MJ. Rabinovitch PS. Mitochondrial proteome remodelling in pressure overload-induced heart failure: The role of mitochondrial oxidative stress. Cardiovasc Res. 2012;93:79–88. doi: 10.1093/cvr/cvr274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai DF. Johnson SC. Villarin JJ. Chin MT. Nieves-Cintrón M. Chen T. Marcinek DJ. Dorn GW., 2nd Kang YJ. Prolla TA. Santana LF. Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Gαq overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Jongh KS. Murphy BJ. Colvin AA. Hell JW. Takahashi M. Catterall WA. Specific phosphorylation of a site in the full-length form of the α1 subunit of the cardiac L-type calcium channel by adenosine 3',5'-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 37.Despa S. Bossuyt J. Han F. Ginsburg KS. Jia LG. Kutchai H. Tucker AL. Bers DM. Phospholemman-phosphorylation mediates the beta-adrenergic effects on Na/K pump function in cardiac myocytes. Circ Res. 2005;97:252–259. doi: 10.1161/01.RES.0000176532.97731.e5. [DOI] [PubMed] [Google Scholar]
- 38.Dipla K. Mattiello JA. Margulies KB. Jeevanandam V. Houser SR. The sarcoplasmic reticulum and the Na+/Ca2+ exchanger both contribute to the Ca2+ transient of failing human ventricular myocytes. Circ Res. 1999;84:435–444. doi: 10.1161/01.res.84.4.435. [DOI] [PubMed] [Google Scholar]
- 39.Dorn GW., 2nd Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duquesnes N. Lezoualc'h F. Crozatier B. PKCδ and PKCɛ: Foes of the same family or strangers? J Mol Cell Cardiol. 2011;51:665–673. doi: 10.1016/j.yjmcc.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Dzhura I. Wu Y. Colbran RJ. Balser JR. Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 42.Eager KR. Dulhunty AF. Activation of the cardiac ryanodine receptor by sulfhydryl oxidation is modified by Mg2+ and ATP. J Membr Biol. 1998;163:9–18. doi: 10.1007/s002329900365. [DOI] [PubMed] [Google Scholar]
- 43.Erickson JR. He BJ. Grumbach IM. Anderson ME. CaMKII in the cardiovascular system: Sensing redox states. Physiol Rev. 2011;91:889–915. doi: 10.1152/physrev.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erickson JR. Joiner ML. Guan X. Kutschke W. Yang J. Oddis CV. Bartlett RK. Lowe JS. O'Donnell SE. Aykin-Burns N. Zimmerman MC. Zimmerman K. Ham AJ. Weiss RM. Spitz DR. Shea MA. Colbran RJ. Mohler PJ. Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Favero TG. Zable AC. Abramson JJ. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1995;270:25557–25563. doi: 10.1074/jbc.270.43.25557. [DOI] [PubMed] [Google Scholar]
- 46.Fearon IM. Palmer AC. Balmforth AJ. Ball SG. Varadi G. Peers C. Modulation of recombinant human cardiac L-type Ca2+ channel α1C subunits by redox agents and hypoxia. J Physiol. 1999;514:629–637. doi: 10.1111/j.1469-7793.1999.629ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frohnwieser B. Chen L. Schreibmayer W. Kallen R. Modulation of the human cardiac sodium channel α-subunit by cAMP-dependent protein kinase and the responsible sequence domain. J Physiol (Lond) 1997;498:309–318. doi: 10.1113/jphysiol.1997.sp021859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukuda K. Davies SS. Nakajima T. Ong BH. Kupershmidt S. Fessel J. Amarnath V. Anderson ME. Boyden PA. Viswanathan PC. Roberts LJ., 2nd Balser JR. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ Res. 2005;97:1262–1269. doi: 10.1161/01.RES.0000195844.31466.e9. [DOI] [PubMed] [Google Scholar]
- 49.Gen W. Tani M. Takeshita J. Ebihara Y. Tamaki K. Mechanisms of Ca2+ overload induced by extracellular H2O2 in quiescent isolated rat cardiomyocytes. Basic Res Cardiol. 2001;96:623–629. doi: 10.1007/s003950170014. [DOI] [PubMed] [Google Scholar]
- 50.Gill JS. McKenna WJ. Camm A. Free radicals irreversibly decrease Ca2+ currents in isolated guinea-pig ventricular myocytes. Eur J Pharmacol. 1995;292:337–340. doi: 10.1016/0926-6917(95)90042-x. [DOI] [PubMed] [Google Scholar]
- 51.Goldhaber JI. Ji S. Lamp ST. Weiss JN. Effects of exogenous free radicals on electromechanical function and metabolism in isolated rabbit and guinea pig ventricle. Implications for ischemia and reperfusion injury. J Clin Invest. 1989;83:1800–1809. doi: 10.1172/JCI114085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldhaber JI. Free radicals enhance Na+/Ca2+ exchange in ventricular myocytes. Am J Physiol. 1996;271:H823–833. doi: 10.1152/ajpheart.1996.271.3.H823. [DOI] [PubMed] [Google Scholar]
- 53.Gopalakrishna R. Anderson W. Ca2+-independent and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Nat Acad Sci. 1989;86:6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grueter CE. Abiria SA. Dzhura I. Wu Y. Ham AJ. Mohler PJ. Anderson ME. Colbran RJ. L-type Ca2+ channel facilitation mediated by phosphorylation of the beta subunit by CaMKII. Mol Cell. 2006;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Haase H. Bartel S. Karczewski P. Morano I. Krause EG. In-vivo phosphorylation of the cardiac L-type calcium channel beta-subunit in response to catecholamines. Mol Cell Biochem. 1996;163–164:99–106. doi: 10.1007/BF00408645. [DOI] [PubMed] [Google Scholar]
- 56.Hallaq H. Yang Z. Viswanathan P. Fukuda K. Shen W. Wang D. Wells K. Zhou J. Yi J. Murray K. Quantitation of protein kinase A-mediated trafficking of cardiac sodium channels in living cells. Cardiovasc Res. 2006;72:250–261. doi: 10.1016/j.cardiores.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 57.Hasenfuss G. Schillinger W. Lehnart SE. Preuss M. Pieske B. Maier LS. Prestle J. Minami K. Just H. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- 58.He BJ. Joiner ML. Singh MV. Luczak ED. Swaminathan PD. Koval OM. Kutschke W. Allamargot C. Yang J. Guan X. Zimmerman K. Grumbach IM. Weiss RM. Spitz DR. Sigmund CD. Blankesteijn WM. Heymans S. Mohler PJ. Anderson ME. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17:1610–1618. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heymes C. Bendall JK. Ratajczak P. Cave AC. Samuel JL. Hasenfuss G. Shah AM. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 60.Hidalgo C. Hudson B. Bogomolovas J. Zhu Y. Anderson B. Greaser M. Labeit S. Granzier H. PKC phosphorylation of titin's PEVK element: A novel and conserved pathway for modulating myocardial stiffness. Circ Res. 2009;105:631–638. doi: 10.1161/CIRCRESAHA.109.198465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hilgemann DW. Nicoll DA. Philipson KD. Charge movement during Na+ translocation by native and cloned cardiac Na+/Ca2+ exchanger. Nature. 1991;352:715–718. doi: 10.1038/352715a0. [DOI] [PubMed] [Google Scholar]
- 62.Hill MF. Singal PK. Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circulation. 1997;96:2414–2420. doi: 10.1161/01.cir.96.7.2414. [DOI] [PubMed] [Google Scholar]
- 63.House C. Kemp BE. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987;238:1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- 64.Hu H. Chiamvimonvat N. Yamagishi T. Marban E. Direct inhibition of expressed cardiac L-type Ca2+ channels by S-nitrosothiol nitric oxide donors. Circ Res. 1997;81:742–752. doi: 10.1161/01.res.81.5.742. [DOI] [PubMed] [Google Scholar]
- 65.Huang L. Wolska BM. Montgomery DE. Burkart EM. Buttrick PM. Solaro RJ. Increased contractility and altered Ca2+ transients of mouse heart myocytes conditionally expressing PKCβ. Am J Physiol Cell Physiol. 2001;280:C1114–1120. doi: 10.1152/ajpcell.2001.280.5.C1114. [DOI] [PubMed] [Google Scholar]
- 66.Hudmon A. Schulman H. Kim J. Maltez JM. Tsien RW. Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hulme JT. Westenbroek RE. Scheuer T. Catterall WA. Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac CaV1.2 channels during β1-adrenergic regulation. Proc Natl Acad Sci USA. 2006;103:16574–16579. doi: 10.1073/pnas.0607294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hund TJ. Koval OM. Li J. Wright PJ. Qian L. Snyder JS. Gudmundsson H. Kline CF. Davidson NP. Cardona N. Rasband MN. Anderson ME. Mohler PJ. A βIV-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120:3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ide T. Tsutsui H. Kinugawa S. Suematsu N. Hayashidani S. Ichikawa K. Utsumi H. Machida Y. Egashira K. Takeshita A. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res. 2000;86:152–157. doi: 10.1161/01.res.86.2.152. [DOI] [PubMed] [Google Scholar]
- 70. This reference has been deleted.
- 71.Ide T. Tsutsui H. Kinugawa S. Utsumi H. Kang D. Hattori N. Uchida K. Arimura K. Egashira K. Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 72.Iwamoto T. Pan Y. Wakabayashi S. Imagawa T. Yamanaka HI. Shigekawa M. Phosphorylation-dependent regulation of cardiac Na+/Ca2+ exchanger via protein kinase C. J Biol Chem. 1996;271:13609–13615. doi: 10.1074/jbc.271.23.13609. [DOI] [PubMed] [Google Scholar]
- 73.Kang SW. Chae HZ. Seo MS. Kim K. Baines IC. Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-a. J Biol Chem. 1998;273:6297–6302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- 74.Kassmann M. Hansel A. Leipold E. Birkenbeil J. Lu SQ. Hoshi T. Heinemann SH. Oxidation of multiple methionine residues impairs rapid sodium channel inactivation. Pflugers Arch. 2008;456:1085–1095. doi: 10.1007/s00424-008-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kato M. Kako KJ. Na+/Ca2+ exchange of isolated sarcolemmal membrane: Effects of insulin, oxidants and insulin deficiency. Mol Cell Biochem. 1988;83:15–25. doi: 10.1007/BF00223194. [DOI] [PubMed] [Google Scholar]
- 76.Kim MS. Akera T. O2 free radicals: Cause of ischemia-reperfusion injury to cardiac Na+-K+-ATPase. Am J Physiol. 1987;252:H252–257. doi: 10.1152/ajpheart.1987.252.2.H252. [DOI] [PubMed] [Google Scholar]
- 77.Kohlhaas M. Liu T. Knopp A. Zeller T. Ong MF. Böhm M. O'Rourke B. Maack C. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 2010;121:1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koval OM. Guan X. Wu Y. Joiner ML. Gao Z. Chen B. Grumbach IM. Luczak ED. Colbran RJ. Song LS. Hund TJ. Mohler PJ. Anderson ME. CaV1.2 β-subunit coordinates CaMKII-triggered cardiomyocyte death and afterdepolarizations. Proc Natl Acad Sci USA. 2010;107:4996–5000. doi: 10.1073/pnas.0913760107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kukreja RC. Okabe E. Schrier GM. Hess ML. Oxygen radical-mediated lipid peroxidation and inhibition of Ca2+ ATPase activity of cardiac sarcoplasmic reticulum. Arch Biochem Biophys. 1988;261:447–457. doi: 10.1016/0003-9861(88)90361-x. [DOI] [PubMed] [Google Scholar]
- 80.Kukreja RC. Weaver AB. Hess ML. Sarcolemmal Na+-K+-ATPase: Inactivation by neutrophil-derived free radicals and oxidants. Am J Physiol. 1990;259:H1330–1336. doi: 10.1152/ajpheart.1990.259.5.H1330. [DOI] [PubMed] [Google Scholar]
- 81.Kuret J. Schulman H. Mechanism of autophosphorylation of the multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem. 1985;260:6427–6433. [PubMed] [Google Scholar]
- 82.Kuroda J. Ago T. Matsushima S. Zhai P. Schneider MD. Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lacampagne A. Duittoz A. Bolanos P. Peineau N. Argibay JA. Effect of sulfhydryl oxidation on ionic and gating currents associated with L-type calcium channels in isolated guinea-pig ventricular myocytes. Cardiovasc Res. 1995;30:799–806. [PubMed] [Google Scholar]
- 84.Liu G. Abramson JJ. Zable AC. Pessah IN. Direct evidence for the existence and functional role of hyperreactive sulfhydryls on the ryanodine receptor–triadin complex selectively labeled by the coumarin maleimide 7-diethylamino-3-(4-Vmaleimidylphenyl)-4- methylcoumarin. Mol Pharmacol. 1994;45:189–200. [PubMed] [Google Scholar]
- 85.Liu M. Liu H. Dudley SC., Jr. Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel. Circ Res. 2010;107:967–974. doi: 10.1161/CIRCRESAHA.110.220673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maack C. Dabew ER. Hohl M. Schäfers HJ. Böhm M. Endogenous activation of mitochondrial KATP channels protects human failing myocardium from hydroxyl radical-induced stunning. Circ Res. 2009;105:811–817. doi: 10.1161/CIRCRESAHA.109.206359. [DOI] [PubMed] [Google Scholar]
- 87.Maack C. Kartes T. Kilter H. Schäfers HJ. Nickenig G. Böhm M. Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation. 2003;108:1567–1574. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 88.Maier LS. Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007;73:631–640. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 89.Maier LS. Zhang T. Chen L. DeSantiago J. Brown JH. Bers DM. Transgenic CaMKIIδC overexpression uniquely alters cardiac myocyte Ca2+ handling: Reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 90.Maier LS. CaMKII regulation of voltage-gated sodium channels and cell excitability. Heart Rhythm. 2011;8:474–477. doi: 10.1016/j.hrthm.2010.09.080. [DOI] [PubMed] [Google Scholar]
- 91.Malhotra A. Kang BP. Opawumi D. Belizaire W. Meggs LG. Molecular biology of protein kinase C signaling in cardiac myocytes. Mol Cell Biochem. 2001;225:97–107. doi: 10.1023/a:1012261903611. [DOI] [PubMed] [Google Scholar]
- 92.Mallat Z. Philip I. Lebret M. Chatel D. Maclouf J. Tedgui A. Elevated levels of 8-iso-prostaglandin F2α in pericardial fluid of patients with heart failure: A potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;97:1536–1539. doi: 10.1161/01.cir.97.16.1536. [DOI] [PubMed] [Google Scholar]
- 93.Marengo JJ. Hidalgo C. Bull R. Sulfhydryl oxidation modifies the calcium dependence of ryanodine-sensitive calcium channels of excitable cells. Biophys J. 1998;74:1263–1277. doi: 10.1016/S0006-3495(98)77840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mikami A. Imoto K. Tanabe T. Niidome T. Mori Y. Takeshima H. Narumiya S. Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- 95.Mochly-Rosen D. Khaner H. Lopez J. Identification of intracellular receptor proteins for activated protein kinase C. Proc Natl Acad Sci USA. 1991;88:3997–4000. doi: 10.1073/pnas.88.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morris TE. Sulakhe PV. Sarcoplasmic reticulum Ca2+-pump dysfunction in rat cardiomyocytes briefly exposed to hydroxyl radicals. Free Radic Biol Med. 1997;22:37–47. doi: 10.1016/s0891-5849(96)00238-9. [DOI] [PubMed] [Google Scholar]
- 97.Murphy AJ. Sulfhydryl group modification of sarcoplasmic reticulum membranes. Biochemistry. 1976;15:4492–4496. doi: 10.1021/bi00665a025. [DOI] [PubMed] [Google Scholar]
- 98.Murphy BJ. Rogers J. Perdichizzi AP. Colvin AA. Catterall WA. cAMP-dependent phosphorylation of two sites in the α subunit of the cardiac sodium channel. J Biol Chem. 1996;271:28837–28843. doi: 10.1074/jbc.271.46.28837. [DOI] [PubMed] [Google Scholar]
- 99.Murray KT. Hu N. Daw JR. Shin H-G. Watson MT. Mashburn AB. George AL., Jr Functional effects of protein kinase C activation on the human cardiac Na+ channel. Circ Res. 1997;80:370–376. doi: 10.1161/01.res.80.3.370. [DOI] [PubMed] [Google Scholar]
- 100.Nicoll DA. Ottolia M. Lu L. Lu Y. Philipson KD. A new topological model of the cardiac sarcolemmal Na+-Ca2+ exchanger. J Biol Chem. 1999;274:910–917. doi: 10.1074/jbc.274.2.910. [DOI] [PubMed] [Google Scholar]
- 101.Pacher P. Beckman JS. Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Palace V. Kumar D. Hill MF. Khaper N. Singal PK. Regional differences in non-enzymatic antioxidants in the heart under control and oxidative stress conditions. J Mol Cell Cardiol. 1999;31:193–202. doi: 10.1006/jmcc.1998.0859. [DOI] [PubMed] [Google Scholar]
- 103.Perrelli MG. Pagliaro P. Penna C. Ischemia/reperfusion injury and cardioprotective mechanisms: Role of mitochondria and reactive oxygen species. World J Cardiol. 2011;3:186–200. doi: 10.4330/wjc.v3.i6.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Piacentino V., 3rd Weber CR. Chen X. Weisser-Thomas J. Margulies KB. Bers DM. Houser SR. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 105.Pieske B. Maier LS. Piacentino V., 3rd Weisser J. Hasenfuss G. Houser S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation. 2002;106:447–453. doi: 10.1161/01.cir.0000023042.50192.f4. [DOI] [PubMed] [Google Scholar]
- 106.Puri TS. Gerhardstein BL. Zhao XL. Ladner MB. Hosey MM. Differential effects of subunit interactions on protein kinase A- and C-mediated phosphorylation of L-type calcium channels. Biochemistry. 1997;36:9605–9615. doi: 10.1021/bi970500d. [DOI] [PubMed] [Google Scholar]
- 107.Qu Y. Rogers J. Tanada T. Catterall W. Scheuer T. Phosphorylation of S1505 in the cardiac Na+ channel inactivation gate is required for modulation by protein kinase C. J Gen Physiol. 1996;108:375–379. doi: 10.1085/jgp.108.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qu Y. Rogers J. Tanada T. Scheuer T. Catterall W. Modulation of cardiac Na+ channels expressed in a mammalian cell line and in ventricular myocytes by protein kinase C. Proc Nat Acad Sci. 1994;91:3289–3293. doi: 10.1073/pnas.91.8.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ramirez-Correa GA. Cortassa S. Stanley B. Gao WD. Murphy AM. Calcium sensitivity, force frequency relationship and cardiac troponin I: Critical role of PKA and PKC phosphorylation sites. J Mol Cell Cardiol. 2010;48:943–953. doi: 10.1016/j.yjmcc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reeves JP. Bailey CA. Hale CC. Redox modification of sodium–calcium exchange activity in cardiac sarcolemmal vesicles. J Biol Chem. 1986;261:4948–4955. [PubMed] [Google Scholar]
- 111.Rozanski GJ. Xu Z. Sulfhydryl modulation of K+ channels in rat ventricular myocytes. J Mol Cell Cardiol. 2002;34:1623–1632. doi: 10.1006/jmcc.2002.2112. [DOI] [PubMed] [Google Scholar]
- 112.Sabri A. Byron KL. Samarel AM. Bell J. Lucchesi PA. Hydrogen peroxide activates mitogen-activated protein kinases and Na+-H+ exchange in neonatal rat cardiac myocytes. Circ Res. 1998;82:1053–1062. doi: 10.1161/01.res.82.10.1053. [DOI] [PubMed] [Google Scholar]
- 113.Sag CM. Köhler AC. Anderson ME. Backs J. Maier LS. CaMKII-dependent SR Ca leak contributes to doxorubicin-induced impaired Ca handling in isolated cardiac myocytes. J Mol Cell Cardiol. 2011;51:749–759. doi: 10.1016/j.yjmcc.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Santacruz-Toloza L. Ottolia M. Nicoll DA. Philipson KD. Functional analysis of a disulfide bond in the cardiac Na+-Ca2+ exchanger. J Biol Chem. 2000;275:182–188. doi: 10.1074/jbc.275.1.182. [DOI] [PubMed] [Google Scholar]
- 115.Scherer NM. Deamer DW. Oxidative stress impairs the function of sarcoplasmic reticulum by oxidation of sulfhydryl groups in the Ca2+-ATPase. Arch Biochem Biophys. 1986;246:589–601. doi: 10.1016/0003-9861(86)90314-0. [DOI] [PubMed] [Google Scholar]
- 116.Schulman H. Greengard P. Stimulation of brain membrane protein phosphorylation by calcium and an endogenous heat-stable protein. Nature. 1978;271:478–479. doi: 10.1038/271478a0. [DOI] [PubMed] [Google Scholar]
- 117.Schworer CM. Colbran RJ. Soderling TR. Reversible generation of a Ca2+-independent form of Ca2+/calmodulin-dependent protein kinase II by an autophosphorylation mechanism. J Biol Chem. 1986;261:8581–8584. [PubMed] [Google Scholar]
- 118.Shattock MJ. Matsuura H. Measurement of Na+-K+ pump current in isolated rabbit ventricular myocytes using the whole-cell voltage-clamp technique. Inhibition of the pump by oxidant stress. Circ Res. 1993;72:91–101. doi: 10.1161/01.res.72.1.91. [DOI] [PubMed] [Google Scholar]
- 119.Sirker A. Zhang M. Shah AM. NADPH oxidases in cardiovascular disease: Insights from in vivo models and clinical studies. Basic Res Cardiol. 2011;106:735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Song Y. Shryock J. Wagner S. Maier LS. Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 121.Song YH. Cho H. Ryu SY. Yoon JY. Park SH. Noh CI. Lee SH. Ho WK. L-type Ca2+ channel facilitation mediated by H2O2-induced activation of CaMKII in rat ventricular myocytes. J Mol Cell Cardiol. 2010;48:773–780. doi: 10.1016/j.yjmcc.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 122.Studer R. Reinecke H. Bilger J. Eschenhagen T. Böhm M. Hasenfuss G. Just H. Holtz J. Drexler H. Gene expression of the cardiac Na+-Ca2+ exchanger in end-stage human heart failure. Circ Res. 1994;75:443–453. doi: 10.1161/01.res.75.3.443. [DOI] [PubMed] [Google Scholar]
- 123.Sumandea MP. Pyle WG. Kobayashi T. de Tombe PP. Solaro RJ. Identification of a functionally critical protein kinase C phosphorylation residue of cardiac troponin T. J Biol Chem. 2003;278:35135–35144. doi: 10.1074/jbc.M306325200. [DOI] [PubMed] [Google Scholar]
- 124.Swaminathan PD. Purohit A. Soni S. Voigt N. Singh MV. Glukhov AV. Gao Z. He BJ. Luczak ED. Joiner ML. Kutschke W. Yang J. Donahue JK. Weiss RM. Grumbach IM. Ogawa M. Chen PS. Efimov I. Dobrev D. Mohler PJ. Hund TJ. Anderson ME. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011;121:3277–3288. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Takeishi Y. Ping P. Bolli R. Kirkpatrick DL. Hoit BD. Walsh RA. Transgenic overexpression of constitutively active protein kinase C epsilon causes concentric cardiac hypertrophy. Circ Res. 2000;86:1218–1223. doi: 10.1161/01.res.86.12.1218. [DOI] [PubMed] [Google Scholar]
- 126.Takimoto E. Champion HC. Li M. Ren S. Rodriguez ER. Tavazzi B. Lazzarino G. Paolocci N. Gabrielson KL. Wang Y. Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tao L. Gao E. Bryan NS. Qu Y. Liu HR. Hu A. Christopher TA. Lopez BL. Yodoi J. Koch WJ. Feelisch M. Ma XL. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: Role of S-nitrosation. Proc Natl Acad Sci. 2004;101:11471–11476. doi: 10.1073/pnas.0402941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Temsah RM. Netticadan T. Chapman D. Takeda S. Mochizuki S. Dhalla NS. Alterations in sarcoplasmic reticulum function and gene expression in ischemic-reperfused rat heart. Am J Physiol. 1999;277:H584–594. doi: 10.1152/ajpheart.1999.277.2.H584. [DOI] [PubMed] [Google Scholar]
- 129.Tsutsui H. Ide T. Hayashidani S. Suematsu N. Utsumi H. Nakamura R. Egashira K. Takeshita A. Greater susceptibility of failing cardiac myocytes to oxygen free radical-mediated injury. Cardiovasc Res. 2001;49:103–109. doi: 10.1016/s0008-6363(00)00197-8. [DOI] [PubMed] [Google Scholar]
- 130.Tsutsui H. Kinugawa S. Matsushima S. Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovasc Res. 2009;81:449–456. doi: 10.1093/cvr/cvn280. [DOI] [PubMed] [Google Scholar]
- 131.Tsutsui H. Kinugawa S. Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 132.Valdivia CR. Ueda K. Ackerman MJ. Makielski JC. GPD1L links redox state to cardiac excitability by PKC-dependent phosphorylation of the sodium channel SCN5A. Am J Physiol Heart Circ Physiol. 2009;297:H1446–1452. doi: 10.1152/ajpheart.00513.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vigil D. Blumenthal DK. Brown S. Taylor SS. Trewhella J. Differential effects of substrate on type I and type II PKA holoenzyme dissociation. Biochemistry. 2004;43:5629–5636. doi: 10.1021/bi0499157. [DOI] [PubMed] [Google Scholar]
- 134.Viste K. Kopperud RK. Christensen AE. Døskeland SO. Substrate enhances the sensitivity of type I protein kinase A to cAMP. J Biol Chem. 2005;280:13279–13284. doi: 10.1074/jbc.M413065200. [DOI] [PubMed] [Google Scholar]
- 135.Wagner S. Dybkova N. Rasenack ECL. Jacobshagen C. Fabritz L. Kirchhof P. Maier SKG. Zhang T. Hasenfuss G. Brown JH. Bers DM. Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wagner S. Ruff HM. Weber SL. Bellmann S. Sowa T. Schulte T. Anderson ME. Grandi E. Bers DM. Backs J. Belardinelli L. Maier LS. Reactive oxygen species-activated Ca/calmodulin kinase IIδ is required for late INa augmentation leading to cellular Na and Ca overload. Circ Res. 2011;108:555–565. doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wagner S. Seidler T. Picht E. Maier LS. Kazanski V. Teucher N. Schillinger W. Pieske B. Isenberg G. Hasenfuss G. Kögler H. Na+-Ca+ exchanger overexpression predisposes to reactive oxygen species-induced injury. Cardiovasc Res. 2003;60:404–412. doi: 10.1016/j.cardiores.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 138.Wang H. Grant JE. Doede CM. Sadayappan S. Robbins J. Walker JW. PKC-βII sensitizes cardiac myofilaments to Ca2+ by phosphorylating troponin I on threonine-144. J Mol Cell Cardiol. 2006;41:823–833. doi: 10.1016/j.yjmcc.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 139.Wang J. Liu X. Sentex E. Takeda N. Dhalla NS. Increased expression of protein kinase C isoforms in heart failure due to myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284:H2277–2287. doi: 10.1152/ajpheart.00142.2002. [DOI] [PubMed] [Google Scholar]
- 140.Ward CA. Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol. 1997;500:631–642. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Weber CR. Piacentino V., 3rd Ginsburg KS. Houser SR. Bers DM. Na+-Ca2+ exchange current and submembrane [Ca2+] during the cardiac action potential. Circ Res. 2002;90:182–189. doi: 10.1161/hh0202.103940. [DOI] [PubMed] [Google Scholar]
- 142.Weber CR. Piacentino V., 3rd Houser SR. Bers DM. Dynamic regulation of sodium/calcium exchange function in human heart failure. Circulation. 2003;108:2224–2229. doi: 10.1161/01.CIR.0000095274.72486.94. [DOI] [PubMed] [Google Scholar]
- 143.Weisser-Thomas J. Piacentino V., 3rd Gaughan JP. Margulies K. Houser SR. Calcium entry via Na/Ca exchange during the action potential directly contributes to contraction of failing human ventricular myocytes. Cardiovasc Res. 2003;57:974–985. doi: 10.1016/s0008-6363(02)00732-0. [DOI] [PubMed] [Google Scholar]
- 144.Xiang Y. Kobilka BK. Myocyte adrenoceptor signaling pathways. Science. 2003;300:1530–1532. doi: 10.1126/science.1079206. [DOI] [PubMed] [Google Scholar]
- 145.Xie ZJ. Wang YH. Askari A. Huang WH. Klaunig JE. Askari A. Studies on the specificity of the effects of oxygen metabolites on cardiac sodium pump. J Mol Cell Cardiol. 1990;22:911–920. doi: 10.1016/0022-2828(90)90122-i. [DOI] [PubMed] [Google Scholar]
- 146.Xu KY. Zweier JL. Becker LC. Hydroxyl radical inhibits sarcoplasmic reticulum Ca2+-ATPase function by direct attack on the ATP binding site. Circ Res. 1997;80:76–81. doi: 10.1161/01.res.80.1.76. [DOI] [PubMed] [Google Scholar]
- 147.Xu L. Eu JP. Meissner G. Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-s-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 148.Yamawaki H. Haendeler J. Berk BC. Thioredoxin: A key regulator of cardiovascular homeostasis. Circ Res. 2003;93:1029–1033. doi: 10.1161/01.RES.0000102869.39150.23. [DOI] [PubMed] [Google Scholar]
- 149.Yan Y. Liu J. Wei C. Li K. Xie W. Wang Y. Cheng H. Bidirectional regulation of Ca2+ sparks by mitochondria-derived reactive oxygen species in cardiac myocytes. Cardiovasc Res. 2008;77:432–441. doi: 10.1093/cvr/cvm047. [DOI] [PubMed] [Google Scholar]
- 150.Yang L. Doshi D. Morrow J. Katchman A. Chen X. Marx SO. Protein kinase C isoforms differentially phosphorylate CaV1.2 α1c. Biochemistry. 2009;48:6674–6683. doi: 10.1021/bi900322a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang L. Liu G. Zakharov SI. Morrow JP. Rybin VO. Steinberg SF. Marx SO. Ser1928 is a common site for CaV1.2 phosphorylation by protein kinase C isoforms. J Biol Chem. 2005;280:207–214. doi: 10.1074/jbc.M410509200. [DOI] [PubMed] [Google Scholar]
- 152.Zhang M. Brewer AC. Schroder K. Santos CX. Grieve DJ. Wang M. Anilkumar N. Yu B. Dong X. Walker SJ. Brandes RP. Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA. 2011;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zima AV. Copello JA. Blatter LA. Effects of cytosolic NADH/NAD+ levels on sarcoplasmic reticulum Ca2+ release in permeabilized rat ventricular myocytes. J Physiol. 2004;555:727–741. doi: 10.1113/jphysiol.2003.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ziolo MT. Maier LS. Piacentino V., 3rd Bossuyt J. Houser SR. Bers DM. Myocyte nitric oxide synthase 2 contributes to blunted β-adrenergic response in failing human hearts by decreasing Ca2+ transients. Circulation. 2004;109:1886–1891. doi: 10.1161/01.CIR.0000124231.98250.A8. [DOI] [PubMed] [Google Scholar]
- 155.Zissimopoulos S. Docrat N. Lai FA. Redox sensitivity of the ryanodine receptor interaction with FK506-binding protein. J Biol Chem. 2007;282:6976–6983. doi: 10.1074/jbc.M607590200. [DOI] [PubMed] [Google Scholar]