Abstract
Significance: Proangiogenic therapy appeared a promising strategy for the treatment of patients with acute myocardial infarction (MI), as de novo formation of microvessels, has the potential to salvage ischemic myocardium at early stages after MI, and is also essential to prevent the transition to heart failure through the control of cardiomyocyte hypertrophy and contractility. Recent Advances: Exciting preclinical studies evaluating proangiogenic therapies for MI have prompted the initiation of numerous clinical trials based on protein or gene transfer delivery of growth factors and administration of stem/progenitor cells, mainly from bone marrow origin. Nonetheless, these clinical trials showed mixed results in patients with acute MI. Critical Issues: Even though methodological caveats, such as way of delivery for angiogenic growth factors (e.g., protein vs. gene transfer) and stem/progenitor cells or isolation/culture procedure for regenerative cells might partially explain the failure of such trials, it appears that delivery of a single growth factor or cell type does not support angiogenesis sufficiently to promote cardiac repair. Future Directions: Optimization of proangiogenic therapies might include stimulation of both angiogenesis and vessel maturation and/or the use of additional sources of stem/progenitor cells, such as cardiac progenitor cells. Experimental unraveling of the mechanisms of angiogenesis, vessel maturation, and endothelial cell/cardiomyocyte cross talk in the ischemic heart, analysis of emerging pathways, as well as a better understanding of how cardiovascular risk factors impact endogenous and therapeutically stimulated angiogenesis, would undoubtedly pave the way for the development of novel and hopefully efficient angiogenesis targeting therapeutics for the treatment of acute MI. Antioxid. Redox Signal. 18, 1100–1113.
Introduction
Heart failure following myocardial infarction (MI) remains one of the major causes of death and disability worldwide, and its treatment is a major challenge of today's cardiovascular medicine (99). Despite a wide therapeutic arsenal, recovery of cardiac function and prevention of the transition to heart failure in MI patients remains unsatisfactory, urging the need for the development of novel therapeutic alternatives (99). In the past two decades, proangiogenic therapy to promote reperfusion and function of the ischemic heart appeared a promising strategy, but so far, clinical trials have failed to meet the expectations raised by exciting preclinical studies (69, 112). These disappointing results highlight the need for a comprehensive understanding of the mechanisms of angiogenesis in the ischemic heart as a prerequisite for the development of novel proangiogenic therapies for the treatment of MI.
Why Should We Target Angiogenesis?
Angiogenesis represents the emergence of newly formed microvessels from pre-existing capillaries. When exposed to angiogenic signals, such as hypoxia, growth factors, or nitric oxide (NO), quiescent endothelial cells become activated. Junctions between endothelial cells loosen and mural cells (pericytes, smooth muscle cells) detach from the vascular wall, resulting in increased vascular permeability. Extravasation of plasma protein allows formation of a provisional matrix onto which endothelial cells migrate. The microvascular sprout is guided by a specialized endothelial cell termed Tip Cell, while the neighboring endothelial cell (termed stalk cell) proliferates to elongate the sprout. Ultimately, a vessel lumen is formed and mural cells are recruited to ensure neovessel stability (19).
This process is to be opposed to arteriogenesis, the maturation and enlargement of blood vessels, and collateral growth, which represents flow-mediated remodeling and enlargement of pre-existing arteries (90). In this review, we will focus on the microcirculation in the ischemic heart, and mainly address angiogenesis and the maturation of newly formed capillaries. Further information regarding collateral growth after MI can be found in other reviews (96).
Ischemia-induced tissue damages and the cardiomyocyte loss depend on several factors, such as the extent of the ischemic injury—namely, the size of the initial infarct-, duration of ischemia and efficiency of reperfusion (121). Early reperfusion of the occluded epicardial coronary artery has substantially improved the outcome of MI patients by restoring blood supply to the infarcted area, hence reducing myocardial necrosis (99, 121). However, some patients remain ineligible for such therapy, and microvascular rarefaction and/or dysfunction in the ischemic heart prevent efficient reperfusion of the entire myocardium (114). Hence, de novo formation of microvessels, namely angiogenesis, has the potential to salvage ischemic myocardium at early stages after MI, and is also essential for long-term left ventricular remodeling to prevent the transition to heart failure (99, 114). After the initial ischemic event, the infarcted myocardium undergoes a vast process of tissue remodeling, which can be separated in three distinct, but overlapping phases (34). During the early inflammatory phase, macrophages and neutrophils clear the wound of necrotic cardiomyocytes. This is followed by a proliferative phase, where endothelial cells and fibroblasts proliferate to form a vascularized granulation tissue, which then matures into a collagen-rich scar after endothelial cell and fibroblast apoptosis (34). This whole process, in turn, tends to increase the physical load on the neighboring viable myocardium (34). Although angiogenesis occurs in the granulation tissue that will ultimately form the infarct scar, neovascularization of surrounding, viable myocardium in the infarct border zone is also crucial during this process of tissue remodeling. Indeed, hypoperfusion of otherwise viable myocardial areas, consecutive to microvascular dysfunction or increased metabolic demand due to mechanical overload, can promote regional decrease of contractile function, and the extent of microvascular rarefaction or dysfunction is associated with irreversibility of myocardial hibernation. Moreover, efficient perfusion provided by microvessels is required to prevent cardiomyocyte death, which can lead to infarct expansion, left ventricular dilation, and heart failure (101).
Cardiomyocyte growth, survival, and contractile function are dependent upon microvascular function and angiogenesis (Fig. 1), as illustrated by mice deficient in the laminin-alpha4 chain (Lama4−/− mice), an extracellular matrix protein abundantly expressed in the basement membrane of blood vessels in the myocardium. In Lama4−/− mice, enlargement of the perivascular space around microvessels triggers cardiomyocyte hypoxia and necrosis, and leads to the development of heart failure (116). In pressure overload cardiac hypertrophy, angiogenesis is essential in preventing the transition to heart failure, as inhibition of angiogenesis in hearts undergoing transgenically induced hypertrophy precipitates heart failure (101). On the other hand, angiogenesis itself promotes cardiomyocyte hypertrophy (111). Cardiac-specific overexpression of a secreted proangiogenic growth factor, PR39, produces massive angiogenesis in normal mouse myocardium, spontaneously triggering cardiomyocyte hypertrophy. Most interestingly, angiogenesis-induced cardiomyocyte hypertrophy ameliorates cardiac function after experimental MI (111). In humans, angiogenic imbalance during peripartum cardiomyopathy has recently been described and modeled in mice with cardiac-specific deficiency of PGC-1α, a strong inducer of Vascular Endothelial Growth Factor (VEGF) expression, and provides a striking example of cardiomyocyte dependence upon the microvasculature, even in the absence of underlying pathology (87).
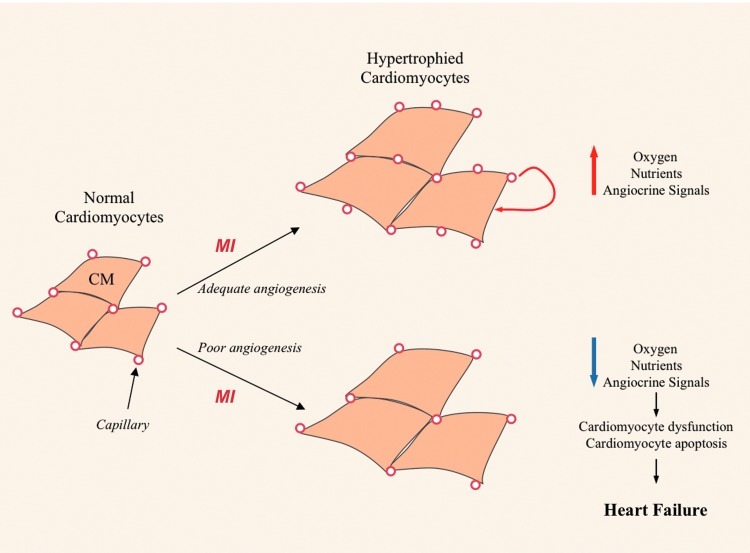
FIG. 1.
Model of angiogenesis/cardiomyocyte function coupling after myocardial infarction (MI). After the onset of MI, mechanichal overload of cardiomyocyte (CM) in the vicinity of ischemic areas leads to CM hypertrophy. Adequate angiogenesis provides CM necessary perfusion to deliver oxygen and nutrient, and Endothelial Cell/CM cross talk through angiocrine signals could also promote CM function. In contrast, poor angiogenesis leads to CM hypoperfusion, dysfunction, and death, which eventually precipitates heart failure. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Endothelial cell/cardiomyocyte cross talk also controls cardiomyocyte contractility (118), and promotes pharmacological protection of cardiomyocytes after ischemia and reperfusion in an NO-dependent manner (64). Similarly, microvascular endothelial cell support organ regeneration of the liver (24) and lung (25) through so called angiocrine signals, which include expression of Hepatocyte Growth Factor (HGF), wnt2, and metalloproteinase (MMP)-14-mediated activation of the Epithelial Growth Factor receptor. This concept might be relevant to heart disease, and angiocrine signals from cardiac microvessels might promote cardiomyocyte survival and function (Fig. 1).
Major Angiogenic Pathways and Their Role in Myocardial Angiogenesis After Infarction
Hypoxia inducible factors
Hypoxia-inducible transcription factors, such as hypoxia-inducible factor 1-α (HIF1-α), mediate cellular adaptation to hypoxia and control both developmental and postnatal angiogenesis (91). HIF1-α is activated by hypoxia and targets a wide array of genes, which include proangiogenic genes, such as VEGF or the progenitor cell mobilizing chemokine Stromal Cell-Derived Factor 1-α (SDF1-α or CXCL12), placing it as an upstream initiator of ischemia-induced angiogenesis (Fig. 2) (91). HIF1-α and the related HIF isoform HIF2-α proteins are expressed in cardiomyocytes, endothelial, and inflammatory cells early after MI, and their expression can persist up to 4 weeks after MI in rats (54). Mice constitutively expressing HIF1-α in cardiomyocytes display improved cardiac function after MI, associated with increased VEGF expression and angiogenesis in the myocardium (55). The prominent role of the HIF pathway in postischemic angiogenesis has further been shown through its indirect activation by Prolyl Hydroxylase Domain proteins (PHDs) and Factor Inhibiting HIF (FIH) inhibition. Oral administration of a nonisoform-specific PHD inhibitor in rats improved microvascular density in the peri-infarct area, preventing deterioration of cardiac function and left ventricle (LV) dilation after MI (8). Moreover, combined shRNA-induced knockdown of PHD-2 and FIH in mice improved LV function after MI, and also enhanced neovascularization (49). In a model of hindlimb ischemia, shRNA-induced knockdown of PHD-1, -2, or -3 enhanced neovascularization through upregulation of VEGF and endothelial Nitric Oxide Synthase (eNOS) as well as recruitment of proangiogenic myeloid cells (67), further demonstrating the pleïotropic role of the HIF1-α pathway during tissue ischemia angiogenesis. Along this line, HIF1-α controls the extent of endothelial progenitor cell (EPC) recruitment to ischemic areas and angiogenesis through the creation of SDF-1 gradients (20).
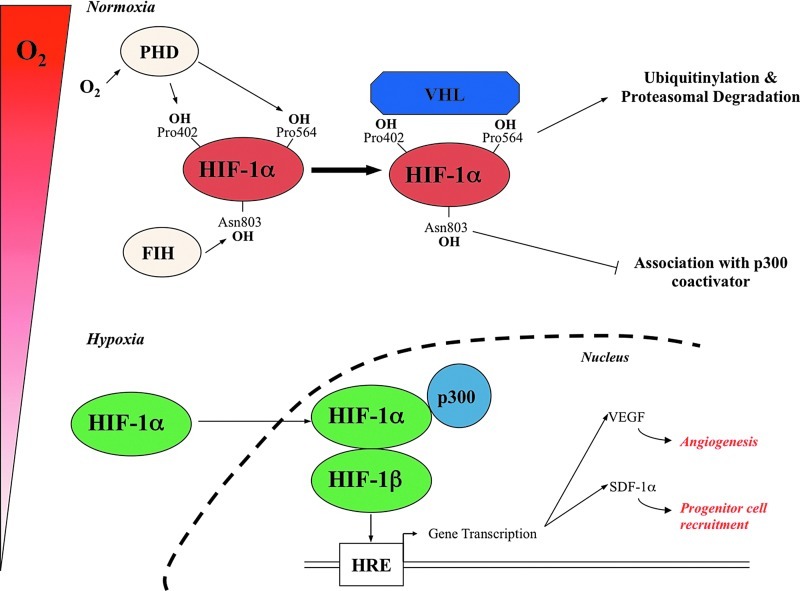
FIG. 2.
Oxygen-dependent post-translational modifications of hypoxia inducible factor (HIF)-1α levels. Under normoxic conditions, HIF-1α is hydroxylated (OH) on proline residues 402 and 564 (Pro) in the oxygen-dependent degradation domain by specific Prolyl Hydroxylase Domain proteins (PHDs). Once hydroxylated, HIF-1α is recognised by the von Hippel-Lindau protein (VHL), which is part of an ubiquitin–ligase complex known as E3 ligase complex, which targets HIF-1α for polyubiquitination and subsequent proteasomal degradation. The asparagine 803 (Asn 803) residue of HIF1α is also hydroxylated by FIH, which impairs the interaction with the transcriptional coactivator p300/CREB binding protein (p300) with the HIF-1α C-terminal transactivation domain. When oxygen becomes limited, the proline residues are no longer hydroxylated and HIF-1αɛσχαπɛσ δɛγραδατιoν, is translocated into the nucleus, where it dimerises with HIF-1β ανδ binds to hypoxia-responsive elements (HRE) to the promoter or enhancer sequences of target genes. In addition, interaction with p300/CREB binding protein, due to inhibition of Asn 803 hydroxylation, increases the transcriptional activity of HIF. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
HIF-2α is also able to induce expression of various angiogenic genes, such as VEGF or angiopoietins (92), but study of its role in postnatal angiogenesis has been mostly restricted to tumor models so far. Nevertheless, endothelial-specific HIF-2α has recently been shown to be essential for ischemia-induced angiogenesis (104). Interestingly, HIF-2α appeared to possess different, but complementary properties with HIF1-α: HIF-2α deletion in endothelial cells provoked enhanced angiogenesis, but the newly formed vessels failed to mature, thus leading to poor tissue perfusion (104). These properties of endothelial HIF-2α would be of particular relevance in post-MI angiogenesis, as functionality and persistence of the newly formed microvasculature are required for efficient tissue perfusion.
Growth factors
Numerous growth factors are expressed in the ischemic heart where they promote angiogenesis. For instance, HGF is induced in the myocardium following ischemia (86), and its proangiogenic potential in experimental models of MI is well established (78, 86). Other growth factors, such as Nerve Growth Factor (76) or Insulin-like Growth Factor-1 (26) activate angiogenesis and alleviate cardiac dysfunction after MI, but to date, the most studied growth factors in post-MI angiogenesis are growth factors of the VEGF and Fibroblast Growth Factor (FGF) families.
VEGF family of growth factors
Members of the VEGF family have a prominent role in post-MI angiogenesis (Fig. 3). VEGF acts through binding to its receptor VEGF receptor 2 (VEGFR2), promoting endothelial cell survival, proliferation, and migration (29). The angiogenic properties of VEGF during vascular development or tumor angiogenesis (29) have rapidly led to the evaluation of its role in post-MI angiogenesis. VEGF is rapidly induced in the ischemic heart in humans and rodents (43, 62), not only by hypoxia, but also by mechanical stretch (65). Several experimental reports demonstrated the proangiogenic activity and the associated benefit on cardiac function of VEGF administration in porcine (88) or rodent (97) models of MI. Nevertheless, VEGF alone stimulates the formation of immature, leaky, and disorganized blood vessels (16), and can also inhibit Platelet-Derived growth Factor (PDGF)-BB signaling, an indispensible pathway of vessel maturation. Indeed, VEGF-R2 activated by VEGF complexes with the PDGF- receptor β to block signal transduction, hence altering mural cells recruitment and vessel stabilization (40). This phenomenon emphasizes the need of timely regulated angiogenic and arteriogenic signals for the formation of a stable microvascular network.
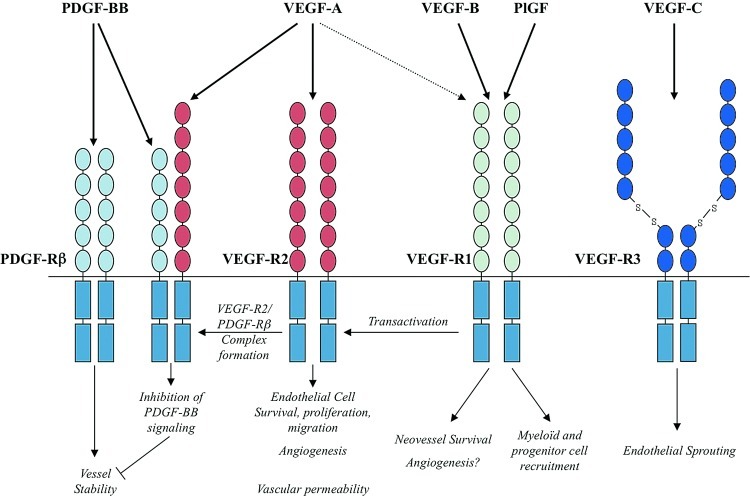
FIG. 3.
Vascular endothelial growth factor (VEGF) family of growth factors-dependent angiogenic pathways. Members of the VEGF family have a prominent role in post-MI angiogenesis through direct effect on endothelial cell survival and proliferation, control of new vessel formation and permeability as well as recruitment of inflammatory and regenerative cells. PDGF, platelet-derived growth factor; PlGF, placental growth factor. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Another member of the VEGF family of growth factor that preferentially binds to the VEGF Receptor 1 (VEGFR1), Placental Growth Factor (PlGF), also activates angiogenesis in ischemic tissues through two main mechanisms. First, PlGF-mediated activation of VEGFR1 leads to transphosphorylation of VEGFR2 and amplification of VEGF-dependent signaling (6). Second, PlGF promotes the recruitment of proangiogenic VEGFR1 expressing myelomonocytic cells (72) and Sca1+ progenitor cells (52) to ischemic tissues.
The role of VEGF-B in post-MI angiogenesis remains controversial. Although an initial report demonstrated a VEGFR1-dependent proangiogenic effect of VEGF-B in a murine model of hindlimb ischemia (103), subsequent studies have suggested that VEGF-B might induce angiogenesis specifically in the murine infarcted heart, but not in other tissues (66), in a VEGFR1- and neuropilin-1-dependent manner (58). Last, using transgenic mice and rats overexpressing VEGF-B in cardiomyocytes, Bry et al. finally suggested that VEGF-B only induced coronary artery growth, but not angiogenesis, in transgenic rat hearts, but not in transgenic mouse hearts (14). Besides these confusing results, a study by Zhang et al. interestingly demonstrated that VEGF-B was not required for vessel growth in mice, but was essential for their survival through the induction of various antiapoptotic signals in endothelial cells, but also in mural cells, such as pericytes and smooth muscle cells (126). Hence, one might speculate that although VEGF-B might not be necessary for angiogenesis per se, its prosurvival actions may allow newly formed vessels to persist and mature in the ischemic myocardium, providing long-term blood supply to cardiomyocytes and delaying adverse remodeling of the LV.
Finally, the VEGF-C isoform, although mainly known to promote lymphangiogenesis via its receptor VEGFR-3 (2), has also been shown to activate angiogenesis after ischemia (119) and might participate to post-MI angiogenesis. VEGF-C angiogenic effect might be indirect, as VEGF-C promotes PDGF-B expression and vessel maturation in ischemic tissues (85). Nevertheless, data indicate that VEGF-C binding to VEGFR-3 directly induces vascular sprouting in vivo (108) and promotes tumor angiogenesis (57). Still, the role of the VEGF-C/VEGFR-3 pathway in myocardial angiogenesis has yet to be properly evaluated.
FGF family of growth factors
Several members of the FGF family have the ability to promote angiogenesis, but the comprehension of the mechanisms mediating the angiogenic effects of FGF family growth factors is hindered by the substantial amount of FGF ligands (22 in mice and humans), the existence of four tyrosine kinase receptors and relative redundancy in the FGF system (79). First, acidic FGF (aFGF or FGF1) was identified as an endothelial cell mitogen expressed in bovine brain (79). Basic FGF (bFGF or FGF2) was then shown to be expressed by microvascular endothelial cells and to promote their proliferation and survival in a putatively autocrine manner (79). Although FGF2 seems to induce endothelial cell proliferation and angiogenesis in vivo, its effect might be at least partially indirect. Indeed, FGF-2 induces VEGF expression in endothelial cells, and VEGF neutralization blocks the proangiogenic effects of FGF-2 (79). FGF-2 can also induce expression of other proangiogenic cytokines, such as HGF or Monocyte Chemoattractant Protein (MCP-1/CCL2) (79). Several reports have demonstrated that FGF-1 (7, 94) or FGF-2 (9, 117) induced angiogenesis in the ischemic heart and promoted cardiac repair after MI.
Interestingly, in the mouse cornea, FGF-2-induced angiogenesis leads to the formation of stable and mature blood vessels, in contrast to VEGF-stimulated neovascularization, which results in disorganized and leaky microvessels (16). This might stem from the synergy between FGF-2 and PDGF-BB signaling, which improves blood vessel stability and maturation, presumably through induction of PDGF-BB receptors expression (15). Coadministration of FGF-2 and PDGF-BB leads to increased angiogenesis and vascular stability in a model of hindlimb ischemia (15) and in a porcine MI model, where it increased angiogenesis, perfusion of the ischemic myocardium, and subsequently improved cardiac function (70).
Other members of the FGF family promote postischemic angiogenesis. In a porcine model of MI, intracoronary gene transfer of FGF-5 promoted recovery of blood flow and cardiac function (39). Recently, a major role of FGF-9 in vessel maturation and stability has been evidenced (36). FGF-9 was shown to be upregulated in vascular smooth muscle cells during neovessel maturation, and to promote neovessel muscularization rather than endothelial proliferation and angiogenesis per se. Interestingly, delivery of FGF-9 in a hindlimb ischemia model stimulated the formation of perfused, multilayered, and long-lasting neovessels (36). Although the effect of FGF-9 has yet to be proven in preclinical models of MI, its properties make it an interesting target for proangiogenic therapies, alone or in combination with other factors targeting endothelial cells.
Nitric Oxide Synthases
The nitric oxide synthase (NOS) enzymes generate NO and are intimately involved in the angiogenic response to ischemia and hypoxia, in part through HIF1-α and VEGF-dependent pathways described above. Most of the evidence supporting these mechanisms comes from studies of peripheral limb ischemia, but the NOS enzymes also play a role in angiogenesis and ventricular remodeling after MI. In mice with deletion of eNOS (eNOS−/− mice), angiogenesis after MI is reduced, and the ability of statins, known to induce angiogenesis through eNOS-dependent mobilization of EPCs, is reduced in eNOS−/− mice (59). Moreover, treatment of rats with the transcriptional eNOS activator, AVE9488, after MI reduced infarct size, increased eNOS levels, and maintained circulating EPC numbers. The effects of AVE9488 were not seen in eNOS−/− mice compared with wild-type mice after MI (33). More recent evidence suggests that the NOS enzymes and their regulation are more widely involved in myocardial angiogenesis, beyond the well-described role of eNOS. In mouse hearts after MI, inducible NOS and neuronal NOS were both substantially upregulated. Nitrotyrosine formation after MI was reduced in inducible NOS−/− mice, and the ability of the NOS cofactor, tetrahydrobiopterin (BH4), to improve myocardial angiogenesis after MI required the presence of inducible NOS (100), suggesting that inducible NOS coupling is the most important determinant of the effect of BH4 in myocardial angiogenesis and remodeling after MI.
Inflammation
MI induces a rapid and massive influx of inflammatory cells into ischemic areas. These inflammatory cells mainly consist of innate immune cells, such as neutrophils and monocytes (MO) (80). Leukocytes infiltration acts as a double-edged sword, as it can promote beneficial processes, such as dead cell phagocytosis and angiogenesis, as well as induce tissue damage, cardiomyocyte death, and LV dilation (Fig. 4) (35). This is best exemplified by the role of leukocyte-derived proteases urokinase Plasminogen Activator and MMP-9 in the ischemic heart: deletion of urokinase Plasminogen Activator or MMP-9 protects mice subjected to MI from LV dilation and cardiac rupture, but impairs angiogenesis and functional recovery (47).
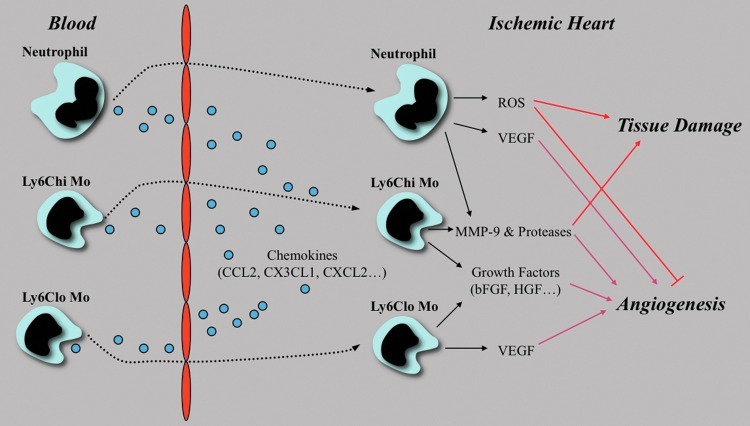
FIG. 4.
Multi-faceted role of innate immune cells in the ischemic heart. Ly6Chi and Ly6Clo monocytes (MO) infiltrate the ischemic heart sequentially through the control of chemokines, Ly6Chi monocytes being predominant in the early phase of myocardial healing, while Ly6Clo monocyte infiltration peaks at later time points. Although the respective role of these subsets in post-MI angiogenesis remains unclear, they promoted angiogenesis through growth factor and proteases releases. The role of neutrophils in post-MI angiogenesis is also ill defined. Neutrophils secrete high amounts of elastase, which induces endothelial cell apoptosis and inhibits the proangiogenic effect of progenitor cells Neutrophils are also a major source of reactive oxygen species (ROS), which, in high quantities, also induce endothelial cell apoptosis. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Historically, MO have first been proposed to promote arteriogenesis and collateral growth in ischemic tissues. Indeed, their presence around collateral vessels is associated with endothelial and mural cells proliferation (3), and the extent of collateral growth in rabbits with hindlimb ischemia is dependent on monocyte circulating levels (46). Nevertheless, MO can also promote angiogenesis through the secretion of various growth factors, such as FGF-2 and VEGF (45) or proteases, such as MMP-9 (53). In mice, monocyte comprises two mains subsets, Ly6Chi and Ly6Clo MO, which roughly correspond to human CD14+CD16- and CD14loCD16+ MO, respectively (37). Ly6Chi and Ly6Clo MO infiltrate the ischemic heart sequentially, Ly6Chi MO being predominant in the early phase of myocardial healing, while Ly6Clo monocyte infiltration peaks at later time points (80). The respective role of these subsets in post-MI angiogenesis remains unclear. Although Nahrendorf et al. proposed that Ly6Clo MO promoted angiogenesis through VEGF secretion, depletion of either subset in mice hampers neovascularization after MI (80). Moreover, several line of evidence suggest that Ly6Chi, but not Ly6Clo MO promote angiogenesis in hindlimb ischemia models (17, 22), presumably in a MMP-9 dependent manner (22). Hence, the role of mouse monocyte subsets in post-MI angiogenesis, as well as that of their human counterparts, needs to be clarified. Moreover, the impact of monocyte-derived VEGF, initially thought to mediate their proangiogenic effect, appears more complicated than expected. Indeed, monocyte-derived VEGF seems to favor unproductive angiogenesis, as myeloid cell-specific deletion of VEGF produces a stable and mature vascular network in tumors, thus reducing hypoxia in the surrounding tumor cells (106). The relevance of this mechanism has to be evaluated in the setting of post-MI angiogenesis.
The role of neutrophils in post-MI angiogenesis is also ill defined. Even though Granulocyte Colony Stimulating Factor-stimulated neutrophils produce VEGF to stimulate angiogenesis (84), neutrophils secrete high amounts of elastase, which induces endothelial cell apoptosis (120) and inhibits the proangiogenic effect of progenitor cells (51). Neutrophils are also a major source of reactive oxygen species, which, in high quantities, also induce endothelial cell apoptosis (102). Along this line, neutrophils are major mediators of microvascular dysfunction after MI, and notably promote ischemia/reperfusion injury and the no-reflow phenomenon (10, 121).
Adult Progenitor Cells
Endothelial progenitor cells
Discovery of putative circulating EPCs in adults in 1997 (4) has triggered a massive amount of research regarding EPCs biology and their therapeutic potential for ischemic diseases, and in particular MI, in the beginning of the past decade (27). EPCs mainly originate from the bone marrow, but extramedullary EPCs can also be recruited toward ischemic tissues (1). Consequently, whole bone marrow-derived mononuclear cells or medullar cell selected on different markers (CD34+, CXCR4+, Lin-ckit+…) have often been used as source of EPCs for preclinical studies of therapeutic cell therapy for angiogenesis. Although a substantial number of studies have demonstrated the proangiogenic and therapeutic effect of EPCs in experimental models of MI (27), the mechanisms of EPC-induced neovascularization and the cellular identity of EPCs remain poorly defined (27).
After the seminal discovery of Asahara, the first mechanism of EPC-induced angiogenesis to be proposed has been incorporation of EPCs into a newly formed vascular structure, a process referred to as postnatal vasculogenesis (4). Subsequent studies have shown that EPCs incorporation into neovessels was generally low, and it appears nowadays that the stem cell-like behavior of EPCs and postnatal vasculogenesis cannot solely account for their proangiogenic effects (27). Nevertheless, differentiation of bone marrow progenitors into cells of the vascular lineage is required for their long-term beneficial effect after MI, as elimination of bone marrow progenitors expressing an inducible suicide gene under the control of an endothelial or smooth muscle cells specific gene promoter late after transplantation reverses the original therapeutic benefit (123). The proangiogenic effect of EPCs might at least partially depend on paracrine signals, as EPCs express various proangiogenic cytokines, such as VEGF or Interleukin-8 (50), and injection of a culture medium conditioned by EPCs recapitulates the proangiogenic and therapeutic effects of cell transplantation (23). EPCs can also promote cardiac repair and post-MI angiogenesis by inducing recruitment of endogenous bone marrow-derived cells (BMCs) (21).
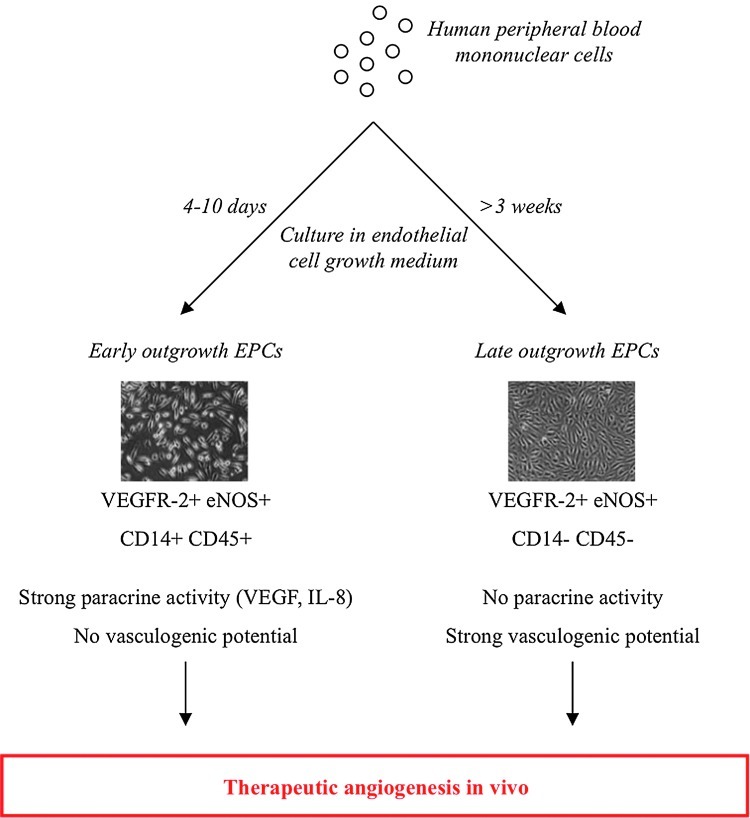
One of the major hurdles in EPCs research is the lack of consensus regarding their precise identity. Although efforts have recently been made to standardize the cell surface markers, isolation procedure and phenotypic properties that define bona-fide EPCs (27, 48), a large number of different EPCs, or EPC-like populations have been used in experimental or clinical studies, and hamper a comprehensive understanding of the existing literature. At least two types of EPCs with divergent properties can be obtained in vitro (50). Early EPCs possess a strong paracrine activity, but no vasculogenic potential, while Late EPCs have low paracrine activity, but can incorporate into newly formed vessels (50) (Fig. 5). Interestingly, both cell types can promote postischemic angiogenesis, and act synergistically when cotransplanted (122).
FIG. 5.
Two types of endothelial progenitor cells (EPCs) promote therapeutic angiogenesis with different mechanisms. From human peripheral blood mononuclear cells cultured in endothelial cell growth medium, spindle-shaped Early EPCs appear as soon as 4 days of culture, while cobblestone-shaped Late EPCs appear around 3 weeks. Early EPCs express hematopoietic markers abd possess strong proangiogenic paracrine, but not vasculogenic properties, while Late EPCs are devoid of hematopoietic markers, have a weak paracrine potential, but strong proliferative and vasculogenic ability. When transplanted in models of tissue ischemia, both cell types can promote therapeutic angiogenesis in vivo. Images adapted from Hur et al., (50). (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Resident cardiac progenitor cells
Although the myocardium is generally considered to possess poor regenerative ability, the description of multipotent resident cardiac progenitor cells (CPCs) isolated from human and mammalian adult hearts (63) has offered a new alternative for regenerative therapeutics for MI. Similarly to EPCs, consensus is lacking regarding the true identity of CPCs as several markers (Lin-Ckit+, sca1+, Isl+) or isolation procedures (direct isolation followed by expansion or isolation of CPCs arising from cardiospheres during in vitro culture of myocardial biopsies) have been used so far (63). Nevertheless, it is generally recognized that CPCs have the ability to differentiate in several cardiac lineages, including myocytes, vascular endothelial and smooth muscle cells in vitro and in vivo, and might thus promote neovascularization as well as cardiomyocyte repopulation of the injured myocardium. Although the therapeutic effect of CPCs in animal models of MI has been documented (11, 109), the mechanisms mediating their therapeutic effect, and mainly their ability to differentiate into cardiomyocytes or vascular cells in vivo is still controversial as preclinical studies have reported inconsistent results (73). The therapeutic benefit of CPCs transplantation might mostly rely on their paracrine activity, as intracoronary infusion of CPCs in a rat model of MI resulted in very low rates of exogenous cell incorporation, but activated endogenous CPCs to promote functional recovery (109).
Induced pluripotent stem cells
A potential breakthrough in regenerative medicine has emerged from the discovery that adult somatic stem cells, such as skin fibroblasts could be reprogrammed to a pluripotent stem cell state by as few as 4 distinct factors (107). These cells, termed induced Pluripotent Stem cells (iPS), were subsequently shown to possess the ability to differentiate in cardiac lineage cells in vitro, including cardiomyocytes, vascular endothelial, and mural cells (81). iPS were notably able to promote repair of ischemic myocardium after infarction, where they differentiated into cardiomyocytes, endothelial cells, and smooth muscle cells (82). Endothelial cells differentiated in vitro from iPS were also shown to promote angiogenesis in a hindlimb ischemia model (93). Although iPS obviously represent a potentially valuable tool for cell therapy after MI, as they would provide an autologous source of cells with multilineage differentiation capacity, further research is warranted before therapeutic clinical use can be considered. One important concern is the ability of iPS to form teratomas in vivo. Moreover, patient-specific generation of iPS is a timely process, which might not compute with the requirement for acute therapy after an ischemic event (83).
Other endogenous adult progenitor cell types, and in particular Mesenchymal Stem Cells and Adipose Tissue-Derived Stem Cells (89) have been endowed with vasculogenic or at least proangiogenic potential and have been successfully used to promote post-MI angiogenesis in experimental studies (56).
miRNA
Micro RNAs (miR) are short RNA molecules that regulate gene expression at the post-transcriptional level. In the past few years, they have emerged has prominent actors in cardiovascular biology, and particularly in post-MI angiogenesis (61). miR-92 thus regulates post-MI angiogenesis through modulation of integrin-α5 expression in endothelial cells (13). Human miR-424 and its murine homologue miR-322 are induced by hypoxia and indirectly favor HIF-1α stabilization, hence promoting postischemic angiogenesis (38), and miR-100 can inhibit angiogenesis by repressing mammalian Target of Rapamycin expression in ischemic tissues (41). Several other miR, such as miR-126 (115), miR-503 (18), or miR-24 (30) regulate postischemic angiogenesis, highlighting the importance of these molecules in the neovascularization process after MI. Targeting miRs for therapeutic angiogenesis after MI could represent a relevant alternative, as miR supplementation or inhibition is technically feasible.
Clinical applications of therapeutic angiogenesis for MI
Exciting preclinical studies evaluating proangiogenic therapies for MI have prompted the initiation of numerous clinical trials (42). Several phase I and a few phase II studies using protein or gene transfer delivery of FGF or VEGF family growth factors have, so far, failed to demonstrate any substantial benefit on LV function in MI patients (42). After the discovery of EPCs and a tremendous amount of experimental evidence pointing toward EPCs or BMCs therapeutic potential for the treatment of MI (68), clinical trials evaluating the benefit of intracoronary administration of BMCs for therapeutic angiogenesis have been conducted in patients with acute MI (5, 71, 77, 95). Although Schachinger et al. demonstrated that intracoronary BMCs delivery early after MI (3 to 7 days) improved cardiac function at 4 months and reduced death, recurrence of MI and need for a revascularization procedure at 1 year (95), a simultaneously published trial did not show any benefit of early BMCs transplantation at 6 months of follow-up (71). Moreover, the BOOST trial evidenced a benefit of BMCs transplantation after 6 months that was lost at 18 months follow-up (77). Recently, intracoronary transplantation of BMCs 2 to 3 weeks after MI did not produce any functional benefits (113). Nevertheless, a 2008 meta-analysis of 13 trials with a total of more than 800 patients has shown that BMCs transplantation led to a modest (+2.99%), but significant increase in LV ejection fraction in patients receiving BMCs when compared to placebo (75).
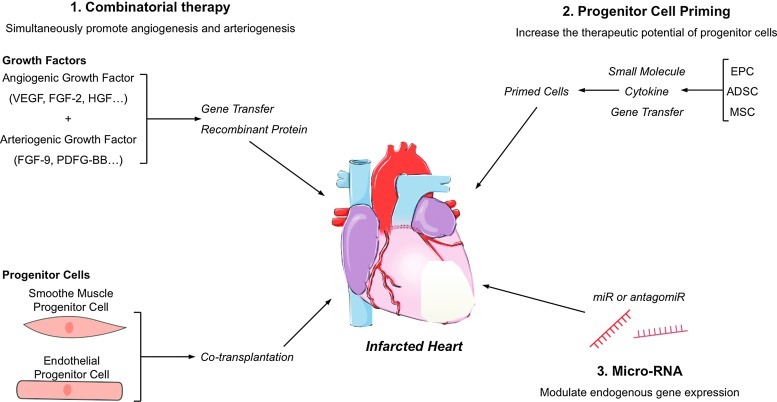
Nonetheless, clinical results of proangiogenic therapies remain globally disappointing. Even though methodological caveats, such as way of delivery for angiogenic growth factors (e.g., protein vs. gene transfer) (42) or isolation procedure for BMCs (98) might partially explain the failure of such trials, it appears that delivery of a single growth factor or cell type does not support angiogenesis sufficiently to promote cardiac repair. Along this line, several experimental reports have proposed alternatives to optimize proangiogenic therapies, and strategies aiming at simultaneously promoting angiogenesis and vessel maturation appear promising (Fig. 6). As previously mentioned in this review, combined administration of an angiogenic and an arteriogenic growth factor, namely FGF-2 and PDGF-BB, synergistically enhances angiogenesis in the ischemic heart (70), but this concept is also relevant to cell-based therapy. Hence, coadministration of EPCs and smooth muscle progenitor cells is superior to EPCs alone for therapeutic angiogenesis, and produces a mature vascular network in ischemic tissues (31). Ex vivo progenitor cell priming with various agents could also increase their proangiogenic and therapeutic potential (32, 125) (Fig. 6).
FIG. 6.
Future avenues for therapeutic angiogenesis. [1]. Combinatorial therapy with a proangiogenic and a proarteriogenic factor, or cotransplantation of smooth muscle cell and endothelial cell progenitors could promote the formation of a functional and sustainable microvascular network. [2]. Enhancing the endogenous proangiogenic potential of adult progenitor cell through small molecule or cytokine priming, or in vitro gene transfer, could increase their therapeutic potential. [3]. Specific microRNA supplementation or inhibition through antagomiR could modulate gene expression to activate endogenous pathways of angiogenesis. ADSC, adipose tissue-derived stem cell; MSC, mesenchymal stem cell. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Recently, the therapeutic use of CPCs in MI patients has been evaluated in two phase 1 trials (12, 74). Although the primary end point of these studies was to assess the safety of CPCs transplantation, both reported encouraging results. Indeed, the SCIPIO trial reported increased LV ejection fraction in the CPCs treated patients 1 year after cell infusion, while in the CADUCEUS study, CPCs delivery led to reduced scar mass, increased viable heart mass, regional contractility and regional systolic wall thickening without significantly improving ejection fraction.
Cardiovascular Risk Factors Limit Endogenous and Therapeutic Angiogenesis
More than being just causes for coronary artery disease and the onset of MI, cardiovascular risk factors, such as hypertension (28), diabetes (105), or hyperlipidemia profoundly impair endogenous and therapeutically induced post-MI angiogenesis, and represent a major difficulty to circumvent in proangiogenic therapy (60). Along this line, several reports have highlighted bone marrow progenitor cell deficiency in diabetic (110), hypertensive (124), or dyslipidemic (44) patients, and might partially explain the disappointing results of proangiogenic BMC therapy for patients with acute MI.
Innovation
Proangiogenic therapy appeared a promising strategy for the treatment of patients with acute myocardial infarction (MI). Exciting preclinical studies have prompted the initiation of numerous clinical trials based on growth factors delivery and administration of stem/progenitor cells. Nonetheless, these clinical trials showed mixed results paving the way for the optimization of proangiogenic therapies, including stimulation of both angiogenesis and vessel maturation and/or the use of additional sources of stem/progenitor cells. Experimental unraveling of the mechanisms of angiogenesis, vessel maturation, and endothelial cell/cardiomyocyte cross talk in the ischemic heart would undoubtedly increase the efficiency of angiogenesis targeting therapeutics for the treatment of acute MI.
Concluding Remarks
Despite the relative failure of proangiogenic therapies for MI patients so far, targeting angiogenesis to prevent heart failure after MI still appears as a promising strategy, but further mechanistic insight are required to optimize such therapy. Experimental unraveling of the mechanisms of angiogenesis, vessel maturation, and endothelial cell/cardiomyocyte cross talk in the ischemic heart, analysis of emerging pathways, such as miRNA, as well as a better understanding of how cardiovascular risk factors impact endogenous and therapeutically stimulated angiogenesis, would undoubtedly pave the way for the development of novel and hopefully efficient angiogenesis targeting therapeutics for the treatment of acute MI.
Abbreviations Used
- ADSC
adipose tissue-derived stem cell
- Asn
asparagine
- BMC
bone marrow-derived cells
- CM
cardiomyocyte
- CPCs
cardiac progenitor cells
- eNOS
endothelial nitric oxide synthase
- EPC
endothelial progenitor cells
- FGF
fibroblast growth factor
- FIH
factor inhibiting HIF
- HGF
hepatocyte growth factor
- HIF
hypoxia inducible factor
- HRE
hypoxia-responsive elements
- iPS
induced pluripotent stem cells
- Lama4
laminin-alpha4 chain
- LV
left ventricle
- MCP-1
monocyte chemoattractant protein-1
- MI
myocardial infarction
- miR
micro RNAs
- MMP
metalloproteinase
- MSC
mesenchymal stem cell
- MO
monocytes
- NO
nitric oxide
- NOS
nitric oxide synthase
- OH
hydroxylated
- p300
p300/CREB binding protein
- PDGF
platelet-derived growth factor
- PHD
prolyl hydroxylase domain proteins
- PlGF
placental growth factor
- Pro
proline
- ROS
reactive oxygen species
- SDF-1
stromal cell-derived factor 1
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
- VHL
von Hippel-Lindau protein
Acknowledgments
J-S.S is supported by grants from Fondation de la Recherche Médicale, ANR Chemrepair (2010 BLAN 1127 02). J-S.S. is a recipient of a Contrat d'Interface from Assistance Publique-Hôpitaux de Paris. J-S.S, C.C, K.C are supported by fondation Leducq transatlantic network (09-CVD-01).
References
- 1.Aicher A. Rentsch M. Sasaki K. Ellwart JW. Fandrich F. Siebert R. Cooke JP. Dimmeler S. Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007;100:581–589. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- 2.Alitalo K. Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 3.Arras M. Ito WD. Scholz D. Winkler B. Schaper J. Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T. Witzenbichler B. Schatteman G. Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 5.Assmus B. Schachinger V. Teupe C. Britten M. Lehmann R. Dobert N. Grunwald F. Aicher A. Urbich C. Martin H. Hoelzer D. Dimmeler S. Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 6.Autiero M. Waltenberger J. Communi D. Kranz A. Moons L. Lambrechts D. Kroll J. Plaisance S. De Mol M. Bono F. Kliche S. Fellbrich G. Ballmer-Hofer K. Maglione D. Mayr-Beyrle U. Dewerchin M. Dombrowski S. Stanimirovic D. Van Hummelen P. Dehio C. Hicklin DJ. Persico G. Herbert JM. Shibuya M. Collen D. Conway EM. Carmeliet P. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 7.Banai S. Jaklitsch MT. Casscells W. Shou M. Shrivastav S. Correa R. Epstein SE. Unger EF. Effects of acidic fibroblast growth factor on normal and ischemic myocardium. Circ Res. 1991;69:76–85. doi: 10.1161/01.res.69.1.76. [DOI] [PubMed] [Google Scholar]
- 8.Bao W. Qin P. Needle S. Erickson-Miller CL. Duffy KJ. Ariazi JL. Zhao S. Olzinski AR. Behm DJ. Pipes GC. Jucker BM. Hu E. Lepore JJ. Willette RN. Chronic inhibition of hypoxia-inducible factor prolyl 4-hydroxylase improves ventricular performance, remodeling, and vascularity after myocardial infarction in the rat. J Cardiovasc Pharmacol. 2010;56:147–155. doi: 10.1097/FJC.0b013e3181e2bfef. [DOI] [PubMed] [Google Scholar]
- 9.Battler A. Scheinowitz M. Bor A. Hasdai D. Vered Z. Di Segni E. Varda-Bloom N. Nass D. Engelberg S. Eldar M, et al. Intracoronary injection of basic fibroblast growth factor enhances angiogenesis in infarcted swine myocardium. J Am Coll Cardiol. 1993;22:2001–2006. doi: 10.1016/0735-1097(93)90790-8. [DOI] [PubMed] [Google Scholar]
- 10.Bekkers SC. Yazdani SK. Virmani R. Waltenberger J. Microvascular obstruction: underlying pathophysiology and clinical diagnosis. J Am Coll Cardiol. 2010;55:1649–1660. doi: 10.1016/j.jacc.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 11.Beltrami AP. Barlucchi L. Torella D. Baker M. Limana F. Chimenti S. Kasahara H. Rota M. Musso E. Urbanek K. Leri A. Kajstura J. Nadal-Ginard B. Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 12.Bolli R. Chugh AR. D'Amario D. Loughran JH. Stoddard MF. Ikram S. Beache GM. Wagner SG. Leri A. Hosoda T. Sanada F. Elmore JB. Goichberg P. Cappetta D. Solankhi NK. Fahsah I. Rokosh DG. Slaughter MS. Kajstura J. Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Bonauer A. Carmona G. Iwasaki M. Mione M. Koyanagi M. Fischer A. Burchfield J. Fox H. Doebele C. Ohtani K. Chavakis E. Potente M. Tjwa M. Urbich C. Zeiher AM. Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 14.Bry M. Kivela R. Holopainen T. Anisimov A. Tammela T. Soronen J. Silvola J. Saraste A. Jeltsch M. Korpisalo P. Carmeliet P. Lemstrom KB. Shibuya M. Yla-Herttuala S. Alhonen L. Mervaala E. Andersson LC. Knuuti J. Alitalo K. Vascular endothelial growth factor-B acts as a coronary growth factor in transgenic rats without inducing angiogenesis, vascular leak, or inflammation. Circulation. 2010;122:1725–1733. doi: 10.1161/CIRCULATIONAHA.110.957332. [DOI] [PubMed] [Google Scholar]
- 15.Cao R. Brakenhielm E. Pawliuk R. Wariaro D. Post MJ. Wahlberg E. Leboulch P. Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 16.Cao R. Eriksson A. Kubo H. Alitalo K. Cao Y. Thyberg J. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ Res. 2004;94:664–670. doi: 10.1161/01.RES.0000118600.91698.BB. [DOI] [PubMed] [Google Scholar]
- 17.Capoccia BJ. Gregory AD. Link DC. Recruitment of the inflammatory subset of monocytes to sites of ischemia induces angiogenesis in a monocyte chemoattractant protein-1-dependent fashion. J Leukoc Biol. 2008;84:760–768. doi: 10.1189/jlb.1107756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caporali A. Meloni M. Vollenkle C. Bonci D. Sala-Newby GB. Addis R. Spinetti G. Losa S. Masson R. Baker AH. Agami R. le Sage C. Condorelli G. Madeddu P. Martelli F. Emanueli C. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation. 2011;123:282–291. doi: 10.1161/CIRCULATIONAHA.110.952325. [DOI] [PubMed] [Google Scholar]
- 19.Carmeliet P. Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceradini DJ. Kulkarni AR. Callaghan MJ. Tepper OM. Bastidas N. Kleinman ME. Capla JM. Galiano RD. Levine JP. Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 21.Cho HJ. Lee N. Lee JY. Choi YJ. Ii M. Wecker A. Jeong JO. Curry C. Qin G. Yoon YS. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochain C. Rodero MP. Vilar J. Recalde A. Richart AL. Loinard C. Zouggari Y. Guerin C. Duriez M. Combadiere B. Poupel L. Levy BI. Mallat Z. Combadiere C. Silvestre JS. Regulation of monocyte subset systemic levels by distinct chemokine receptors controls post-ischaemic neovascularization. Cardiovasc Res. 2010;88:186–195. doi: 10.1093/cvr/cvq153. [DOI] [PubMed] [Google Scholar]
- 23.Di Santo S. Yang Z. Wyler von Ballmoos M. Voelzmann J. Diehm N. Baumgartner I. Kalka C. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One. 2009;4:e5643. doi: 10.1371/journal.pone.0005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding BS. Nolan DJ. Butler JM. James D. Babazadeh AO. Rosenwaks Z. Mittal V. Kobayashi H. Shido K. Lyden D. Sato TN. Rabbany SY. Rafii S. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding BS. Nolan DJ. Guo P. Babazadeh AO. Cao Z. Rosenwaks Z. Crystal RG. Simons M. Sato TN. Worgall S. Shido K. Rabbany SY. Rafii S. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobrucki LW. Tsutsumi Y. Kalinowski L. Dean J. Gavin M. Sen S. Mendizabal M. Sinusas AJ. Aikawa R. Analysis of angiogenesis induced by local IGF-1 expression after myocardial infarction using microSPECT-CT imaging. J Mol Cell Cardiol. 2010;48:1071–1079. doi: 10.1016/j.yjmcc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fadini GP. Losordo D. Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feihl F. Liaudet L. Levy BI. Waeber B. Hypertension and microvascular remodelling. Cardiovasc Res. 2008;78:274–285. doi: 10.1093/cvr/cvn022. [DOI] [PubMed] [Google Scholar]
- 29.Ferrara N. Gerber HP. LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 30.Fiedler J. Jazbutyte V. Kirchmaier BC. Gupta SK. Lorenzen J. Hartmann D. Galuppo P. Kneitz S. Pena JT. Sohn-Lee C. Loyer X. Soutschek J. Brand T. Tuschl T. Heineke J. Martin U. Schulte-Merker S. Ertl G. Engelhardt S. Bauersachs J. Thum T. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124:720–730. doi: 10.1161/CIRCULATIONAHA.111.039008. [DOI] [PubMed] [Google Scholar]
- 31.Foubert P. Matrone G. Souttou B. Lere-Dean C. Barateau V. Plouet J. Le Ricousse-Roussanne S. Levy BI. Silvestre JS. Tobelem G. Coadministration of endothelial and smooth muscle progenitor cells enhances the efficiency of proangiogenic cell-based therapy. Circ Res. 2008;103:751–760. doi: 10.1161/CIRCRESAHA.108.175083. [DOI] [PubMed] [Google Scholar]
- 32.Foubert P. Silvestre JS. Souttou B. Barateau V. Martin C. Ebrahimian TG. Lere-Dean C. Contreres JO. Sulpice E. Levy BI. Plouet J. Tobelem G. Le Ricousse-Roussanne S. PSGL-1-mediated activation of EphB4 increases the proangiogenic potential of endothelial progenitor cells. J Clin Invest. 2007;117:1527–1537. doi: 10.1172/JCI28338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fraccarollo D. Widder JD. Galuppo P. Thum T. Tsikas D. Hoffmann M. Ruetten H. Ertl G. Bauersachs J. Improvement in left ventricular remodeling by the endothelial nitric oxide synthase enhancer AVE9488 after experimental myocardial infarction. Circulation. 2008;118:818–827. doi: 10.1161/CIRCULATIONAHA.107.717702. [DOI] [PubMed] [Google Scholar]
- 34.Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006;8:1907–1939. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- 35.Frangogiannis NG. Smith CW. Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 36.Frontini MJ. Nong Z. Gros R. Drangova M. O'Neil C. Rahman MN. Akawi O. Yin H. Ellis CG. Pickering JG. Fibroblast growth factor 9 delivery during angiogenesis produces durable, vasoresponsive microvessels wrapped by smooth muscle cells. Nat Biotechnol. 2011;29:421–427. doi: 10.1038/nbt.1845. [DOI] [PubMed] [Google Scholar]
- 37.Geissmann F. Jung S. Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh G. Subramanian IV. Adhikari N. Zhang X. Joshi HP. Basi D. Chandrashekhar YS. Hall JL. Roy S. Zeng Y. Ramakrishnan S. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-alpha isoforms and promotes angiogenesis. J Clin Invest. 2010;120:4141–4154. doi: 10.1172/JCI42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giordano FJ. Ping P. McKirnan MD. Nozaki S. DeMaria AN. Dillmann WH. Mathieu-Costello O. Hammond HK. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nat Med. 1996;2:534–539. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- 40.Greenberg JI. Shields DJ. Barillas SG. Acevedo LM. Murphy E. Huang J. Scheppke L. Stockmann C. Johnson RS. Angle N. Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grundmann S. Hans FP. Kinniry S. Heinke J. Helbing T. Bluhm F. Sluijter JP. Hoefer I. Pasterkamp G. Bode C. Moser M. MicroRNA-100 regulates neovascularization by suppression of mammalian target of rapamycin in endothelial and vascular smooth muscle cells. Circulation. 2011;123:999–1009. doi: 10.1161/CIRCULATIONAHA.110.000323. [DOI] [PubMed] [Google Scholar]
- 42.Haider H. Akbar SA. Ashraf M. Angiomyogenesis for myocardial repair. Antioxid Redox Signal. 2009;11:1929–1944. doi: 10.1089/ars.2009.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashimoto E. Ogita T. Nakaoka T. Matsuoka R. Takao A. Kira Y. Rapid induction of vascular endothelial growth factor expression by transient ischemia in rat heart. Am J Physiol. 1994;267:H1948–H1954. doi: 10.1152/ajpheart.1994.267.5.H1948. [DOI] [PubMed] [Google Scholar]
- 44.Heeschen C. Lehmann R. Honold J. Assmus B. Aicher A. Walter DH. Martin H. Zeiher AM. Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 45.Heil M. Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis) Circ Res. 2004;95:449–458. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 46.Heil M. Ziegelhoeffer T. Pipp F. Kostin S. Martin S. Clauss M. Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002;283:H2411–H2419. doi: 10.1152/ajpheart.01098.2001. [DOI] [PubMed] [Google Scholar]
- 47.Heymans S. Luttun A. Nuyens D. Theilmeier G. Creemers E. Moons L. Dyspersin GD. Cleutjens JP. Shipley M. Angellilo A. Levi M. Nube O. Baker A. Keshet E. Lupu F. Herbert JM. Smits JF. Shapiro SD. Baes M. Borgers M. Collen D. Daemen MJ. Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–1142. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 48.Hirschi KK. Ingram DA. Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang M. Nguyen P. Jia F. Hu S. Gong Y. de Almeida PE. Wang L. Nag D. Kay MA. Giaccia AJ. Robbins RC. Wu JC. Double knockdown of prolyl hydroxylase and factor-inhibiting hypoxia-inducible factor with nonviral minicircle gene therapy enhances stem cell mobilization and angiogenesis after myocardial infarction. Circulation. 2011;124:S46–S54. doi: 10.1161/CIRCULATIONAHA.110.014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hur J. Yoon CH. Kim HS. Choi JH. Kang HJ. Hwang KK. Oh BH. Lee MM. Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 51.Iba O. Matsubara H. Nozawa Y. Fujiyama S. Amano K. Mori Y. Kojima H. Iwasaka T. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002;106:2019–2025. doi: 10.1161/01.cir.0000031332.45480.79. [DOI] [PubMed] [Google Scholar]
- 52.Iwasaki H. Kawamoto A. Tjwa M. Horii M. Hayashi S. Oyamada A. Matsumoto T. Suehiro S. Carmeliet P. Asahara T. PlGF repairs myocardial ischemia through mechanisms of angiogenesis, cardioprotection and recruitment of myo-angiogenic competent marrow progenitors. PLoS One. 2011;6:e24872. doi: 10.1371/journal.pone.0024872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson C. Sung HJ. Lessner SM. Fini ME. Galis ZS. Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues: potential role in capillary branching. Circ Res. 2004;94:262–268. doi: 10.1161/01.RES.0000111527.42357.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jurgensen JS. Rosenberger C. Wiesener MS. Warnecke C. Horstrup JH. Grafe M. Philipp S. Griethe W. Maxwell PH. Frei U. Bachmann S. Willenbrock R. Eckardt KU. Persistent induction of HIF-1alpha and -2alpha in cardiomyocytes and stromal cells of ischemic myocardium. FASEB J. 2004;18:1415–1417. doi: 10.1096/fj.04-1605fje. [DOI] [PubMed] [Google Scholar]
- 55.Kido M. Du L. Sullivan CC. Li X. Deutsch R. Jamieson SW. Thistlethwaite PA. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol. 2005;46:2116–2124. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 56.Kumar AH. Caplice NM. Clinical potential of adult vascular progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:1080–1087. doi: 10.1161/ATVBAHA.109.198895. [DOI] [PubMed] [Google Scholar]
- 57.Laakkonen P. Waltari M. Holopainen T. Takahashi T. Pytowski B. Steiner P. Hicklin D. Persaud K. Tonra JR. Witte L. Alitalo K. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007;67:593–599. doi: 10.1158/0008-5472.CAN-06-3567. [DOI] [PubMed] [Google Scholar]
- 58.Lahteenvuo JE. Lahteenvuo MT. Kivela A. Rosenlew C. Falkevall A. Klar J. Heikura T. Rissanen TT. Vahakangas E. Korpisalo P. Enholm B. Carmeliet P. Alitalo K. Eriksson U. Yla-Herttuala S. Vascular endothelial growth factor-B induces myocardium-specific angiogenesis and arteriogenesis via vascular endothelial growth factor receptor-1- and neuropilin receptor-1-dependent mechanisms. Circulation. 2009;119:845–856. doi: 10.1161/CIRCULATIONAHA.108.816454. [DOI] [PubMed] [Google Scholar]
- 59.Landmesser U. Engberding N. Bahlmann FH. Schaefer A. Wiencke A. Heineke A. Spiekermann S. Hilfiker-Kleiner D. Templin C. Kotlarz D. Mueller M. Fuchs M. Hornig B. Haller H. Drexler H. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 60.Lassaletta AD. Chu LM. Sellke FW. Therapeutic neovascularization for coronary disease: current state and future prospects. Basic Res Cardiol. 2011;106:897–909. doi: 10.1007/s00395-011-0200-1. [DOI] [PubMed] [Google Scholar]
- 61.Latronico MV. Condorelli G. Therapeutic use of microRNAs in myocardial diseases. Curr Heart Fail Rep. 2011;8:193–197. doi: 10.1007/s11897-011-0068-2. [DOI] [PubMed] [Google Scholar]
- 62.Lee SH. Wolf PL. Escudero R. Deutsch R. Jamieson SW. Thistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342:626–633. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- 63.Leri A. Kajstura J. Anversa P. Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res. 2011;109:941–961. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Leucker TM. Bienengraeber M. Muravyeva M. Baotic I. Weihrauch D. Brzezinska AK. Warltier DC. Kersten JR. Pratt PF., Jr. Endothelial-cardiomyocyte crosstalk enhances pharmacological cardioprotection. J Mol Cell Cardiol. 2011;51:803–811. doi: 10.1016/j.yjmcc.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J. Hampton T. Morgan JP. Simons M. Stretch-induced VEGF expression in the heart. J Clin Invest. 1997;100:18–24. doi: 10.1172/JCI119510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X. Tjwa M. Van Hove I. Enholm B. Neven E. Paavonen K. Jeltsch M. Juan TD. Sievers RE. Chorianopoulos E. Wada H. Vanwildemeersch M. Noel A. Foidart JM. Springer ML. von Degenfeld G. Dewerchin M. Blau HM. Alitalo K. Eriksson U. Carmeliet P. Moons L. Reevaluation of the role of VEGF-B suggests a restricted role in the revascularization of the ischemic myocardium. Arterioscler Thromb Vasc Biol. 2008;28:1614–1620. doi: 10.1161/ATVBAHA.107.158725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loinard C. Ginouves A. Vilar J. Cochain C. Zouggari Y. Recalde A. Duriez M. Levy BI. Pouyssegur J. Berra E. Silvestre JS. Inhibition of prolyl hydroxylase domain proteins promotes therapeutic revascularization. Circulation. 2009;120:50–59. doi: 10.1161/CIRCULATIONAHA.108.813303. [DOI] [PubMed] [Google Scholar]
- 68.Losordo DW. Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 69.Losordo DW. Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation. 2004;109:2487–2491. doi: 10.1161/01.CIR.0000128595.79378.FA. [DOI] [PubMed] [Google Scholar]
- 70.Lu H. Xu X. Zhang M. Cao R. Brakenhielm E. Li C. Lin H. Yao G. Sun H. Qi L. Tang M. Dai H. Zhang Y. Su R. Bi Y. Cao Y. Combinatorial protein therapy of angiogenic and arteriogenic factors remarkably improves collaterogenesis and cardiac function in pigs. Proc Natl Acad Sci U S A. 2007;104:12140–12145. doi: 10.1073/pnas.0704966104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lunde K. Solheim S. Aakhus S. Arnesen H. Abdelnoor M. Egeland T. Endresen K. Ilebekk A. Mangschau A. Fjeld JG. Smith HJ. Taraldsrud E. Grogaard HK. Bjornerheim R. Brekke M. Muller C. Hopp E. Ragnarsson A. Brinchmann JE. Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 72.Luttun A. Tjwa M. Moons L. Wu Y. Angelillo-Scherrer A. Liao F. Nagy JA. Hooper A. Priller J. De Klerck B. Compernolle V. Daci E. Bohlen P. Dewerchin M. Herbert JM. Fava R. Matthys P. Carmeliet G. Collen D. Dvorak HF. Hicklin DJ. Carmeliet P. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 73.Lyngbaek S. Schneider M. Hansen JL. Sheikh SP. Cardiac regeneration by resident stem and progenitor cells in the adult heart. Basic Res Cardiol. 2007;102:101–114. doi: 10.1007/s00395-007-0638-3. [DOI] [PubMed] [Google Scholar]
- 74.Makkar RR. Smith RR. Cheng K. Malliaras K. Thomson LE. Berman D. Czer LS. Marban L. Mendizabal A. Johnston PV. Russell SD. Schuleri KH. Lardo AC. Gerstenblith G. Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin-Rendon E. Brunskill SJ. Hyde CJ. Stanworth SJ. Mathur A. Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 76.Meloni M. Caporali A. Graiani G. Lagrasta C. Katare R. Van Linthout S. Spillmann F. Campesi I. Madeddu P. Quaini F. Emanueli C. Nerve growth factor promotes cardiac repair following myocardial infarction. Circ Res. 2010;106:1275–1284. doi: 10.1161/CIRCRESAHA.109.210088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meyer GP. Wollert KC. Lotz J. Steffens J. Lippolt P. Fichtner S. Hecker H. Schaefer A. Arseniev L. Hertenstein B. Ganser A. Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 78.Miyagawa S. Sawa Y. Taketani S. Kawaguchi N. Nakamura T. Matsuura N. Matsuda H. Myocardial regeneration therapy for heart failure: hepatocyte growth factor enhances the effect of cellular cardiomyoplasty. Circulation. 2002;105:2556–2561. doi: 10.1161/01.cir.0000016722.37138.f2. [DOI] [PubMed] [Google Scholar]
- 79.Murakami M. Simons M. Fibroblast growth factor regulation of neovascularization. Curr Opin Hematol. 2008;15:215–220. doi: 10.1097/MOH.0b013e3282f97d98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nahrendorf M. Swirski FK. Aikawa E. Stangenberg L. Wurdinger T. Figueiredo JL. Libby P. Weissleder R. Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Narazaki G. Uosaki H. Teranishi M. Okita K. Kim B. Matsuoka S. Yamanaka S. Yamashita JK. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 82.Nelson TJ. Martinez-Fernandez A. Yamada S. Perez-Terzic C. Ikeda Y. Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oh Y. Wei H. Ma D. Sun X. Liew R. Clinical applications of patient-specific induced pluripotent stem cells in cardiovascular medicine. Heart. 2012;98:443–449. doi: 10.1136/heartjnl-2011-301317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohki Y. Heissig B. Sato Y. Akiyama H. Zhu Z. Hicklin DJ. Shimada K. Ogawa H. Daida H. Hattori K. Ohsaka A. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. Faseb J. 2005;19:2005–2007. doi: 10.1096/fj.04-3496fje. [DOI] [PubMed] [Google Scholar]
- 85.Onimaru M. Yonemitsu Y. Fujii T. Tanii M. Nakano T. Nakagawa K. Kohno R. Hasegawa M. Nishikawa S. Sueishi K. VEGF-C regulates lymphangiogenesis and capillary stability by regulation of PDGF-B. Am J Physiol Heart Circ Physiol. 2009;297:H1685–H1696. doi: 10.1152/ajpheart.00015.2009. [DOI] [PubMed] [Google Scholar]
- 86.Ono K. Matsumori A. Shioi T. Furukawa Y. Sasayama S. Enhanced expression of hepatocyte growth factor/c-Met by myocardial ischemia and reperfusion in a rat model. Circulation. 1997;95:2552–2558. doi: 10.1161/01.cir.95.11.2552. [DOI] [PubMed] [Google Scholar]
- 87.Patten IS. Rana S. Shahul S. Rowe GC. Jang C. Liu L. Hacker MR. Rhee JS. Mitchell J. Mahmood F. Hess P. Farrell C. Koulisis N. Khankin EV. Burke SD. Tudorache I. Bauersachs J. del Monte F. Hilfiker-Kleiner D. Karumanchi SA. Arany Z. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333–338. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pearlman JD. Hibberd MG. Chuang ML. Harada K. Lopez JJ. Gladstone SR. Friedman M. Sellke FW. Simons M. Magnetic resonance mapping demonstrates benefits of VEGF-induced myocardial angiogenesis. Nat Med. 1995;1:1085–1089. doi: 10.1038/nm1095-1085. [DOI] [PubMed] [Google Scholar]
- 89.Planat-Benard V. Silvestre JS. Cousin B. Andre M. Nibbelink M. Tamarat R. Clergue M. Manneville C. Saillan-Barreau C. Duriez M. Tedgui A. Levy B. Penicaud L. Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 90.Potente M. Gerhardt H. Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 91.Pugh CW. Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 92.Qing G. Simon MC. Hypoxia inducible factor-2alpha: a critical mediator of aggressive tumor phenotypes. Curr Opin Genet Dev. 2009;19:60–66. doi: 10.1016/j.gde.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rufaihah AJ. Huang NF. Jame S. Lee JC. Nguyen HN. Byers B. De A. Okogbaa J. Rollins M. Reijo-Pera R. Gambhir SS. Cooke JP. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2011;31:e72–e79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Safi J., Jr. DiPaula AF., Jr. Riccioni T. Kajstura J. Ambrosio G. Becker LC. Anversa P. Capogrossi MC. Adenovirus-mediated acidic fibroblast growth factor gene transfer induces angiogenesis in the nonischemic rabbit heart. Microvasc Res. 1999;58:238–249. doi: 10.1006/mvre.1999.2165. [DOI] [PubMed] [Google Scholar]
- 95.Schachinger V. Erbs S. Elsasser A. Haberbosch W. Hambrecht R. Holschermann H. Yu J. Corti R. Mathey DG. Hamm CW. Suselbeck T. Assmus B. Tonn T. Dimmeler S. Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 96.Schirmer SH. van Nooijen FC. Piek JJ. van Royen N. Stimulation of collateral artery growth: travelling further down the road to clinical application. Heart. 2009;95:191–197. doi: 10.1136/hrt.2007.136119. [DOI] [PubMed] [Google Scholar]
- 97.Schwarz ER. Speakman MT. Patterson M. Hale SS. Isner JM. Kedes LH. Kloner RA. Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat—angiogenesis and angioma formation. J Am Coll Cardiol. 2000;35:1323–1330. doi: 10.1016/s0735-1097(00)00522-2. [DOI] [PubMed] [Google Scholar]
- 98.Seeger FH. Tonn T. Krzossok N. Zeiher AM. Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 99.Shah AM. Mann DL. In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet. 2011;378:704–712. doi: 10.1016/S0140-6736(11)60894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shimazu T. Otani H. Yoshioka K. Fujita M. Okazaki T. Iwasaka T. Sepiapterin enhances angiogenesis and functional recovery in mice after myocardial infarction. Am J Physiol Heart Circ Physiol. 2011;301:H2061–H2072. doi: 10.1152/ajpheart.00525.2011. [DOI] [PubMed] [Google Scholar]
- 101.Shiojima I. Sato K. Izumiya Y. Schiekofer S. Ito M. Liao R. Colucci WS. Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Silvestre JS. Mallat Z. Tedgui A. Levy BI. Post-ischaemic neovascularization and inflammation. Cardiovasc Res. 2008;78:242–249. doi: 10.1093/cvr/cvn027. [DOI] [PubMed] [Google Scholar]
- 103.Silvestre JS. Tamarat R. Ebrahimian TG. Le-Roux A. Clergue M. Emmanuel F. Duriez M. Schwartz B. Branellec D. Levy BI. Vascular endothelial growth factor-B promotes in vivo angiogenesis. Circ Res. 2003;93:114–123. doi: 10.1161/01.RES.0000081594.21764.44. [DOI] [PubMed] [Google Scholar]
- 104.Skuli N. Majmundar AJ. Krock BL. Mesquita RC. Mathew LK. Quinn ZL. Runge A. Liu L. Kim MN. Liang J. Schenkel S. Yodh AG. Keith B. Simon MC. Endothelial HIF-2alpha regulates murine pathological angiogenesis and revascularization processes. J Clin Invest. 2012;122:1427–1443. doi: 10.1172/JCI57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spinetti G. Kraenkel N. Emanueli C. Madeddu P. Diabetes and vessel wall remodelling: from mechanistic insights to regenerative therapies. Cardiovasc Res. 2008;78:265–273. doi: 10.1093/cvr/cvn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stockmann C. Doedens A. Weidemann A. Zhang N. Takeda N. Greenberg JI. Cheresh DA. Johnson RS. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 108.Tammela T. Zarkada G. Wallgard E. Murtomaki A. Suchting S. Wirzenius M. Waltari M. Hellstrom M. Schomber T. Peltonen R. Freitas C. Duarte A. Isoniemi H. Laakkonen P. Christofori G. Yla-Herttuala S. Shibuya M. Pytowski B. Eichmann A. Betsholtz C. Alitalo K. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 109.Tang XL. Rokosh G. Sanganalmath SK. Yuan F. Sato H. Mu J. Dai S. Li C. Chen N. Peng Y. Dawn B. Hunt G. Leri A. Kajstura J. Tiwari S. Shirk G. Anversa P. Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tepper OM. Galiano RD. Capla JM. Kalka C. Gagne PJ. Jacobowitz GR. Levine JP. Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 111.Tirziu D. Chorianopoulos E. Moodie KL. Palac RT. Zhuang ZW. Tjwa M. Roncal C. Eriksson U. Fu Q. Elfenbein A. Hall AE. Carmeliet P. Moons L. Simons M. Myocardial hypertrophy in the absence of external stimuli is induced by angiogenesis in mice. J Clin Invest. 2007;117:3188–3197. doi: 10.1172/JCI32024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tongers J. Losordo DW. Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur Heart J. 2011;32:1197–1206. doi: 10.1093/eurheartj/ehr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Traverse JH. Henry TD. Ellis SG. Pepine CJ. Willerson JT. Zhao DX. Forder JR. Byrne BJ. Hatzopoulos AK. Penn MS. Perin EC. Baran KW. Chambers J. Lambert C. Raveendran G. Simon DI. Vaughan DE. Simpson LM. Gee AP. Taylor DA. Cogle CR. Thomas JD. Silva GV. Jorgenson BC. Olson RE. Bowman S. Francescon J. Geither C. Handberg E. Smith DX. Baraniuk S. Piller LB. Loghin C. Aguilar D. Richman S. Zierold C. Bettencourt J. Sayre SL. Vojvodic RW. Skarlatos SI. Gordon DJ. Ebert RF. Kwak M. Moye LA. Simari RD. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van der Laan AM. Piek JJ. van Royen N. Targeting angiogenesis to restore the microcirculation after reperfused MI. Nat Rev Cardiol. 2009;6:515–523. doi: 10.1038/nrcardio.2009.103. [DOI] [PubMed] [Google Scholar]
- 115.van Solingen C. de Boer HC. Bijkerk R. Monge M. van Oeveren-Rietdijk AM. Seghers L. de Vries MR. van der Veer EP. Quax PH. Rabelink TJ. van Zonneveld AJ. MicroRNA-126 modulates endothelial SDF-1 expression and mobilization of Sca-1(+)/Lin(−) progenitor cells in ischaemia. Cardiovasc Res. 2011;92:449–455. doi: 10.1093/cvr/cvr227. [DOI] [PubMed] [Google Scholar]
- 116.Wang J. Hoshijima M. Lam J. Zhou Z. Jokiel A. Dalton ND. Hultenby K. Ruiz-Lozano P. Ross J., Jr. Tryggvason K. Chien KR. Cardiomyopathy associated with microcirculation dysfunction in laminin alpha4 chain-deficient mice. J Biol Chem. 2006;281:213–220. doi: 10.1074/jbc.M505061200. [DOI] [PubMed] [Google Scholar]
- 117.Watanabe E. Smith DM. Sun J. Smart FW. Delcarpio JB. Roberts TB. Van Meter CH., Jr. Claycomb WC. Effect of basic fibroblast growth factor on angiogenesis in the infarcted porcine heart. Basic Res Cardiol. 1998;93:30–37. doi: 10.1007/s003950050059. [DOI] [PubMed] [Google Scholar]
- 118.Winegrad S. Henrion D. Rappaport L. Samuel JL. Vascular endothelial cell-cardiac myocyte crosstalk in achieving a balance between energy supply and energy use. Adv Exp Med Biol. 1998;453:507–514. doi: 10.1007/978-1-4684-6039-1_56. [DOI] [PubMed] [Google Scholar]
- 119.Witzenbichler B. Asahara T. Murohara T. Silver M. Spyridopoulos I. Magner M. Principe N. Kearney M. Hu JS. Isner JM. Vascular endothelial growth factor-C (VEGF-C/VEGF-2) promotes angiogenesis in the setting of tissue ischemia. Am J Pathol. 1998;153:381–394. doi: 10.1016/S0002-9440(10)65582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang JJ. Kettritz R. Falk RJ. Jennette JC. Gaido ML. Apoptosis of endothelial cells induced by the neutrophil serine proteases proteinase 3 and elastase. Am J Pathol. 1996;149:1617–1626. [PMC free article] [PubMed] [Google Scholar]
- 121.Yellon DM. Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 122.Yoon CH. Hur J. Park KW. Kim JH. Lee CS. Oh IY. Kim TY. Cho HJ. Kang HJ. Chae IH. Yang HK. Oh BH. Park YB. Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 123.Yoon CH. Koyanagi M. Iekushi K. Seeger F. Urbich C. Zeiher AM. Dimmeler S. Mechanism of improved cardiac function after bone marrow mononuclear cell therapy: role of cardiovascular lineage commitment. Circulation. 2010;121:2001–2011. doi: 10.1161/CIRCULATIONAHA.109.909291. [DOI] [PubMed] [Google Scholar]
- 124.You D. Cochain C. Loinard C. Vilar J. Mees B. Duriez M. Levy BI. Silvestre JS. Hypertension impairs postnatal vasculogenesis: role of antihypertensive agents. Hypertension. 2008;51:1537–1544. doi: 10.1161/HYPERTENSIONAHA.107.109066. [DOI] [PubMed] [Google Scholar]
- 125.Zemani F. Silvestre JS. Fauvel-Lafeve F. Bruel A. Vilar J. Bieche I. Laurendeau I. Galy-Fauroux I. Fischer AM. Boisson-Vidal C. Ex vivo priming of endothelial progenitor cells with SDF-1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol. 2008;28:644–650. doi: 10.1161/ATVBAHA.107.160044. [DOI] [PubMed] [Google Scholar]
- 126.Zhang F. Tang Z. Hou X. Lennartsson J. Li Y. Koch AW. Scotney P. Lee C. Arjunan P. Dong L. Kumar A. Rissanen TT. Wang B. Nagai N. Fons P. Fariss R. Zhang Y. Wawrousek E. Tansey G. Raber J. Fong GH. Ding H. Greenberg DA. Becker KG. Herbert JM. Nash A. Yla-Herttuala S. Cao Y. Watts RJ. Li X. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci U S A. 2009;106:6152–6157. doi: 10.1073/pnas.0813061106. [DOI] [PMC free article] [PubMed] [Google Scholar]