Abstract
Aims
Autoantibodies against second extracellular loops of β1-adrenergic receptors frequent in dilated cardiomyopathy confer myocardial dysfunction presumably via cAMP stimulation. Here, we investigate the autoantibody impact on receptor conformation and function.
Methods and results
IgG was prepared from patients with dilated cardiomyopathy, matched healthy donors (10 each) or commercial IgG preparations (2). IgG binding to β1-adrenergic receptor peptides was detected in 5 of 10 patients and 2 of 10 controls. IgG colocalization with the native receptor was detected in 8 of 10 patients and 1 of 10 controls (10 of 10 patients and 7 of 10 controls at >30 mg IgG/L). All IgGs exhibiting receptor colocalization triggered changes in receptor conformation (determined with fluorescent sensors) not stringently correlated to cAMP stimulation, suggesting the induction of more or less active receptor conformations. Receptor-activating IgG was detected in 8 of 10 patients but only 1 of 10 controls. In addition, IgG from 8 of 10 patients and 3 of 10 controls attenuated receptor internalization (measured by total internal reflection fluorescence microscopy). IgG-inducing inactive receptor conformations had no effect on subsequent cAMP stimulation by isoproterenol. IgG-inducing active receptor conformations dampened or augmented subsequent cAMP stimulation by isoproterenol, depending on whether receptor internalization was attenuated or not. Corresponding IgG effects on the basal beating rate and chronotropic isoproterenol response of embryonic human cardiomyocytes were observed.
Conclusions
(i) Autoantibodies trigger conformation changes in the β1-adrenergic receptor molecule. (ii) Some also attenuate receptor internalization. (iii) Combinations thereof increase the basal beating rate of cardiomyocytes and optionally entail dampening of their chronotropic catecholamine responses. (iv) The latter effects seem specific for patient autoantibodies, which also have higher levels.
Keywords: β1-Adrenergic receptors, Receptor conformation, Autoantibodies, Dilated cardiomyopathy, Human embryonic cardiomyocytes
1. Introduction
Antibodies against β1-adrenergic receptors (β1AR) are frequently found in the serum of patients with chronic heart failure. Their prevalence is associated with Chagas' disease,1 dilated cardiomyopathy (DCM),2,3 or ischaemic heart disease,3 but not cardiomyopathies of other aetiology4,5. Epitopes most frequently targeted are located in the second extracellular loop of the human β1AR.6,7 Human autoantibodies and corresponding antibodies raised in rodents against peptides cross-react with native β1ARs.3,5,8 They exert positive ino- and chronotropic effects on cardiomyocytes5,9 by a mechanism presumed to involve the modulation of receptor conformation.4,10 Immunization of rodents against the second extracellular β1AR loop induce left ventricular dilation and dysfunction11–13 among other effects compatible with chronic cardiac dysfunction.14–16 Such effects are reversible upon removal of the antibodies,17 transferable via serum transfusions,12,16,18,19 and partially abrogated by β1AR antagonists20 or synthetic mimics of the receptor epitope.21–23 In DCM patients, the prevalence3 and cAMP-stimulatory efficacy24 of β1AR autoantibodies are correlated to more reduced cardiac function,3 increased mortality,25 more severe ventricular arrhythmia,26 and sudden cardiac death.27,28 Therefore, autoimmunity against the second extracellular loop of the β1AR is considered a cause or cofactor of chronic left ventricular dysfunction and a potential therapy target.17,21–23,29–31 Interleucin 6 is suspected to play a key role in β1AR-induced autoimmune cardiomyopathy through enhancing antibody production.32 Mechanisms, however, by which the antibodies modulate receptor function are poorly understood.33 Normal βAR activation involves a conformational switch enabling G-protein coupling.34 Since β1AR autoantibodies recognize conformational epitopes,3 they are thought to modulate receptor conformation4,10 or influence the stability of transient conformational states induced by true ligands.16 Here, we investigate whether β1AR autoantibodies derived from DCM patients indeed trigger changes in β1AR conformation.
2. Methods
2.1. Expression of biofluorescent human β1AR in HEK293 cells
The human β1AR cDNA was fused to yellow fluorescence protein (YFP) at the C-terminus to provide a fluorescent signal of receptor abundance and localization; cyan fluorescence protein (CFP) was additionally inserted into the third intracellular loop (between Pro292 and Gly304) to constitute a fluorescence resonance energy transfer (FRET)-donor/-acceptor pair reporting the activation-associated conformational switch.35 These constructs were stably expressed in human embryonic kidney 293 cells (HEK293, DSMZ ACC 305, Braunschweig, Germany) and characterized in the intact cell by binding isotherms and isoproterenol displacement of the βAR antagonist [3H]-(-)-CGP12,177 (Amersham Biosciences, Freiburg, Germany). The βAR subtype was determined by competition-binding studies with the β1-selective adrenergic antagonist CGP20712A and the β2-selective adrenergic antagonist ICI118551. Stable expression resulted in 8 ± 0.66 and 4.8 ± 0.89 million β1-adrenergic-binding sites/cell for YFP-fused and YFP/CFP-fused β1AR, respectively, when compared with 100 ± 20 endogenous β2AR (<0.001%). An appropriate size and localization of the overexpressed β1AR was confirmed by YFP-directed immunoblotting and immunofluorescence microscopy, respectively. Further characteristics of the cell model are summarized in Supplementary material online, Figure S1 and Table S2).
2.2. Selection and preparation of IgG samples
Ten patients with DCM were randomly selected upon presentation for regular outpatient follow-up. At the time of blood sampling, all patients had received stable oral heart failure medication for >3 months; active infections, known autoimmune diseases, cancer, chronic alcoholism, or heart failure of known origins were excluded; coronary heart disease and acute myocarditis were excluded by angiography and endo-myocardial biopsy according to Dallas criteria, respectively. Ten healthy volunteers with no symptoms of heart disease and the exclusion of left ventricular dysfunction by echocardiography were selected based on age and gender. Characteristics of patients and controls are summarized in Supplementary material online, Table S1. The investigation conforms to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the University Greifswald, Germany. Each patient provided written consent. IgG was isolated from the serum36 or randomly sampled from commercial preparations used for therapeutic IgG substitution (Privigen and Sandoglobulin; CSL Plasma, Marburg, Germany). Total and subclass IgG concentrations were determined by procedures accredited for clinical diagnosis. Peptide analogues of the first (VWGRWEYGSFFCEL, denominated Pep 1) or the second (LMHWWRAESDEARRCYNDPKCCD, denominated Pep 2) extracellular loop of the human β1AR used for pre-absorption and enzyme-linked immunosorbent assay (ELISA) came from nanoTools, Freiburg, Germany.
2.3. Detection quantification of autoantibodies
IgG binding to a peptide analogue of the second extracellular β1AR loop (Pep 2) was quantified by ELISA following published procedures.3 IgG-colocalization with the native receptor was determined in HEK293 cells stably expressing YFP-fused β1AR. Cells were incubated with IgG (20 min, 37°C), followed by washing, fixation (acetone, 5 min, −20°C), and counterstaining with fluorescent goat anti-human IgG (Dianova, Hamburg, Germany). Colocalization of receptor- and antibody-associated fluorescence was visualized by dual colour fluorescence microscopy (LSM 510 Meta confocal microscope, 63x/1.3 DIC oil immersion objective, Carl Zeiss, Jena, Germany) (Figure 1A) and quantified by serial IgG dilution starting with the equivalent of a 1:100 dilution of normal serum (134 mg IgG/L) (Figure 1B). The ratio of red (bound IgG) and green (β1AR abundance) fluorescence emission intensities (FLR/G) in the outer cell membrane was normalized to background FLR/G measured with secondary antibody alone. IgG-β1AR colocalization was thus detected/quantified as a FLR/G increase above background with the detection limit delineated by the confidence limit of FLR/G background scatter (Figure 1C, horizontal dashed line). Rabbit antibodies against the second extracellular loop of the human β1AR4,10,13 were used as a positive control to demonstrate the quantitative correlation of FLR/G increases with the amount (dilution) of β1AR antibodies in the assay (Figure 1C, closed squares). The specificity cut-off for β1AR-related FLR/G increases (Figure 1C, dashed vertical line) was defined by the IgG dilution at and above which commercial IgG preparations used as a bona fide β1AR antibody-negative control (Figure 1C, open squares) resulted in FLR/G values within the background. It was determined at serum-equivalent dilutions of ≥1:500 (26.8 mg IgG/L). Consistent with this cut-off, IgG signals at cell surfaces observed upon staining at 1:500 dilution (Figure 2A, left) were absent in cells not overexpressing β1AR (Figure 2A, right) or overexpressing C-terminally green fluorescence protein (GFP)-fused human EGF receptors (Figure 2B, middle) or stained with second antibody alone (Figures 1A and B, 2B, left) or pretreated with a molar excess of peptide corresponding to the second extracellular loop of the β1AR (Figure 2B, right). In conclusion, FLR/G increases observed at IgG dilutions of 1:500–2000 were taken to indicate β1AR autoantibody levels scored +, ++, and +++, respectively (Figure 1C, arrows).
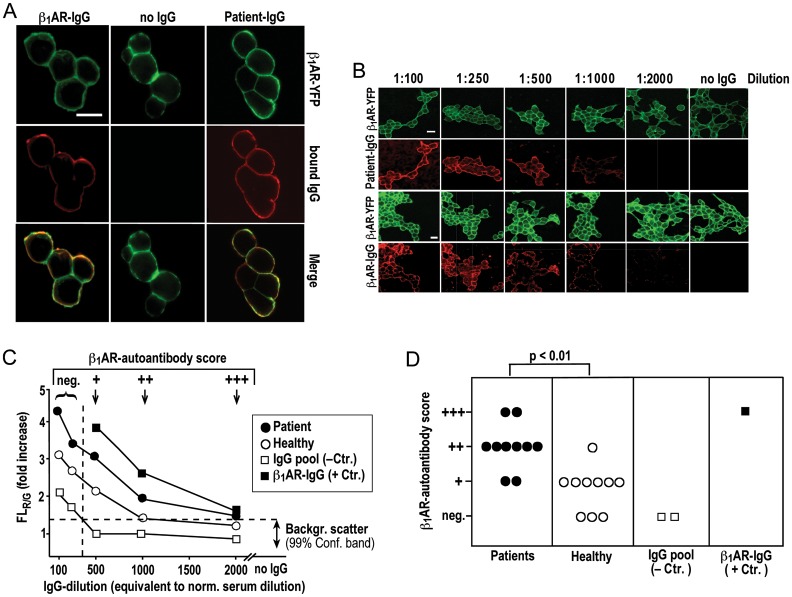
Figure 1.
Colocalization of IgG with native YFP-fused β1AR on the surface of human cells. (A) Colocalization (bottom) of β1AR- (top) and IgG-associated fluorescence (middle) on native cells incubated with IgG (26.8 mg/L) from a DCM patient (right), secondary antibody alone (middle) or rabbit β1AR-IgG (left); visualization at 630-fold magnification, size bar (30 μm) at top left applies to all panels. (B) Serial dilution of patient IgG (top) or rabbit β1AR-IgG (bottom) starting with 134 mg IgG/L (equivalent to 1:100 dilution of normal serum); green and red images show receptor- and IgG-associated fluorescence, respectively, at 200-fold magnification. Size bar (30 μm) at top left applies to all panels. (C) Cells stained with serial IgG dilutions and imaged as in (B); the ratio of emission intensities of red (bound IgG) and green (β1AR abundance) fluorescence intensities (FLR/G) is normalized to FLR/G background with second antibody alone. FLR/G increases above background (mean values from 10 fields of vision, SEM <20% not shown) are plotted over dilution of IgG from a DCM patient (filled circle), a healthy volunteer (open circle), a commercial human-IgG preparation (open square) or rabbit antibodies against the second extracelluar loop of the human β1AR13 (filled square). Horizontal dashed line: confidence limit of FLR/G background scatter (determined in 10 independent cell preparations, 30 fields of vision each). Vertical dashed line: specificity cut-off. (D) Scoring of β1AR autoantibody positivity [criteria at the top of (C)]; means of triplicate determinations of individual IgGs done on different days with different cell preparations; SEM was less than one dilution step; Bracket: U test.
Figure 2.
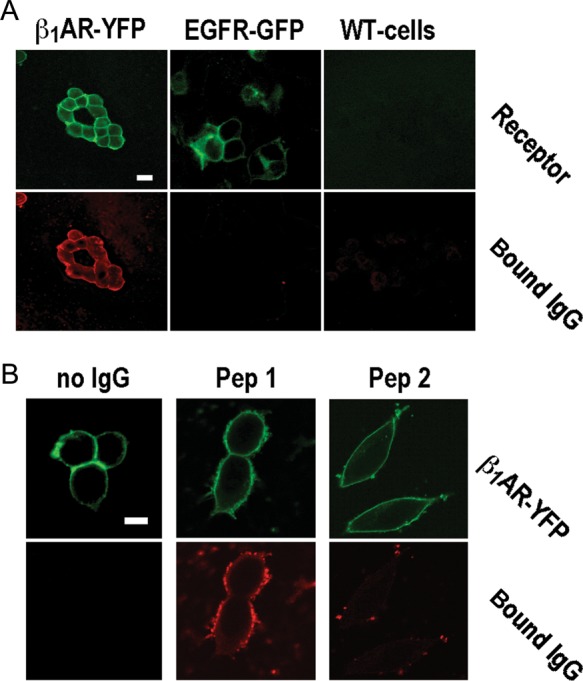
Specificity of IgG-β1ARs colocalization at specificity cut-off. (A) β1AR- (top) and IgG-associated fluorescence (bottom) in wild-type cells (right) and cells expressing YFP-fused human β1ARs (left) or GFP-fused human EGF receptors (middle), all stained with patient IgG at 1:500 dilution (26.8 mg/L). Images obtained at 400-fold magnification, size bar of 30 μm at top left applies to all panels. (B) β1AR- (top) and IgG-associated fluorescence (bottom) in cells expressing YFP-fused human β1ARs and stained with secondary antibody alone (left) or human autoimmune IgG (26.8 mg/L) pre-absorbed with a 1000-fold molar excess of peptide analogues of the first (middle) or the second (right) extracellular loop of the human β1AR. Images obtained at 630-fold magnification. Size bar of 30 μm at top left applies to all panels.
2.4. Determination of changes in β1AR conformation by FRET
Cells expressing the YFP/CFP-fused β1AR FRET reporter or co-expressing β1ARs, each bearing just one component of the fluorescent donor/acceptor pair, were cultured under an LSM 510 Meta inverted confocal microscope equipped with a 63x/1.3 DIC oil immersion objective and placed inside an XL-incubator (Carl Zeiss, Jena, Germany). The donor CFP was excited at 458 nm and fluorescent emission was recorded using the Meta detector. CFP and YFP emission spectra were separated by emission fingerprinting and corrected for bleaching during image acquisition. FRET efficiency was expressed as an emission intensity ratio IYFP/ICFP. The mean fluorescence lifetime τm of the donor CFP was analysed to ascertain that decreases in IYFP/ICFP were due to alterations in FRET and not to unrelated artefacts that alter IYFP/ICFP but not τm. For this purpose, cells were cultured under a DCS-120 Confocal Scanning FLIM system (Becker & Hickl GmbH, Berlin, Germany) mounted on an IX-71 inverted microscope equipped with a 60x (NA 1.2) water immersion objective (Olympus Deutschland GmbH, Hamburg, Germany) and placed inside a cage incubator (Okolab, Naples, Italy). CFP was excited using a 405 nm diode laser (Becker & Hickl GmbH) with a pulse repetition rate of 50 MHz. Fluorescence signals in the CFP channel (BP 460–500 nm) and the YFP channel (BP 520–550 nm) were detected using H7422P-40 photomultipliers (Hamamatsu, Herrsching, Germany) connected to two TCSPC-150 modules (Becker & Hickl GmbH). Fluorescence lifetime imaging of live cells was performed by continuous fast scanning and fitting TCSPC data by a double exponential decay function using the SPC Image software (version 2.9.1, Becker & Hickl GmbH). τm was calculated from the multi-exponential decay of each pixel. It was ascertained that a drop in IYFP/ICFP and a prolongation of τm indicated intramolecular changes of β1AR conformation (Supplementary material online, Figures S1 and S2 and Table S2 and S3). Maximal decreases (Δmax) of IYFP/ICFP upon stimulation of the receptor are stated as the mean of three determinations done on different days with different cell preparations each based on measurements in the plasma membranes of 10 individual cells.
2.5. Measurement of β1AR internalization
β1AR internalization was determined by total internal reflection fluorescence (TIRF)37 in cells stably expressing C-terminally YFP-fused β1AR. An IX-81 inverted microscope equipped with a fast CCD camera and 60× (NA 1.49) oil immersion objective (Olympus, Hamburg, Germany) was used. The illumination angle was adjusted to create an evanescent wave providing excitation in a 200 nm cytosolic slice above but not including the basal cell membrane. Following the addition of isoproterenol and/or IgG, continuous quantifications of biofluorescent receptosomes in the cell slice were monitored (typical recording see Supplementary material online, Movie) and averaged during 1000 s using ImageJ 1.4.2Q (National Institute of Health, Bethesda, USA). The mean of 10 measurements in individual cells is stated.
2.6. cAMP measurements and ERK1/2 phosphorylation
cAMP measurements in HEK293 cells expressing YFP-fused β1AR used published procedures.38 Briefly, cells were kept in serum-free medium for 24 h, exposed to stimulators and extracted with ethanol (98%, −20°C). Phosphodiesterase inhibitors were omitted from incubations unless stated otherwise. cAMP was measured in the extracts with a commercial kit (GE Healthcare, Freiburg, Germany). The detection threshold for cAMP stimulation by isoproterenol was at 5 nmol/L (Supplementary material online, Figure S1E), which was sufficient to demonstrate cAMP stimulation by all patient IgG studied (Figure 4A). ERK1/2 phosphorylation was analysed in HEK293 cells grown to 80% confluence, kept for 24 h in serum-free medium and then subjected to treatment with receptor ligands for 10 min at 37°C. Western blots were probed with ERK antibodies (Cell Signaling, Boston, USA) and mouse monoclonal antibodies against phospho-p42/p44 MAP kinase (Thr 202/Tyr 204).
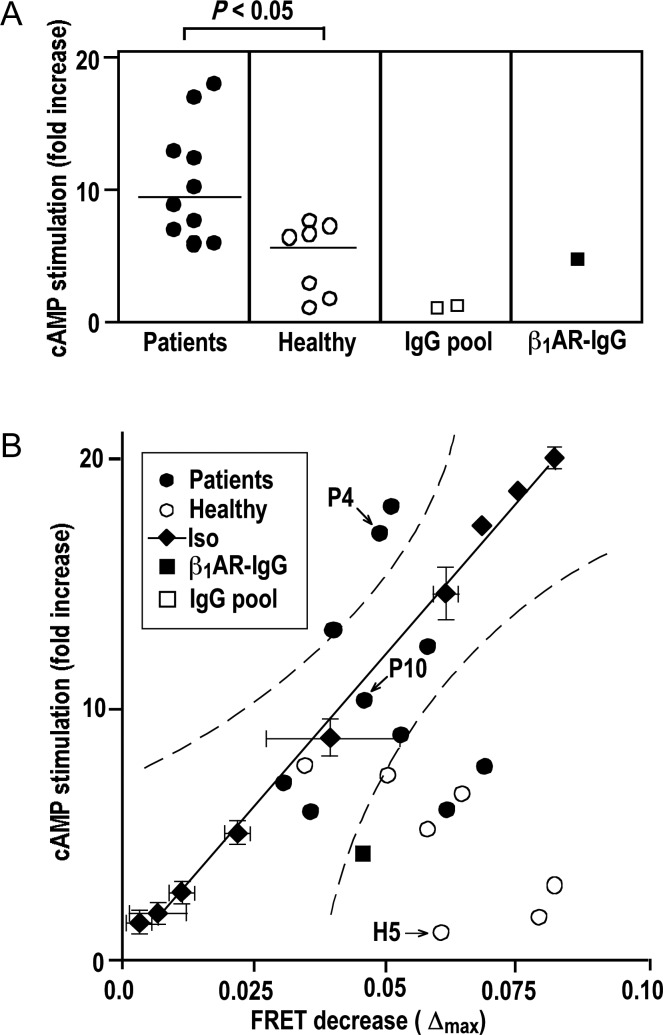
Figure 4.
cAMP stimulation by IgG and corresponding changes in β1AR conformation. (A) Fold increments of cAMP over basal levels in cells overexpressing human β1ARs in response to IgG (26.8 mg/L, 20 min, 37°C) from DCM patients (filled circle), matched healthy volunteers (open circle), commercial human-IgG preparations (open square) and rabbit β1AR antibodies (filled square). Lines show medians, symbols shown means (n = 6, SEM <10% not shown). Bracket: U test. (B) Correlation of cAMP and FRET responses to IgG from DCM patients (filled circle), healthy volunteers (open circle), and rabbit β1AR antibodies (filled square) (mean, n = 3, SEM <10% not shown), compared with responses to isoproterenol (5 × 10–11 – 5 × 10−4 mol/L, for each concentration mean ± SEM, n = 3) (filled diamond). Solid and dashed lines: linear regression and 99% confidence band of the isoproterenol data. P4, P10, and H5: index samples for comparison with Figures 5 and 6.
2.7. Beating rates of human embryonic cardiomyocytes
Rates of spontaneous synchronized rhythmic cardiomyocyte contraction were monitored with the xCELLigence RTCA Cardio Instrument (Roche Diagnostics, Penzberg, Germany) as published.39 Human embryonic cardiomyocytes (CMC-100-110-001, cellular dynamics, WI, USA) were plated on E-plate Cardio 96 wells and grown for 5 days until the beating rate was stable. For suppression of β2-adrenergic chronotropic effects, cells were pretreated with 10 µM atropine (Sigma, Munich, Germany) and 1 µM ICI 118551 (Sigma, Munich, Germany) for 2 h.40 Cells were exposed to IgG (50 mg/L, 2 h) and then isoproterenol (10 μM) was added. IgG effects on the basal beating rate and the chronotropic isoproterenol response were derived from these recording as shown in Supplementary material online, Figure S6 using the xCELLigence Cardio Software (Roche Diagnostics). Quantitative results were derived from triplicate determinations on different days with different cell plates and simultaneous recordings in five wells per plate subjected to the same experimental condition.
2.8. Statistics
Results of continuous variables are stated as mean ± SEM. GraphPad PRISM 4.0a (GraphPad Software, Inc., USA) was used for linear data regression, the exclusion of normal data distribution by the Shapiro–Wilk test, and the calculation of significances by the Mann–Whitney U test. P-values <0.05 were considered statistically significant.
3. Results
3.1. Receptor autoantibodies detected by colocalization with native β1AR are frequently present in DCM patients and healthy volunteers
We detected β1AR autoantibodies in 5 of 10 samples from DCM patients and 2 of 10 samples from healthy subjects using peptide-based ELISA (Supplementary material online, Figure S3), which conforms to previous studies.3,8,28,41 Cross-reactivity with the native β1AR was addressed by analysing IgG colocalization with β1ARs on the surface of cells stably overexpressing YFP-fused human β1ARs that were incubated with IgG prior to fixation (Figure 1A). β1AR specificity of IgG signals thus obtained on the cell surface was ascertained by their absence upon the omission of first antibody (Figure 1A), pre-adsorption with a 1000-fold molar excess of a peptide homologue of the second extracellular loop of the human β1AR (Figure 2B) or in cells not overexpressing the β1AR or overexpressing GFP-fused human EGF receptors instead (Figure 2A). IgG- β1AR colocalization was quantified by serial IgG dilution, resulting in a dose-dependent decline of β1AR-associated IgG signal (Figure 1B) and corresponding shifts of FLR/G in the cell membrane (Figure 1C). FLR/G increases above background were quantitatively correlated to the level (dilution) of bona fide β1AR antibodies4,10,13 (Figure 1C, closed squares) and not obtained upon incubation with bona fide autoantibody-negative IgG at dilutions ≥1:500 (Figure 1C, open squares). In conclusion, we used FLR/G increases at 1:500–2000 dilutions for scoring levels of IgG- β1AR colocalization (Figure 1C). At the highest sensitivity level (+), all 10 DCM patients and 7 of 10 healthy subjects were autoantibody positive. Quantitative autoantibody scores seemed significantly (P < 0.5) higher in the patients (Figure 1D). Cross-comparison with the quantitative results of peptide ELISA (Supplementary material online, Figure S3) suggested a rough correlation of the two methods with the peptide ELISA exhibiting a lesser sensitivity. Moreover, IgG- β1AR colocalization was significantly diminished upon cell fixation before IgG staining (Supplementary material online, Figure S4) suggesting β1AR autoantibodies bind a labile conformational epitope possibly inadequately represented by immobilized peptides. Therefore, we decided to include all IgG exhibiting β1AR colocalization at the highest sensitivity level (+) into functional analyses, even though a more stringent assessment of receptor-antibody positivity at the (++) level fits better to previous evaluations3,8,28,41 and is probably more relevant in clinical terms.
3.2. β1AR-colocalizing IgG trigger conformational changes of the receptor molecule not stringently correlated to cAMP stimulation
An IgG impact on receptor conformation was studied in cells stably expressing β1ARs bearing an intramolecular FRET sensor capable of reporting the activation-associated conformational switch by a rapid decrease in the emission ratio of FRET donor and acceptor fluorophores (IYFP/ICFP, Figure 3A). All IgG exhibiting β1AR colocalization (including the rabbit β1AR antibody) also decreased IYFP/ICFP, which could be blocked by pre-incubation with a peptide homologue of the second, but not of the first extracellular loop of the β1AR (Figure 3B). Maximal decreases in IYFP/ICFP (Δmax) triggered by patient or control IgG had similar ranges and medians. The rabbit β1AR antibody had a similar effect (Figure 3C). Effects of individual IgG samples on receptor conformation did not add to each other or the effect of isoproterenol. Submaximal IgG effects did not preclude subsequent, maximal effects by isoproterenol (Figure 3D). Thus, the autoantibodies seemed to trigger a similar conformational switch as isoproterenol by targeting epitopes in the second extracellular β1AR loop, which conforms to published results of peptide pre-adsorption.6,7
Figure 3.
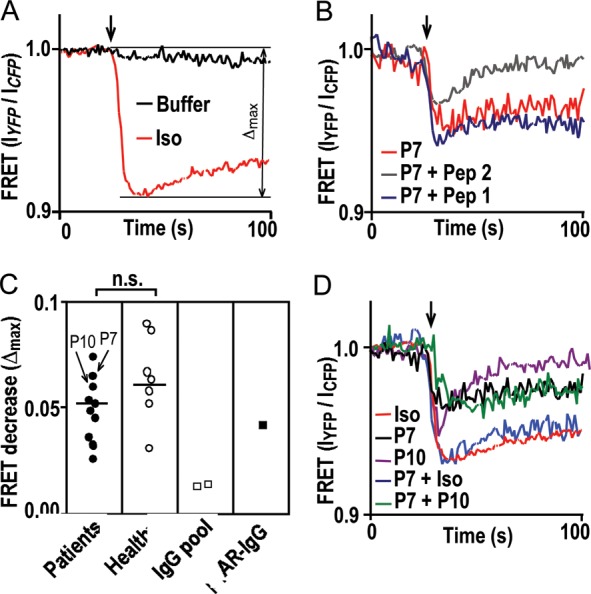
IgG impact on receptor conformation measured by a drop in FRET efficiency. (A) FRET response to 10−5 mol/L isoproterenol (red) or buffer (black). (B) FRET response to patient IgG (26.8 mg/L) without (red) or with pre-adsorption (1000-fold molar excess, 10 min, 37°C) with peptide analogues of the first (Pep 1, blue) or second (Pep 2, grey) extracellular loop of the human β1AR. (C) Comparison of FRET responses [quantification of Δmax demonstrated in (A)] to individual IgG (26.8 mg/L) from DCM patients (filled circle), matched healthy volunteers (open circle), commercial human-IgG preparations (open square) and rabbit β1AR antibodies13 (filled square) by the U test (bracket). P7 and P10 identify corresponding IgG samples in (B) and (D). Lines show medians. (D) FRET responses to simultaneous incubation with two individual patient IgGs (green) or sequential incubation with IgG and then isoproterenol (blue), compared with each agent alone [black (P7), magenta (P10), and red (Iso)].
The rabbit antibody, all patient IgG, and most β1AR-colocalizing IgG from healthy volunteers increased intracellular cAMP to some extent (Figure 4A). In agreement with previous findings,3,24 patient IgGs had a significantly higher cAMP-stimulatory efficacy, although their impact on receptor conformation was not significantly different from that of β1AR-colocalizing IgG from healthy volunteers or the rabbit β1AR antibodies (Figure 3C). To address this discrepancy, we created a correlative matrix of cAMP stimulation and corresponding changes in receptor conformation (Δmax of IYFP/ICFP decrease) and used dose–response curves of isoproterenol (Supplementary material online, Figure S1E and J) to determine the 99% confidence band of receptor activation by a normal agonist (Figure 4B, dotted lines). Eight of ten patient-derived IgG samples exhibited values within or above the isoproterenol-delimited confidence band (Figure 4B, closed circles), suggesting that they were as good as, or even superior to, isoproterenol in inducing receptor conformations coupled to cAMP. In contrast, six of seven β1AR-colocalizing IgG from healthy volunteers and the rabbit antibody were below the isoproterenol-delimited confidence band (Figure 4B, open circles and closed square, respectively), suggesting that they induced receptor conformations not or inefficiently coupled to cAMP.
3.3. Inhibition of agonist triggered β1AR internalization by autoantibodies
In the steady state of isoproterenol saturation, 20% of the biofluorescent β1ARs were relocated to cytosolic endosomes (Figure 6A, top, right), indicative of receptor endocytosis42 and recycling to the cell surface.37 cAMP-stimulatory receptor autoantibodies failed to induce receptor internalization (Figure 5A, bottom, left), and moreover, attenuated receptor internalization inducible by subsequent exposure to isoproterenol (Figure 5A, bottom, right) despite the overruling isoproterenol effect on receptor conformation (Figure 3D). This observation suggests that autoantibodies inhibited receptor internalization. Given the low-internalization rate of the β1AR,43 this effect could not be quantified by cell fractionation. Instead, we used TIRF37 to monitor the appearance of biofluorescent β1AR receptosomes in a 200 nm cytosolic section immediately above the basal membrane. Such receptosomes started to appear 1–2 min after isoproterenol exposure and increased in density over time (Supplementary material online, Movie). IgG pre-incubation significantly delayed onset and decreased rates of agonist-induced receptosome trafficking (Figure 5B). Going by the mean rate of receptosome appearance during the first 17 min of agonist exposure, receptor internalization was significantly more extensively and frequently reduced by patient-derived IgG samples than by β1AR-colocalizing IgG from healthy volunteers, whereas commercial IgG preparations and rabbit β1AR antibodies had no effect on internalization (Figure 5C). The inhibitory effect was abolished by pre-adsorption with a peptide analogue of the second (but not the first) extracellular loop of the human β1AR (Figure 5D), suggesting that it is triggered by IgG interactions with the same receptor domain as cAMP stimulation. However, it seems unlikely that the same epitopes are involved, because the two effects were not correlated between individual IgGs (Figure 5E).
Figure 6.
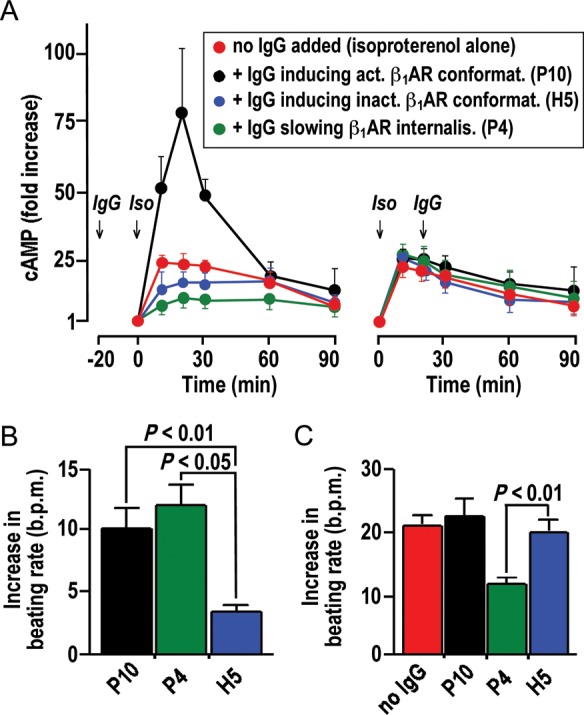
Modulation of cAMP stimulation and cardiomyocyte beating rate by IgG. (A) cAMP-time courses in HEK293 cells overexpressing human β1AR upon exposure to isoproterenol (10−6 mol/L). Left: IgG pre-incubation (50 mg/L, 20 min, 37°C). Right: IgG addition 20 min after isoproterenol. Red symbols in left and right: cAMP-time courses without IgG. Means ± SEM of triplicate determinations (for isoproterenol alone) or independent measurements with three independent IgG samples with similar properties. (B and C) IgG effects on basal beating rates (B) and chronotropic isoproterenol responses (C) of human embryonic cardiomyocytes subjected to sequential exposure to IgG (50 mg/L) and isoproterenol (10 μM). Data derived from continuous recordings, as shown in Supplementary material online, Figure S6. Mean ± SEM of measurements with three independent IgG samples with similar properties. P4, P10, H5: indexes referring IgG properties to corresponding data points in Figures 4 and 5.
Figure 5.
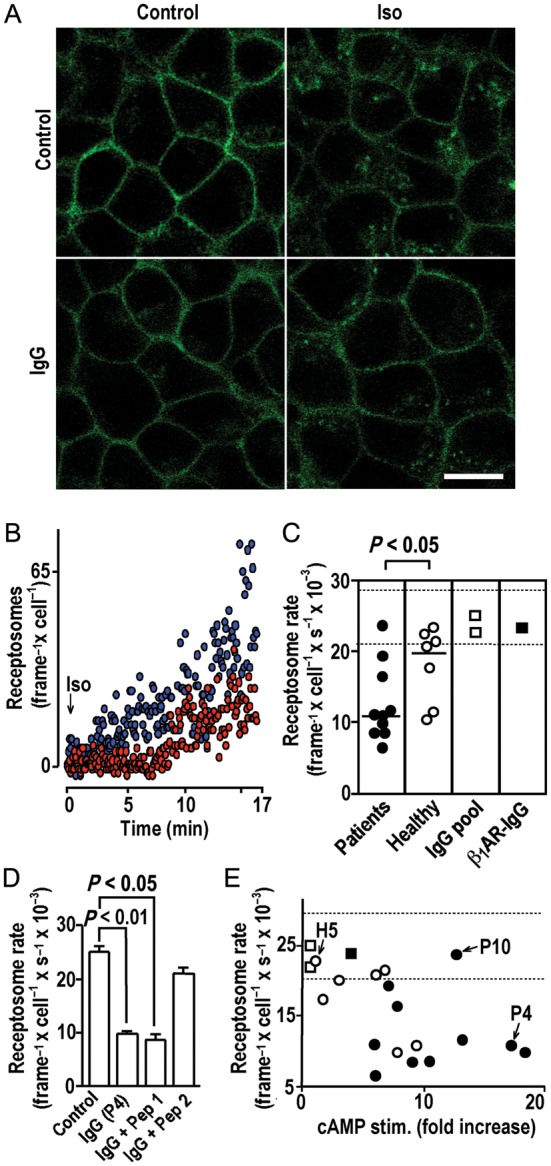
IgG inhibition of agonist-induced receptor internalization. (A) Confocal images (630-fold magnification, size bar of 30 μm at bottom right applies to all panels) of cells expressing YFP-fused human β1ARs before (top, left) or after (top, right) exposure to isoproterenol (10−5 mol/L, 37°C, 30 min) or after pre-incubation with autoimmune IgG (26.8 mg/L, 20 min, 37°C) (bottom, left) and subsequent isoproterenol exposure (bottom, right). (B) Internalized biofluorescent receptosomes quantified by TIRF following isoproterenol exposure (10−5 mol/L) without (blue) or with (red) IgG pre-incubation as in (A). (C) Impacts of IgG pre-incubation [as in (A)] on mean receptosome frequency averaged over 16.7 min of isoproterenol exposure. Bracket: U test. Dashed lines: 98% confidence band of the receptosome rate without IgG pre-incubation (n = 5). (D) Decrease of the isoproterenol-induced receptosome rate [mean ± SEM determined as in (C)] after IgG pre-incubation [as in (A)] with and without pre-absorption (1000-fold molar excess, 10 min, 37°C) with peptide analogues of first or second extracellular receptor loops (Pep 1 and 2, respectively). (E) IgG efficacies to stimulate cAMP and decrease agonist-triggered receptor internalization. H5, P4, and P10: index samples for comparison with Figures 4 and 6. Dashed lines as in (C).
3.4. Modulation of agonist stimulated cAMP production and cardiomyocyte stimulation by β1AR autoantibodies
The correlative matrices shown in Figure 4B and 5E suggest that different types of β1AR autoantibodies can be distinguished: (i) IgG (here mostly found in healthy individuals) that induce inactive receptor conformation (indexed ‘H5’); (ii) IgG (here mostly found in patients) that induce active receptor conformations (indexed ‘P10’); (iii) IgG (here mostly found in patients) that strongly attenuate receptor internalization (indexed ‘P4’). These three IgG types were analysed with respect to their impact on isoproterenol-stimulated cAMP production. Two settings were tested: (i) IgG pre-incubation (20 min) followed by isoproterenol stimulation (Figure 6A left) and (ii) isoproterenol stimulation followed after 20 min by the addition of IgG (Figure 6A right). cAMP-time courses upon exposure to isoproterenol alone (red symbols) were consistent with initial stimulation followed by desensitization. They were not significantly changed by the addition of any of the above types of β1AR autoantibodies subsequent to isoproterenol (Figure 6A right), which conforms to the data in Figure 3D and indicates that isoproterenol overrules subsequent IgG effects on the receptor. However, pre-incubation of unliganded β1AR with autoantibodies notably affected subsequent stimulation by isoproterenol. Moreover, the three types of β1AR autoantibodies had different effects in this setting (Figure 6A, left): IgG-inducing inactive receptor conformations had no significant effect on the cAMP response to isoproterenol, whereas IgG-inducing active receptor conformations augmented the initial cAMP response to isoproterenol. Interestingly, β1AR-stimulating IgG that, in addition, strongly attenuated receptor internalization had the opposite effect; it decreased the initial cAMP response.
The pathophysiological relevance of these divergent IgG effects was corroborated in human embryonic cardiomyocytes, using real-time impedance monitoring of the spontaneous beating rate as a readout of chronotropic effects39 and restricting the responsiveness of the model to β1AR-mediated regulation.40 As expected, patient-derived IgG capable of β1AR-mediated cAMP stimulation (indexed ‘P10’ and P4’) significantly stimulated the basal beating rate of the cardiomyocytes, whereas IgG from healthy individuals inducing inactive receptor conformations (indexed ‘H5’) had no significant effect (Figure 6B). Most notably, the divergent effect of the two types of β1AR-stimulating IgG on isoproterenol stimulation of cAMP (Figure 6A, left) was faithfully reflected by their modulatory effect on cardiomyocyte stimulation: pre-incubation with stimulating IgG increased or decreased a subsequent chronotropic isoproterenol response depending on whether the autoantibodies in addition inhibited β1AR internalization (indexed ‘P4’) or not (indexed ‘P10’) (Figure 6C). This finding allows the conclusion that β1AR-stimulating IgG increases the basal beating rate of cardiomyocytes (which was known). In addition, they can either enhance or restrict the amplitude of the chronotropic chatecholamine response in a manner related to their inhibitory effect on receptor internalization, which is a new finding. It should be noted, however, that the enhancing effect was very minor and not significant in cardiomyocytes.
4. Discussion
It is thought that β1AR autoantibodies are specifically induced in conjunction with DCM pathogenesis, because they are detected in 26–60% of DCM patients, but only 1–10% of healthy subjects.3,23,28,41 Here, we detected β1AR autoantibodies with comparable frequency by peptide-based ELISA or high stringency scoring of IgG interactions with the native receptor. However, our data suggest that β1AR autoantibodies possibly occur with a much higher frequency (here in all patients and 7 of 10 healthy subjects) when a more sensitive scoring of IgG interactions with native β1AR is applied. We blame this discrepancy on the (fixation-) labile conformation of the autoantibody epitope, which may be inadequately represented by immobilized peptides3 even though soluble peptides are capable of antibody neutralization.6,7 On the premise that IgG-β1AR colocalization is an adequate measure of autoantibody binding to native β1ARs, our data suggest that such autoantibodies could occur more frequently in patients and healthy individuals than autoantibodies interacting with immobilized peptides.3,28,41 or denatured β1AR.23 This notion underscores the previous postulate3,10 that DCM patients are possibly distinguished not by the mere presence of β1AR autoantibodies but a higher level and different functional impact of their autoantibodies. However, most β1AR-directed IgG uncovered here in healthy individuals by high sensitivity scoring of IgG interactions with native β1AR had little or no effect on cAMP production or the beating rate of human embryonic cardiomyocytes suggesting that they are irrelevant in pathophysiological terms.
DCM-associated IgG stimulates cAMP production24 and MAP kinase signalling44 via the β1/β2AR, inhibits cAMP signalling via the M2-muscarinic receptor,45 and exerts negative inotropic effects via Fcγ receptors.46 These pleiotropic actions have previously been discriminated by selective antagonists and/or proteins presenting the respective binding site.6,7,40,45,47 In addition, we use here a purely β1AR regulated cell model, in which effects on MAP kinase signalling are excluded (see Supplementary material online, Figure S5) and Fcγ receptors are not present. In this restricted model, all autoantibodies colocalizing with native β1AR triggered conformation changes in the receptor molecule. For DCM-associated autoantibodies, such changes were mostly matched by increases in cAMP production, consistent with the induction or stabilization of active receptor conformations.10 Pre-incubation with most cAMP-stimulatory autoantibodies also augmented subsequent isoproterenol stimulation of intracellular cAMP suggesting that they possibly promote the agonist-coupled high-affinity state of the receptor.16 In contrast, most autoantibodies detected in healthy individuals induced conformation changes of the receptor molecule that were inadequately matched by increased cAMP production. This inefficiency could be due to the lower levels of these antibodies precluding a sufficient binding equilibrium or indicate stabilization of distinct alternative receptor conformations that are inactive per se. In any case such antibodies had no effect on the basal beating rate or chronotropic catecholamine response of human cardiomyocytes suggesting they are functionally irrelevant.
In many but not all DCM-derived IgG samples, we detected β1AR autoantibodies that attenuate receptor internalization triggered by isoproterenol. Our observation contradicts an earlier report on the loss of surface-binding sites in response to β1AR autoantibodies,48 but seemingly conforms to the long-standing observations that cAMP-stimulatory autoantibodies do not induce β1AR desensitization and inhibit desensitization by catecholamines.2 However, autoantibodies that significantly slowed receptor internalization also attenuated the initial cAMP response to isoproterenol, which seems more consistent with retardation of receptor regeneration following desensitization (which requires internalization42) than with the inhibition of desensitization itself. Interestingly, the effect of such autoantibodies on the unliganded β1AR entailed stimulation of basal cAMP production, suggesting an overall bimodal effect, which was clearly recapitulated in the effect of such antibodies on the basal beat rate and the chronotropic isoproterenol response of human cardiomyocytes. It should be noted that similar bimodal IgG effects were previously observed in subgroups of DCM patients.9,10
In summary, we present here a detailed mechanistic pilot study on differential β1AR autoantibody effects at the level of the receptor. Our data are of limited relevance for the general population because only 10 patients and healthy individuals were analysed. However, we furnish direct evidence for the hypothesis3,10 that β1AR autoantibodies trigger conformation changes in the receptor molecule. We present the new finding that β1AR autoantibodies can attenuate receptor internalization. We demonstrate that combinations of these properties can result in bimodal effects on receptor activity that are meaningful for basal activity and chronotropic catecholamine responses of human cardiomyocytes.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft [collaborative research centers SFB 612 and SFB 728 and research training group GK1089 to F.B. and collaborative research center SFB TR 19 to S.B.F.].
Supplementary Material
Acknowledgements
We gratefully acknowledge the gift of GFP-fused human EGF receptor to Donna Arndt-Jovin, Max-Planck-Institute for Biophysical Medicine, Goettingen, Germany.
Conflict of interest: none declared.
References
- 1.Sterin-Borda L, Cossio PM, Gimeno MF, Gimeno AL, Diez C, Laguens RP, et al. Effect of chagasic sera on the rat isolated atrial preparation: immunological, morphological and function aspects. Cardiovasc Res. 1976;10:613–622. doi: 10.1093/cvr/10.6.613. doi:10.1093/cvr/10.6.613. [DOI] [PubMed] [Google Scholar]
- 2.Magnusson Y, Wallukat G, Waagstein F, Hjalmarson A, Hoebeke J. Autoimmunity in idiopathic dilated cardiomyopathy. Characterization of antibodies against the beta1-adrenoceptor with positive chronotropic effect. Circulation. 1994;89:2760–2767. doi: 10.1161/01.cir.89.6.2760. doi:10.1161/01.CIR.89.6.2760. [DOI] [PubMed] [Google Scholar]
- 3.Jahns R, Boivin V, Siegmund C, Inselmann G, Lohse MJ, Boege F. Autoantibodies activating human beta1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation. 1999;99:649–654. doi: 10.1161/01.cir.99.5.649. doi:10.1161/01.CIR.99.5.649. [DOI] [PubMed] [Google Scholar]
- 4.Jahns R, Boivin V, Siegmund C, Boege F, Lohse MJ, Inselmann G. Activating beta1-adrenoceptor antibodies are not associated with cardiomyopathies secondary to valvular or hypertensive heart disease. J Am Coll Cardiol. 1999;34:1545–1551. doi: 10.1016/s0735-1097(99)00381-2. doi:10.1016/S0735-1097(99)00381-2. [DOI] [PubMed] [Google Scholar]
- 5.Magnusson Y, Hjalmarson A, Hoebeke J. Beta1-adrenoceptor autoimmunity in cardiomyopathy. Int J Cardiol. 1996;54:137–141. doi: 10.1016/0167-5273(96)02590-9. doi:10.1016/0167-5273(96)02590-9. [DOI] [PubMed] [Google Scholar]
- 6.Magnusson Y, Marullo S, Hoyer S, Waagstein F, Andersson B, Vahlne A, et al. Mapping of a functional autoimmune epitope on the beta1-adrenergic receptor in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1990;86:1658–1663. doi: 10.1172/JCI114888. doi:10.1172/JCI114888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallukat G, Wollenberger A, Morwinski R, Pitschner HF. Anti-beta1-adrenoceptor autoantibodies with chronotropic activity from the serum of patients with dilated cardiomyopathy: mapping of epitopes in the first and second extracellular loops. J Mol Cell Cardiol. 1995;27:397–406. doi: 10.1016/s0022-2828(08)80036-3. doi:10.1016/S0022-2828(08)80036-3. [DOI] [PubMed] [Google Scholar]
- 8.Holthoff HP, Zeibig S, Boivin V, Bauer J, Lohse MJ, Kaab S, et al. Detection of anti beta1-AR auto-antibodies in heart failure by a cell-based competition ELISA. Circ Res. doi: 10.1161/CIRCRESAHA.112.272682. Advance Access published July 20, 2012, doi:10.1161/CIRCRESAHA.112.272682. [DOI] [PubMed] [Google Scholar]
- 9.Christ T, Schindelhauer S, Wettwer E, Wallukat G, Ravens U. Interaction between autoantibodies against the beta1-adrenoceptor and isoprenaline in enhancing L-type Ca2+ current in rat ventricular myocytes. J Mol Cell Cardiol. 2006;41:716–723. doi: 10.1016/j.yjmcc.2006.06.011. doi:10.1016/j.yjmcc.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Jahns R, Boivin V, Krapf T, Wallukat G, Boege F, Lohse MJ. Modulation of beta1-adrenoceptor activity by domain-specific antibodies and heart failure-associated autoantibodies. J Am Coll Cardiol. 2000;36:1280–1287. doi: 10.1016/s0735-1097(00)00881-0. doi:10.1016/S0735-1097(00)00881-0. [DOI] [PubMed] [Google Scholar]
- 11.Buvall L, Bollano E, Chen J, Shultze W, Fu M. Phenotype of early cardiomyopathic changes induced by active immunization of rats with a synthetic peptide corresponding to the second extracellular loop of the human beta-adrenergic receptor. Clin Exp Immunol. 2006;143:209–215. doi: 10.1111/j.1365-2249.2005.02986.x. doi:10.1111/j.1365-2249.2005.02986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jahns R, Boivin V, Hein L, Triebel S, Angermann CE, Ertl G, et al. Direct evidence for a beta1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest. 2004;113:1419–1429. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jahns R, Siegmund C, Jahns V, Reilander H, Maidhof A, Muller-Esterl W, et al. Probing human beta 1- and beta 2-adrenoceptors with domain-specific fusion protein antibodies. Eur J Pharmacol. 1996;316:111–121. doi: 10.1016/s0014-2999(96)00654-1. doi:10.1016/S0014-2999(96)00654-1. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda Y, Miyoshi S, Tanimoto K, Oota K, Fujikura K, Iwata M, et al. Autoimmunity against the second extracellular loop of beta1-adrenergic receptors induces early afterdepolarization and decreases in K-channel density in rabbits. J Am Coll Cardiol. 2004;43:1090–1100. doi: 10.1016/j.jacc.2003.09.057. doi:10.1016/j.jacc.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 15.Matsui S, Fu ML, Hayase M, Katsuda S, Yamaguchi N, Teraoka K, et al. Active immunization of combined beta1-adrenoceptor and M2-muscarinic receptor peptides induces cardiac hypertrophy in rabbits. J Card Fail. 1999;5:246–254. doi: 10.1016/s1071-9164(99)90009-x. doi:10.1016/S1071-9164(99)90009-X. [DOI] [PubMed] [Google Scholar]
- 16.Jane-wit D, Altuntas CZ, Johnson JM, Yong S, Wickley PJ, Clark P, et al. Beta1-adrenergic receptor autoantibodies mediate dilated cardiomyopathy by agonistically inducing cardiomyocyte apoptosis. Circulation. 2007;116:399–410. doi: 10.1161/CIRCULATIONAHA.106.683193. doi:10.1161/CIRCULATIONAHA.106.683193. [DOI] [PubMed] [Google Scholar]
- 17.Matsui S, Larsson L, Hayase M, Katsuda S, Teraoka K, Kurihara T, et al. Specific removal of beta1-adrenoceptor autoantibodies by immunoabsorption in rabbits with autoimmune cardiomyopathy improved cardiac structure and function. J Mol Cell Cardiol. 2006;41:78–85. doi: 10.1016/j.yjmcc.2006.04.016. doi:10.1016/j.yjmcc.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Mao W, Iwai C, Fukuoka S, Vulapalli R, Huang H, et al. Adoptive passive transfer of rabbit beta1-adrenoceptor peptide immune cardiomyopathy into the Rag2-/- mouse: participation of the ER stress. J Mol Cell Cardiol. 2008;44:304–314. doi: 10.1016/j.yjmcc.2007.11.007. doi:10.1016/j.yjmcc.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsui S, Fu M, Hayase M, Katsuda S, Yamaguchi N, Teraoka K, et al. Transfer of immune components from rabbit autoimmune cardiomyopathy into severe combined immunodeficiency (SCID) mice induces cardiomyopathic changes. Autoimmunity. 2006;39:121–128. doi: 10.1080/08916930500314855. doi:10.1080/08916930500314855. [DOI] [PubMed] [Google Scholar]
- 20.Matsui S, Persson M, Fu HM, Hayase M, Katsuda S, Teraoka K, et al. Protective effect of bisoprolol on beta-1 adrenoceptor peptide-induced autoimmune myocardial damage in rabbits. Herz. 2000;25:267–270. doi: 10.1007/s000590050018. doi:10.1007/s000590050018. [DOI] [PubMed] [Google Scholar]
- 21.Haberland A, Wallukat G, Dahmen C, Kage A, Schimke I. Aptamer neutralization of beta1-adrenoceptor autoantibodies isolated from patients with cardiomyopathies. Circ Res. 2011;109:986–992. doi: 10.1161/CIRCRESAHA.111.253849. doi:10.1161/CIRCRESAHA.111.253849. [DOI] [PubMed] [Google Scholar]
- 22.Jahns R, Schlipp A, Boivin V, Lohse MJ. Targeting receptor antibodies in immune cardiomyopathy. Semin Thromb Hemost. 2010;36:212–218. doi: 10.1055/s-0030-1251506. doi:10.1055/s-0030-1251506. [DOI] [PubMed] [Google Scholar]
- 23.Munch G, Boivin-Jahns V, Holthoff HP, Adler K, Lappo M, Truol S, et al. Administration of the cyclic peptide COR-1 in humans (phase I study): ex vivo measurements of anti-beta1-adrenergic receptor antibody neutralization and of immune parameters. Eur J Heart Fail. doi: 10.1093/eurjhf/hfs118. Advance Access published September 13, 2012, doi:10.1093/eurjhf/hfs/118. [DOI] [PubMed] [Google Scholar]
- 24.Nikolaev VO, Boivin V, Stork S, Angermann CE, Ertl G, Lohse MJ, et al. A novel fluorescence method for the rapid detection of functional beta1-adrenergic receptor autoantibodies in heart failure. J Am Coll Cardiol. 2007;50:423–431. doi: 10.1016/j.jacc.2007.03.051. doi:10.1016/j.jacc.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 25.Stork S, Boivin V, Horf R, Hein L, Lohse MJ, Angermann CE, et al. Stimulating autoantibodies directed against the cardiac beta1-adrenergic receptor predict increased mortality in idiopathic cardiomyopathy. Am Heart J. 2006;152:697–704. doi: 10.1016/j.ahj.2006.05.004. doi:10.1016/j.ahj.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Chiale PA, Ferrari I, Mahler E, Vallazza MA, Elizari MV, Rosenbaum MB, et al. Differential profile and biochemical effects of antiautonomic membrane receptor antibodies in ventricular arrhythmias and sinus node dysfunction. Circulation. 2001;103:1765–1771. doi: 10.1161/01.cir.103.13.1765. doi:10.1161/01.CIR.103.13.1765. [DOI] [PubMed] [Google Scholar]
- 27.Iwata M, Yoshikawa T, Baba A, Anzai T, Mitamura H, Ogawa S. Autoantibodies against the second extracellular loop of beta1-adrenergic receptors predict ventricular tachycardia and sudden death in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2001;37:418–424. doi: 10.1016/s0735-1097(00)01109-8. doi:10.1016/S0735-1097(00)01109-8. [DOI] [PubMed] [Google Scholar]
- 28.Pei J, Li N, Chen J, Li X, Zhang Y, Wang Z, et al. The predictive values of beta1-adrenergic and M2 muscarinic receptor autoantibodies for sudden cardiac death in patients with chronic heart failure. Eur J Heart Fail. 2012;14:887–894. doi: 10.1093/eurjhf/hfs082. doi:10.1093/eurjhf/hfs082. [DOI] [PubMed] [Google Scholar]
- 29.Felix SB, Staudt A, Landsberger M, Grosse Y, Stangl V, Spielhagen T, et al. Removal of cardiodepressant antibodies in dilated cardiomyopathy by immunoadsorption. J Am Coll Cardiol. 2002;39:646–652. doi: 10.1016/s0735-1097(01)01794-6. doi:10.1016/S0735-1097(01)01794-6. [DOI] [PubMed] [Google Scholar]
- 30.Ronspeck W, Brinckmann R, Egner R, Gebauer F, Winkler D, Jekow P, et al. Peptide based adsorbers for therapeutic immunoadsorption. Ther Apher Dial. 2003;7:91–97. doi: 10.1046/j.1526-0968.2003.00017.x. doi:10.1046/j.1526-0968.2003.00017.x. [DOI] [PubMed] [Google Scholar]
- 31.Wallukat G, Muller J, Hetzer R. Specific removal of beta1-adrenergic autoantibodies from patients with idiopathic dilated cardiomyopathy. N Engl J Med. 2002;347:1806. doi: 10.1056/NEJM200211283472220. doi:10.1056/NEJM200211283472220. [DOI] [PubMed] [Google Scholar]
- 32.Ma LP, Premaratne G, Bollano E, Lindholm C, Fu M. Interleukin-6-deficient mice resist development of experimental autoimmune cardiomyopathy induced by immunization of beta1-adrenergic receptor. Int J Cardiol. 2012;155:20–25. doi: 10.1016/j.ijcard.2011.01.085. doi:10.1016/j.ijcard.2011.01.085. [DOI] [PubMed] [Google Scholar]
- 33.Herda LR, Felix SB, Boege F. Drug-like actions of autoantibodies against receptors of the autonomous nervous system and their impact on human heart function. Br J Pharmacol. 2012;166:847–857. doi: 10.1111/j.1476-5381.2012.01828.x. doi:10.1111/j.1476-5381.2012.01828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. doi:10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Granier S, Kim S, Shafer AM, Ratnala VR, Fung JJ, Zare RN, et al. Structure and conformational changes in the C-terminal domain of the beta2-adrenoceptor: insights from fluorescence resonance energy transfer studies. J Biol Chem. 2007;282:13895–13905. doi: 10.1074/jbc.M611904200. doi:10.1074/jbc.M611904200. [DOI] [PubMed] [Google Scholar]
- 36.Staudt A, Staudt Y, Dorr M, Bohm M, Knebel F, Hummel A, et al. Potential role of humoral immunity in cardiac dysfunction of patients suffering from dilated cardiomyopathy. J Am Coll Cardiol. 2004;44:829–836. doi: 10.1016/j.jacc.2004.04.055. doi:10.1016/j.jacc.2004.04.055. [DOI] [PubMed] [Google Scholar]
- 37.Yudowski GA, Puthenveedu MA, Henry AG, von Zastrow M. Cargo-mediated regulation of a rapid Rab4-dependent recycling pathway. Mol Cell Biol. 2009;20:2774–2784. doi: 10.1091/mbc.E08-08-0892. doi:10.1091/mbc.E08-08-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoonakker ME, Ruiterkamp N, Hendriksen CF. The cAMP assay: a functional in vitro alternative to the in vivo Histamine Sensitization test. Vaccine. 2010;28:1347–1352. doi: 10.1016/j.vaccine.2009.11.009. doi:10.1016/j.vaccine.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Nguemo F, Saric T, Pfannkuche K, Watzele M, Reppel M, Hescheler J. In vitro model for assessing arrhythmogenic properties of drugs based on high-resolution impedance measurements. Cell Phys Biochem. 2012;29:819–832. doi: 10.1159/000188069. [DOI] [PubMed] [Google Scholar]
- 40.Wallukat G, Munoz Saravia SG, Haberland A, Bartel S, Araujo R, Valda G, et al. Distinct patterns of autoantibodies against G-protein-coupled receptors in Chagas’ cardiomyopathy and megacolon. Their potential impact for early risk assessment in asymptomatic Chagas’ patients. J Am Coll Cardiol. 2010;55:463–468. doi: 10.1016/j.jacc.2009.06.064. doi:10.1016/j.jacc.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 41.Liu HR, Zhao RR, Zhi JM, Wu BW, Fu ML. Screening of serum autoantibodies to cardiac beta1-adrenoceptors and M2-muscarinic acetylcholine receptors in 408 healthy subjects of varying ages. Autoimmunity. 1999;29:43–51. doi: 10.3109/08916939908995971. doi:10.3109/08916939908995971. [DOI] [PubMed] [Google Scholar]
- 42.Gagnon AW, Kallal L, Benovic JL. Role of clathrin-mediated endocytosis in agonist-induced down-regulation of the beta2-adrenergic receptor. J Biol Chem. 1998;273:6976–6981. doi: 10.1074/jbc.273.12.6976. doi:10.1074/jbc.273.12.6976. [DOI] [PubMed] [Google Scholar]
- 43.Liang W, Austin S, Hoang Q, Fishman PH. Resistance of the human beta1-adrenergic receptor to agonist-mediated down-regulation. Role of the C terminus in determining beta-subtype degradation. J Biol Chem. 2003;278:39773–39781. doi: 10.1074/jbc.M304482200. doi:10.1074/jbc.M304482200. [DOI] [PubMed] [Google Scholar]
- 44.Tutor AS, Penela P, Mayor F., Jr Anti-beta1-adrenergic receptor autoantibodies are potent stimulators of the ERK1/2 pathway in cardiac cells. Cardiovasc Res. 2007;76:51–60. doi: 10.1016/j.cardiores.2007.05.022. doi:10.1016/j.cardiores.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 45.Stavrakis S, Kem DC, Patterson E, Lozano P, Huang S, Szabo B, et al. Opposing cardiac effects of autoantibody activation of beta-adrenergic and M2 muscarinic receptors in cardiac-related diseases. Int J Cardiol. 2011;148:331–336. doi: 10.1016/j.ijcard.2009.11.025. doi:10.1016/j.ijcard.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staudt A, Eichler P, Trimpert C, Felix SB, Greinacher A. Fcγ receptors IIa on cardiomyocytes and their potential functional relevance in dilated cardiomyopathy. J Am Coll Cardiol. 2007;49:1684–1692. doi: 10.1016/j.jacc.2006.11.051. doi:10.1016/j.jacc.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 47.Fu LX, Magnusson Y, Bergh CH, Liljeqvist JA, Waagstein F, Hjalmarson A, et al. Localization of a functional autoimmune epitope on the muscarinic acetylcholine receptor-2 in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1993;91:1964–1968. doi: 10.1172/JCI116416. doi:10.1172/JCI116416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Limas CJ, Goldenberg IF, Limas C. Effect of antireceptor antibodies in dilated cardiomyopathy on the cycling of cardiac beta receptors. Am Heart J. 1991;122:108–114. doi: 10.1016/0002-8703(91)90766-b. doi:10.1016/0002-8703(91)90766-B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.