Abstract
Aims
We have reported that either toll-like receptor 4 deficiency (TLR4−/−) or TLR2 modulation protects against myocardial ischaemia/reperfusion (I/R) injury. The mechanisms involve attenuation of I/R-induced nuclear factor KappaB (NF-κB) activation. MicroRNA-146a (miR-146a) has been reported to target interleukin-1 receptor-associated kinase 1 (IRAK1) and tumor necrosis factor (TNF) receptor associated factor 6 (TRAF6), resulting in inhibiting NF-κB activation. This study examined the role of microRNA-146a in myocardial I/R injury.
Methods and results
We constructed lentivirus expressing miR-146a (LmiR-146a). LmiR-146a was transfected into mouse hearts through the right common carotid artery. The lentivirus vector (LmiR-Con) served as vector control. Untransfected mice served as I/R control. Sham operation served as sham control. Seven days after transfection, the hearts were subjected to ischaemia (60 min) followed by reperfusion (4 h). Myocardial infarct size was analysed by triphenyltetrazolium chloride (TTC) staining. In separate experiments, the hearts were subjected to ischaemia (60 min) followed by reperfusion for up to 7 days. Cardiac function was measured by echocardiography prior to I/R, 3 and 7 days after myocardial I/R. LmiR-146a transfection significantly decreased I/R-induced myocardial infarct size by 55% and prevented I/R-induced decreases in ejection fraction (EF%) and fractional shortening (%FS). LmiR-146a transfection attenuated I/R-induced myocardial apoptosis and caspase-3/7 and -8 activities. LmiR-146a transfection suppresses IRAK1 and TRAF6 expression in the myocardium. In addition, transfection of LmiR-146a prevented I/R-induced NF-κB activation and inflammatory cytokine production.
Conclusions
MicroRNA-146a protects the myocardium from I/R injury. The mechanisms may involve attenuation of NF-κB activation and inflammatory cytokine production by suppressing IRAK1 and TRAF6.
Keywords: MicroRNA-146a myocardial I/R, Toll-like receptors, NF-κB activation
1. Introduction
MicroRNAs are non-coding 12–23 nucleotide RNA molecules which regulate post-transcriptional gene expression by binding to target messenger RNAs (mRNAs). There is evidence that the expression of miRs affects many biological systems, including the mammalian immune system1–3 and the cardiovascular system.4–6 Several miRs have been reported to play a role in ischaemic heart disease.4,7,8 For example, miR-21 protects cells from oxidative stress-induced damage9 and the myocardium from ischaemic injury.9,10 MiR-320 is involved in I/R-induced cardiac injury and dysfunction via regulation of Hsp20.11
MicroRNAs have been reported to play a critical role in the negative regulation of innate immune and inflammatory responses.1–3 MiR-146 was first identified as a negative regulator in innate immune and inflammatory responses that are mediated by Toll-like receptors (TLRs). Taganov et al.12 have reported that stimulation of human monocytic THP-1 cells with lipopolysaccharides (LPS) rapidly induces the expression of both miR-146a and miR-146b. Interestingly, miR-146a directly targets IRAK1 and TRAF6, which are the key adapter molecules in the TLR/nuclear factor KappaB (NF-κB) pathway. This data suggest that miR-146a plays a negative regulatory role in the TLR-mediated NF-κB activation pathway.12
TLR-mediated innate immune and inflammatory responses are involved in myocardial ischaemia/reperfusion (I/R) injury.13 TLR-mediated signalling predominately activates nuclear factor KappaB (NF-κB), which is an important transcription factor controlling innate immune and inflammatory cytokine gene expression.14 We and other investigators have reported that modulation of the TLR4-mediated signalling pathway, or TLR4 deficiency, results in protection against myocardial I/R injury.15–17 TLR4 deficiency significantly decreases NF-κB binding activity following myocardial I/R injury.16,17 We have also reported that administration of the TLR2 ligands decreased myocardial I/R injury and attenuated I/R-induced NF-κB binding activity.18 Recently, miR-146a has been reported to serve as a negative regulator in inhibiting NF-κB activation.12 However, it is unclear whether TLR4 deficiency and TLR2 ligand will increase miR-146a expression in the myocardium, leading to down-regulation of NF-κB binding activity following myocardial I/R. In addition, the role of miR-146a in myocardial I/R injury has not been investigated.
In the present study, we examined whether increased expression of miR-146a will protect against myocardial I/R injury. We have observed that transfection of lentivirus expressing miR-146a (LmiR-146a) into the myocardium significantly reduced myocardial infarct size and prevented I/R-induced cardiac dysfunction. The data suggest that miR-146a could be the target for protection against myocardial I/R injury.
2. Methods
2.1. Animals
TLR4-deficient (TLR4−/−) and wild-type (WT) genetic background control mice were obtained from Jackson Laboratory as described in our previous studies.16,19 Mice were maintained in the Division of Laboratory Animal Resources, East Tennessee State University (ETSU). The experiments outlined in this manuscript conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication, 8th Edition, 2011). The animal care and experimental protocols were approved by the ETSU Committee on Animal Care.
2.2. qPCR assay of microRNAs
MicroRNAs were isolated from heart tissues or cultured cells using the mirVanaTM miR isolation kit (Ambion) in accordance with the manufacturer's protocol.20 Quantitative real-time (qPCR) was conducted using a 4800 Real-time PCR machine (Bio-Rad). MicroRNA levels were quantified by qPCR using specific Taqman assays for miR (Applied Biosystems, USA) and Taqman Universal Master Mix (Applied Biosystems). Specific primers for miR-146a were obtained from Applied Biosystems [primer identification numbers: 000468 for hsa-miR-146a and 001973 for U6 small nucleolar RNA (snRU6)]. MicroRNA-146a levels were quantified with the 2(-ΔΔct) relative quantification method that was normalized to the snRU6.
2.3. Construction of miR-146a into lentivirus expressing system
MicroRNA-146a was constructed into the lentivirus expression vector using a lentivirus expressing system (Invitrogen corporation) as described previously.20 Briefly, the oligonucleotides for miR-146a were synthesized at Integrated DNA Technologies, annealed and ligated into pcDNATM6.2-GW/ EmGFP-miR. The pcDNATM6.2-GW/EmGFP-miR cassette was subsequently transferred to pDONR221TM and finally pLenti6/V5-DEST by two sequential Gateway BP and LR recombinations. The lentiviral control vector contains a non-sense miR sequence that allows formation of a pre-miRNA hairpin predicted not to target any known vertebrate gene (Invitrogen Corporation). The viral particles were produced by third-generation packaging in 293FT cells and Lentiviral stocks were concentrated using ultracentrifugation.20
2.4. In vitro experiments
H9C2 cardiomyoblasts were plated in six-well plates at 1 × 105 cells/well. The cells were transfected with LmiR-146a or lentivirus expressing vector that served as control (LmiR-Con). Stably transfected cells were selected using a Blasticidin-resistant marker. The cells were subjected to hypoxia/reoxygenation as described previously.20 Briefly, the medium was changed to hypoxia-equilibrated medium (5% CO2 and 0.1% O2) immediately before the cells were incubated at 37°C with 5% CO2 and 0.1% O2 in a hypoxia chamber (Pro-Ox Model C21, BioSpherix Ltd, Redfield NY) for 2 h followed by reoxygenation for 24 h in an incubator with 5% CO220. The cells that were not subjected to H/R were incubated at 37°C with 5% CO2 for the same time periods and served as control (normoxia). There were six replicates in each group. The cells were harvested at 24 h for isolation of cellular protein.
2.5. In vivo transfection of lentivirus expressing miR-146a into mouse hearts
Mice were intubated and anaesthetized with mechanical ventilation using 5% isoflurane. Anaesthesia was maintained by inhalation of 1.5–2% isoflurane in 100% oxygen. The adequacy of anaesthesia was monitored by measuring heart rate and the response to tail stimulation. Body temperature was maintained at 37°C by surface water heating. An incision was made in the middle of the neck and the right common carotid artery was carefully exposed. The common carotid artery was isolated by temporary ligation of the proximal common carotid artery and proximal internal carotid artery. A micro-catheter was introduced into the isolated common carotid artery and positioned into the aortic root. One hundred microlitres of LmiR-146a (1 × 108 PFU) or LmiR-Con was injected through the micro-catheter. The micro-catheter was gently removed and the common carotid artery was tightened before the skin was closed. Intramuscular injection of LmiR-146a or LmiR-Con was performed as described previously.21 Briefly, after the left ventricle (LV) was exposed, a 30-gauge needle was advanced from the apex of LV along to the left anterior free wall adjacent to the ligated area. There were four injections with a total volume of 20 µL of LimR-146a or LmiR-Con. Seven days after transfection, the hearts were harvested for isolation of microRNAs. The expression of miR-146a in the heart tissues was examined by qPCR.
2.6. Induction of myocardial I/R injury
Myocardial I/R injury was induced as described previously.15,16,18 Briefly, TLR4−/− mice or mice that were treated with the TLR2 agonist, Pam3CSK4 (50 µg/25 g body weight) by ip injection for 1h18,22 were anaesthetized by 5.0% isoflurane, intubated, and ventilated with room air using a rodent ventilator. Anaesthesia was maintained by inhalation of 1.5–2% isoflurane driven by 100% oxygen flow. The adequacy of anaesthesia was monitored by measuring heart rate and the response to tail stimulation. Body temperature was regulated at 37°C by surface water heating. Following the skin incision, the hearts were exposed through a left thoracotomy in the fourth intercostal space. The left anterior descending (LAD) coronary artery was ligated with an 8–0 silk ligature over a 1 mm polyethylene tube (PE-10). After completion of 60 min of occlusion, the coronary artery was reperfused by pulling on the exteriorized suture to release the knot. The skin was closed, anaesthesia was discontinued, and the animals were allowed to recover in pre-warmed cages. After reperfusion for indicated time, the mice were euthanized by CO2 inhalation and the hearts were harvested.
2.7. Determination of myocardial infarct size
Infarct size was evaluated by triphenyltetrazolium chloride (TTC, Sigma-Aldrich) staining as described previously.15,16,18 Briefly, the hearts were perfused with saline on a Langendorff system to wash blood from the coronary vasculature. The LAD was re-ligated at the previous site of ligation prior to staining with 1% Evans Blue in order to assess area at risk. Each heart was then sliced horizontally to yield five slices. The slices were incubated in 1% TTC for 15 min at 37°C and fixed by immersion in 10% neutral buffered formalin. The area of infarction on both sides of each slice was determined by an image analyser, corrected for the weight of each slice, and summed for each heart. Ratios of risk area (RA) to LV area and infarct area to RA were calculated and expressed as a percentage.
2.8. Echocardiography
M-mode tracings were used to measure LV wall thickness, LV end-systolic diameter, and LV end-diastolic diameter. Per cent fractional shortening (%FS) and ejection fraction (EF) were calculated as described previously.18,22
2.9. In situ apoptosis assay
Myocardial apoptosis was examined as described previously15,16,18,23 using the in situ cell death detection kit, fluorescein (Roche, USA). Briefly, hearts were harvested and slices cut horizontally. One slice was immersion-fixed in 4% buffered paraformaldehyde, embedded in paraffin, and cut at a 5 µm thickness. The sections were incubated at 37°C for 1 h with the commercially prepared labelling mixture supplied by the manufacturer. The nuclei of living and apoptotic cells were counterstained with Hoechst 33342 (Invitrogen). Three slides from each block were evaluated for percentage of apoptotic cells and four fields on each slide were examined at the border areas using a defined rectangular field area with ×20 magnification. Numbers of apoptotic cardiac myocytes are presented as the percentage of total cells counted.
2.10. Accumulation of neutrophils
Neutrophil accumulation in the heart tissues was examined by staining with anti-neutrophil marker antibody (NIMO-R14, Santa Cruz Biotechnology) as described previously.24 Three slides from each block were evaluated and three different areas of each section were evaluated. The results are expressed as the numbers of macrophages/field (×40).
2.11. Western blot
Western blot was performed as described previously.15,16,18 Briefly, the cellular proteins were separated by SDS–polyacrylamide gel electrophoresis and transferred onto Hybond ECL membranes (Amersham Pharmacia, Piscataway, NJ, USA). The ECL membranes were incubated with the appropriate primary antibody anti-IRAK1 (sc-7883, Santa Cruz Biotechnology) and anti-TRAF6 (sc-7221, Santa Cruz Biotechnology), respectively, followed by incubation with peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Inc.) and analysis by the ECL system (Amersham Pharmacia, Piscataway). The signals were quantified using the G:Box gel imaging system by Syngene (Syngene, USA, Fredrick, MD, USA).
2.12. Electrophoretic mobility shift assay
Nuclear proteins were isolated from heart samples as previously described.15,16,18 NF-κB binding activity was measured using a LightShift Chemiluminescent EMSA kit (Thermo Fisher Scientific, Waltham, MA, USA).
2.13. Caspase-activity
Caspase-3/7 and -8 activities in heart tissues were measured as described previously25 using a Caspase-Glo assay kit (Promega).
2.14. ELISA for cytokine assay
The levels of cytokines (TNFα and IL-1β) were measured by ELISA using OptEIA cytokine kits (BD Biosciences).
2.15. Statistical analysis
The data are expressed as mean ± SE. Comparisons of data between groups were made using one-way analysis of variance (ANOVA), and Tukey's procedure for multiple-range tests was performed. P < 0.05 was considered to be significant.
3. Results
3.1. TLR4 deficiency and the TLR2 ligand, Pam3CSK4, increased the expression of miR-146a in the myocardium
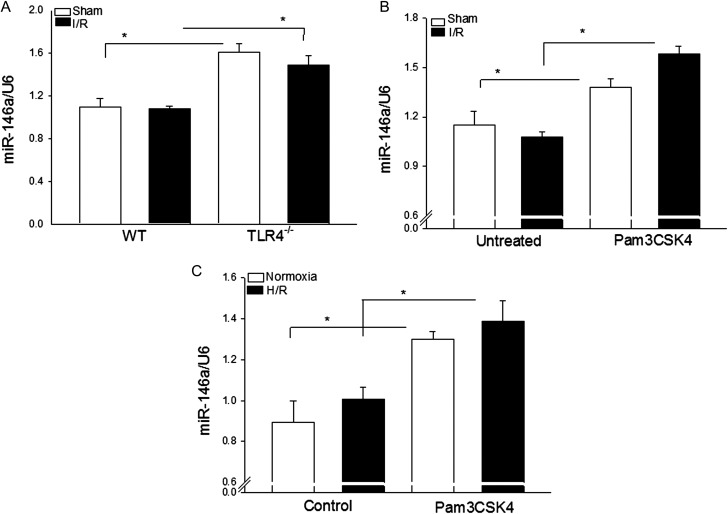
We examined the effect of TLR4 deficiency and TLR2 activation with Pam3CSK4 on miR-146a expression in the myocardium in the presence and absence of I/R. As shown in Figure 1, the levels of miR-146a in TLR4-deficient mice (A) and in Pam3CSK4-treated mice (B) were significantly increased in the presence or absence of I/R injury. I/R injury had no additional effect on myocardial miR-146a expression in both TLR4-deficient and Pam3CSK4-treated mice.
Figure 1.
TLR4 deficiency or TLR2 modulation with Pam3CSK4 increases the levels of miR-146a in the myocardium. (A) TLR4−/− (n = 4) and WT mice (n = 6) were subjected to myocardial ischaemia (60 min) followed by reperfusion (4 h). Sham surgery served as sham control (n = 4/group). (B) Mice were treated with and without Pam3CSK4 (50 µg/25 g body weight) 1h prior to myocardial ischaemia (60 min) followed by reperfusion (4 h) (n = 6–8/group). Sham surgical-operated mice treated with and without Pam3CSK4 served as sham control (n = 4/group). Hearts were harvested for qPCR analysis of miR-146a expression. (C) H9C2 cells were treated with or without Pam3CSK4 (1 µg/mL) 30 min before the cells were subjected to hypoxia (2 h) followed by reoxygenation (12 h). The cells were harvested for qPCR analysis of miR-146a expression. There were four duplicates. *P < 0.05 compared with indicated groups.
We examined whether the effect of Pam3CSK4 on myocardial miR-146a expression was direct or indirect. H9C2 cardiomyoblasts were treated with or without Pam3CSK4 (1 µg/mL) 30 min before the cells were subjected to hypoxia (2 h) followed by reoxygenation (12 h). The cells were harvested and miR-146a levels were measured by qPCR. Figure 1C shows that Pam3CSK4 treatment significantly increased the levels of miR-146a in H9C2 cells in the presence or absence of H/R, compared with the untreated control groups.
3.2. Transfection of LmiR-146a attenuates hypoxia/reoxygenation-induced injury in cardiomyoblasts
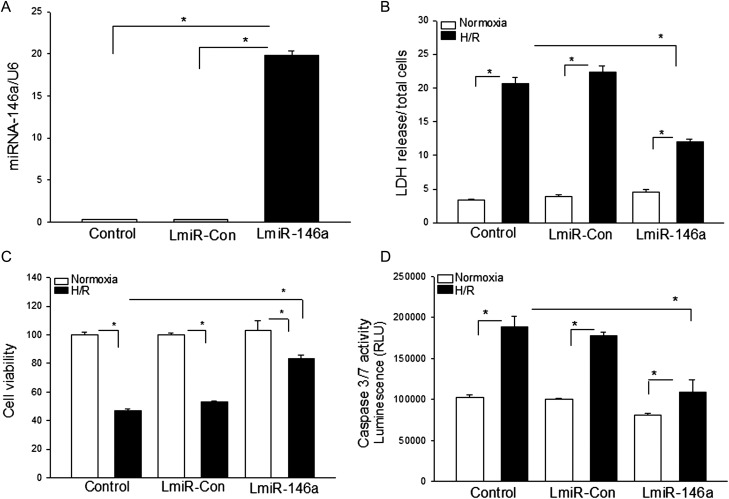
We evaluated the effect of increased expression of miR-146a on H/R-induced cellular injury. H9C2 cells were transfected with LmiR-146a. The lentivirus vector (LmiR-Con) served as control. A stably transfected cell line was established using antibiotic selection. qPCR data showed that the levels of miR-146a in LmiR-146a-transfected cells were significantly greater compared with miR-Con-transfected cells (Figure 2A). The cells were subjected to hypoxia (2 h) followed by reoxygenation (24 h). Untransfected H9C2 cells served as control. Figure 2B shows that H/R significantly increased LDH activity 5.1-fold compared with untransfected control (normoxia). H/R also markedly increased LDH activity 1.6-fold in LmiR-146a-transfected cells. However, LDH activity in LmiR-146a-transfected cells was significantly lower than in untreated H/R control cells, indicating that increased expression of miR-146a attenuated H/R-induced LDH activity. Transfection of LmiR-Con did not alter H/R-induced LDH activity.
Figure 2.
Transfection of LmiR-146a attenuated hypoxia/reoxygenation-induced cell injury and apoptosis in H9C2 cardiomyoblasts. H9C2 cardiomyoblasts were transfected with LmiR-146a or LmiR-Con. Stably transfected cell lines were selected using a Blasticidin-resistant marker. (A) The levels of miR-146a were significantly increased in LmiR-146a-transfected cells. (B–E) H9C2 cells and stably transfected cells were subjected to hypoxia (2 h) followed by reoxygenation (24 h) (H/R). Transfection of LmiR-146a attenuated H/R-increased LDH activity (B), decreased cell viability (C), and increased caspase-3/7 activity (D). There were four to six replicates in each group. *P < 0.05 compared with indicated groups.
H/R also significantly decreased cell viability by (50%) compared with the normoxic control group (Figure 2C). The cell viability in miR-146a-transfected cells was also significantly reduced by 25% following H/R compared with miR-126a-transfected non-H/R cells. However, H/R-induced decreases in cell viability were significantly attenuated by LmiR-146a transfection. In addition, transfection of LmiR-146a attenuated H/R-induced caspase-3 activity by 42.5% (Figure 2D). Transfection of LmiR-Con did not affect H/R-decreased cell viability and increased caspase-3/7 activity.
3.3. Transfection of LmiR-146a suppresses the expression of IRAK and TRAF6 in H9C2 cardiomyoblasts
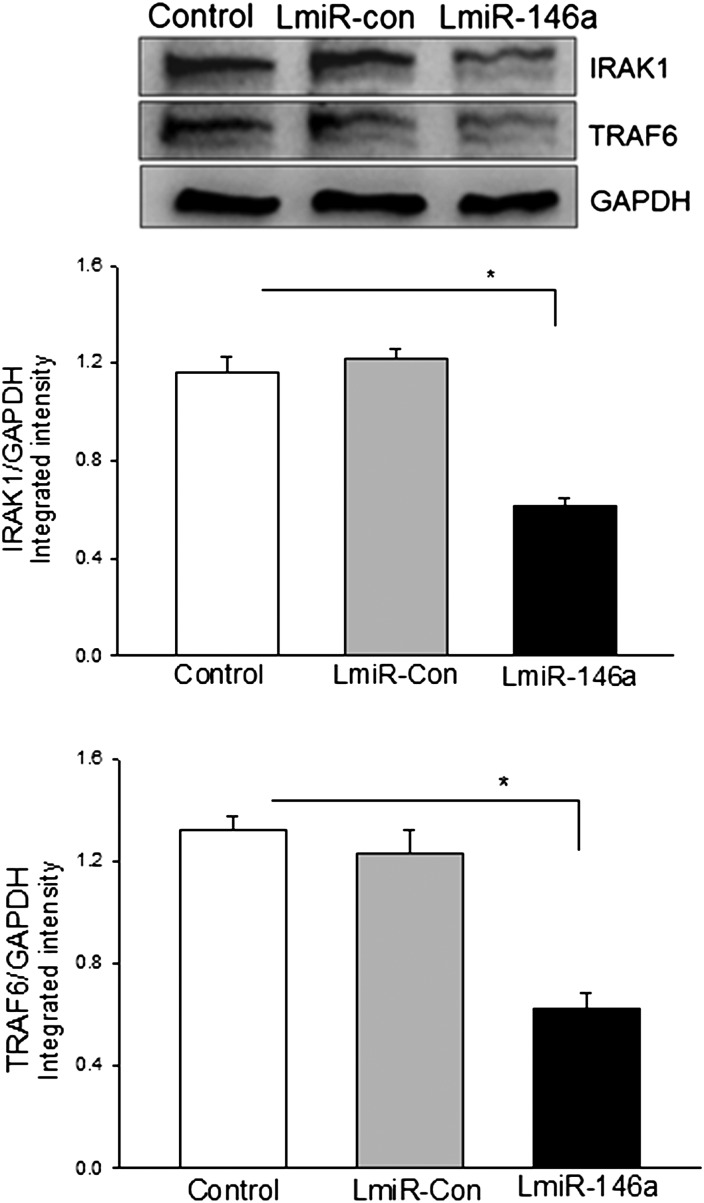
We examined the effect of increased expression of miR-146a by transfection of LmiR-146a on IRAK and TRAF6 expression in H9C2 cells. H9C2 cells were transfected with LmiR-146a or LmiR-Con. A stably transfected cell line was established using antibiotic selection. Western blot showed that the cells transfected with LmiR-146a had relatively less IRAK1 and TRAF6 compared with LmiR-Con-transfected cells (Figure 3).
Figure 3.
Transfection of LmiR-146a suppresses the expression of IRAK1 and TRAF6 in H9C2 cardiomyoblasts. Western blot showed that transfection of LmiR-146a suppresses IRAK1 and TRAF6 in H9C2 cells. n = 4/group. *P < 0.05 compared with indicated groups.
3.4. Increased miR-146a expression in the myocardium following LmiR-146a transfection
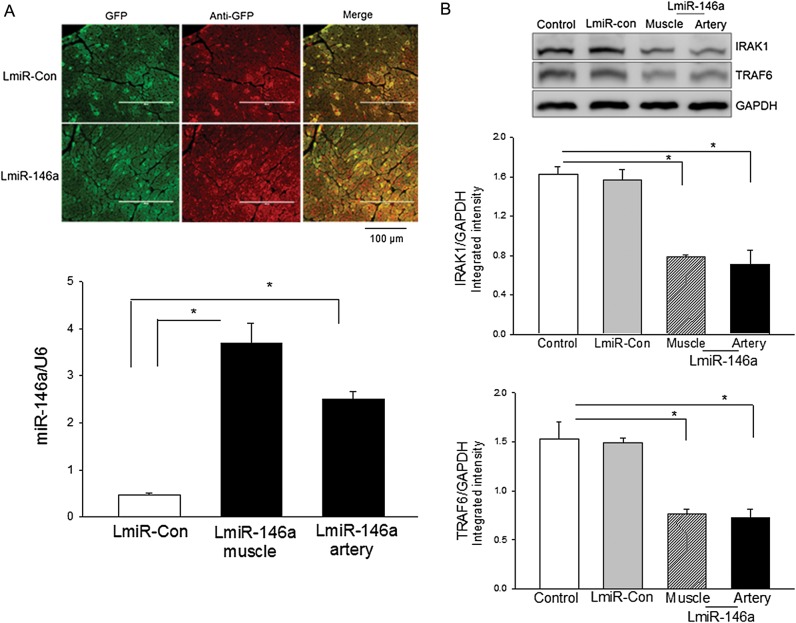
To evaluate whether increased expression of miR-146a will protect against myocardial I/R injury, we transfected mouse hearts with LmiR-146a through the right common carotid artery or directly injected into cardiac muscles. LmiR-Con served as transfection control. Seven days after transfection, we examined the transfection efficiency. Figure 4A shows that the expression of green fluorescent protein that was carried by the lentiviral vector appeared in the myocardium following transfection of LmiR-146a or LimR-Con. qPCR data show that the levels of miR-146a were significantly increased 6.9-fold in intramuscular injection of LmiR-146a and 4.4-fold in delivery of LmiR-146a via the right carotid artery compared with LmiR-Con transfected hearts.
Figure 4.
Myocardial transfection of LmiR-146a suppresses IRAK1 and TRAF6 expression. LmiR-146a or LmiR-Con was transfected into mouse hearts through the right common carotid artery or via direct injection into heart muscle. (A) The expression of GFP carried by the lentiviral vector appeared in the myocardium (top). The levels of miR-146a were increased following LmiR-146a transfection (bottom). (B) Transfection of LmiR-146a suppresses IRAK1 and TRAF6 in the myocardium. n = 3/group. *P < 0.05 compared with indicated groups. Scale bar = 100 µm.
We examined the effect of overexpression of miR-146a on the expression of IRAK1 and TRAF6 in the myocardium. Figure 4B shows that the levels of IRAK1 and TRAF6 in LmiR-146a transfected hearts, either by artery delivery or by direct muscle injection, were significantly lower than in LmiR-Con-transfected hearts. The data suggest that transfection of LmiR-146a suppresses the expression of IRAK1 and TRAF6. The data also suggest that miR-146a transfection efficiency is sufficient to down-regulate IRAK1 and TRAF6 expression in the myocardium.
3.5. Transfection of LmiR-146a into the myocardium protects against myocardial I/R injury and cardiac dysfunction
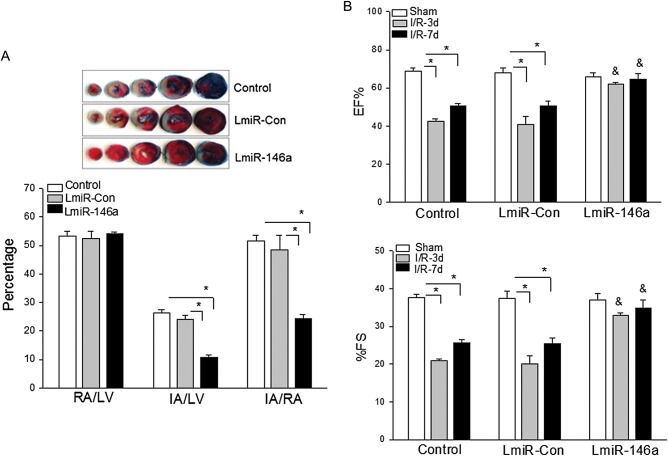
We examined whether increased expression of miR-146a will protect against myocardial I/R-induced injury. LmiR-146a and LmiR-Con were transfected into mouse hearts via the right carotid artery. Untransfected hearts served as control. The hearts were subjected to ischaemia (60 min) followed by reperfusion (4 h). The hearts were harvested for evaluation of infarct size. As shown in Figure 5A, I/R induced significant infarct size in untransfected I/R control hearts. However, LmiR-146a transfection markedly reduced infarct size by 50% compared with the untransfected I/R control group. LmiR-Con transfection did not alter I/R-induced myocardial infarct size.
Figure 5.
Transfection of LmiR-146a protects the myocardium from I/R injury. Mouse hearts were transfected with either LmiR-146a or LmiR-Con. Seven days after transfection, the hearts were subjected to I/R. (A) Transfection of LmiR-146a reduced myocardial infarct size. The infarct area (white) and the area at risk (red + white) from each section were measured using an image analyser. Ratios of risk area to left ventricle area (RA/LV) and infarct area to risk area (IA/RA) were calculated and are presented in the graphs. Photographs of representative heart sections are shown above. (B) Transfection of LmiR-146a attenuated I/R-induced cardiac dysfunction. Cardiac function was examined by echocardiography before I/R (Baseline) and at 3 and 7 days after I/R. n = 6–9/group. *P < 0.05 compared with indicated groups. &P < 0.05 compared with untransfected I/R control. EF, ejection fraction; FS: fractional shortening.
We also examined the effect of transfection of LmiR-146a on cardiac function following myocardial I/R. Cardiac function was measured by echocardiography prior to I/R (baseline) as well as 3 and 7 days after I/R. Figure 5B shows that EF% and %FS in the untransfected I/R control hearts were significantly reduced by 39.7 and 46.4% on Day 3 and by 28.1 and 34.8% on Day 7 after myocardial I/R compared with baseline. In contrast, transfection of LmiR-146a prevented I/R-induced cardiac dysfunction. EF% and %FS values in LmiR-146a-transfected mice were not significantly decreased at 3 and 7 days after myocardial I/R compared with the baseline of LmiR-146a-transfected hearts. Transfection of LmiR-Con did not alter I/R-induced cardiac dysfunction.
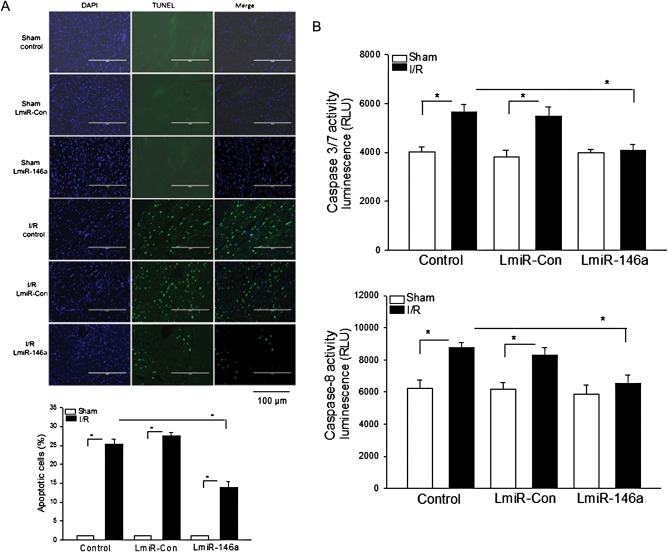
3.6. LmiR-146a transfection attenuated I/R-induced myocardial apoptosis
Cardiac myocyte apoptosis contributes to myocardial I/R injury.26 We evaluated whether increased expression of miR-146a by transfection of LmiR-146a would have anti-apoptotic effects during myocardial I/R. Figure 6A shows that I/R significantly induced myocardial apoptosis by 24% compared with the untransfected sham control. In LmiR-146a-transfected mice, the numbers of apoptotic cells in the myocardium were markedly less (11%) than in the untransfected I/R group (24%). LmiR-Con transfection did not affect I/R-induced myocardial apoptosis.
Figure 6.
Transfection of LmiR-146a attenuates I/R-induced myocardial apoptosis. Mouse hearts were transfected with either LmiR-146a or LmiR-Con. Seven days after transfection, the hearts were subjected to myocardial ischaemia (60 min) followed by reperfusion (4 h). Myocardial apoptosis was examined by the TUNEL assay in the heart sections. (A) DAPI-stained nuclei are blue and TUNEL-positive cells show green fluorescence. The bar graph shows the per cent of apoptotic cells. (B) Transfection of LmiR-146a attenuated I/R-induced caspase-3/7 and -8 activities in the myocardium. n = 5–6/group. *P < 0.05 compared with indicated groups. Scale bar = 100 µm.
I/R increased caspase-3/7 (31.4%) and caspase-8 (40.5%) activities in the myocardium compared with sham control (Figure 6B). In contrast, transfection of LmiR-146a prevented I/R-induced caspase-3/7 and -8 activities compared with the untransfected I/R control group. There was no significant difference in caspase-3/7 and -8 activities between LmiR-Con I/R mice and the untransfected I/R control group.
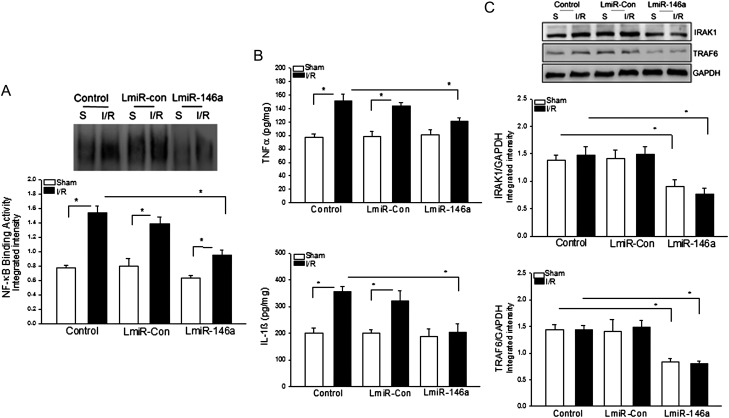
3.7. Transfection of LmiR-146a suppresses IRAK and TRAF6 expression and attenuates NF-κB activation and inflammatory cytokine production following I/R
I/R induces NF-κB activation, which plays a critical role in myocardial injury and cardiac dysfunction.27 We examined the effect of LmiR-146a transfection on NF-κB binding activity following myocardial I/R. Figure 7A shows that I/R significantly increased myocardial NF-κB binding activity compared with the untransfected sham control. Transfection of hearts with LmiR-Con did not decrease I/R-induced NF-κB binding activity. In contrast, transfection of LmiR-146a blunted I/R-induced NF-κB binding activity.
Figure 7.
Transfection of LmiR-146a attenuated I/R-induced NFκB activation and inflammatory cytokine production. Mouse hearts were transfected with either LmiR-146a or LmiR-Con. Seven days after transfection, the hearts were subjected to myocardial ischaemia (60 min) followed by reperfusion (4 h). Transfection of LmiR-146a attenuated I/R-induced NF-κB binding activity (A) and TNFα and IL-1β production (B), and suppressed IRAK1 and TRAF6 in the myocardium (C). n = 5–6/group. *P < 0.05 compared with indicated groups.
NF-κB activation regulates inflammatory cytokine production.14 Figure 7B shows that I/R significantly increased TNFα and IL-1β production in the serum, which positively correlated with NF-κB binding activity. In LmiR-146a-transfected mice, I/R-induced increases in TNFα and IL-1β production were attenuated. In contrast, there was no significant difference in cytokine production between the LmiR-Con-transfected I/R mice and the untransfected I/R control group.
To determine whether the inhibitory effect of transfection of LmiR-146a on NF-κB activation and cytokine production is due to suppression of IRAK1 and TRAF6 during myocardial I/R, we analysed the levels of IRAK1 and TRAF6 in the myocardium after myocardial I/R. Figure 7C shows that transfection of LmiR-146a significantly suppresses myocardial IRAK1 and TRAF6 expression in the presence and absence of I/R, compared with untransfected sham and I/R control groups. There is no significant difference in the levels of IRAK1 and TRAF6 between untransfected control mice and LmiR-Con transfected groups.
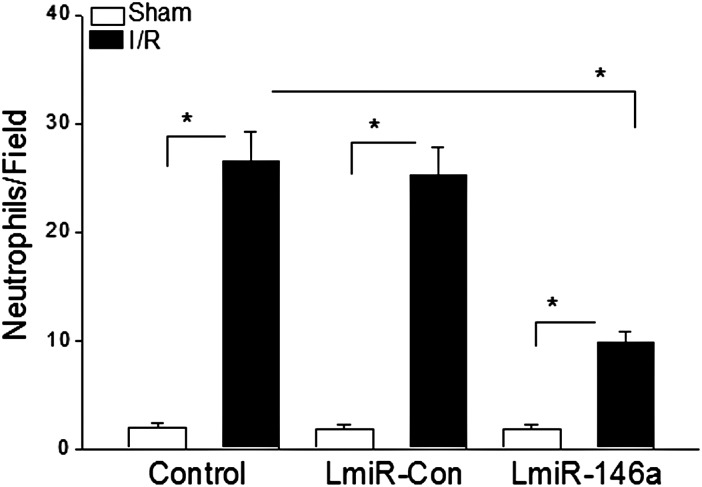
3.8. LmiR-146a transfection attenuated neutrophil infiltration into the myocardium following myocardial I/R
Neutrophil infiltration into the myocardium plays an important role in myocardial I/R injury. We examined the effect of LmiR-146a transfection on I/R-induced neutrophil infiltration into the myocardium. Figure 8 shows that there was more neutrophil infiltration in the untransfected I/R control mice than in the untransfected sham control group. I/R also increased the number of neutrophils in the LmiR-146a-transfected I/R hearts compared with the LmiR-146a-transfected sham control. However, the numbers of neutrophils in the LimiR-146a-transfected I/R hearts were significantly lower than in the untransfected I/R control group. Transfection of LmiR-Con did not alter I/R-induced increases in the numbers of neutrophil infiltration into the myocardium.
Figure 8.
Transfection of LmiR-146a attenuated I/R-induced neutrophil infiltration into the myocardium. Mouse hearts were transfected with either LmiR-146a or LmiR-Con. Seven days after transfection, the hearts were subjected to myocardial ischaemia (60 min) followed by reperfusion (4 h). The hearts were harvested and sectioned for immunohistochemical staining of neutrophils with anti-neutrophil antibody. The bar graph shows the numbers of neutrophils in examined fields. n = 3/group. *P < 0.05 compared with indicated groups.
4. Discussion
In the present study, we have demonstrated that transfection of LmiR-146a significantly decreases infarct size and improves cardiac function following myocardial I/R injury. To the best of our knowledge, this is the first report that miR-146a exerts a protective role in myocardial I/R injury. The protective mechanisms may involve attenuation of NF-κB activation via suppression of IRAK1 and TRAF6 expression by transfection of LmiR-146a.
Innate immune and inflammatory responses mediated by TLRs have been demonstrated to participate in the pathophysiological mechanisms of myocardial I/R injury.15–17 With the exception of TLR3, TLR-mediated signalling directly activates NF-κB28, which plays an important role in myocardial I/R injury.27 We and others have reported that TLR4 deficiency protects against myocardial I/R injury15–17 by attenuation of I/R-induced NF-κB activation.14 However, in addition to TLR4, other TLRs also activate NF-κB pathways.28 Why does TLR4 deficiency or TLR2 modulation attenuate NF-κB activity during myocardial I/R injury? We hypothesized that TLR4 deficiency or TLR2 modulation induces cardioprotection via up-regulation of miRs that target the TLR-mediated NF-κB signalling pathway. Indeed, we have found that the levels of miR-146a are significantly greater in the TLR4−/− hearts than in WT hearts. Similarly, TLR2 modulation by administration of Pam3CSK4 also significantly increased miR-146a levels in the myocardium. To evaluate the role of increased expression of miR-146a in the TLR-mediated NF-κB activation pathway, we transfected H9C2 cardiomyoblasts with either LimR-146a or LmiR-Con. We observed that LmiR-146a transfection significantly suppresses IRAK1 and TRAF6. Similarly, in vivo transfection of LmiR-146a markedly decreased the levels of IRAK1 and TRAF6 in the myocardium. The data indicated that increased expression of miR-146a by transfection of LmiR-146a suppresses IRAK1 and TRAF6 expression, resulting in attenuation of NF-κB activation during myocardial I/R.
Our observation is consistent with recent reports showing that miR-146a targets IRAK1 and TRAF6 expression.12 Taganov et al.12 reported that stimulation of THP-1 cells with LPS and cytokines significantly increased the expression of mature miR-146a. These authors demonstrated that there are several NF-κB binding sites in the promoter of miR-146a, suggesting that miR-146a expression induced by LPS and cytokines is NF-κB dependent.12,29 Importantly, miR-146a suppresses the expression of IRAK1 and TRAF6, resulting in inhibition of NF-κB binding activity and suppression of inflammatory cytokine production. The data suggest that miR-146a is a negative regulator in TLR-mediated innate immune and inflammatory responses via a finely tuned negative feedback regulatory loop.12,29
On the basis of published literature12,29 and our observation in vitro, we hypothesized that miR-146a plays a protective role in myocardial I/R injury via targeting the TLR-mediated NF-κB activation pathway.30 Indeed, we have found that transfection of LmiR-146a significantly decreases myocardial infarct size and improves cardiac function following myocardial I/R. LmiR-146a transfection also significantly attenuated I/R-induced myocardial NF-κB binding activity and serum levels of TNFα and IL-1β. Importantly, myocardial IRAK1 and TRAF6 levels were markedly decreased following LmiR-146a transfection. The data indicate that the suppression of IRAK and TRAF6 may be the mechanisms by which LmiR-146a transfection protects against myocardial I/R injury. We have previously reported that myocardial I/R increases IRAK phosphorylation and activation, which positively correlates with myocardial I/R injury.15 Thomas et al.31 reported that LPS administration induces IRAK1 activation in the heart. Hearts isolated from IRAK1-deficient mice showed a resistance to LPS-induced contractile dysfunction and attenuation of LPS-induced activation of NF-κB pathway.31 Collectively, the data suggest that IRAK1 plays a role in myocardial I/R injury and that it may be a potential target for the protection against myocardial I/R injury.
TRAF6 plays a crucial role in the induction of inflammatory responses via activation of IKK, leading to NF-κB activation.14,28 TRAF6 is utilized by TLR/IL-1R to activate NF-κB and MAPK signalling pathways.14,28 TRAF6 also contains a RING domain that confers E3 ligase activity32 which induces TRAF6 autoubiquitination via catalysation of lysine-63 (K63) polyubiquitination. K63 polyubiquitination activates IKK and MAPKs, as well as RIG-1 signal transduction at multiple points.32 Stimulation of RIG-I signalling also activates IKK, leading to NF-κB activation.32 Therefore, suppression of TRAF6 will significantly down-regulate inflammatory responses mediated by NF-κB and MAPK signalling pathways. We have observed that transfection of LmiR-146a decreases the levels of TRAF6 in the myocardium, which could be an important mechanism by which LmiR-146 transfection protects against myocardial I/R injury.
We have observed that transfection of LmiR-146a significantly attenuated I/R-induced myocardial apoptosis and caspase-3/7 and -8 activities in the myocardium. The data indicate that increased expression of miR-146a exerts anti-apoptotic properties. At present, we do not fully understand the anti-apoptotic mechanisms of miR-146a. However, a recent study by Suzuki et al.33 reported that diazoxide significantly improved mesenchymal stem cell survival via NF-κB-dependent miR-146a expression. Blockade of miR-146a expression by an antisense miR-146a inhibitor abolished diazoxide-induced cytoprotective effect. These authors found that overexpression of miR-146a down-regulates Fas expression via targeting the 3′ untranslated region of Fas mRNA.33 Targeting Fas by miR-146a may be a mechanism for attenuation of myocardial apoptosis during myocardial I/R by transfection of LmiR-146a.
In summary, the present study demonstrated that increased expression of miR-146a protects the myocardium from I/R injury. The mechanisms may involve suppression of IRAK1 and TRAF6 as well as NF-κB activation and cytokine production. MicroRNA-146a could be a target for protection against acute myocardial I/R injury.
Funding
This work was supported by NIH HL071837 to C.L., GM083016 to C.L., and D.L.W., GM53522 to D.L.W.
Conflict of interest: none declared.
Reference
- 1.Taganov KD, Boldin MP, Baltimore D. MicroRNAs and immunity: tiny players in a big field. Immunity. 2007;26:133–137. doi: 10.1016/j.immuni.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Sheedy FJ, O'Neill LAJ. Adding fuel to fire: microRNAs as a new class of mediators of inflammation. Ann Rheum Dis. 2008;67:iii50–iii55. doi: 10.1136/ard.2008.100289. [DOI] [PubMed] [Google Scholar]
- 3.Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol. 2008;18:131–140. doi: 10.1016/j.semcancer.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 4.van Rooij E, Marshall WS, Olson EN. Toward MicroRNA-based therapeutics for heart disease: the sense in antisense. Circ Res. 2008;103:919–128. doi: 10.1161/CIRCRESAHA.108.183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroen B, Heymans S. MicroRNAs and beyond: the heart reveals its treasures. Hypertension. 2009;54:1189–1194. doi: 10.1161/HYPERTENSIONAHA.109.133942. [DOI] [PubMed] [Google Scholar]
- 6.Fishtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 7.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Zhang X, Ren XP, Chen J, Liu H, Yang J, et al. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardial injury. Circulation. 2010;122:1308–1318. doi: 10.1161/CIRCULATIONAHA.110.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H(2)O(2)-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol. 2009;47:5–14. doi: 10.1016/j.yjmcc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, et al. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–2925. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, et al. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taganov KD, Boldin MP, Chang K-J, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. PNAS. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linde A, Mosier D, Blecha F, Melgarejo T. Innate immunity and inflammation: new frontiers in comparative cardiovascular pathology. Cardiovasc Res. 2007;73:26–36. doi: 10.1016/j.cardiores.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Ha T, Kelley J, Gao X, Qiu Y, Kao RL, et al. Modulating Toll-like receptor mediated signaling by (1–>3)-b-D-glucan rapidly induces cardioprotection. Cardiovasc Res. 2003;61:538–547. doi: 10.1016/j.cardiores.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, et al. Protection against myocardial ischemia/reperfusion injury in TLR4 deficient mice is mediated through a phosphoinositide 3-kinase dependent mechanism. J Immunol. 2007;178:7317–7324. doi: 10.4049/jimmunol.178.11.7317. [DOI] [PubMed] [Google Scholar]
- 17.Oyama J, Blais C, Jr, Liu X, Pu M, Kobzik L, Kelly RA, et al. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–9. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 18.Ha T, Hu Y, Liu L, Lu C, McMullen JR, Shioi T, et al. TLR2 ligands induce cardioprotection against ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Cardiovasc Res. 2010;87:694–703. doi: 10.1093/cvr/cvq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hua F, Ma J, Ha T, Xia Y, Kelley J, Williams DL, et al. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol. 2007;190:101–111. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren D, Wang X, Ha T, Liu L, Kalbfleisch J, Gao X, et al. SR-A deficiency reduces myocardial ischemia/reperfusion injury; involvement of increased microRNA-125b expression in macrophages. Biochim Biophys Acta. 2012;1832:336–346. doi: 10.1016/j.bbadis.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L, Ma W, Yang Z, Zhang F, Lu L, Ding Z, et al. VEGF165 and angiopoietin-1 decreased myocardium infarct size through phosphatidylinositol-3 kinase and Bcl-2 pathways. Gene Therapy. 2004;12:196–202. doi: 10.1038/sj.gt.3302416. [DOI] [PubMed] [Google Scholar]
- 22.Ha T, Lu C, Liu L, Hua F, Hu Y, Kelley J, et al. TLR2 ligands attenuate cardiac dysfunction in polymicrobial sepsis via a phosphoinositide-3-kinase dependent mechanism. Am J Physiol Heart Circ Physiol. 2010;298:H984–H991. doi: 10.1152/ajpheart.01109.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, et al. Lipopolysaccharide-induced myocardial protection against ischemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovascr Res. 2008;78:546–553. doi: 10.1093/cvr/cvn037. [DOI] [PubMed] [Google Scholar]
- 24.Gao M, Ha T, Zhang X, Liu L, Wang X, Kelley J, et al. Toll-like receptor 3 plays a central role in cardiac dysfunction during polymicrobial sepsis. Crit Care Med. 2012;40:2390–2399. doi: 10.1097/CCM.0b013e3182535aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu C, Hua F, Liu L, Ha T, Kalbfleisch J, Schweitzer J, et al. Scavenger receptor class-A has a central role in cerebral ischemia/reperfusion injury. J Cereb Blood Flow Metab. 2010;30:1972–1981. doi: 10.1038/jcbfm.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narula J, Hajjar RJ, Dec GW. Apoptosis in the failing heart. Cardiol Clin. 1998;16:691–710. doi: 10.1016/s0733-8651(05)70045-x. ix. [DOI] [PubMed] [Google Scholar]
- 27.Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, et al. In vivo transfection of cis element ‘decoy’ against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med. 1997;3:894–899. doi: 10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]
- 28.Zhang G, Ghosh S. Toll-like receptor-mediated NF-κB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest. 2001;107:13–19. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao JL, Rao D, Boldin MP, Taganov KD, O'Connell RM, Baltimore D. NF-kB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. PNAS. 2011;108:9184–9189. doi: 10.1073/pnas.1105398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma X, Buscaglia LEB, Barker JR, Li Y. MicroRNAs in NF-kB signaling. J Molecu C Bio. 2011;3:159–166. doi: 10.1093/jmcb/mjr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas JA, Haudek SB, Koroglu T, Tsen MF, Bryant DD, White DJ, et al. IRAK1 deletion disrupts cardiac Toll/IL-1 signaling and protect against contractile dysfunction. Am J Physiol Heart Circ Physiol. 2003;285:H597–H606. doi: 10.1152/ajpheart.0655.2001. [DOI] [PubMed] [Google Scholar]
- 32.Chen ZJ. Ubiquitination in signaling to and activation if IKK. Immunol Rev. 2012;246:95–106. doi: 10.1111/j.1600-065X.2012.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki Y, Kim HW, Ashraf M, Haider HKh. Diazoxide potentiates mesenchymal stem cell survival via NF-kappaB-dependent miR-146a expression by targeting Fas. Am J Physiol Heart Circ Physiol. 2010;299:H1077–H1082. doi: 10.1152/ajpheart.00212.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]