Abstract
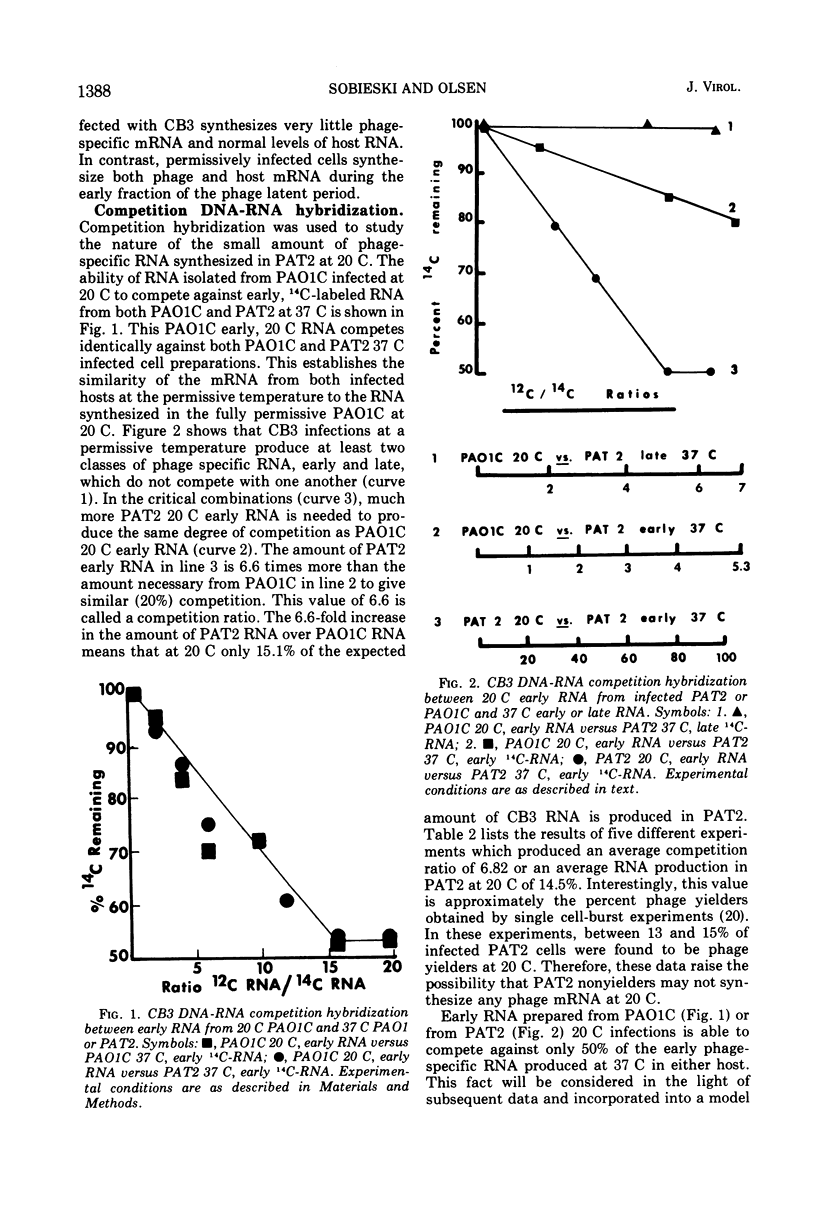
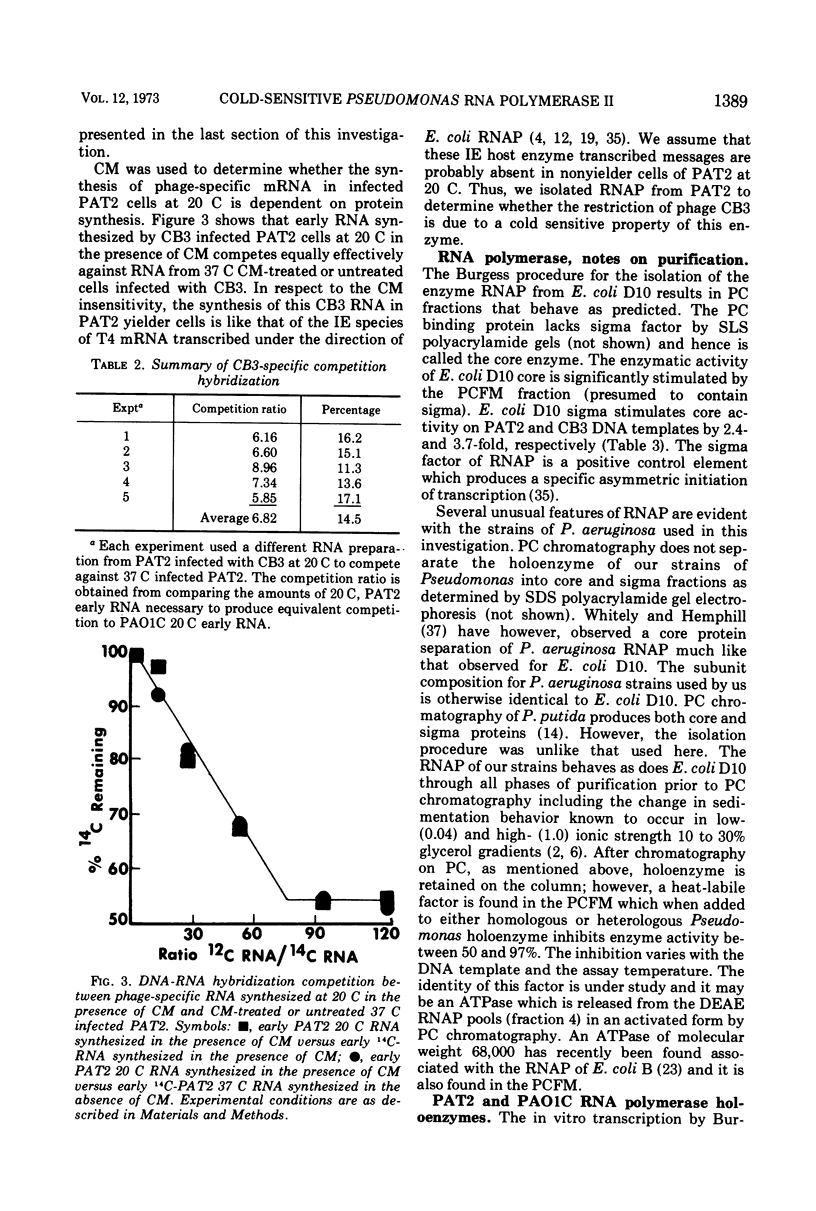
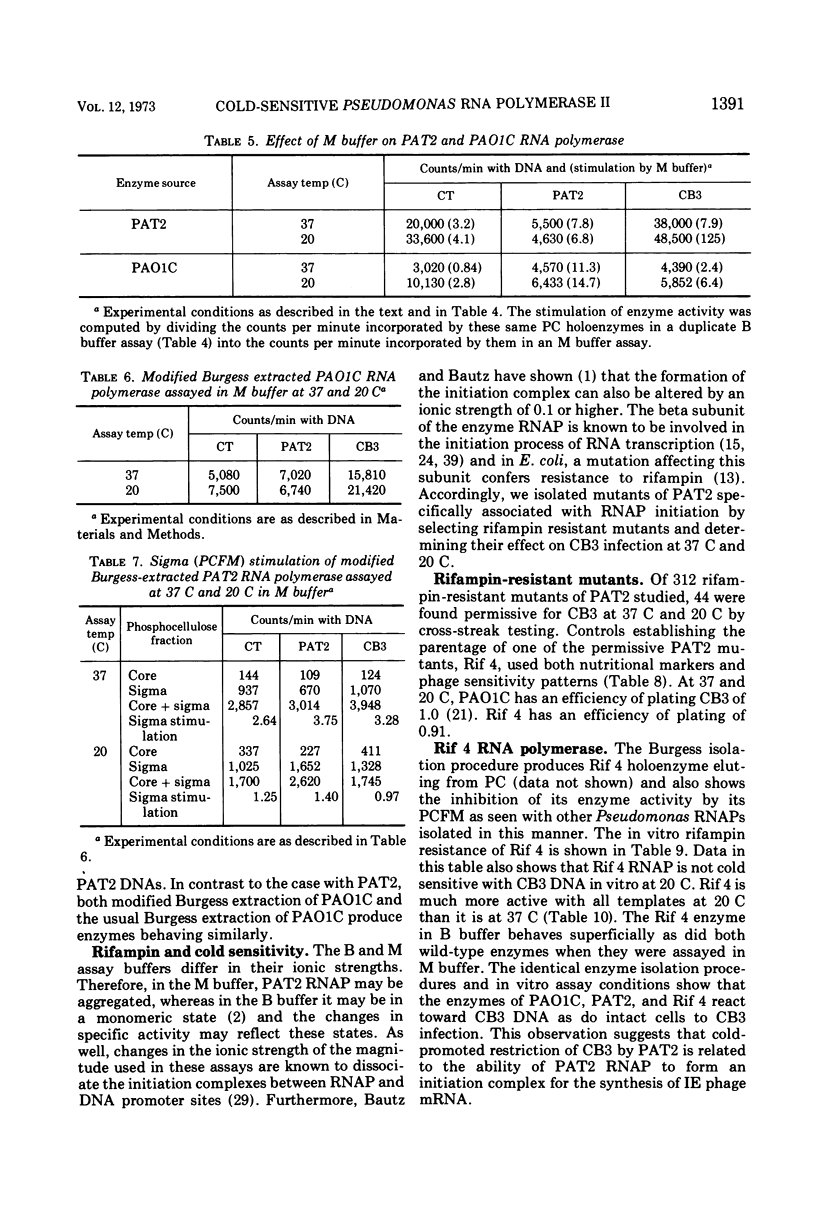
Cold-sensitive restriction of Pseudomonas phage CB3 by Pseudomonas aeruginosa strain PAT2 involves some aspect of CB3 specific RNA synthesis at 20 C. Experiments using chloramphenicol treatment and RNA-DNA hybridization establish that the amount of CB3 RNA present at 20 C is consistent with the known percentage of phage yielder cells at 20 C. Thus, it appears that nonyielder cells of PAT2 synthesize little or no phage-specific mRNA. Burgess technique extracted PAT2 RNA polymerase (RNAP) is cold sensitive when assayed in vitro with CB3 DNA at 20 C. However, it is not cold sensitive when either calf thymus or PAT2 DNA are the templates for transcription. Low ionic strength assay conditions eliminate the cold sensitivity of PAT2 RNAP. The effect of low ionic environments on transcription initiation along with the in vivo and in vitro suppression of cold sensitivity by host rifampin resistance suggests that the inability of CB3 to reproduce in PAT2 at 20 C is a cold-sensitive step in host RNAP initiation. Our modified RNAP extraction procedure for PAT2 and PAO1C also results in the recovery of cold-sensitive PAT2 RNAP with respect to CB3 DNA templates and points to basic enzymological differences between the two hosts. A model is presented for the unusual influence of temperature on the initiation process of both PAT2 and PAO1C on RNAP transcription.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bautz E. K., Bautz F. A. Initiation of RNA synthesis: the function of sigma in the binding of RNA polymerase to promoter sites. Nature. 1970 Jun 27;226(5252):1219–1222. doi: 10.1038/2261219a0. [DOI] [PubMed] [Google Scholar]
- Berg D., Chamberlin M. Physical studies on ribonucleic acid polymerase from Escherichia coli B. Biochemistry. 1970 Dec 22;9(26):5055–5064. doi: 10.1021/bi00828a003. [DOI] [PubMed] [Google Scholar]
- Bremer H., Konrad M., Bruner R. Capacity of T4 DNA to serve as template for purified Escerichia coli RNA polymerase. J Mol Biol. 1966 Mar;16(1):104–117. doi: 10.1016/s0022-2836(66)80266-8. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Weber C. S. Gene linkage by RNA-DNA hybridization. I. Unique DNA sequences homologous to 4 s RNA, 5 s RNA and ribosomal RNA. J Mol Biol. 1968 Jun 28;34(3):661–680. doi: 10.1016/0022-2836(68)90188-5. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Fuchse, Millette R. L., Zillig W., Walter G. Influence of salts on RNA synthesis by DNA-dependent RNA-polymerase from Escherichia coli. Eur J Biochem. 1967 Dec;3(2):183–193. doi: 10.1111/j.1432-1033.1967.tb19514.x. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Grasso R. J., Buchanan J. M. Synthesis of early RNA in bacteriophage T4-infected Escherichia coli B. Nature. 1969 Nov 29;224(5222):882–885. doi: 10.1038/224882a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. C., DeBacker M., Boezi J. A. Deoxyribonucleic acid-dependent ribonucleic acid polymerase of Pseudomonas putida. J Biol Chem. 1971 Mar 10;246(5):1222–1232. [PubMed] [Google Scholar]
- Losick R., Shorenstein R. G., Sonenshein A. L. Structural alteration of RNA polymerase during sporulation. Nature. 1970 Aug 29;227(5261):910–913. doi: 10.1038/227910a0. [DOI] [PubMed] [Google Scholar]
- Matsukage A. The effects of KC1 concentration on the transcription by E. coli RNA polymerase. I. Specific effect of the combination of nucleoside triphosphates. Mol Gen Genet. 1972;118(1):11–22. doi: 10.1007/BF02428328. [DOI] [PubMed] [Google Scholar]
- Matsukage A. The effects of KC1 concentration on the transcription by E. coli RNA polymerase. II. Effect on the specificity of T7 transcription. Mol Gen Genet. 1972;118(1):23–31. doi: 10.1007/BF02428329. [DOI] [PubMed] [Google Scholar]
- Milanesi G., Brody E. N., Grau O., Geiduschek E. P. Transcriptions of the bacteriophage T4 template in vitro: separation of "delayed early" from "immediate early" transcription. Proc Natl Acad Sci U S A. 1970 May;66(1):181–188. doi: 10.1073/pnas.66.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- Olsen R. H., Metcalf E. S., Brandt C. Conditional temperature-sensitive restriction of Pseudomonas bacteriophge CB3. J Virol. 1968 Dec;2(12):1393–1399. doi: 10.1128/jvi.2.12.1393-1399.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H., Metcalf E. S., Todd J. K. Characteristics of bacteriophages attacking psychrophilic and mesophilic pseudomonads. J Virol. 1968 Apr;2(4):357–364. doi: 10.1128/jvi.2.4.357-364.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paetkau V., Coy G. On the purification of DNA-dependent RNA polymerase from E. coli: removal of an ATPase. Can J Biochem. 1972 Feb;50(2):142–150. doi: 10.1139/o72-018. [DOI] [PubMed] [Google Scholar]
- Rabussay D., Zillig W. A rifampicin resistent rna-polymerase from E. coli altered in the beta-subunit. FEBS Lett. 1969 Oct 21;5(2):104–106. doi: 10.1016/0014-5793(69)80305-4. [DOI] [PubMed] [Google Scholar]
- Reid P. Isolation of cold sensitive-rifampicin resistant RNA polymerase mutants of Escherichia coli. Biochem Biophys Res Commun. 1971 Aug 6;44(3):737–744. doi: 10.1016/s0006-291x(71)80145-6. [DOI] [PubMed] [Google Scholar]
- Richardson J. P. Reinitiation of RNA chain synthesis in vitro. Nature. 1970 Mar 21;225(5238):1109–1112. doi: 10.1038/2251109a0. [DOI] [PubMed] [Google Scholar]
- Richardson J. P. Some physical properties of RNA polymerase. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1616–1623. doi: 10.1073/pnas.55.6.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So A. G., Davie E. W., Epstein R., Tissières A. Effects of cations on DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1739–1746. doi: 10.1073/pnas.58.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So A. G., Downey K. M. Studies on the mechanism of ribonucleic acid synthesis. II. Stabilization of the deoxyribonucleic acid-ribonucleic acid polymerase complex by the formation of a single phosphodiester bond. Biochemistry. 1970 Nov 24;9(24):4788–4793. doi: 10.1021/bi00826a024. [DOI] [PubMed] [Google Scholar]
- Sobieski R. J., Olsen R. H. Cold-sensitive Pseudomonas RNA polymerase. I. Characterization of the host dependent cold-sensitive restriction of phage CB3. J Virol. 1973 Dec;12(6):1375–1383. doi: 10.1128/jvi.12.6.1375-1383.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M., Okamoto T., Takanami M. RNA polymerase sigma-factor and the selection of initiation site. Nature. 1970 Feb 14;225(5233):598–600. doi: 10.1038/225598a0. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Siegel R. B. Control of template specificity of E. coli RNA polymerase by a phage-coded protein. Nature. 1969 Sep 13;223(5211):1111–1113. doi: 10.1038/2231111a0. [DOI] [PubMed] [Google Scholar]
- Taylor K., Hradecna Z., Szybalski W. Asymmetric distribution of the transcribing regions on the complementary strands of coliphage lambda DNA. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1618–1625. doi: 10.1073/pnas.57.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Whiteley H. R., Hemphill H. E. The interchangeability of stimulatory factors isolated from three microbial RNA polymerases. Biochem Biophys Res Commun. 1970 Nov 9;41(3):647–654. doi: 10.1016/0006-291x(70)90062-8. [DOI] [PubMed] [Google Scholar]
- Witmer H. J. Effect of ionic strength and temperature on the in vitro transcription of T 4 DNA. Biochim Biophys Acta. 1971 Aug 12;246(1):29–43. doi: 10.1016/0005-2787(71)90069-4. [DOI] [PubMed] [Google Scholar]


