Abstract
The puzzle piece-shaped Arabidopsis leaf pavement cells (PCs) with interdigitated lobes and indents is a good model system to investigate the mechanisms that coordinate cell polarity and shape formation within a tissue. Auxin has been shown to coordinate the interdigitation by activating ROP GTPase-dependent signaling pathways. To identify additional components or mechanisms, we screened for mutants with abnormal PC morphogenesis and found that cytokinin signaling regulates the PC interdigitation pattern. Reduction in cytokinin accumulation and defects in cytokinin signaling (such as in ARR7-over-expressing lines, the ahk3cre1 cytokinin receptor mutant, and the ahp12345 cytokinin signaling mutant) enhanced PC interdigitation, whereas over-production of cytokinin and over-activation of cytokinin signaling in an ARR20 over-expression line delayed or abolished PC interdigitation throughout the cotyledon. Genetic and biochemical analyses suggest that cytokinin signaling acts upstream of ROPs to suppress the formation of interdigitated pattern. Our results provide novel mechanistic understanding of the pathways controlling PC shape and uncover a new role for cytokinin signaling in cell morphogenesis.
Keywords: Arabidopsis, pavement cells, cytokinin, cell morphogenesis
Introduction
Cytokinin is a crucial phytohormone that modulates many important developmental processes and responses to the environment including embryogenesis, seed development, organogenesis, vascular patterning, senescence, and stress tolerance1,2,3,4,5,6,7,8. At the cellular level, cytokinin is known to promote cell division, stem-cell specification, and chloroplast biogenesis9,10,11,12. The major cytokinin biosynthetic pathway is mediated by adenosine phosphate-isopentenyltransferase (IPT), which catalyzes the first and rate-limiting step13,14. The structural diversity of natural cytokinins and modifications of the adenine moiety confer specificity of the cytokinin receptor interaction14. Cytokinin homeostasis is achieved through regulated conjugation or irreversible degradation15, and cytokinin oxidase/dehydrogenase (CKX) catalyzes the irreversible degradation by cleavage of the side chain14. In Arabidopsis, three Arabidopsis thaliana histidine kinases serve as cytokinin receptors: AHK2, AHK3, and AHK4/CRE1/WOL16,17,18. The receptors are activated by autophosphorylation at a conserved histidine residue, and the phosphoryl group is transferred to Arabidopsis histidine phosphotransfer proteins (AHPs)19. AHPs (AHP1-AHP5) and nuclear response regulators serve as cytokinin signaling transcriptional regulators3. Based on domain structures and amino acid sequence similarities, the 22 Arabidopsis response regulators (ARRs) can be divided into two classes, type-A ARRs (ARR3-ARR9, ARR15-ARR17, ARR22) and type-B ARRs (ARR1, ARR2, ARR10-ARR14, ARR18-ARR21)18,20. In general, type-B ARRs positively regulate the expression of cytokinin-induced genes, whereas type-A ARRs negatively regulate the gene expression21,22,23,24.
Arabidopsis leaf pavement cells (PCs) form an interdigitated pattern with complementary lobes and indentations through the development of interdigitated multi-polarity and subsequent local expansion at each polar site25. Thus, the generation of interdigitated lobes and indentations in the two-dimensional plane of the leaf epidermis provides a nice model system to investigate the planar coordination of cell polarity and morphogenesis among cells within a tissue25,26,27,28,29,30,31. Our recent studies suggest that auxin locally coordinates lobe formation with indentation by activating two counteracting Rho GTPase-based signaling pathways: the lobe-promoting ROP2 pathway localized to the lobe-forming region, and the lobe-restricting (indentation-promoting) ROP6 pathway localized to the indenting region of the plasma membrane (PM)28,29,30. The ROP2 pathway induces the formation of cortical F-actin, inhibiting the internalization of PIN1 and leading to its polarized enrichment at the lobing region28,30,32. The ROP2 and ROP6 pathways mutually inhibit each other, maintaining the separation of ROP2 and ROP6 function and coordinating PM lobe and indentation formation within the PC28,29,30. The interdigitation of lobes and indentations is also coordinated between neighboring PCs30. Through stimulation of the putative cell surface auxin receptor ABP1, PIN1-exported extracellular auxin is proposed to activate not only the ROP2 pathway in the lobing regions, but also the ROP6 pathway in the complementary indenting regions of neighboring cells28,29,30. Thus, auxin acts as a self-organizing signal to locally coordinate the establishment of multi-polarity for the generation of the interdigitated cell pattern in the leaf epidermis33. However, it is unknown whether other signaling pathways also regulate the PC interdigitation and cell morphogenesis.
In this report, we demonstrate that AHK3/CRE1- and ARR-dependent cytokinin signaling modulates the PC interdigitation in Arabidopsis. Our findings reveal a new mechanism for the regulation of cell polarity and morphogenesis as well as a novel function for cytokinin in the modulation of these fundamental cellular processes.
Results
Identification of cytokinin-signaling components regulating the interdigitated cell pattern in PCs
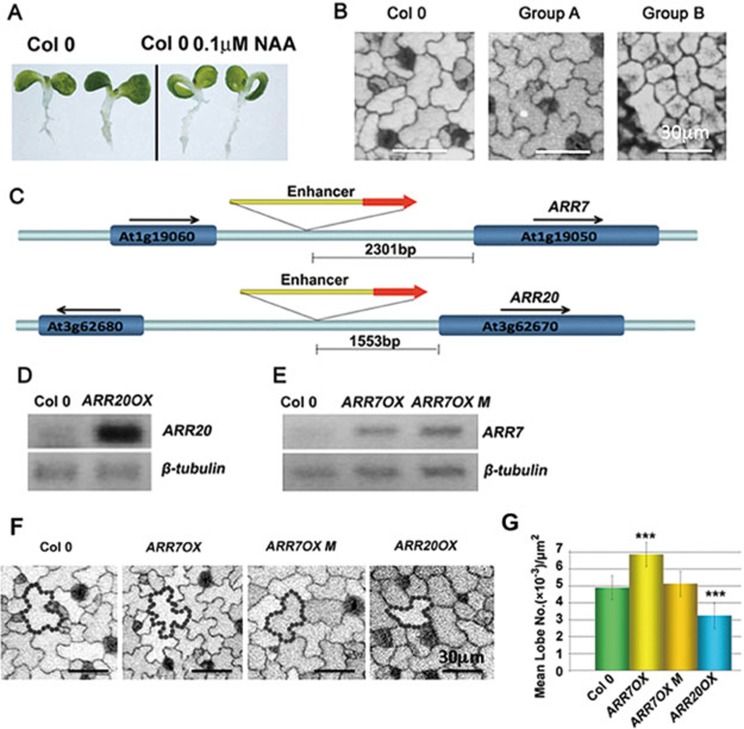
To investigate the mechanisms underlying the interdigitated pattern of PC expansion, we screened for T-DNA insertion mutations altering the interdigitation pattern in cotyledon PCs. We previously found that bathing germinating seedlings in a liquid medium containing 5-20 nM synthetic auxin Naphthalene-1-acetic acid (NAA) induced rapid interdigitation of all PCs in cotyledons/leaves30. In addition, we observed the downward curling of cotyledons from one-week-old seedlings after incubation with liquid medium containing auxin (Figure 1A; Supplementary information, Figure S1A and S1B). Thus, the progressive activation of PC interdigitation, which is accompanied by extensive cell expansion, may be important to maintain the flat cotyledon surface. We reasoned that mutations that cause insensitivity to the auxin induction of cotyledon curling might identify mutations affecting the coordination of PC interdigitation. Consistent with this notion, we found that the auxin receptor tir1 afb1 afb2 afb3 quadruple mutant and previously studied PC-defective mutants abp1-5 and rop2-1 rop4-130 showed a relatively flat cotyledon surface phenotype after NAA treatment (Supplementary information, Figure S2). Therefore, we screened an activation-tagged T-DNA insertional mutant library for such mutants and then analyzed their PC interdigitation. We screened 240 000 seedlings that covered 62 000 individual lines from 208 activation-tagging pools. This screen, along with a screen for abnormal leaf shape mutants, identified two groups of mutants with altered PC interdigitation patterning: group A with PCs having premature and enhanced interdigitation and group B with PCs showing reduced interdigitation (Figure 1B). From each group of more than 100 candidates, we cloned 11 genes belonging to group A and 12 genes in group B.
Figure 1.
Genetic screen leading to the isolation of cytokinin signaling mutants that are altered in the PC interdigitation pattern. (A) Treatment with 0.1 μM NAA in liquid medium induced downward curling of cotyledons in 1-week-old Col-0 seedlings. (B) Representative images of 2-DAG (Days After Germination) cotyledons with altered PC interdigitation patterns in two groups of mutants. Group A mutants displayed more lobes, while group B mutants showed fewer lobes. Bars = 30 μm. (C) T-DNA insertion sites in ARR7 (At1g19050) and ARR20 (At3g62670) genes. The 35S enhancer sequences were inserted 2 301 bp and 1 553 bp upstream of the start codons of ARR7 and ARR20, respectively. (D) RT-PCR analysis of ARR20 transcript levels in the ARR20OX T-DNA insertion line. (E) RT-PCR analysis of ARR7OX and ARR7OX M transcript levels in the ARR70X and ARR7OX M lines. ARR7OX M (D85N) contains a point mutation in the D85 phosphorylation site that abolishes the function of ARR724. (F) Representative images of PC morphology in 2-DAG cotyledons of the ARR7OX, ARR7OX M and ARR20OX lines in the absence of benzyladenine (BA). Bars = 30 μm. (G) Quantitative analysis of lobe number per area (mean ± SEM) in the lines described in (F). Quantitative analysis of cotyledon PC shape in greater than 400 PCs. The quantification of PC interdigitation is according to a previously described method30. Asterisks indicate significant differences from the wild-type control (P < 0.001, t test).
TAIL-PCR analysis revealed that each group contained an ARR gene that participates in cytokinin signaling9,22,34. Group A includes ARR7 (Supplementary information, Figure S2), a type-A ARR, that negatively regulates cytokinin signaling through repression of type-B ARRs. Group B includes ARR20, a type-B ARR, that positively regulates cytokinin signaling. The T-DNAs were inserted 2 301 bp and 1 553 bp upstream of the ARR7 and ARR20 start codons, respectively, suggesting that both genes were activation-tagged (Figure 1C). A semi-quantitative RT-PCR analysis confirmed that ARR20 was strongly over-expressed in the activation-tagged line designated as ARR20OX (Figure 1D). Quantitative analysis of the lobing phenotype showed that the number of lobes was greatly reduced in cotyledon PCs of the ARR20OX line (Figure 1F and 1G). In the T-DNA insertion line from group A, a moderate increase in ARR7 mRNA level was observed in the T-DNA insertion line. To determine whether the enhanced lobing phenotype was due to ARR7 over-expression, we analyzed PC shapes in a previously described p35S::ARR7 line designated as ARR7OX24. ARR7OX PCs exhibited a significantly increased number of lobes (Figure 1E-1G). Over-expression of the ARR7 (D85N) mutant designated as ARR7OX M, which has a defective phospho-accepting site and is unable to transmit cytokinin signal24, did not alter the PC interdigitation pattern (Figure 1F and 1G). Therefore, we hypothesize that cytokinin signaling might regulate PC interdigitation.
Regulation of cytokinin levels affects PC interdigitation
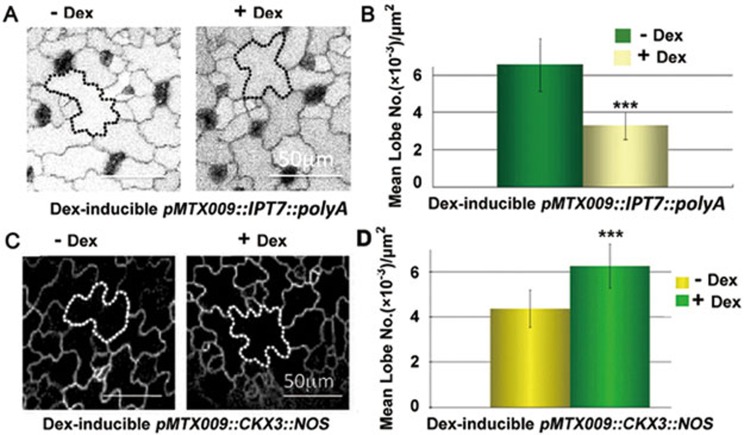
Cytokinin is a multi-functional hormone that modulates many growth and developmental processes in plants. To determine whether cytokinin directly regulates PC interdigitation patterns, we induced changes in cytokinin levels in cotyledons through the dexamethasone (DEX)-induced expression of adenylate isopentyltransferase 7 (IPT7) or cytokinin oxidase 3 (CKX3). IPT7 catalyzes the rate-limiting first step of cytokinin biosynthesis14, while CKX3 catalyzes cytokinin degradation14. DEX induction of IPT7 expression in cotyledons greatly reduced PC lobe formation (Figure 2A and 2B). Treatment of cotyledons with exogenous cytokinin (50 nM) also inhibited PC interdigitation (Figure 3C and 3D). On the contrary, DEX induction of CKX3 expression in cotyledons significantly enhanced PC interdigitation (Figure 2C and 2D). These results, together with the effects of ARR7 and ARR20 over-expression, demonstrate that cytokinin inhibits PC interdigitation.
Figure 2.
Cytokinin biosynthesis inhibits PC interdigitation. (A) Representative images of PC interdigitation in the 2 days presence or absence of DEX induced expression of IPT7 (cytokinin biosynthetic enzyme) after 3-DAG pMTX009::IPT7::polyA seedlings. Bars = 50 μm. (B) Quantitative analysis of lobe number per area (mean ± SEM) after induction of IPT7 expression by DEX treatment as described in (A). Asterisks indicate significant differences from untreated seedlings (P < 0.001, t test). (C) Representative images of PC interdigitation in the 2 days presence or absence of DEX induced expression of CKX3 (cytokinin degradation enzyme) after 2-DAG pMTX009::CKX3::NOS seedlings. Bars = 50 μm. (D) Quantitative analysis of lobe number per area (mean ± SEM) after induction of CKX3 expression by DEX treatment as described in (C). Asterisks indicate significant differences from untreated seedlings (P < 0.001, t test).
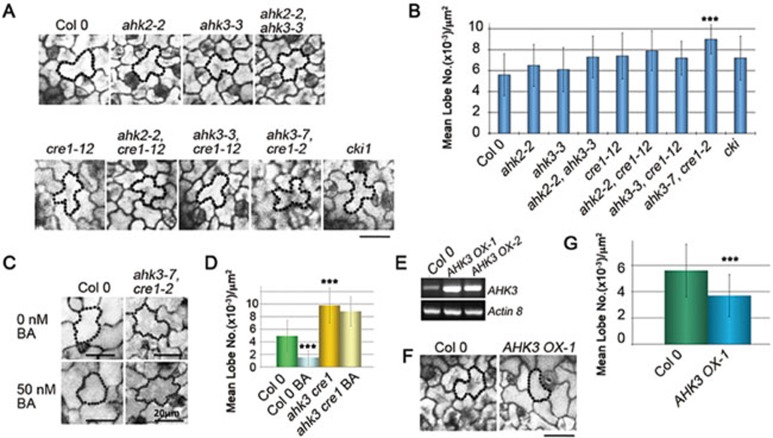
Figure 3.
Cytokinin receptors regulate PC interdigitation. (A) Representative PC images of 2-DAG cotyledon PC shapes in Col 0 and different cytokinin receptor mutants. (B) Quantitative analysis of lobe number per area (mean ± SEM) in (A). Asterisks indicate significant differences from Col 0 control (P < 0.001, t test). (C) Representative images of 2-DAG cotyledon PC shapes in wild type (WT) and the ahk3 cre1 cytokinin receptor mutant both with and without BA treatment. Bars = 20 μm. (D) Quantitative analysis of lobe number per area (mean ± SEM) in the treatments described in (C). Asterisks indicate a significant differences from untreated seedlings and WT (P <0.001, t test). The mean lobe number of ahk3 cre1 PCs is 9.8×10−3/μm2, whereas WT PCs at the same stage have a mean lobe number of 4.7×10−3/μm2. (E) RT-PCR analysis of AHK3 transcript level in two over-expression AHK3 lines: AHK3 OX-1 and AHK3 OX-2. (F) The representative PC images of 2-DAG AHK3 OX-1 cotyledons compared with Col 0. Bars = 20 μm. (G) Quantitative analysis of lobe number per area (mean ± SEM) in (F). Asterisks indicate significant differences from Col 0 seedlings (P < 0.001, t test).
The CRE1 and AHK cytokinin receptors regulate cytokinin-mediated cell interdigitation
We next examined whether cytokinin regulates PC interdigitation through the known histidine kinase (AHK) cytokinin receptor family comprised of three functionally redundant/overlapping members, CRE1/AHK4, AHK2, and AHK3, which activate cytokinin signaling by phospho-relaying to downstream ARRs16,35. Based on the “Arabidopsis eFP Browser”36, AHK2, AHK3, and AHK4 are all expressed in cotyledons, although AHK4 expression is quite weak; and similar expression patterns in rosette leaves for these AHKs have been reported37. We analyzed available single and double mutants of AHKs, including ahk2-2, ahk3-3, cre1-12, ahk2-2 ahk3-3, ahk2-2 cre1-12, ahk3-3 cre1-12, ahk3-7 cre1-2, and cki1 (Figure 3A and 3B). Among these mutants, the double loss-of-function mutant ahk3-7 cre1-2 showed a clear change in the PC interdigitation pattern with precocious interdigitation in un-germinated cotyledons and greatly enhanced interdigitation in 2-DAG cotyledons (Figure 3A and 3B). The ahk3-7 cre1-2 (ahk3 cre1) PCs were insensitive to exogenously applied synthetic cytokinin benzyladenine (BA), which severely inhibited PC interdigitation in wild-type cotyledons (Figure 3C and 3D). In addition, over-expression of AHK3 (AHK3 OX) (Figure 3E) greatly decreased PC interdigitation in both cotyledons (Figure 3F and 3G) and true leaves (Supplementary information, Figure S3A and S3B). These results show that cytokinin acts through the functionally overlapping AHK3 and CRE1 to negatively regulate PC interdigitation and to prevent precocious interdigitation in the embryonic leaves during emobryogenesis.
Cytokinin suppresses PC interdigitation through a subset of ARR genes
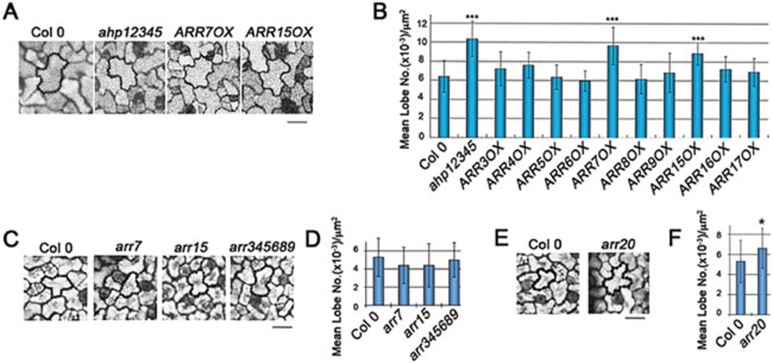
A well-characterized mechanism downstream of the CRE1/AHK receptors involves phosphoryl relay from AHKs to ARRs through the histidine phospho-transfer proteins (AHPs) in Arabidopsis. Loss-of-function mutations in the five AHP genes (ahp12345 quintuple mutant) abolishes the sensitivity to cytokinin in its regulation of various developmental processes19. Since we have shown that ARRs regulate PC interdigitation (Figure 1), we expect that AHPs would also affect this process. Indeed, the ahp12345 mutant exhibited precocious and enhanced PC interdigitation in cotyledons, indicating that PC interdigitation is also regulated by the AHP-dependent mechanism (Figure 4A and 4B).
Figure 4.
Components of the cytokinin signaling pathway regulate PC interdigitation. (A) Representative images of the PC morphology of 2-DAG cotyledons in Col 0, ahp12345, ARR7OX, and ARR15OX. Bar = 20 μm. (B) Quantitative analysis of lobe number per area (mean ± SEM) in cotyledon PCs of lines with an impaired regulation of cytokinin signaling, including the ahp12345 pentuple loss-of-function mutant for the positive regulator AHPs, and transgenic lines over-expressing the indicated negative regulator type-A ARRs. Asterisks indicate a significant increase relative to wild-type PCs (P < 0.001, t test). (C) Representative images of the PC morphology of 2-DAG cotyledons in Col 0, arr7, arr15 and arr345689 loss-of-function mutants. Bar = 20 μm. (D) Quantitative analysis of lobe number per area (mean ± SEM) in (C). (E) Representative images of the PC morphology of 2-DAG cotyledons in Col 0 and arr20 loss-of-function mutants. Bar = 20 μm. (F) Quantitative analysis of lobe number per area (mean ± SEM) in (E). Asterisks indicate a significant increase relative to wild-type PCs (P < 0.05, t test).
Both type-A and type-B ARRs are encoded by a gene family, and evidence suggests that certain subsets of ARRs have evolved to regulate a specific cytokinin-mediated process23. Because type-B, positive regulators of cytokinin signaling also controls the transcription of the type-A ARRs23, we observed PC interdigitation in various type-A ARR over-expression lines. Among various type-A ARRs that negatively regulate cytokinin signaling34, only over-expression of ARR7 or its paralog ARR15 enhanced PC interdigitation (Figure 4A and 4B), suggesting that ARR7/ARR15 have diversified to regulate PC interdigitation patterns. These two ARRs are closely related, and represent an ARR clade that has evolved with a specific role for the regulation of PC interdigitation. In support of this, we found that the type-A arr345689 hextuple mutant did not show obvious PC interdigitation defects, although it was reported to have other developmental defects, e.g. shorter roots, and to be more sensitive to cytokinin and less sensitive to red light than the wild type22. Neither arr7 nor arr15 single mutants showed obvious PC defects (Figure 4C and 4D; Supplementary information, Figure S4A), likely due to functional redundancy of these two ARRs, since both genes are expressed in cotyledons (Arabidopsis eFP Browser)36.
Moreover, we analyzed various type-B ARR loss-of-function alleles in PC morphogenesis, and found that arr20 shows increased PC interdigitation (Figure 4E and 4F; Supplementary information, Figure S4B), which is opposite to ARR20OX, with defects in PC interdigitation (Figure 1F and 1G). These data support the notion that ARR20 has evolved to specify the function in PC morphogenesis. In addition, we noticed that arr11-1 shows a significant increase in PC interdigitation comparing to wild type (WT) (Supplementary information, Figure S4B), suggesting that ARR11 may have overlapping functions with ARR20. Nonetheless, we do not exclude the possibility that the other ARRs may also participate in the cytokinin signaling pathway that regulates PC interdigitation.
Cytokinin signaling acts upstream of ROPs in suppressing PC interdigitation
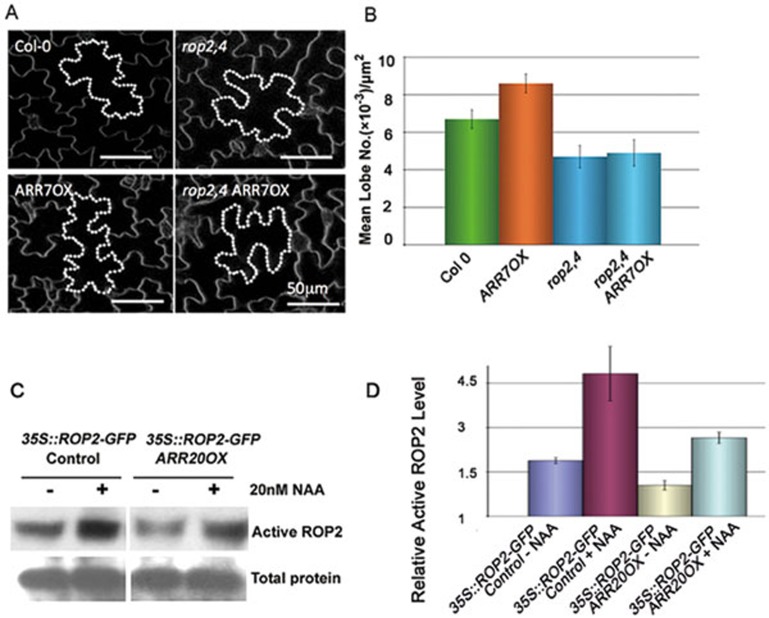
Since the ROP signaling pathways are required for PC interdigitation, we next asked whether cytokinin acts through or independent of these pathways. To assess the relationship between cytokinin and ROP signaling, we generated the rop2−/−, rop4+/− and ARR7OX triple mutant (rop2,4 ARR7OX). PCs of the triple mutant line showed defects similar to those in the rop2,4 mutant (Figure 5A and 5B). Furthermore, we found that ROP2 activity is decreased in ARR20OX (Figure 5C and 5D). Taken together these results suggest that cytokinin signaling acts upstream of ROPs to suppress the formation of the interdigitated pattern.
Figure 5.
Cytokinin signaling acts upstream of ROPs to suppress the PC interdigitation. (A) Representative images of 4-DAG cotyledon PC shapes in WT, ARR7OX, rop2,4, and rop2,4 ARR7OX. Bars = 50 μm. (B) Quantitative analysis of lobe number per area (mean ± SEM) in WT, ARR7OX, rop2,4, and rop2,4 ARR7OX. (C) Auxin rescued the defect in ROP2 activation in ARR20OX. ROP2 activity was assayed using a pull-down assay as previously described (see Materials and Methods). ARR20 over-expression reduced the endogenous ROP2 activity, but exogenously applied NAA (20 nM) greatly increased ROP2 activity to a level similar to or higher than that in untreated wild-type seedlings. (D) Quantitative analysis of relative ROP2 activity in ARR20OX.
Discussion
The cellular basis for the phytohormonal control of developmental and morphogenetic processes has been linked to the regulation of cell growth, cell division, and cell fate specification. Our results expand the known roles of cytokinin signaling in plant development to include the regulation of cell morphogenesis. Our findings here, together with several recent findings on the new roles of auxin and cytokinin, suggest that the small molecule phytohormones play an important role in the spatial regulation of fundamental cellular processes such as cell polarity formation and cell morphogenesis. This adds a new dimension to cellular mechanisms for phytohormone action in plant growth and development. Auxin has recently been shown to regulate PC morphogenesis and polar distribution of PIN proteins by activating ROP GTPases30,32,38. Auxin has also been implicated in the regulation of the orientation of cell division by affecting microtubule organization39. Cytokinin is known to interact with auxin through the regulation of genes that are involved in the TIR1/AFB-based auxin signaling9,40. However, recent studies suggest that cytokinin also modulates auxin function by directly regulating PIN degradation, consequently influencing PIN polar distribution and polar auxin transport8,41. Our results, showing that cytokinin signaling regulates PC morphogenesis and ROP GTPase activity, further support a direct role for cytokinin signaling in the spatial regulation of fundamental cellular processes.
Our results suggest that cytokinin signaling provides a new mechanism for the regulation of PC morphogenesis, adding another developmental process regulated by auxin and cytokinin. We previously showed that auxin promotes PC interdigitation by activating ROP GTPases30. Interestingly, we found that cytokinin signaling suppresses PC interdigitation, suggesting that cytokinin and auxin have opposing roles in this process in a concentration-dependent manner (Supplementary information, Figure S5). Indeed our results showed that an increasing concentration of NAA could rescue PC interdigitation defects induced by cytokinin (Supplementary information, Figure S5). Therefore, cytokinin regulation of the fundamental cellular process (cell morphogensis) appears to follow the common theme for the coordination of various developmental and morphogenetic processes in different tissues/organs involving a balancing act between cytokinin and auxin, although detailed mechanisms for the auxin-cytokinin balancing act may differ in different processes1,5,6,8,9,10,11,42.
How does cytokinin antagonize the action of auxin in PC morphogenesis? Our data suggest that cytokinin acts upstream of ROP GTPases (Figure 5), and thus it is likely that cytokinin suppresses PC interdigitation by directly down-regulating ROP GTPase activity, as we have shown that ROP2 activity is inhibited by cytokinin signaling. However, our data do not exclude the possibility that cytokinin regulates ROP activity through the modulation of auxin levels, which are critical for PC interdigitation and ROP activation30. In addition, there are other possible mechanisms for cytokinin's action in suppressing PC interdigitation. Polar PIN1 distribution to the lobe tips in PCs has been shown to be required for PC interdigitation30. Cytokinin is known to affect PIN1 levels and PIN distribution in root morphogenesis8,41. Thus a similar regulation of PIN1 in PCs might also participate in the control of PC interdigitation. Future research should be directed at exploring the mechanistic connection between cytokinin and auxin signaling in the regulation of PC morphogenesis.
Materials and Methods
NAA treatment and mutant screen
NAA (Sigma, St. Louis, MO) was dissolved in DMSO as a 0.5 M stock solution prior to dilution in liquid Murashige-Skoog (MS) media. Seeds were germinated in liquid MS media containing the indicated concentrations of NAA. Per 150 mm plate containing a sterilized filter paper at the bottom, approximately 1 000 seeds were added prior to addition of ½ MS media containing 100 nM NAA to cover the seeds. At 10 DAG (Days after germination), most of the seedlings would have expanded and curved cotyledons (Figure 1A). Seedlings with flat cotyledons were selected as the mutant candidates. Prior to transfer to soil, candidates were further grown in fresh auxin liquid medium for a few days to ensure the cotyledon did not curve after extended auxin treatment.
Materials and growth conditions
Arabidopsis plants were grown at 22 °C on ½ MS agar or in soil with 16 h-light /8 h-dark cycles. The majority of the mutant or transgenic lines used in this work were previously described, including ARR7OX (35S::ARR7) and ARR7OX M (D85N)24, ahk3 cre1 (ahk3-7 cre1-2)2, ahp12345 (ahp1,2-2,3,4,5 quintuple mutant)43, various type-A ARR over-expression lines (ARR3OX, ARR4OX, ARR5OX, ARR6OX, ARR7OX, ARR8OX, ARR9OX, ARR15OX, ARR16OX, ARR17OX)34, and cre1-12, ahk2-2, ahk3-3, ahk2-2 ahk3-3, ahk2-2 cre1-12, ahk3-3 cre1-1237. ARR20OX (At3g62670) was obtained from the Ohio State University Arabidopsis Biological Resource Center. The type-A and type-B ARR loss-of-function Salk lines were obtained from ABRC or NASC: arr1 (CS6971), arr1-3 arr12-1 (N6981), arr1-3 arr10-5 arr12-144, arr2-1 (SALK_043107), arr2-4 (CS6975), arr3 (SALK_042068C), arr4 (CS25266), arr5 (CS25267), arr6 (SALK_133123C and SALK_080024C), arr3456 (CS25276), arr345689 (CS25279), arr7 (N858131), arr8 (SALK_057940C), arr8 arr9 (CS25275), arr10-1 (CS6990, Ws background), arr11-1 (N6370, Ws background), arr12-1 (CS6978), arr13 (SALK_042719C), arr14 (SALK_630_D09), arr15 (GK_123D02.01), arr16 (N873779), arr17 (N873297), and cki1 (CS6360). The primers' sequences for genotyping type A and type B ARRs can be found in Supplementary information, Table S1. The accession of pMTX009::IPT7::polyA and pMTX009::CKX3::NOS lines are Ler (Landsberg erecta).
Microscopic analysis of PC shape
Cell outlines of PCs were visualized using UV laser (at 351 nm or 364 nm, 50% laser power and emission at 400-600 nm) under a Leica SP2 confocal microscope30, including Figures 1B, 1F, 2A, 3A, 3C, 3F, 4A, 4C, and 4E. Alternatively, cell outlines were visualized by staining the PM with 5 μg/ml FM4-64 or FM1-43 dye for half an hour, and imaged using confocal microscopy with TRITC or FITC channels, respectively (Figures 2C and 5A). Stack images (1.0- to 2.0-μm increments) were collected from at least 200 cells from 5 individual plants, as described previously30. Additional image analysis was conducted by using MetaMorph software30. Adobe Photoshop CS was used for text editing of all images. Lobe numbers were quantified by number/area. Results are shown as mean numbers with standard error bars. For t test, the significance (triple asterisks) was set at P < 0.001, and one asterisk was set at P < 0.05.
We analyzed cotyledon PC phenotypes at different stages of seedlings, from 1-DAG seedling to plants with true leaves; the data we present in figures are the most pronounced phenotypes during the developmental stages. However, in most of the cases, the 2-DAG seedlings show the best phenotype and it is easy to quantify lobe numbers before the secondary lobes start to occur. Based on treatment and purpose of experiment, different developmental stages of PC are required. For example, Figure 2A, in order to see the decreasing lobes after treatment, we had to choose the stage, which had already formed enough lobes, for DEX treatment to observe maximal changes induced by cytokinin overaccumulation. In Figure 2B, we had to choose a relatively early stage when there were not many lobes for each cell. This allowed the observation of the differences induced by cytokinin degradation. The different developmental stages of seedlings we presented in figures are mentioned in each figure legend.
Regarding different confocal methods, we had to use FM4-64 staining only when it is very difficult to observe by UV, for example, large seedlings or plants with true leaves, for which the UV scanning signal was weak and unclear. We have attempted to use the same method as much as possible, however, in order to present the readers the clearest image, we had to use FM staining in some cases. Generally speaking, the UV laser is suitable for very small seedlings. We use staining method for those samples that give difficulties for observation by UV laser.
RT-PCR
Total RNA from cotyledons was isolated using RNeasy Plant Mini Kit (Qiagen). Reverse transcription was performed using SuperScript II (Invitrogen). Specific transcript levels were analyzed by RT-PCR relative to a tubulin loading control. Primer pairs used were tubulin_F: TTCCGTACCCTCAAGCTCGCTAAT, tubulin_R: ATCCTCTCGATGTCAATGGTGCGA. ARR7_F: AGAGTGGAACTAGGGCTTTGCAGT, ARR7_R: CTCCTTCTTTGAGACATTCTTGTATACGAGG. ARR20_F: CATCTCCAGAAGTACCGTCAAAG, ARR20_R: GGCTGCAAGAGTGACATCTG. AHK3 RT-F: GGAGGTGCGGTTTGATTTG, AHK3 RT-R: GGCTGGTTGTTGTCATTCTTTC. Actin 8F: CACATGCTATCCTCCGTCTC, Actin 8 R: CAATGCCTGGACCTGCTT.
Cytokinin treatment
Cytokinin treatments were conducted by germinating seeds in MS media containing cytokinin. A final concentration of 50 nM synthetic cytokinin BA was used.
Plasmid construction
The IPT7 and CKX3 genes were cloned from cDNA generated by RT-PCR. The cDNAs were first cloned into the pENTR D-TOPO vector (Invitrogen) and were sequenced to confirm error-free amplification. By employing Gateway methods the polyA terminator of the 35S gene was added to IPT7 and the NOS terminator was added to CKX3. Subsequently, the combined gene and terminator cassettes were inserted into the KpnI and partially digested XhoI sites of pPOPOFF2 (HYG)45 to generate pMTX009::IPT7::polyA and pMTX009::CKX3::NOS constructs.
For AHK3 OX-1 and AHK3 OX-2, the AHK3 (At1g27320) gene has been cloned into pFGC5941 by using the Asc I and XBA I cleavage sites. AHK3- Asc I: ggcgcgccATGAGTCTGTTCCATGTGCTAGG, AHK3-XBA I: tctagaTTATGATTCTGTATCTGAAGGCG.
DEX treatment
To induce IPT7 or CKX3 expression, a final concentration of 3 μM DEX was sprayed on pMTX009::IPT7::polyA or pMTX009::CKX3::NOS seedlings. The DEX spraying was repeated twice a day for two days, and the control seedlings had been sprayed by water. Treated seedlings and control were used for cotyledon PC shape analysis.
ROP2 activity assay
GFP-tagged active ROP2 was pulled down by use of MBP-RIC1 as described previously30. Protoplasts were isolated from leaves of 2- to 3-week-old 35S::GFP-ROP2 Arabidopsis plants. Second or third pair rosette leaves were used to prepare protoplasts as described previously46. Protoplasts were counted by using a hemacytometer (Hausser scientific, Cat # 1483). Isolated protoplasts were treated with 20 nM NAA for 2 min and frozen by liquid nitrogen. Total protein was extracted from 105–106 treated protoplasts by extraction buffer (25 mM HEPEs pH 7.4; 10 mM MgCl2; 10 mM KCl; 5 mM DTT; 5 mM Na3VO4; 5 mM NaF; 1 mM PMSF; 1% protease inhibitor from Sigma, St. Louis; 1% TritonX-100). A part of total proteins was used as control to determine the total amount of GFP-ROP2 (GDP-bound and GTP-bound). A saturated amount of MBP-RIC1-conjugated beads was added to the same amount of protoplast extracts from mutants with/without NAA treatment, which were then gently shaken at 4 °C for 3 h. Beads were washed in a washing buffer (25 mM HEPEs pH 7.4; 1 mM EDTA; 5 mM MgCl2; 1 mM DTT; 0.5% TritonX-100) for three times at 4 °C (5 min each). Western blotting with anti-GFP antibody was used for analysis of the GTP-bound active form of GFP-ROP2 that was associated with the MBP-RIC1 beads (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Acknowledgments
We thank Dr Thomas Schmülling from Free University of Berlin, Germany for ahk3-7 cre1-2 mutant. We thank Dr Tatsuo Kakimoto from Osaka University, Japan for cre1-12, ahk2-2, ahk3-3, ahk2-2 ahk3-3, ahk2-2 cre1-12, ahk3-3 cre1-12 mutants. We are grateful to members of the Yang group for stimulating discussion during the course of this work. We thank Xinyan Zhang in Guo lab for sharing part of the SALK lines. This work was supported by grants from the US National Institute of General Medical Sciences (GM081451 and GM081451-03S2) to ZY. We thank National Science Foundation grant (IOS-1147250) to GVR and MX. HL and DL were partially supported by the Chinese Scholarship Council.
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Material
Auxin insensitive mutant (AIM) screen method.
Auxin response of AIM mutants compared to tir quadruple, abp1-5, and rop2 rop4 mutants.
Cytokinin receptor regulates PC interdigitation.
PC shapes of type-A and type-B ARR loss of function alleles and their relative expression level.
The PC shape varies under different combinations of NAA and BA treatment.
Sequences of primers for genotyping type A and type B ARRs.
References
- Aloni R, Aloni E, Langhans M, Ullrich CI. Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot. 2006;97:883–893. doi: 10.1093/aob/mcl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riefler M, Novak O, Strnad M, Schmulling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell. 2006;18:40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller B, Sheen J. Arabidopsis cytokinin signaling pathway. Sci STKE. 2007;2007:cm5. doi: 10.1126/stke.4072007cm5. [DOI] [PubMed] [Google Scholar]
- To JP, Kieber JJ. Cytokinin signaling: two-components and more. Trends Plant Sci. 2008;13:85–92. doi: 10.1016/j.tplants.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Bishopp A, Help H, El-Showk S, et al. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr Biol. 2011;21:917–926. doi: 10.1016/j.cub.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Bishopp A, Lehesranta S, Vatén A, et al. Phloem-transported cytokinin regulates polar auxin transport and maintains vascular pattern in the root meristem. Curr Biol. 2011;21:927–932. doi: 10.1016/j.cub.2011.04.049. [DOI] [PubMed] [Google Scholar]
- Kushwah S, Jones AM, Laxmi A. Cytokinin-induced root growth involves actin filament reorganization. Plant Signal Behav. 2011;6:1848–1850. doi: 10.4161/psb.6.11.17641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhavy P, Bielach A, Abas L, et al. Cytokinin modulates endocytic trafficking of PIN1 auxin efflux carrier to control plant organogenesis. Dev Cell. 2011;21:796–804. doi: 10.1016/j.devcel.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Muller B, Sheen J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature. 2008;453:1094–1097. doi: 10.1038/nature06943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moubayidin L, Di Mambro R, Sabatini S. Cytokinin-auxin crosstalk. Trends Plant Sci. 2009;14:557–562. doi: 10.1016/j.tplants.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Ruzicka K, Simásková M, Duclercq J, et al. Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc Natl Acad Sci USA. 2009;106:4284–4289. doi: 10.1073/pnas.0900060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Baba S, Obayashi T, et al. Regulation of root greening by light and auxin/cytokinin signaling in Arabidopsis. Plant Cell. 2012;24:1081–1095. doi: 10.1105/tpc.111.092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto T. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate: ATP/ADP isopentenyltransferases. Plant Cell Physiol. 2001;42:677–685. doi: 10.1093/pcp/pce112. [DOI] [PubMed] [Google Scholar]
- Sakakibara H. Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol. 2006;57:431–449. doi: 10.1146/annurev.arplant.57.032905.105231. [DOI] [PubMed] [Google Scholar]
- Werner T, Kollmer I, Bartrina I, Holst K, Schmulling T. New insights into the biology of cytokinin degradation. Plant Biol (Stuttg) 2006;8:371–381. doi: 10.1055/s-2006-923928. [DOI] [PubMed] [Google Scholar]
- Inoue T, Higuchi M, Hashimoto Y, et al. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature. 2001;409:1060–1063. doi: 10.1038/35059117. [DOI] [PubMed] [Google Scholar]
- Ueguchi C, Sato S, Kato T, Tabata S. The AHK4 gene involved in the cytokinin-signaling pathway as a direct receptor molecule in Arabidopsis thaliana. Plant Cell Physiol. 2001;42:751–755. doi: 10.1093/pcp/pce094. [DOI] [PubMed] [Google Scholar]
- Kakimoto T. Perception and signal transduction of cytokinins. Annu Rev Plant Biol. 2003;54:605–627. doi: 10.1146/annurev.arplant.54.031902.134802. [DOI] [PubMed] [Google Scholar]
- Hutchison CE, Li J, Argueso C, et al. The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell. 2006;18:3073–3087. doi: 10.1105/tpc.106.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T, Oka A. Cytokinin signal transduction in plant cells. J Plant Res. 2003;116:221–231. doi: 10.1007/s10265-003-0094-6. [DOI] [PubMed] [Google Scholar]
- Kiba T, Yamada H, Sato S, et al. The type-A response regulator, ARR15, acts as a negative regulator in the cytokinin-mediated signal transduction in Arabidopsis thaliana. Plant Cell Physiol. 2003;44:868–874. doi: 10.1093/pcp/pcg108. [DOI] [PubMed] [Google Scholar]
- To JP, Haberer G, Ferreira FJ, et al. Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell. 2004;16:658–671. doi: 10.1105/tpc.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To JP, Deruère J, Maxwell BB, et al. Cytokinin regulates type-A Arabidopsis response regulator activity and protein stability via two-component phosphorelay. Plant Cell. 2007;19:3901–3914. doi: 10.1105/tpc.107.052662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Kim S, Ha YM, Kim J. Phosphorylation of Arabidopsis response regulator 7 (ARR7) at the putative phospho-accepting site is required for ARR7 to act as a negative regulator of cytokinin signaling. Planta. 2008;227:577–587. doi: 10.1007/s00425-007-0640-x. [DOI] [PubMed] [Google Scholar]
- Yang Z. Cell polarity signaling in Arabidopsis. Annu Rev Cell Dev Biol. 2008;24:551–575. doi: 10.1146/annurev.cellbio.23.090506.123233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Li H, Yang Z. The ROP2 GTPase controls the formation of cortical fine F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell. 2002;14:777–794. doi: 10.1105/tpc.001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Blanchoin L, Yang Z, Lord EM. The putative Arabidopsis arp2/3 complex controls leaf cell morphogenesis. Plant Physiol. 2003;132:2034–2044. doi: 10.1104/pp.103.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell. 2005;120:687–700. doi: 10.1016/j.cell.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Fu Y, Xu T, Zhu L, Wen M, Yang Z. A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Curr Biol. 2009;19:1827–1832. doi: 10.1016/j.cub.2009.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Wen M, Nagawa S, et al. Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell. 2010;143:99–110. doi: 10.1016/j.cell.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lin D, Dhonukshe P, et al. Phosphorylation switch modulates the interdigitated pattern of PIN1 localization and cell expansion in Arabidopsis leaf epidermis. Cell Res. 2011;21:970–978. doi: 10.1038/cr.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagawa S, Xu T, Lin D, et al. ROP GTPase-dependent actin microfilaments promote PIN1 polarization by localized inhibition of clathrin-dependent endocytosis. PLoS Biol. 2012;10:e 1001299. doi: 10.1371/journal.pbio.1001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Nagawa S, Yang Z. Uniform auxin triggers the Rho GTPase-dependent formation of interdigitation patterns in pavement cells. Small Gtpases. 2011;2:227–232. doi: 10.4161/sgtp.2.4.16702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Liang Y, Deng Y, et al. Genome-wide comparative analysis of type-A Arabidopsis response regulator genes by overexpression studies reveals their diverse roles and regulatory mechanisms in cytokinin signaling. Cell Res. 2009;19:1178–1190. doi: 10.1038/cr.2009.88. [DOI] [PubMed] [Google Scholar]
- Sheen J. Phosphorelay and transcription control in cytokinin signal transduction. Science. 2002;296:1650–1652. doi: 10.1126/science.1071883. [DOI] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS One. 2007;2:e718. doi: 10.1371/journal.pone.0000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Pischke MS, Mähönen AP, et al. In planta functions of the Arabidopsis cytokinin receptor family. Proc Natl Acad Sci USA. 2004;101:8821–8826. doi: 10.1073/pnas.0402887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Kleine-Vehn J, Barbez E, et al. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell. 2010;143:111–121. doi: 10.1016/j.cell.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Weits DA, Cruz-Ramirez A, et al. A PLETHORA-Auxin transcription module controls cell division plane rotation through MAP65 and CLASP. Cell. 2012;149:383–396. doi: 10.1016/j.cell.2012.02.051. [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Nakamura K, Moubayidin L, et al. A genetic framework for the control of cell division and differentiation in the root meristem. Science. 2008;322:1380–1384. doi: 10.1126/science.1164147. [DOI] [PubMed] [Google Scholar]
- Zhang W, To JP, Cheng CY, Eric Schaller G, Kieber JJ. Type-A response regulators are required for proper root apical meristem function through post-transcriptional regulation of PIN auxin efflux carriers. Plant J. 2011;68:1–10. doi: 10.1111/j.1365-313X.2011.04668.x. [DOI] [PubMed] [Google Scholar]
- Jones B, Gunnerås SA, Petersson SV, et al. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell. 2010;22:2956–2969. doi: 10.1105/tpc.110.074856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Dong H, Mu J, et al. Arabidopsis histidine kinase CKI1 acts upstream of histidine phosphotransfer proteins to regulate female gametophyte development and vegetative growth. Plant Cell. 2010;22:1232–1248. doi: 10.1105/tpc.108.065128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyros RD, Mathews DE, Chiang YH, et al. Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. Plant Cell. 2008;20:2102–2116. doi: 10.1105/tpc.108.059584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielopolska A, Townley H, Moore I, Waterhouse P, Helliwell C. A high-throughput inducible RNAi vector for plants. Plant Biotechnol J. 2005;3:583–590. doi: 10.1111/j.1467-7652.2005.00149.x. [DOI] [PubMed] [Google Scholar]
- Sheen J. Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol. 2001;127:1466–1475. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Auxin insensitive mutant (AIM) screen method.
Auxin response of AIM mutants compared to tir quadruple, abp1-5, and rop2 rop4 mutants.
Cytokinin receptor regulates PC interdigitation.
PC shapes of type-A and type-B ARR loss of function alleles and their relative expression level.
The PC shape varies under different combinations of NAA and BA treatment.
Sequences of primers for genotyping type A and type B ARRs.