Abstract
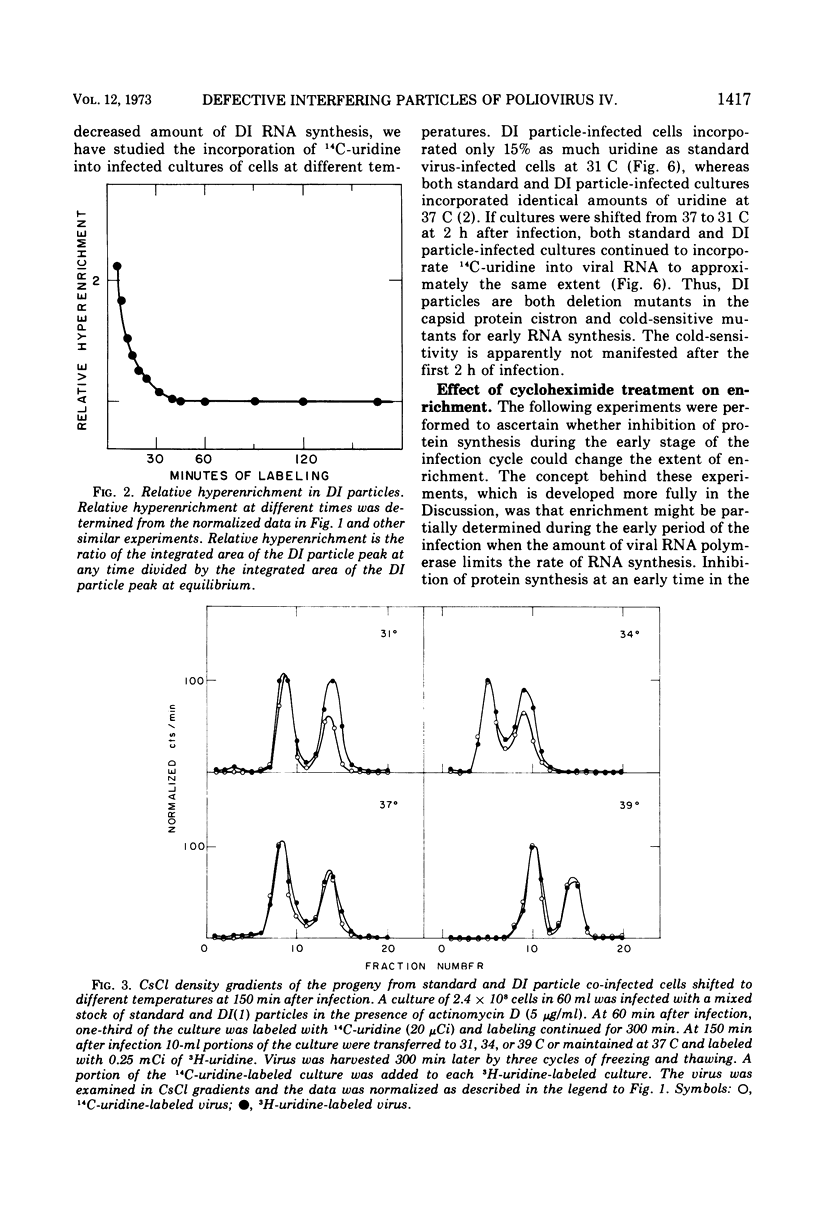
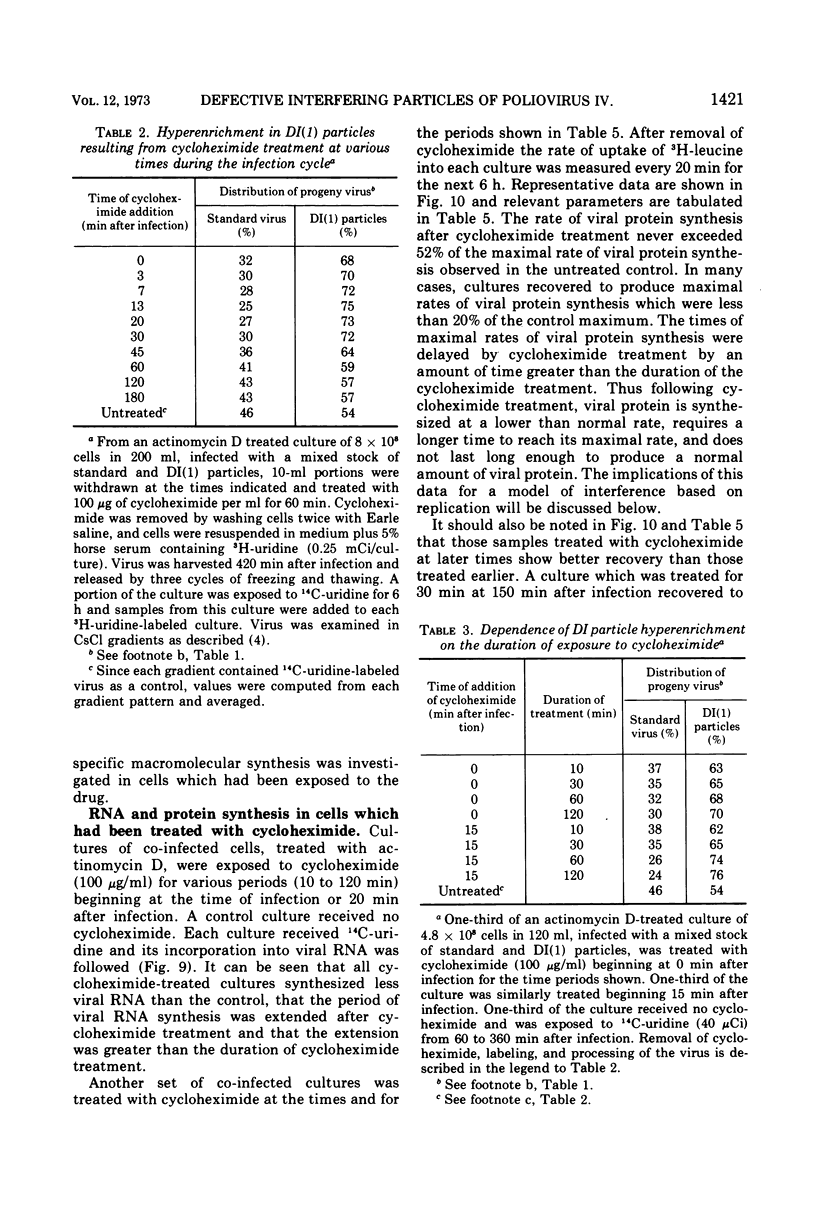
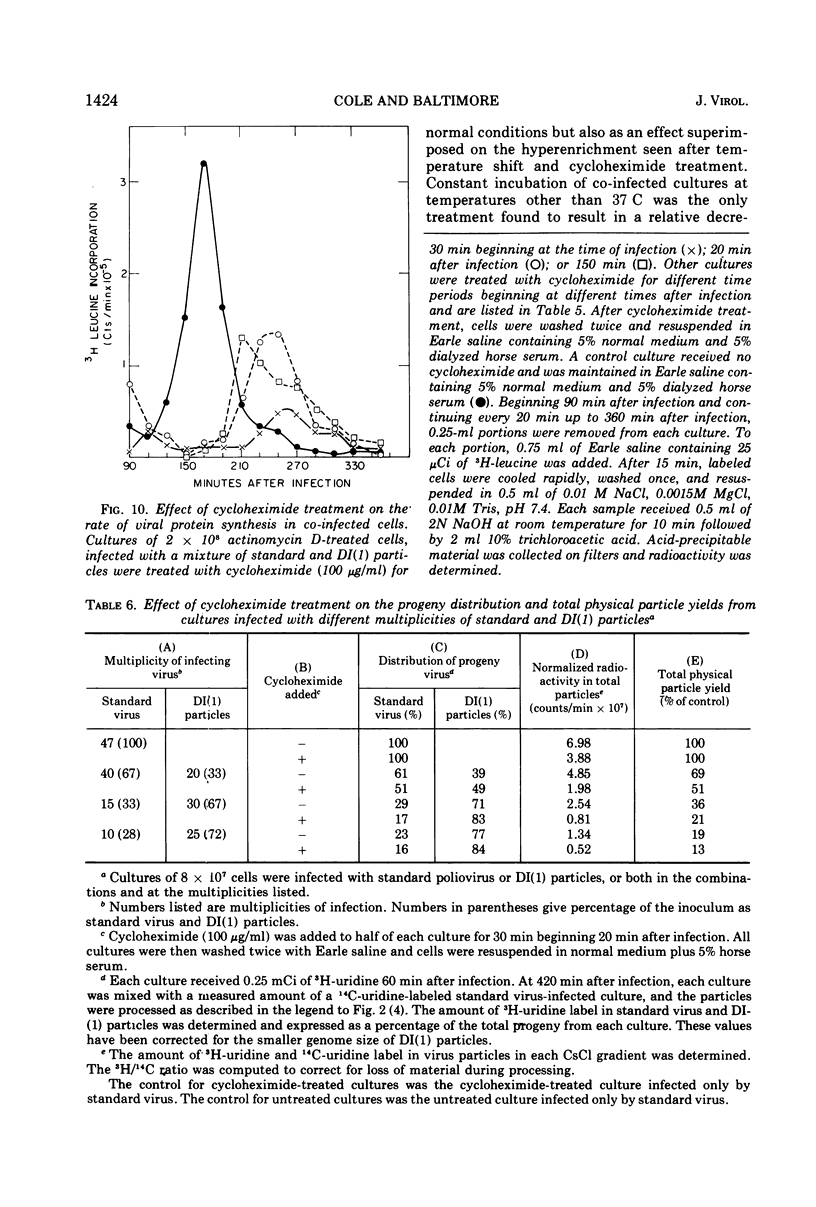
Infection of HeLa cells by mixtures of standard poliovirus and defective, interfering (DI) poliovirus particles leads to a higher ratio of DI particles in the progeny than in the inoculum. The extent of this enrichment could be varied by various manipulations of the co-infected cells. At any time during the infection cycle, virions made within short times after addition of radioactive uridine were hyperenriched in DI particles; this transient hyperenrichment fell to the equilibrium enrichment level within 45 min after uridine addition. A shift of the temperature of infection from 37 to 31 C also led to a hyperenrichment of DI particles and pulse-labeling revealed a superimposed transient hyperenrichment. By contrast, cells continuously infected at 31 C showed a severe decrement in DI particles apparently because poliovirus DI particles behave as cold-sensitive mutants for RNA synthesis. Cycloheximide treatment early in the infection cycle also led to hyperenrichment. Study of the cycloheximide effect showed that the drug acted as if to change the input ratio of standard to DI particles. These effects on enrichment can be explained as aspects of two different phenomena: enrichment due to preferential DI RNA synthesis and enrichment due to preferential encapsidation of DI RNA. Both mechanisms probably play a role in the normal level of enrichment.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Girard M., Darnell J. E. Aspects of the synthesis of poliovirus RNA and the formation of virus particles. Virology. 1966 Jun;29(2):179–189. doi: 10.1016/0042-6822(66)90024-9. [DOI] [PubMed] [Google Scholar]
- COLOMBO B., FELICETTI L., BAGLIONI C. INHIBITION OF PROTEIN SYNTHESIS BY CYCLOHEXIMIDE IN RABBIT RETICULOCYTES. Biochem Biophys Res Commun. 1965 Feb 3;18:389–395. doi: 10.1016/0006-291x(65)90719-9. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. 3. Interference and enrichment. J Mol Biol. 1973 May 25;76(3):345–361. doi: 10.1016/0022-2836(73)90509-3. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. II. Nature of the defect. J Mol Biol. 1973 May 25;76(3):325–343. doi: 10.1016/0022-2836(73)90508-1. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Smoler D., Wimmer E., Baltimore D. Defective interfering particles of poliovirus. I. Isolation and physical properties. J Virol. 1971 Apr;7(4):478–485. doi: 10.1128/jvi.7.4.478-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Tomas C. B., Baltimore D. Morphogenesis of poliovirus. II. Demonstration of a new intermediate, the proviron. J Virol. 1973 Nov;12(5):1122–1130. doi: 10.1128/jvi.12.5.1122-1130.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- KERRIDGE D. The effect of actidione and other antifungal agents on nucleic acid and protein synthesis in Saccharomyces carlsbergensis. J Gen Microbiol. 1958 Dec;19(3):497–506. doi: 10.1099/00221287-19-3-497. [DOI] [PubMed] [Google Scholar]



