Summary
Oncogenic mutations in the small Ras GTPases KRas, HRas, or NRas render the encoded proteins constitutively GTP-bound and active, which promote cancer [1]. Ras proteins share ~85% amino acid identity [2], are activated by [3] and signal through [4] the same proteins, and can exhibit functional redundancy [5][6]. Nevertheless, manipulating expression or activation of each isoform yields different cellular responses [7–10] and tumorigenic phenotypes [11–13], even when different ras genes are expressed from the same locus [6]. We now report a novel regulatory mechanism hardwired into the very sequence of RAS genes that underlies how such similar proteins impact tumorigenesis differently. Specifically, despite their high sequence similarity, KRAS is poorly translated compared to HRAS due to enrichment in genomically underrepresented, or rare, codons. Converting rare to common codons increased KRas expression and tumorigenicity to mirror that of HRas. Furthermore, in a genome-wide survey similar gene pairs with opposing codon bias were identified that not only manifested dichotomous protein expression, but were also enriched in key signaling protein classes and pathways. Thus, synonymous nucleotide differences affecting codon usage account for differences between HRas and KRas expression and function, and may represent a broader regulation strategy in cell signaling.
Keywords: Ras, codon bias, cancer
Results
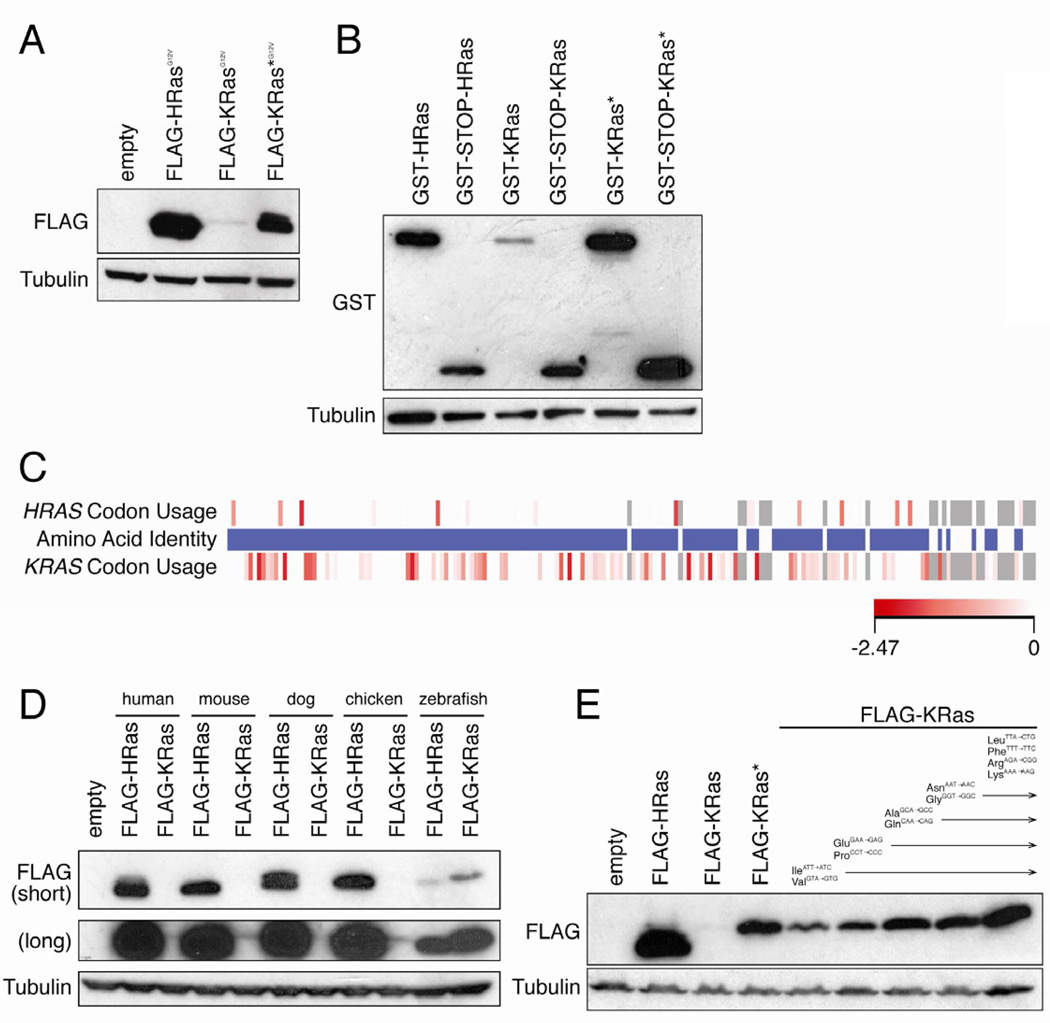
Oncogenic HRAS cDNA, when ectopically over-expressed in human cells consistently produced twenty-fold more protein than the identically tagged oncogenic version of the major splice form (4B) of KRAS cDNA (termed KRAS), regardless of the method of introducing DNA, cell type, epitope tag, promoter, exogenous UTRs, introns, mutation, or membrane targeting (Figures 1A and S1A–H). To explore this difference further, KRAS* was generated by fusing HRAS cDNA encoding the first 158 amino acids (mutated at nine residues to match KRas) to KRAS cDNA encoding the terminal hypervariable 30 amino acids (Figure S1I). KRAS* produced more KRas protein than KRAS, regardless of other parameters, indicating KRAS nucleotide sequence limits protein expression. KRas* protein was still expressed less than HRas protein (Figures 1A and S1A–C, E–G), whether this is a consequence of the remaining KRAS sequence or due to other mechanisms remains to be determined. Additionally, pERK and pAKT levels, measures of Ras effector activation, were similar in cells expressing oncogenic HRas, KRas or KRas* (Figure S1G). Brief metabolic labeling revealed higher KRas protein expression from KRAS* compared to KRAS (Figure S1J), consistent with a translational etiology. Indeed, GST fused with KRAS cDNA (GST-KRas) expressed little protein unless a STOP codon was inserted between the two (GST-STOP-KRas), or if KRas protein was encoded from KRAS*, suggesting that ribosomes stall upon entering KRAS mRNA (Figure 1B). Moreover, KRAS and KRAS* mRNA levels were within two-fold of HRAS mRNA (Figure S1K), although KRAS was consistently the lowest, perhaps reflecting no-go decay of stalled transcripts [14]. Thus, the nucleotide sequence of KRAS message impedes translation.
Figure 1. Translation of KRAS is limited by rare codons.
(A) Immunoblot of lysates isolated from human HEK-HT cells stably infected with the retrovirus pBabepuro encoding the indicated N-terminal FLAG-epitope tagged oncogenic (G12V) human Ras proteins (FLAG-RasG12V) with an αFLAG or αtubulin antibody. One of three experiments. (B) Immunoblot of lystates isolated from HEK-HT cells transiently transfected with the plasmid pCIneo encoding GST cDNA, a STOP codon where indicated, and in frame the indicated FLAG-Ras cDNAs, with an αGST or αtubulin antibody. One of three experiments. (C) Amino acid identity of HRas and KRas is shown in the middle bar. Blue: identical amino acids. White: non-identical amino acids. Relative codon usage of HRAS versus KRAS is shown in the upper and lower bars, respectively. Shade of red box: relative rarity of the codon. Grey box: gaps in alignment or non-identical amino acids. (D) Immunoblot of lysates isolated from human HEK-HT cells stably infected with the retrovirus pBabepuro encoding the indicated FLAG-Ras proteins isolated from the indicated species with an αFLAG or αtubulin antibody. One of two experiments. (E) Immunoblot of lysates isolated from human HEK-HT cells stably infected with the retrovirus pBabepuro encoding FLAG-HRas, FLAG-KRas or FLAG-KRas* (in which the indicated rare codons were progressively converted to the indicated common codons) with an αFLAG or αtubulin antibody. One of two experiments. Detailed methodologies and regent descriptions are provided in Supplemental Experimental Procedures. See also Figure S1.
Comparing the KRAS and HRAS sequence revealed that the third position of codons was typically an A/T in KRAS, but G/C in HRAS. Based on human genome-wide analysis of the relative frequencies that degenerate codons are used to encode for the same amino acid [15], the A/T bias in KRAS corresponds to underrepresented (rare) codons (Figure 1C). Consistent with rare codons limiting protein expression, KRas levels were increased when translated from KRAS* mRNA (Figure 1A). Similarly, the bias toward rare codons was conserved in mammalian and avian KRAS genes (Figure S1L), and equated with lower expression compared to HRas in mammalian cells (Figure 1D). Moreover, zebrafish hras and kras encoded by a mixture of rare and common codons exhibited comparable protein expression at a level between that of mammalian HRas and KRas (Figures 1D and S1L). Finally, progressively converting rare to common codons proportionally increased human KRas protein expression, even upon changing only nine rare isoleucine and valine codons scattered throughout KRAS to their common counterparts (Figures 1E and S1I). Thus, rare codons limit KRAS translation.
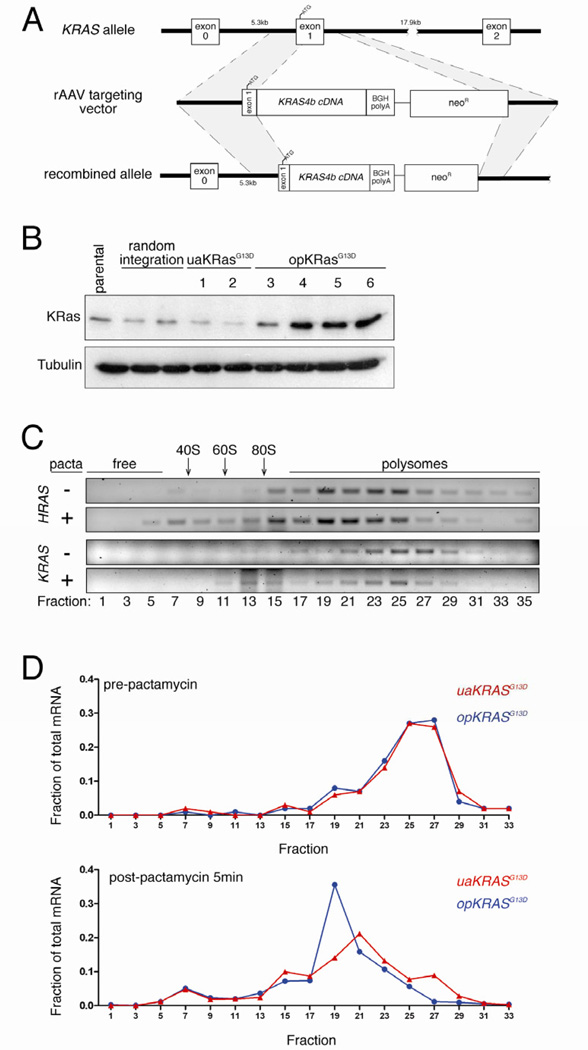
To investigate this phenomenon at the endogenous level, HCT116 human cancer cells (which have a KRASG13D allele) were infected with an AAV targeting vector [16] designed to knock into KRAS exon 1 either opKRASG13D (oncogenic KRASG13D cDNA in which 130 rare codons were optimized to common codons), or uaKRASG13D (unaltered KRASG13D cDNA). The resultant transcripts would be expressed from the endogenous KRAS promoter and retain the 5’ UTR and first 17 coding nucleotides, minimizing effects on translation initiation, but encode only the KRAS4B spliced product (Figure 2A). Screening ~1,800 neomycin-resistant clones revealed four with opKRASG13D and two with uaKRASG13D cDNA successfully knocked into one allele of the KRAS gene. Immunoblot revealed poor expression of endogenous KRas in both the uaKRASG13D and control cell lines exhibiting a random integration event, on par with the parental HCT116 cells. Conversely, the opKRASG13D cell lines exhibited, on average, five-fold higher KRas protein and two-fold higher KRAS mRNA levels (Figures 2B and S2A,B). This increase was lower compared to the ectopic setting, consistent with overexpression systems magnifying the effects of codon bias [17]. Thus, changing rare to common codons increases endogenous KRas protein expression.
Figure 2. Rare codon bias limits endogenous KRAS translation.
(A) Targeting strategy to knock into exon 1 the endogenous KRAS gene in human HCT116 colon cancer cells (ATCC) oncogenic KRASG13D cDNA in which 130 rare codons were either optimized to common codons (opKRASG13D) or left unaltered (uaKRASG13D) by AAV-based recombination [16]. (B) Immunoblot of lysates isolated from parental HCT116 cell line, stable clones with non-homologous vector integration, clones with homologous integration of uaKRASG13D, and clones with homologous integration of opKRASG13D, with an αKRas or αtubulin antibody. One of one to two experiments. (C) Semi-quantitative RT-PCR using primers specific for HRAS and the non-targeted KRAS mRNA of the indicated sedimentation fractions (relative positions of ribosome-free, ribosome subunits 40S, 60S and 80S and polysome fractions) isolated from HCT116 opKRASG13D clone 5 pre- (-) and post- (+) pactamycin (pacta) treatment to halt translation initiation. (D) Quantitative RT-PCR using primers specific for the KRASG13D knock-in mRNA of the indicated sedimentation fractions isolated from HCT116 opKRASG13D clone 5 and uaKRASG13D clone 1 pre- and post-pactamycin treatment to halt translation initiation. Detailed methodologies and regent descriptions are provided in Supplemental Experimental Procedures. See also Figure S2.
Polysome profiling was performed to characterize relative ribosomal kinetics of endogenous HRAS and endogenous untargeted KRAS mRNAs from HCT116 clone 5. Semi-quantitative RT-PCR revealed that both transcripts accumulated in polysome fractions, although KRAS occupied relatively heavier fractions despite nearly equal transcript length (Figure 2C), indicating more dense packing of ribosomes. Pactamycin treatment to halt translation initiation (Figure S2C) led to an accumulation of HRAS mRNA in lighter polysome and ribosome-free fractions, indicative of ribosome translocation. As previously observed [18], there was only a minimal shift of KRAS mRNA in the gradient, with the bulk of the message retained in the heavy fractions (Figure 2C). These results were confirmed in HEK-HT cells (Figure S2D). Polysome profiles of mRNA derived from the opKRASG13D HCT116 clone 5 and uaKRASG13D HCT116 clone 1 were compared. Quantitative RT-PCR analysis revealed that after pactamycin treatment (Figure S2C), opKRASG13D mRNA was modestly shifted to lighter fractions relative to uaKRASG13D mRNA (Figure 2D), consistent with the degree other transcripts are shifted upon alleviating ribosome stalling [19]. These shifts were recapitulated in opKRASG13D HCT116 clone 4 (Figures S2C,E). Thus, rare codons impede translation of endogenous KRAS mRNA.
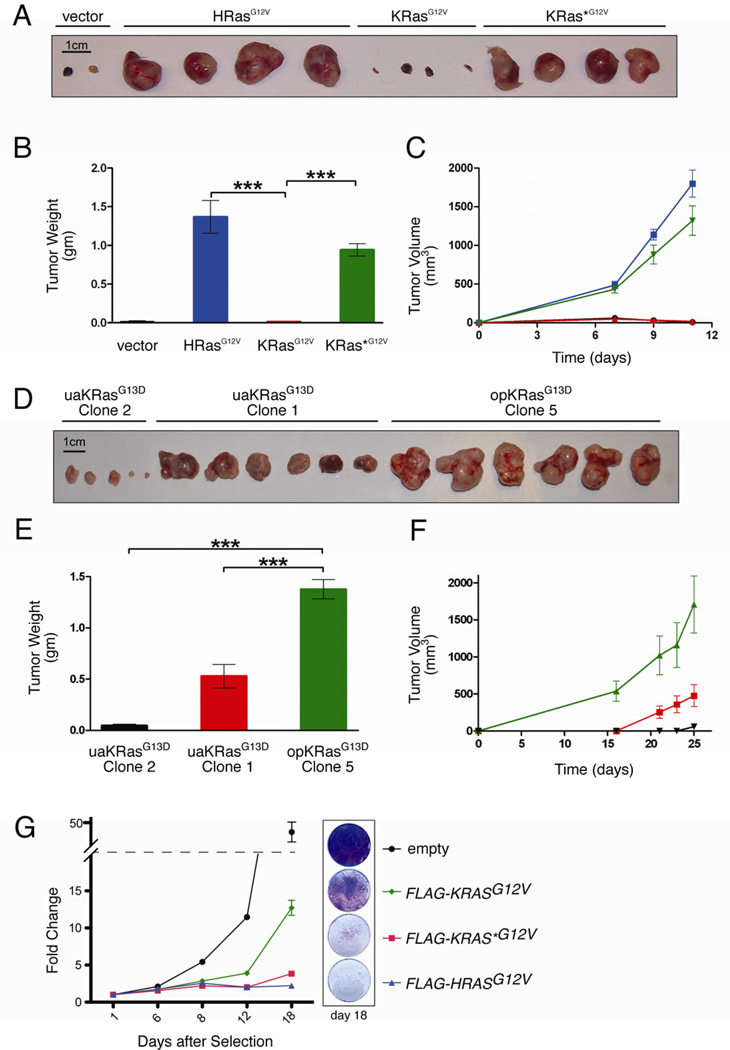
The biological impact of codon bias in mammalian genes is largely unknown. However, targeted replacement of codons in the Drosophila alcohol dehydrogenase gene with either suboptimal [20] or optimal [21] codons can respectively decrease or increase enzyme activity and alcohol tolerance, suggesting codon bias affects gene function in higher eukaryotes. As oncogenic Ras can impart tumorigenic growth to cells, we used tumor growth to assess the biological impact of altering codon bias of human KRAS. HEK-HT cells, which require oncogenic HRas for tumor growth [22], were transduced with a vector expressing no transgene, oncogenic HRasG12V, KRasG12V, or KRas*G12V (Figure 1A) and assayed for tumor growth. KRasG12V, like vector cells, formed tumors with 90-fold smaller masses and reduced kinetics compared to HRasG12V cells, an effect rescued by KRAS*G12V (Figures 3A–C). Tumors that eventually formed 3 months later from KRasG12V cells exhibited elevated KRas expression (Figure S3A). Tumorigenic growth of opKRASG13D and uaKRASG13D knock-in clones in which targeted recombination occurred at the same wild-type KRAS allele were compared. opKRASG13D clone 5 formed tumors with up to 30-fold larger masses and increased kinetics compared to uaKRASG13D clones (Figure 3D–F), although this was not as dramatic as observed in the ectopic setting. Tumors from opKRASG13D cells retained differential KRas protein expression and, interestingly, exhibited higher HRas expression (not shown). While altering rare codons may affect protein folding and processing [17], there is a direct correlation between codon content, protein expression, and tumorigenesis of ectopic and endogenous oncogenic KRAS.
Figure 3. Rare codons limit oncogenic KRas-driven tumorigenesis.
(A) Photograph and (B) mean weight ± SEM of tumors at endpoint, as well as (C) mean size ± SEM tumors over time derived from HEK-HT cells stably expressing: ● empty vector, ■ HRasG12V, ▲ KRasG12V, and ▼ KRas*G12V, n=4. (D) Photograph and (E) mean weight ± SEM of tumors at endpoint, as well as (F) mean size ± SEM tumors over time derived from HCT116 clones expressing ▲ opKRasG13D (clone 5), ■ uaKRasG13D (clone 1), or ▼ uaKRasG13D (clone 2), n=6. One of two experiments. ***P<0.001. (G) Change in IMR90 cell number over time following acute expression of the indicated FLAG-RasG12V constructs. Left: Fold change in crystal violet absorbance at 590nm at the indicated time points after cell plating is normalized to day 1 for each cell line. Relative cell number for IMR90 cells expressing any of the oncogenic FLAG-Ras constructs was significantly lower than empty vector control cells on days 8, 12, and 18 (P<0.01). Additionally, relative cell numbers for both empty vector and FLAG-KRASG12V expressing cells were significantly different from each other and FLAG-KRAS* G12V and FLAG-HRASG12V cells on days 12 and 18 (P<0.0001). Right: Crystal violet staining of intact cells at day 18, representative of four replicates. One of one to two experiments. Detailed methodologies and regent descriptions are provided in Supplemental Experimental Procedures. See also Figure S3.
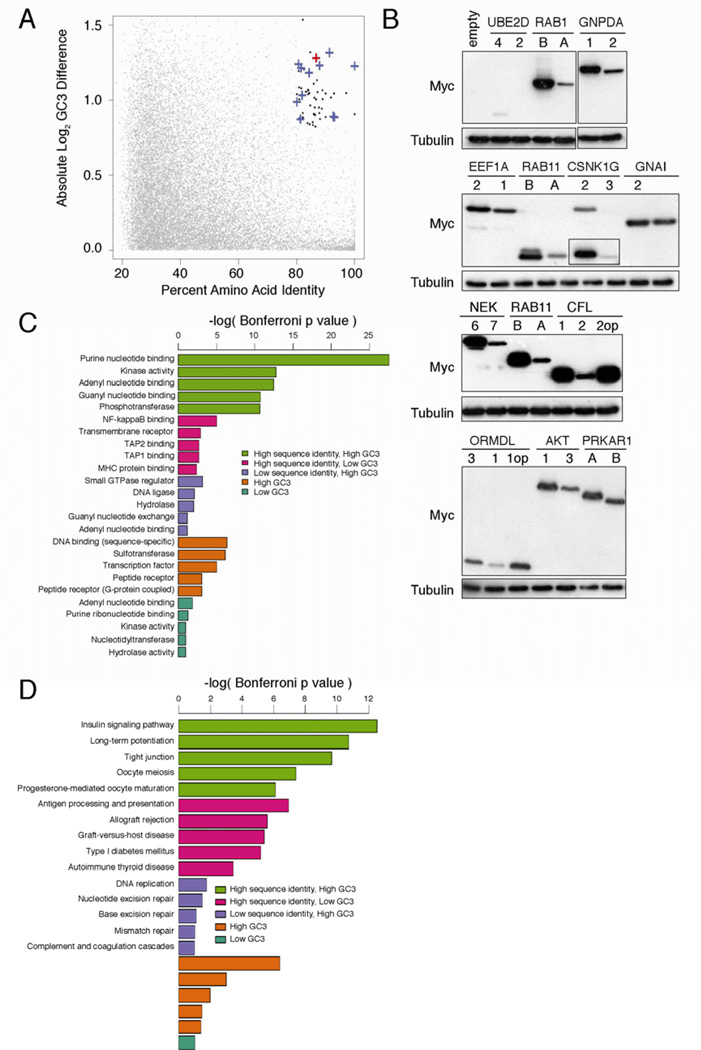
To explore whether the differences in codon bias between RAS genes reflects a broader regulation strategy, we performed a genome-wide survey to identify gene pairs with high similarity and divergent codon usage (Table S1) using G-C content at synonymous sites (GC3) as a proxy for rare codons [15]. Using KRAS and HRAS as a benchmark, the top 60 gene pairs had ≥80% amino acid identity and ≥1.8-fold difference in GC3 content (Figure 4A, Table S1). Twelve such cDNAs pairs were Myc epitope-tagged and expressed in human cells. In every case, the gene enriched in rare codons expressed less protein. Changing rare to common codons in the two genes CFL2 and ORMDL1 augmented protein expression to that of CFL1 and ORMDL3 (Figure 4B). Thus, synonymous differences altering codon usage influence expression of mammalian genes encoding similar proteins.
Figure 4. Gene pairs with divergent codon bias demonstrate correlating differences in expression and cluster in unique signaling protein classes.
(A) Percent amino acid identity versus log difference in CDS GC3 content of individual protein pairs identified by BLAST alignment (grey points). Black points: gene pairs with >80% identity and >1.8-fold difference in GC3 content. Blue cross: gene pair tested for protein expression. Red cross: HRAS-KRAS gene pair. (B) Immunoblot of lysates isolated from human 293 cells (ATCC) stably infected with the retrovirus pBabepuro encoding the indicated N-terminal FLAG-epitope tagged cDNAs corresponding to human genes pairs of high amino acid sequence identity that have a common (first) versus a rare (second) codon bias, or the gene pair enriched in rare codons after rare codons were optizimized (op) to common codons, with an αFLAG or αtubulin antibody. One of one to two experiments. Histogram comparing p values of (C) gene ontology categories or (D) KEGG signaling pathways enriched in lists of gene pairs with high amino acid sequence identity and high GC3 difference (green), high identity and low GC3 difference (pink), low identity and high GC3 difference (purple), or lists of genes with high GC3 content (brown) or low GC3 content (aquamarine). Detailed methodologies and regent descriptions are provided in Supplemental Experimental Procedures. See also Tables S1–3.
To assess the functional significance of this gene set, gene ontology analysis [23] was performed on the top 150 gene pairs sharing high identity and large differences in GC3 content (Table S1). Strikingly, we found that this group of proteins was highly enriched for proteins with purine-nucleotide binding or kinase activity. The enrichment of these proteins was ≥6 orders of magnitude more significant than enrichment of any other functional category from the analysis of gene pairs from complementary combinations of identity and GC3 content criteria (Figure 4C, Table S2). KEGG pathway analysis [24] was also performed to determine if gene pairs reside in pathways. Insulin signaling, long-term potentiation, and tight junction pathways, in particular, were significantly enriched for gene pairs with high protein identity and differential codon usage (Figure 4D, Table S3). Thus, the combination of two seemingly unrelated criteria — high protein identity and opposing codon bias — identified unique functional classes of proteins as well as signaling pathways.
Discussion
While differences in KRas and HRas expression were previously reported in at least some settings [25], the mechanism responsible and effects thereof remained unknown. Here we present a novel regulatory strategy that has been hiding in plain sight: codon bias. Rare codons throughout KRAS message impede translation and correspondingly protein, and to a lesser extent, mRNA levels, and reduce oncogenic activity. This was surprising, as while rare codons impede protein translation in heterologous expression systems [26, 27] and common codon bias exists in highly expressed genes in bacteria and yeast [17], there are few reports of it affecting expression of ectopic homologous transcripts [28–30], let alone endogenous mammalian genes. Moreover, codon bias in mammals has been argued to be a byproduct of genes residing within larger genomic regions of nucleotide bias (isochores) that affect chomatin functions like meiotic recombination [31]. Furthermore, in genome-wide translational assessment, sites of ribosome stalling did not correlate with presence of rare codons [32]. Nevertheless, the effects of codon usage between RAS genes on protein expression and function suggest that are indeed consequences resulting from synonymous differences at the nucleotide level [20, 21, 33].
At face value, the finding that KRAS is an incredibly weak oncogene due to rare codon bias seems at odds with this gene being the most commonly mutated RAS isoform in cancer [34]. However, untransformed cells are sensitive to oncogenic stress [35], and increasing levels of transgenic HrasG12V result in progressively more growth arrested (senescent) cells and fewer mammary lesions in mice [36]. Perhaps rare codons limit the expression of KRas to an ideal range to initiate tumorigenesis — high enough to promote hyperplasia but low enough to avoid excessive senescence. Indeed, IMR90 primary human fibroblasts expressing KRAS*G12V or HRASG12V, but not KRASG12V, appeared to arrest with a senescent morphology (Figure 3G and not shown). Interestingly, there is concordance between the degree of rare codon bias and the mutation frequency amongst RAS family members (Figure S3B,C). However, codon bias cannot alone account for these differences, as Hras cDNA knocked into the Kras locus is mutated at a high frequency in a urethane model of lung cancer [6]. Nevertheless, rare codons clearly crippled the oncogenic activity of KRAS, which could be a barrier to malignant progression. In this regard, it has been hypothesized that up-regulation of oncogenic KRas is a necessary intermediate step in tumor progression after senescence escape [36]. Multiple mechanisms that may increase KRas expression occur during cancer including KRAS gene amplification [37, 38], miRNA down-regulation directly [39, 40] or indirectly by sequestration via the KRAS pseudogene [41], and a general increase in tRNA levels [42, 43].
It was also striking that the divergent tumorigenesis phenotypes of two nearly identical proteins were reconciled by altering codon usage. It is formally possible that changing codon bias affected some other aspect of mRNA regulation, but the multitude of complementary codon modification strategies implemented make this possibility less likely. Codon usage might underlie functional differences between members in other protein families with opposing codon bias. The enrichment of gene pairs with high amino acid sequence identity and divergent codon bias in signaling networks further suggests that codon usage may impact entire signaling pathways. Indeed, differential regulation of functionally redundant genes may add specificity to signal transduction [44], and there are situations in which transcripts enriched in rare codons are preferentially translated in mammalian cells [45]. Thus, codon bias is not only a novel mechanism regulating Ras isoforms, but may reflect a broader regulatory strategy in signaling pathways.
Supplementary Material
Highlights.
Mammalian KRAS is enriched in rare codons that limit its expression.
Changing rare to common codons increases ectopic and endogenous KRAS expression.
KRAS oncogenicity is limited by rare codons.
Other gene pairs exhibit high sequence identity but opposing codon bias.
Acknowledgments
We thank Doug Lowy for critical discussions and comments, and Joshua Brandstadter for technical assistance. This work was supported by grants R01CA123031 (C.M.C.) and 120222-RSG-11-048-01-DMC (D.M.M) and the Edward Spiegel Fund of the Lymphoma Foundation (C.M.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nature Rev. Cancer. 2011;11:761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;2004:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quilliam LA, Rebhun JF, Castro AF. A growing family of guanine nucleotide exchange factors is responsible for activation of Ras-family GTPases. Prog. Nucleic Acid Res. Mol. Biol. 2002;71:391–444. doi: 10.1016/s0079-6603(02)71047-7. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Viciana P, Sabatier C, McCormick F. Signaling specificity by Ras family GTPases is determined by the full spectrum of effectors they regulate. Mol. Cell. Biol. 2004;24:4943–4954. doi: 10.1128/MCB.24.11.4943-4954.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potenza N, Vecchione C, Notte A, De Rienzo A, Rosica A, Bauer L, Affuso A, De Felice M, Russo T, Poulet R, et al. Replacement of K-Ras with H-Ras supports normal embryonic development despite inducing cardiovascular pathology in adult mice. EMBO Rep. 2005;6:432–437. doi: 10.1038/sj.embor.7400397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To MD, Wong CE, Karnezis AN, Del Rosario R, Di Lauro R, Balmain A. Kras regulatory elements and exon 4A determine mutation specificity in lung cancer. Nat. Genet. 2008;40:1240–1244. doi: 10.1038/ng.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ninomiya Y, Kato K, Takahashi A, Ueoka Y, Kamikihara T, Arima T, Matsuda T, Kato H, Nishida J-I, Wake N. K-Ras and H-Ras activation promote distinct consequences on endometrial cell survival. Cancer Res. 2004;64:2759–2765. doi: 10.1158/0008-5472.can-3487-2. [DOI] [PubMed] [Google Scholar]
- 8.Quinlan MP, Quatela SE, Philips MR, Settleman J. Activated Kras, but not Hras or Nras, may initiate tumors of endodermal origin via stem cell expansion. Mol. Cell. Biol. 2008;28:2659–2674. doi: 10.1128/MCB.01661-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fotiadou PP, Takahashi C, Rajabi HN, Ewen ME. Wild-type NRas and KRas perform distinct functions during transformation. Mol. Cell. Biol. 2007;27:6742–6755. doi: 10.1128/MCB.00234-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voice JK, Klemke RL, Le A, Jackson JH. Four human ras homologs differ in their abilities to activate Raf-1, induce transformation, and stimulate cell motility. J. Biol. Chem. 1999;274:17164–17170. doi: 10.1074/jbc.274.24.17164. [DOI] [PubMed] [Google Scholar]
- 11.Parikh C, Subrahmanyam R, Ren R. Oncogenic NRAS, KRAS, and HRAS exhibit different leukemogenic potentials in mice. Cancer Res. 2007;67:7139–7146. doi: 10.1158/0008-5472.CAN-07-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Haigis KM, McDaniel A, Harding-Theobald E, Kogan SC, Akagi K, Wong JCY, Braun BS, Wolff L, Jacks T, et al. Hematopoiesis and leukemogenesis in mice expressing oncogenic NrasG12D from the endogenous locus. Blood. 2011;117:2022–2032. doi: 10.1182/blood-2010-04-280750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haigis KM, Kendall KR, Wang Y, Cheung A, Haigis MC, Glickman JN, Niwa-Kawakita M, Sweet-Cordero A, Sebolt-Leopold J, Shannon KM, et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat. Genet. 2008;40:600–608. doi: 10.1038/ngXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doma MK, Parker R. Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature. 2006;440:561–564. doi: 10.1038/nature04530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura Y, Gojobori T, Ikemura T. Codon usage tabulated from international DNA sequence databases: status for the year 2000. Nucleic Acids Res. 2000;28:292. doi: 10.1093/nar/28.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rago C, Vogelstein B, Bunz F. Genetic knockouts and knockins in human somatic cells. Nat. Protoc. 2007;2:2734–2746. doi: 10.1038/nprot.2007.408. [DOI] [PubMed] [Google Scholar]
- 17.Plotkin JB, Kudla G. Synonymous but not the same: the causes and consequences of codon bias. Nat. Rev. Genet. 2011;12:32–42. doi: 10.1038/nrg2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maroney PA, Yu Y, Fisher J, Nilsen TW. Evidence that microRNAs are associated with translating messenger RNAs in human cells. Nat. Struct. Mol. Biol. 2006;13:1102–1107. doi: 10.1038/nsmb1174. [DOI] [PubMed] [Google Scholar]
- 19.Darnell JC, Van Driesche SJ, Zhang C, Hung KYS, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlini DB. Experimental reduction of codon bias in the Drosophila alcohol dehydrogenase gene results in decreased ethanol tolerance of adult flies. J. Evol. Biol. 2004;17:779–785. doi: 10.1111/j.1420-9101.2004.00725.x. [DOI] [PubMed] [Google Scholar]
- 21.Hense W, Anderson N, Hutter S, Stephan W, Parsch J, Carlini DB. Experimentally increased codon bias in the Drosophila Adh gene leads to an increase in larval, but not adult, alcohol dehydrogenase activity. Genetics. 2010;184:547–555. doi: 10.1534/genetics.109.111294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 23.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32:D277–D280. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis CA, Clark G. The importance of being K-Ras. Cell Signal. 2000;12:425–434. doi: 10.1016/s0898-6568(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 26.Zolotukhin S, Potter M, Hauswirth WW, Guy J, Muzyczka N. A "humanized" green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J. Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fath S, Bauer AP, Liss M, Spriestersbach A, Maertens B, Hahn P, Ludwig C, Schäfer F, Graf M, Wagner R. Multiparameter RNA and codon optimization: a standardized tool to assess and enhance autologous mammalian gene expression. PLoS ONE. 2011;6:e17596. doi: 10.1371/journal.pone.0017596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson F, Jackson RJ, Smith CW. Expression of human nPTB is limited by extreme suboptimal codon content. PLoS One. 2008;3:e1801. doi: 10.1371/journal.pone.0001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim CH, Oh Y, Lee TH. Codon optimization for high-level expression of human erythropoietin (EPO) in mammalian cells. Gene. 1997;199:293–301. doi: 10.1016/s0378-1119(97)00384-3. [DOI] [PubMed] [Google Scholar]
- 30.Valdmanis PN, Gu S, Schüermann N, Sethupathy P, Grimm D, Kay MA. Expression determinants of mammalian argonaute proteins in mediating gene silencing. Nucleic Acids Res. 2012;40:3704–3713. doi: 10.1093/nar/gkr1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duret L, Galtier N. Biased gene conversion and the evolution of mammalian genomic landscapes. Annu. Rev. Genomics Hum. Genet. 2009;10:285–311. doi: 10.1146/annurev-genom-082908-150001. [DOI] [PubMed] [Google Scholar]
- 32.Ingolia NT, Lareau LF, Weissman JS. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell. 2011;147:789–802. doi: 10.1016/j.cell.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. A "silent" polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 34.Prior IA, Lewis PD, Mattos C. A comprehensive survey of ras mutations in cancer. Cancer Res. 2012;72:2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat. Rev. Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat. Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- 37.Yamada H, Sakamoto H, Taira M, Nishimura S, Shimosato Y, Terada M, Sugimura T. Amplifications of both c-Ki-ras with a point mutation and c-myc in a primary pancreatic cancer and its metastatic tumors in lymph nodes. Jpn. J. Cancer Res. 1986;77:370–375. [PubMed] [Google Scholar]
- 38.Soh J, Okumura N, Lockwood WW, Yamamoto H, Shigematsu H, Zhang W, Chari R, Shames DS, Tang X, MacAulay C, et al. Oncogene mutations, copy number gains and mutant allele specific imbalance (MASI) frequently occur together in tumor cells. PLoS ONE. 2009;4:e7464. doi: 10.1371/journal.pone.0007464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA, Jacks T. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc. Natl. Acad. Sci. U.S. A. 2008;105:3903–3908. doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White RJ. RNA polymerase III transcription and cancer. Oncogene. 2004;23:3208–3216. doi: 10.1038/sj.onc.1207547. [DOI] [PubMed] [Google Scholar]
- 43.Marshall L, Kenneth NS, White RJ. Elevated tRNA(iMet) synthesis can drive cell proliferation and oncogenic transformation. Cell. 2008;133:78–89. doi: 10.1016/j.cell.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 44.Kafri R, Springer M, Pilpel Y. Genetic redundancy: new tricks for old genes. Cell. 2009;136:389–392. doi: 10.1016/j.cell.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 45.Zhao K-N, Chen J. Codon usage roles in human papillomavirus. Rev. Med. Virol. 2011;21:397–411. doi: 10.1002/rmv.707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.