Abstract
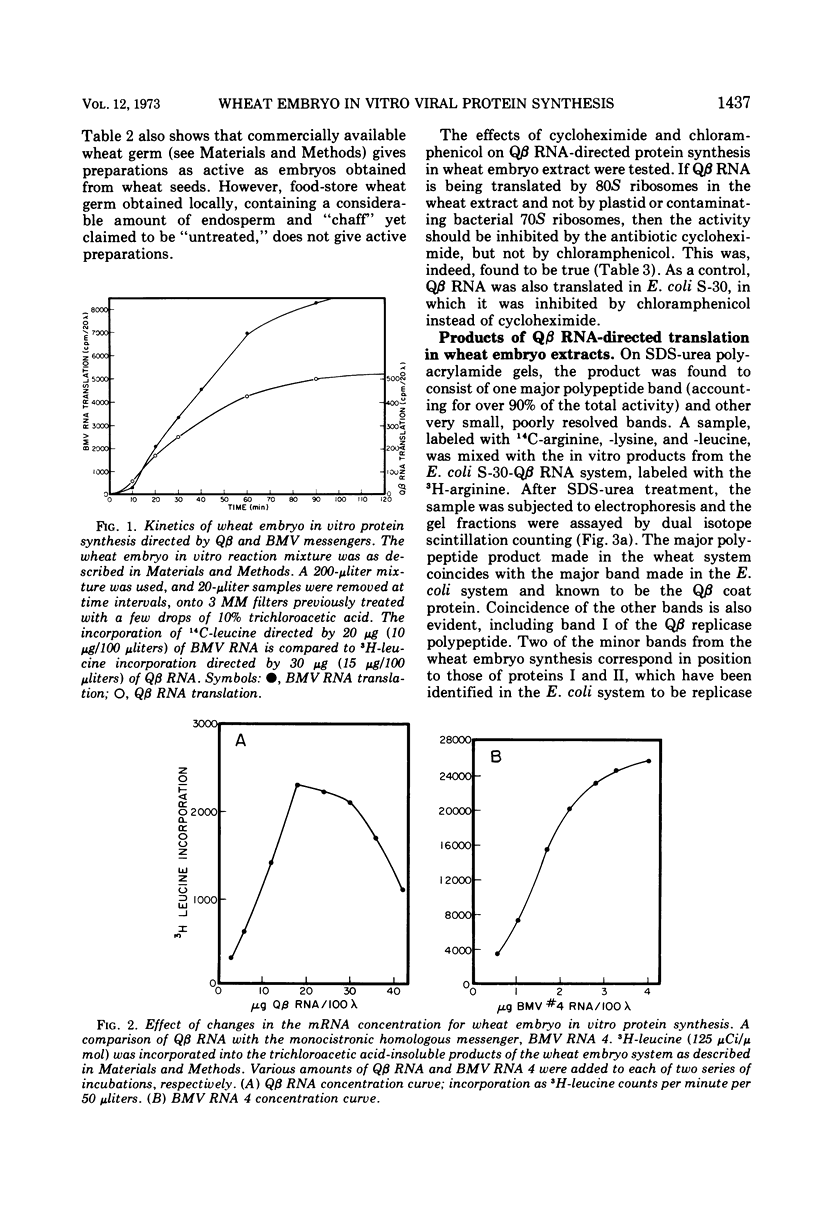
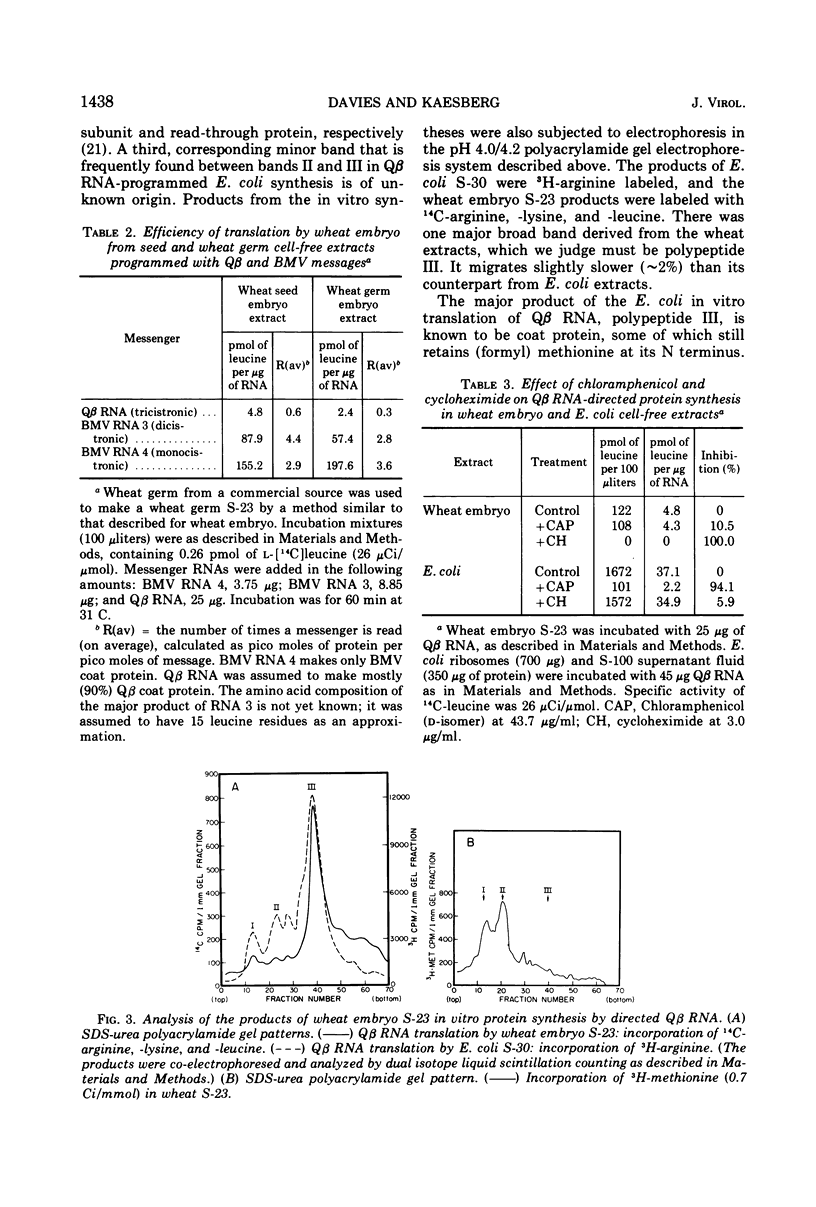
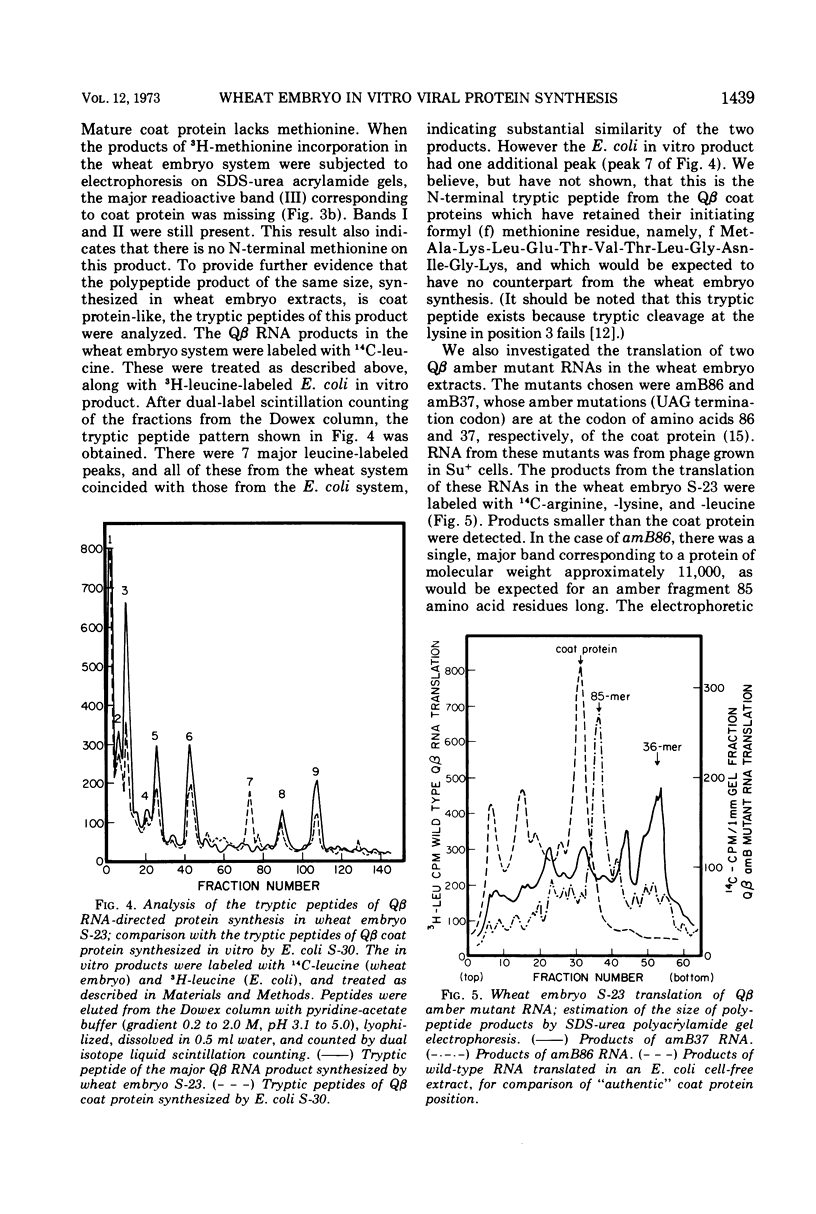
The RNA from bacteriophage Qβ can be translated by cell-free extracts from wheat embryos. This translation, by 80S ribosomes, occurs at a low magnesium ion concentration. Three products are synthesized which coelectrophorese with Qβ proteins synthesized in Escherichia coli extracts. The smallest of these has been identified as coat protein. Although the polycistronic bacteriophage message is translated with fidelity, the efficiency is much less than when the monocistronic brome mosaic virus coat protein message is translated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307. doi: 10.1073/pnas.68.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Boime I., Loyd B., Leder P. Translation of bacteriophage Q messenger RNA in a murine Krebs 2 ascites tumor cell-free system. Science. 1972 Dec 22;178(4067):1293–1295. doi: 10.1126/science.178.4067.1293. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Cell-free protein synthesis programmed with R17 RNA: identification of two phage proteins. J Mol Biol. 1966 Oct 28;21(1):173–193. doi: 10.1016/0022-2836(66)90086-6. [DOI] [PubMed] [Google Scholar]
- Craven G. R., Voynow P., Hardy S. J., Kurland C. G. The ribosomal proteins of Escherichia coli. II. Chemical and physical characterization of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2906–2915. doi: 10.1021/bi00835a032. [DOI] [PubMed] [Google Scholar]
- Efron D., Marcus A. Efficient synthesis of rabbit globin in a cell-free system from wheat embryo. FEBS Lett. 1973 Jun 15;33(1):23–27. doi: 10.1016/0014-5793(73)80150-4. [DOI] [PubMed] [Google Scholar]
- Eggen K. L., Shatkin A. J. In vitro translation of cardiovirus ribonucleic acid by mammalian cell-free extracts. J Virol. 1972 Apr;9(4):636–645. doi: 10.1128/jvi.9.4.636-645.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei W. D., 3rd, Lengyel P. Translation of in vitro synthesized reovirus messenger RNAs into proteins of the size of reovirus capsid proteins in a mouse L cell extract. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1816–1823. doi: 10.1016/0006-291x(72)90056-3. [DOI] [PubMed] [Google Scholar]
- JOHNSTON F. B., STERN H. Mass isolation of viable wheat embryos. Nature. 1957 Jan 19;179(4551):160–161. doi: 10.1038/179160b0. [DOI] [PubMed] [Google Scholar]
- Jockusch H., Ball L. A., Kaesberg P. Synthesis of polypeptides directed by the RNA of phage Q beta. Virology. 1970 Oct;42(2):401–414. doi: 10.1016/0042-6822(70)90283-7. [DOI] [PubMed] [Google Scholar]
- Klein W. H., Nolan C., Lazar J. M., Clark J. M., Jr Translation of satellite tobacco necrosis virus ribonucleic acid. I. Characterization of in vitro procaryotic and eucaryotic translation products. Biochemistry. 1972 May 23;11(11):2009–2014. doi: 10.1021/bi00761a003. [DOI] [PubMed] [Google Scholar]
- Maita T., Konigsberg W. The amino acid sequence of the Q coat protein. J Biol Chem. 1971 Aug 25;246(16):5003–5024. [PubMed] [Google Scholar]
- Marcus A., Luginbill B., Feeley J. Polysome formation with tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1243–1250. doi: 10.1073/pnas.59.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell M. J., Joklik W. K., Villa-Komaroff L., Lodish H. F. Translation of reovirus messenger RNAs synthetesized in vitro into reovirus polypeptides by several mammalian cell-free extracts. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2649–2653. doi: 10.1073/pnas.69.9.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Lodish H. F. Translation of bacteriophage Q RNA by cytoplasmic extracts of mammalian cells. Proc Natl Acad Sci U S A. 1973 Feb;70(2):315–319. doi: 10.1073/pnas.70.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A., Kaesberg P. Amber mutant of bacteriophage Q capable of causing overproduction of Q replicase. J Virol. 1973 Apr;11(4):603–605. doi: 10.1128/jvi.11.4.603-605.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUSS J. H., Jr, SINSHEIMER R. L. Purification and properties of bacteriophage MS2 and of its ribonucleic acid. J Mol Biol. 1963 Jul;7:43–54. doi: 10.1016/s0022-2836(63)80017-0. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T., Gesteland R. F., Spahr P. F. Translation of bacteriophage R17 and Qbeta RNA in a mammalian cell-free system. J Mol Biol. 1973 Apr 15;75(3):575–578. doi: 10.1016/0022-2836(73)90462-2. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Kaesberg P. Translation of brome mosaic viral ribonucleic acid in a cell-free system derived from wheat embryo. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1799–1803. doi: 10.1073/pnas.70.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Lane L. C., Kaesberg P. Origin of the small component of brome mosaic virus RNA. J Mol Biol. 1972 Mar 14;64(2):353–362. doi: 10.1016/0022-2836(72)90503-7. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Kaesberg P. Acrylamide gel electrophoresis of bacteriophage Q beta: electrophoresis of the intact virions and of the viral proteins. Virology. 1970 Oct;42(2):437–452. doi: 10.1016/0042-6822(70)90287-4. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Weber K. Natural read-through at the UGA termination signal of Q-beta coat protein cistron. Nat New Biol. 1971 Sep 15;234(50):206–209. doi: 10.1038/newbio234206a0. [DOI] [PubMed] [Google Scholar]



