Abstract
Breast cancer is a very heterogeneous disease, encompassing several intrinsic subtypes with various morphological and molecular features, natural history and response to therapy. Currently, molecular targeted therapies are available for estrogen receptor (ER)− and human epidermal growth factor receptor 2 (Her2)-positive breast tumors. However, a significant proportion of primary breast cancers are negative for ER, progesterone receptor (PgR), and Her2, comprising the triple negative breast cancer (TNBC) group. Women with TNBC have a poor prognosis because of the aggressive nature of these tumors and current lack of suitable targeted therapies. As a consequence, the identification of novel relevant protein targets for this group of patients is of great importance. Using a systematic two dimensional (2D) gel-based proteomic profiling strategy, applied to the analysis of fresh TNBC tissue biopsies, in combination with a three-tier orthogonal technology (two dimensional PAGE/silver staining coupled with MS, two dimensional Western blotting, and immunohistochemistry) approach, we aimed to identify targetable protein markers that were present in a significant fraction of samples and that could define therapy-amenable sub-groups of TNBCs. We present here our results, including a large cumulative database of proteins based on the analysis of 78 TNBCs, and the identification and validation of one specific protein, Mage-A4, which was expressed in a significant fraction of TNBC and Her2-positive/ER negative lesions. The high level expression of Mage-A4 in the tumors studied allowed the detection of the protein in the tumor interstitial fluids as well as in sera. The existence of immunotherapeutics approaches specifically targeting this protein, or Mage-A protein family members, and the fact that we were able to detect its presence in serum suggest novel management options for TNBC and human epidermal growth factor receptor 2 positive/estrogen receptor negative patients bearing Mage-A4 positive tumors.
Breast cancer, although a very heterogeneous disease, can be divided into three therapeutically relevant fundamental disease entities, simply based on estrogen receptor (ER) and human epidermal growth factor receptor 2 (Her2)1 status (i.e. ER+ and/or Her2+, and ER−Her2−), as the major currently available breast cancer therapeutic options are based on the ability to target these proteins. Hormone receptor positive and hormone receptor negative breast cancers are disease entities with distinct morphological, genetic and biological behavior (1). Hormone receptor negative tumors, which constitute ∼30% of primary breast cancers, tend to be high-grade, more frequently BRCA1 and TP53 mutated, and, more importantly, are not amenable to endocrine therapy. Her2 is amplified in ∼18–20% of breast cancers, and is more frequently observed in hormone receptor negative tumors. Her2 amplification is associated with worse prognosis (higher rate of recurrence and mortality) in patients with newly diagnosed breast cancer who do not receive any adjuvant systemic therapy. Her2 status is also predictive for several systemic therapies, particularly for agents that target Her2. The development of a humanized monoclonal antibody against Her2 (trastuzumab) has resulted in reduction of the risk of recurrence and mortality in patients with Her2 amplification (2, 3). Although trastuzumab is considered one of the most effective targeted therapies currently available in oncology, a significant number of patients with Her2-overexpressing breast cancer do not benefit from it (4, 5).
Breast tumors that do not express ER, PgR, or Her2 (ER− PgR− Her2−), as determined by immunohistochemistry (IHC), are generally referred to as triple negative breast cancers (TNBCs), and they are not candidates for targeted therapies (endocrine therapy or trastuzumab). Although TNBCs account for a relatively small proportion of breast cancer cases (10–15%), they are responsible for a disproportionate number of breast cancer deaths. TNBC tumors form a recognizable prognostic group of breast cancer with aggressive behavior that currently lacks the benefit of available systemic therapy (6–8). Given the need to develop molecular criteria to reproducibly categorize molecular breast tumor subtypes at the protein level and the lack of targeted therapies available to treat patients bearing TNBCs, we have implemented a systematic proteomics approach to identify, characterize, and evaluate proteins present in triple-negative tumors that could constitute an appropriate therapeutic target for the clinical management of this group of patients. To this end, based on the analysis of 78 individual TNBC samples, we have established a large, cumulative, 2D-PAGE database of proteins expressed by TNBCs, including some that could be of potential therapeutic value. Comparison of this TNBC protein database with protein databases of other breast cancer subtypes previously established by our laboratory allowed us to single out a number of proteins preferentially expressed in TNBCs for which targeted therapeutics exist. In this report we further focused on the characterization of one such target, the cancer/testis antigen, melanoma-associated antigen 4 - Mage-A4.
Cancer/testis antigens (CTAs) are expressed in a large variety of tumor types, whereas their expression in normal tissues is restricted to male germ cells, which are immune-privileged because of their lack of or low expression of human leukocyte antigen (HLA) molecules (9). Several studies have shown the existence of natural cellular and humoral responses against some CTAs, indicating that they are appropriate targets for vaccine-based cancer immunotherapy (10–12). So far, the use of CTAs in immunotherapeutic approaches to cancer treatment has been tested in more than 60 early phase clinical trials, with varying success, and a few candidate products have reached late-stage clinical trials. One such candidate vaccine, Astuprotimut-R (GSK-249553), a Mage-A3 antigen-specific cancer immunotherapeutic agent, is currently under clinical evaluation by GlaxoSmithKline in the largest-ever treatment trial in lung cancer, called MAGRIT (Mage-A3 as Adjuvant nonsmall cell lunG canceR ImmunoTherapy) (13).
At present, CTAs comprise about 150 members, more than half of which are encoded by large, recently expanded families on chromosome X (14; see also CTDatabase at www.cta.lncc.br; last accessed 01.09.2012). These genes are organized into clusters and have undergone rapid evolution, possibly because of positive selection. The biological functions of CTAs are not fully understood, but emerging evidence suggest that they direct the proliferation, differentiation, and survival of human germ line cells and may have similar effect in cancer cells. Mage-A4 protein belongs to the Mage-A family of CT antigens. The Mage-A family is composed by 12 proteins (14, 15) and many members of the Mage-A family of CTAs have been associated with cancer, including breast cancer (14, 16, 17). However, past studies reported mostly on MAGE genes rather than protein expression, or on the expression of Mage protein families and not on any given specific protein.
In this paper we describe the identification of Mage-A4 in breast tumor biopsies using 2D PAGE coupled with MS proteomics, and follow the protein localization from the tumor cells, to the tumor microenvironment, and to the serum of a patient. Using a three-tier orthogonal technology approach that combined 2D PAGE silver staining coupled with MS, with 2D Western blotting, and IHC, we showed that high level Mage-A4 expression in breast tumors occurs almost exclusively in the receptor negative disease (TNBC and Her2+ER−PgR−). The existence of immunotherapeutic approaches targeting MAGE protein family members (Mage-A4 specific or with broader specificity) and the fact that we were able to detect its presence in serum suggest novel management options for patients bearing Mage-A4 positive TNBCs and Her2+ER−PgR− tumors.
EXPERIMENTAL PROCEDURES
Sample Collection and Handling
Tissue and serum samples from clinical high-risk patients (high-risk definition according to the Danish Breast Cooperative Group; www.dbcg.dk, last accessed 22.10.2009) that underwent mastectomy between 2003 and 2008, were collected within the Danish Center for Translational Breast Cancer Research program. All patients had no previous surgery to the breast and did not receive preoperative treatment. They presented a unifocal tumor of an estimated size of more than 20 mm. In addition, 78 nonselected retrospective TNBCs and 30 Her2-positive cases were provided by the Department of Pathology at the Copenhagen University Hospital. Fresh breast tissue samples for 2D PAGE were flash-frozen in liquid nitrogen following surgery and were rapidly transported to the Institute of Cancer Biology where they were stored at −80 °C until the time of analysis; on average no more than 15 min elapsed from tissue excision to freezing. Samples for interstitial fluid recovery from tumors and normal specimens were kept in PBS at 4 °C and were routinely prepared within a maximum of 30–45 min from the time of surgical excision. The project was approved (KF 01–069/03) by the Copenhagen and Frederiksberg regional division of the Danish National Committee on Biomedical Research Ethics. Testis samples were provided by the Department of Growth and Reproduction at the Copenhagen University Hospital. Blood samples were drawn preoperatively into endotoxin-free collection tubes without anticoagulation agents and processed as soon as possible (no later than 1.5 h after collection). Samples were centrifuged at 2000 × g for 10 min to separate the plasma from blood cells, and stored frozen at −80 °C until analyzed.
Sample Preparation for 2D PAGE
Twenty to thirty, 6-μm cryostat sections of frozen tissues were resuspended in 0.1 ml lysis solution for 2D-PAGE (18, 19). The resulting lysates were frozen and kept at −20 °C until used, usually within 24–48 h. Twenty to forty microliters were applied to the gel tube for isoelectrofocusing (IEF) as described (19), and each sample was run at least in duplicate. The first and last two sections of each sample were used for hematoxylin and eosin (H&E) staining and immunofluorescence analysis using cytokeratin 19 (CK19) antibodies, respectively. The H&E stained sections were reviewed by a pathologist to do a coarse determination of the percentage of tumors cells. In addition, we analyzed the tissue sections for CK19 positive cells, as this epithelial marker is ubiquitously expressed by mammary epithelial cells (20). The availability of these pictures greatly facilitated the interpretation of the gel data as it gave a rough estimate of the ratio of epithelial cells to stromal tissue. Only specimens with at least 80% tumor cells, evaluated by the combined H&E and CK19 stainings, were used for subsequent analysis. The proportion of cells was only used as inclusion criterion of selection of samples but not as a normalization parameter. Sample preparation for 2D PAGE from cell lines was performed as described previously (21).
Tissue Interstitial Fluid (TIF) preparation
Tissue interstitial fluid was recovered from tumor and normal samples following a previously published procedure (22). For analysis, 100 μl of TIF solution were lyophilized and subsequently solubilized in 0.1 ml lysis solution for 2D PAGE (18, 19).
2D PAGE and 2D Western Immunoblotting
2D PAGE (isoelectric focusing, IEF and nonequilibrium pH gradient electrophoresis, NEPHGE) and 2D gel Western blotting were performed as previously described (23). After running the second dimension, gels were placed in 7.5% acetic acid, 50% ethanol, and 0.05% formalin for 1 h, washed three times for 30 min each in 7.5% acetic acid, 10% ethanol, and stained with silver nitrate according to a procedure compatible with mass spectrometry (23). Silver stained gels were dried between cellophane followed by scanning for comparative protein and MS analysis. 2D gel Western blotting was performed according procedure described elsewhere (24).
Image Analysis
2D gels were scanned and analyzed using the PDQUEST software package from BioRad (version 8.0.1). A comprehensive protein database of TNBC expression profile was prepared based on the analysis of 78 TNBC and using the gel of TNBC 22 as a master image. All identified protein spots selected for the analysis were added to the master image.
Protein Spot Handling and Mass Spectrometry Analysis
All detected protein spots were excised from silver stained dry gels followed by re-hydrating in water. Gel pieces were then detached from the cellophane film and cut into 1 mm2 pieces followed by “in-gel” digestion as previously described (25) followed by a MS analysis that has been reported previously using Anchor target 600 (22). We included in all cases a post-silver de-staining before in-gel tryptic digestion, thus additionally improving peptide recovery from the gel. MALDI-TOF-TOF data were acquired using an Ultraflex ™ III 200 time-of-flight mass spectrometer (Bruker Daltonik, Germany) equipped with a Smart beam™ laser and a LIFT-TOF/TOF unit. Data acquisition and data processing were performed by the Flex Control 3.0 and Flex Analysis 3.0 software (Bruker Daltonik, Germany). All of the spectra were obtained using reflector positive mode with an acceleration voltage of 25 kV, reflector voltage of 26.38 kV and detection suppressed up to 450 Da. A total of 2000 shots in steps of 200 shots were added to one spectrum in the mass range of m/z 600–4000 using peak detection algorithm: SNAP (Sort Neaten Assign and Place); S/N threshold: 3 and Quality Factor Threshold: 50.
Spectral Analysis and Protein Identification
Post-acquisition two step calibration was automatically performed in FlexAnalysis using standard peptide calibration mixture (Bruker Daltonics, Germany) for external calibration followed by an additional post-acquisition internal calibration step to obtain better mass accuracies. Ubiquitous presented auto-digested tryptic mass values visible in all the spectra were used for internal calibration. The background masses (matrix, metal adducts, tryptic peptides from contaminating alpha-keratins) were automatically subtracted from finally generated pick list and were excluded from the further analysis. Additionally, pairwise comparison of all analyzed spectra has been performed to remove irrelevant picks derived from putative contaminations. For protein identification, peptide masses were transferred to the BioTools 3.2 interface (Bruker Daltonik, Germany) to search in the National Center for Biotechnology nonredundant NCBInr (20110301) database using in house MASCOT search engine (version 2.3.0.2, Matrix Science Ltd.). No restriction on the protein molecular mass and taxonomy was applied as a first step. A number of fixed (acrylamide modified cystein, i.e. propionamide/carbamidomethylation) and variable modifications (methionine oxidation and protein N terminus acetylation) were included in the search parameters. The peptide tolerance did not exceed 30 ppm and a maximum of one trypsin missed cleavage was allowed. Protein identifications by peptide-mass fingerprinting were considered to be confident when the protein score of the hit exceeded the threshold significance score of 65 (p < 0.05) and no less than six peptides were recognized.
If peptides match to multiple members of a protein family, and it was impossible to eliminate redundancy of unambiguous identification by applying manual search for unique isoform specific peptides, both family members were specified in the final table (Supplemental Tables S1 and S2). Whenever possible SwissProt accession numbers were assigned (UniProtKB/Swiss-Prot 55.3 (20247 human entries). Protein identification was confirmed by sequence information obtained from MS/MS analysis in “LIFT” mode. Whenever the protein score hit was close to the threshold significance score of 65, the LIFT analysis was performed as an additional mean to confirm the identity of the proteins identified by peptide-mass fingerprinting (PMF) using the same parameters as previously described (22).
Enzyme-linked Immunosorbent Assay (ELISA)
A sensitive and specific sandwich ELISA was developed using two of the Mage-A4 specific antibodies used in this study. The rab Ab 1982 (EP101638) rabbit polyclonal antibody was used for catching and the mouse monoclonal antibody to human Mage-A4 (CPTC-Mage-A4–1) was used for detection of the antigen as this combination provided the best signal-to-noise ratio. Immunoassay 96 MicroWell Solid Plates (Medisorp™; Nunc, Denmark) were coated overnight at 4 °C with 100 μl per well of catching antibody solution (rab Ab 1982) 2 μg/ml in 100 mm bicarbonate/carbonate buffer (3.03 g Na2CO3 and 6.0 g NaHCO3 in 1000 ml distilled water, pH 9.6). The assay wells were rinsed five times with PBS buffer containing 0.05% (v/v) Tween20 (PBS-T). Wells were then treated with 200 μl of Protein-Free T20 (PBS) Blocking Buffer (Pierce, Thermo Scientific) for 1h at 30 °C, and subsequently washed five times with washing buffer (PBS-T buffer) before use. One hundred microliter of diluted samples were added in triplicate to each well and incubated for 1h at 30 °C. Every assay plate also contained a series of standards, consisting of eleven serial dilutions (1:2) of purified full length recombinant human Mage-A4 (ProSpec, Rehovot, Israel) in triplicate. Diluent for standards was PBS with 1% bovine serum albumin and the highest standard concentration was 50 ng/ml. Included on each plate were blank control wells, containing sample dilution buffer only. Before ELISA measurements, serum samples and interstitial fluid samples were diluted 1:4 with Bio-Plex human serum diluent (Bio-Rad, USA), to yield values within the linear range of the standard curve for the assay. After binding of Mage-A4 protein, the wells were washed five times with washing buffer, and 100 μl of mouse monoclonal detection antibody CPTC-Mage-A4–1 (1 μg/ml) were added per well. After incubation for 1 h at 30 °C, wells were washed five times with washing buffer and an anti-mouse immunoglobulins peroxidase-conjugated polymer backbone (Envision™; DAKO, Denmark) dilution 1:200 was used in the secondary detection step. Detection was performed with 3,3′,5,5′-tetramethylbenzidine (TMB). The TMB reaction was stopped with 2 m H2SO4 and read spectrophotometrically at 450 nm using a VersaMax microplate reader (Molecular Devices, Sunnyvale, CA). All samples were determined in triplicate and the mean values were used for statistical analysis. The mean minimum detectable dose (MDD) of Mage-4 protein by this assay was determined to be 0.5 pg/μl. We performed spike-and-recovery experiments using low (10 pg/μl), medium (25 pg/μl), and high (40 pg/μl) spike concentrations of analyte in the various sample matrices. Average % of recovery from serum was 84 (range 77–89%), and for TIF was 92 (range 84–102%) across the concentration range of the assay, demonstrating no quantifiable matrix interference for each sample type. A four-parameter-fitted standard curve was generated using MasterPlex® ReaderFit software (Hitachi Software Engineering America Ltd., MiraiBio Group, USA), by which the concentration of Mage-A4 in samples was calculated.
Antibodies
A commercially available antibody against CK19 (mouse monoclonal; used at a dilution of 1:1000) was obtained from Labvision-ThermoScientific. The CPTC-MageA4–1 hybridoma developed by the Clinical Proteomic Technologies for Cancer initiative was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242, and was used at a dilution of 1:1000. The rabbit polyclonal anti-peptide antibody EP101638 (rab Ab 1982) [N-term peptide immunogen: FTCWRQPNEGSSSQE (aa78–92)] raised against Mage-4 was generated by Eurogentec (Belgium) and was used at a dilution of 1:200. The mouse monoclonal antibody clone 57B (used at a dilution of 1:100), was kindly provided by G. Spagnoli.
Immunohistochemistry of FFPE (Formalin-Fixed, Paraffin-Embedded) Samples
Following surgery, fresh tissue blocks were immediately fixed in neutral buffered formalin and paraffin embedded for archival use. Pellets from exponentially growing breast cancer cell lines (MDA-MB-436 and MCF-7) received from ATCC (USA) and cultured in DMEM/10%FCS, were likewise formalin fixed and paraffin embedded. Five-μm sections were cut from the paraffin blocks and mounted on Super Frost Plus slides (Menzel-Gläser, Braunschweig, Germany), baked at 60 °C for 60 min, deparaffinized, and rehydrated through graded alcohol rinses (24). Heat induced antigen retrieval was performed by immersing the slides in Tris/EDTA pH 9.0 buffer (10 mm Tris, 1 mm EDTA) and heating them in a 750 W microwave oven for 10 min. The slides were then cooled at room temperature for 20 min and rinsed abundantly in tap water. Nonspecific staining of slides was blocked (10% normal goat serum in PBS buffer) for 15 min, and endogenous peroxidase activity quenched using 0.3% H2O2 in methanol for 30 min. Antigen was detected with a relevant primary antibody, followed by a suitable secondary antibody conjugated to a peroxidase complex (HRP conjugated goat anti-rabbit or anti-mouse antibody (DakoCytomation, Glostrup, Denmark). Finally, color development was done with 3, 3′- diaminobenzidine (Pierce, Rockford, IL, USA) as a chromogen to detect bound antibody complex. Slides were counterstained with hematoxylin. Standardization of the dilution, incubation, and development times appropriate for each antibody allowed an accurate comparison of expression levels across samples for each antibody (26–28). Normal rabbit or mouse sera instead of primary antibody were used as a negative control.
Blocking Experiments for IHC Validation
The appropriate amount of antibody was diluted in TBS buffer to the final volume needed for staining of two section slides and divided equally into two tubes. Recombinant Mage-A4 protein was added into one tube to a final concentration of 5 μg/ml giving app. 1:5 molar excess of blocking peptide). Both tubes were incubated at room temperature for 1 h with agitation. Tandem sections of tissue or cell pellets paraffin blocks were stained either with the blocked antibody or antibody alone and processed in parallel for antigen detection with an appropriate secondary antibody as described in the previous section.
Tissue Microarrays (TMAs)
Breast cancer tissue microarray slides were obtained from Pantomics (BRC1501, BRC1502 and BRC1503 - Pantomics Inc., CA, USA). The TMAs contained a total of 210 nonoverlapping breast tumors. We also used a set of four TMAs prepared at the Department of Pathology, Copenhagen University Hospital comprising the 78 samples from the TNBC patients included in our proteomic analysis. For TMA construction, a hematoxylin and eosin-stained (H&E) slide from each block was analyzed by a pathologist (VTW) and regions of interest for each sample were defined. Tissue cores of 2 mm were punched from these defined areas and arrayed into a recipient paraffin block using an automated computer controlled machine (3D Histotech, Budapest, Hungary). Two donor tissue cores were used per case. The slides were stained as above using an appropriate primary antibody. For detection of immune complexes we used a horseradish peroxidase-labeled polymer (Envision+ detection kit, DAKO, Denmark) as a secondary antibody. All slides were independently reviewed by three of the authors (JEC, TC, and JMAM) and in the few discrepant cases a consensus was reached after joint review.
RESULTS AND DISCUSSION
Proteomic Profiling of TNBC
Triple-negative breast cancers are defined by absence of expression of the hormone receptors, ER and PgR, and Her2. Because of a lack of common molecular therapeutic targets, conventional chemotherapy remains the mainstay of treatment strategies for patients with TNBC. The combination of limited treatment options with clinicopathological heterogeneity of the disease makes clinical management of these lesions very demanding (reviewed in 29), and as a consequence, identification of novel therapeutic targets and options for management of TNBCs is of vital importance. We performed 2D PAGE/silver staining profiling coupled with MS-based identification of proteins in a prospective cohort of 78 TNBC patients (defined by lack of ER, PgR, and Her2 expression by IHC analysis) and established a database of proteins comprising all polypeptides identified in the patient cohort by either IEF (Fig. 1, right hand panel: Supplemental Fig. S1) and/or NEPHGE (Fig. 1 left hand panel: Supplemental Fig. S2) 2D PAGE analysis. Taking into account the fact that tumor samples are characterized by a high degree of heterogeneity, such a cumulative protein database could not be constructed on the basis of 2D gel/MS analysis of only one sample. Accordingly, we selected a set of several of the most representative 2D gels of TNBCs and the images were matched using PDQUEST 8.0.1. Then, a master image was prepared that contained all the proteins spots present in the different images. To identify as many proteins as possible we carried out a systematic computer assisted analysis of the 2D gels of the remaining TNBC samples. All matched proteins were identified by MALDI MS-MS as described in the Experimental Procedures section (The data associated with this manuscript may be downloaded from the ProteomeCommons.org Tranche network). Supplemental Tables S1 and S2 list all the proteins identified in the 78 TNBC patient cohort by either IEF (553 proteins) and/or NEPHGE (152 proteins) 2D PAGE analysis. Some polypeptides were identified both in IEF and NEPHGE gels and in addition, some proteins were identified in multiple spots, reflecting posttranslational protein processing. Consequently, the number of unique protein species we could identify in TNBCs was 523. It should be noted that in many cases the matching procedure was encumbered by confounding factors such as: the low abundance of some proteins, the fact that some proteins migrated close to a cluster of proteins, heterogeneity of tissue samples, as well as to the inherent variability in the gel runs. Consequently, it is likely that some proteins expressed by TNBCs escaped identification.
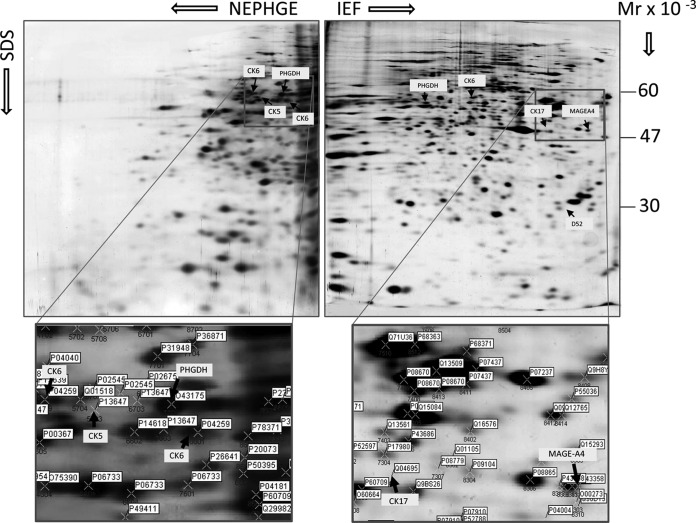
Fig. 1.
Proteomic analysis of TNBCs. Representative silver stained 2D-PAGE NEPHGE (upper left hand panel) and IEF (upper right hand panel) gels of proteins extracted from TNBC patient 22. The positions of the basal markers CK5, CK6, CK17, as well as Mage-A4, PHGDH, and D52 protein spots are indicated for reference. The framed areas in the gels, corresponding to the portions of the gels that contain these proteins, is shown enlarged in the lower panels of the figure, with the positions for the respective spots indicated. The position for each protein spot is marked by a cross, with SSP number (indicated by blue) and UniProt ID. SSP is a unique sample spot protein number assigned by the PDQuest software. Comprehensive information about 2D gel spot position, identity (protein name, UniProt, UniGene) as well as MALDI TOF characteristics is presented in Supplemental Figs. S1, and S2, and Supplemental Tables S1 and S2, respectively.
Inspection of the TNBC protein database, and comparison with proteomic data obtained from other breast cancer subtypes in our laboratory (23,24,30; this study), revealed a number of interesting candidate targets such as: tumor protein D52 (TPD52, IEF SSP# 8114, Supplemental Fig. S1), a protein frequently overexpressed in breast and other cancers (31) and a potential tumor antigen target for immunotherapy (32–34), d-3-phosphoglycerate dehydrogenase (PHGDH) (IEF SSP#2512; NEPHGE SSP# 2601, Supplemental Figs. S1 and S2, respectively), a protein involved in the serine biosynthetic pathway that was recently identified in ER-negative breast cancer (35, 36), and melanoma-associated antigen 4 (Mage-A4) (Fig. 1 right hand panel and Supplemental Fig. S1; IEF SSP#8314), a protein that we selected for further analysis, as well as a number of other, potentially important, candidates that are currently being studied and will be reported in detail elsewhere. We also identified basal cytokeratin (CK) markers such as CK5 (multiple variants in NEPHGE SSP#2602, 3607, 3609, 4706, 5603, 6703, and 7603), CK6 (IEF SSP# 5501; multiple variants in NEPHGE SSP#3608, 4709, and 8601) and CK17 (IEF SSP# 7414) (Fig. 1, and Supplemental Figs. S1 and S2). The basal cytokeratins CK5/6 and CK17 have been proposed as potential IHC-based markers that can distinguish between basal and nonbasal tumor subtypes (37); as such the fact that we were able to identify these foreknown TNBC markers lend some support to the relevancy of our analysis.
Analysis of Mage-A4 Deregulation in Breast Cancer
Selection and Specificity of Mage-A4 Antibodies
Mage-A4 is a cancer testis antigen (CTA), a protein whose expression is restricted to human germ line cells, but which is abnormally expressed in various types of cancer (38–42). A number of studies have previously established the expression of CTAs in breast tumors, and in some cases CTA expression was shown to be associated with hormone-receptor negative breast cancers (41, 43–49). However, these studies reported mostly on MAGE gene expression rather than protein levels or on the expression of Mage protein families rather than on specific proteins. The high degree of homology between different Mage proteins and the limited availability of anti-Mage antibodies recognizing only specific Mage family members, does not allow one to easily distinguish among Mage proteins; for example, the anti-Mage-A1 antibody 6C1 cross-reacts with Mage-A2, -A3, -A4, -A6, -A10, and -A12 (50).
To overcome the inherent difficulties associated with the extensive homology among the various Mage-A protein family members, we implemented a three-tier orthogonal technology approach based on the analysis of the same sample by 1) silver stained 2D PAGE coupled with MS-based identification of spots, 2) 2D Western blot, and 3) IHC using three different antibodies. This strategy combines the intrinsic sensitivity and cellular resolution of IHC, with the specificity of 2D Western and MS-based identification, and the overall protein analysis capability of 2D gels.
Several broad-spectrum vaccination strategies using cross-recognized epitopes, thus targeting various Mage protein family members, have been described (51, 52). But other more specific approaches targeting Mage-A4 also exist (53, 54). To analyze the specific expression of Mage-A4 protein we used a panel of three different antibodies: the first antibody was a rabbit polyclonal raised against a selected peptide of Mage-A4 (rab Ab 1982, raised against Mage-A4 aa78–92; Eurogentec). The second antibody was a monoclonal antibody (clone 57B) that was developed by Spagnoli and colleagues. This antibody was generated using Mage-A3 recombinant protein as immunogen and it recognizes an epitope (Mage-A3 aa16–31 region) that is identical in Mage-A2 and -A6, and differs by only 5, 3, and 1 residues in Mage-A1, -A4, and -A12, respectively (50), permitting cross-reactivity with Mage-A1, -A2, -A3, -A4, -A6, and -A12, as it was shown with cellular overexpression models (50, 55). Although at physiological levels of Mage-A protein family expression, the 57B mAb antibody (dilution 1:20) has shown specificity solely to Mage-A4, both in melanoma and nonsmall cell lung carcinoma tissue samples (55), thus suggesting a higher affinity of the antibody for Mage-A4, or that expression levels of this protein in tumor cells are higher than those of the other Mage-A proteins, any results obtained using this antibody should be interpreted with caution considering the potential for cross-reactivity. The third antibody we examined, was a mouse monoclonal antibody, CPTC-MageA4–1 developed by the U.S. NIH-Clinical Proteomic Technologies for Cancer Program (the epitope recognized by this antibody was not disclosed by the provider).
Determination of Antibody Specificity
Expression of CTAs in normal adult tissues is restricted to male germ cells in the testis. In malignancy, this pattern of regulation is disrupted, resulting in CTA expression in some tumors of various types. However, the spectrum of expression of CTAs in tumors of adult somatic tissues varies from individual tumor to individual tumor, with different CTAs being expressed differently in each case. Consequently, we tested the specificity of the our three antibodies by Western blotting using 2D gels of human normal testicular tissue, which expresses not only Mage-A4 (Fig. 2A), but also the full complement of potential cross-reacting Mage-A family proteins. As shown in Fig. 2B, all three antibodies detected various polypeptides of similar Mw (about 46kDa) in the testis extracts (Fig. 2B, black arrowheads), compatible with multiple post-translational modifications of the protein. This observation is congruent with published data identifying two phosphorylation sites (Ser90 and Ser99), and two ubiquitylation sites (Lys154 and Lys245) for Mage-A4 (56–58). The identity of the Mage-A4 protein spot corresponding to the major form recognized by all three antibodies (Fig. 2B, white arrows) was confirmed by MS in 2D gels of testis proteins (Fig. 2A, right hand panel). Despite an exhaustive analysis of all MS spectra generated from this area we did not find any hits (lower scores in Mascot search results) for other Mage-A proteins. In conclusion, with the one exception of the rab Ab 1982 antibody, which detected an additional smaller polypeptide (Fig. 2B, rab Ab 1982, gray arrow), the three antibodies showed comparable specificities. Interestingly, rab Ab 1982 did not detect this lower Mw polypeptide in multiple 2D blots from TNBCs samples (data not shown). It is possible that the lower Mw polypeptide corresponds to Mage-A5 (13 kDa, the smallest member of the Mage-A family) or to a smaller variant of the Mage-A4 protein present in testicular but not in breast tissue, as the existence of Mage-A4 isoforms under specific conditions has been previously reported (59). Immunohistochemistry analysis of testicular biopsy specimens with normal spermatogenesis using the three Mage-A4 antibodies, showed that in all three cases Mage-A4 immunoreactivity occurred in spermatogonia, giving a characteristic “pearl-necklace” appearance (illustrated in Fig. 2C for rab Ab 1982). This observation reiterated the results previously reported for mAb 57B (55). In each case, this immunoreactivity could be blocked by pre-incubating the antibody with recombinant Mage-A4 protein (Fig. 2C, illustrated with recMage-A4+rab Ab 1982). As expected, samples exhibited strong nuclear and cytoplasmic Mage-A4 staining of spermatogonia and weak staining of spermatocytes. Multiple indirect immunofluorescence analysis of testis samples with the three antibodies confirmed that the antibodies have nearly identical staining patterns (data not shown). These data demonstrated that, under the conditions of the assays, the antibodies showed comparable specificity toward Mage-A4. All subsequent analyses were done primarily using the mAb 57B antibody, and results corroborated with the rab Ab 1982 and CPTC-MageA4–1 antibodies. For simplicity, only the data from the mAb 57B antibody are shown henceforth.
Fig. 2.
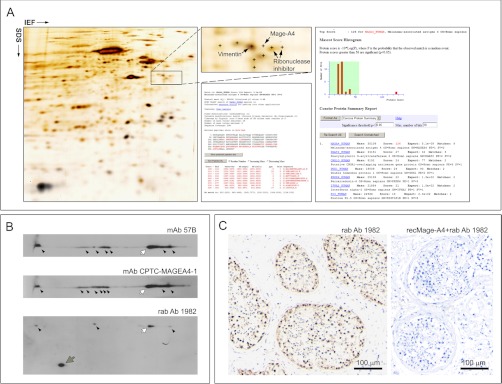
Proteomic analysis of Mage-A4 expression in normal human testicular tissue. A, Silver stained IEF 2D PAGE analysis with MS identification of the Mage-A4 protein (B) 2D Western blot analyses of Mage-A4 expression patterns in normal human testicular tissue lysates using three antibodies (mAb 57B, mAb CPTC-MageA4–1, and rab Ab 1982). Several polypeptides were detected by the three antibodies; the major peptide form corresponding to the MS-identified protein spot is indicated with white arrows and the other potential post-translationally modified forms are indicated with black arrowheads. One additional, lower Mw, polypeptide was observed with rab Ab 1982 and is indicated with a gray arrow (C). Immunohistochemical expression analysis of Mage-A4 in FFPE testicular biopsy specimens with normal spermatogenesis using rab Ab 1982 (left hand panel). Immunoreactivity occurred primarily in spermatogonia, presenting a characteristic “pearl-necklace ” appearance. Pre-incubation control assay with purified recombinant Mage-A4 protein (recMage-A4) blocked immunoreactvity in a tandem section (right hand panel). Scale bar, 100 μm.
Evaluation of Antibody Specificity in Breast Carcinomas
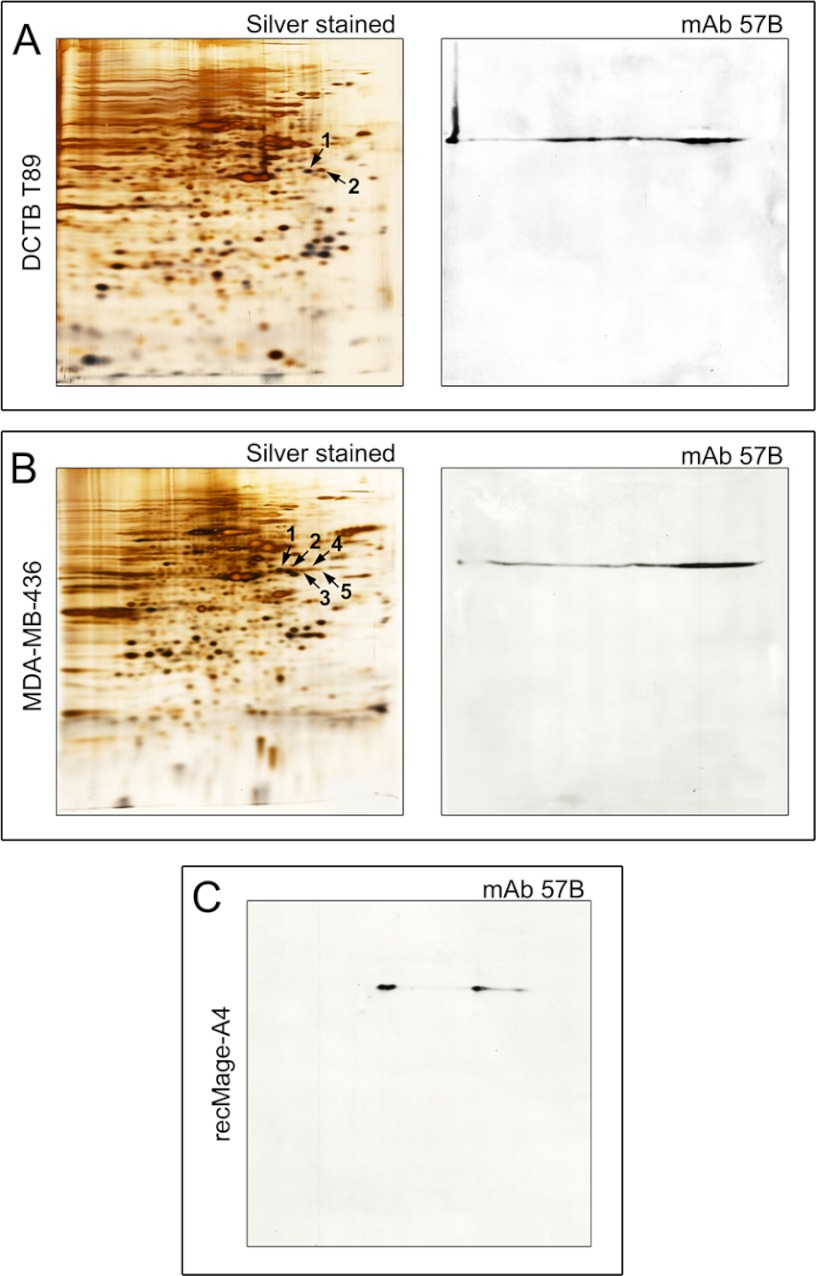
To address the issue of antibody specificity in malignant samples, and given that some potential cross-reactivities may be expressed only under pathological conditions, we performed a comparative analysis of MAGE-A4 expression in breast tumor biopsies and cell lines by 2D Western blotting followed by mass spectrometry analysis of the corresponding 2D gels. A number of Mage-A4 positive TNBC tissue biopsies were analyzed, and one representative example (DCTB T89) is shown in Fig. 3A. In addition, one cell line, the BRCA1-mutant triple-negative MDA-MB-436 cell line, which showed the highest expression of Mage-A4 protein among all lines we tested (data not shown), was also examined (Fig. 3B). As illustrated in Fig. 3, immunoblotting for Mage-A4 in T89 (Fig. 3A, mAb 57B) and MDA-MB-436 cell line (Fig. 3B, mAb 57B) was consistent with the identification of the proteins in corresponding 2D silver stained gels, both for T89 (Fig. 3A, spots 1 and 2 black arrows; Supplemental Table S3) and MDA-MB-436 (Fig. 3B, spots 1–5, black arrows, Supplemental Table S4). Additionally, 2D Western blot detection of purified recombinant human Mage-A4 protein (Fig. 3C, mAb57B) was also performed. The position of the mAb 57B immunoreactive spots on the blots of both breast carcinoma sample T89 and MDA-MB-436 cell line was matched to that of purified recombinant human Mage-A4 protein (compare Figs. 3A, B and C, mAb 57B 2D blots). The slightly different focusing of the Mage-A4 isoforms observed in the case of the purified recombinant protein is not surprising given the fact that a number of additional post-translational modifications are to be expected in mammalian cells and, additionally, the high complexity of tissue/cell lysates can affect IEF protein separation. Interestingly, although all visible spots in this area of the 2D PAGE gels were analyzed by MS (data not shown), we did not hit on any other Mage-A proteins (hits of lower scores in Mascot search results did not show any entries for other Mage-A proteins indicating an absence of peptides belonging to this group of proteins), which is in agreement with previous results showing lower expression level for the other Mage-A protein family members in breast cancer (47). Of note is the complete lack of cross-reactivity to any proteins from both breast tumor and MDA-MB-436 cell line (2D Western blots of full size are presented). No cross-reactivity was observed on the 2D Western blots of basic proteins (NEPHGE 2DPAGE, data not shown). The same results were obtained when rab Ab 1982 as well as CPTC-Mage-A4 antibodies were tested (data not shown).
Fig. 3.
Silver stained 2D-PAGE (IEF) gels and respective 2D Western blots of (A) a representative primary breast tumor (T89), (B) MDA-MB-436 breast cancer cell line, and (C) purified recombinant full length Mage-A4 protein. A, Silver stained 2D IEF gel of proteins extracted from T89 (left hand panel). Protein spots 1 and 2 identified by mass spectrometry as Mage-A4 are indicated by black arrows. 2D Western blot of T89 developed with the anti-Mage-A4 mAb 57B antibody (right hand panel) matched the MS identities. B, Silver stained 2D IEF gel of proteins extracted from MDA-MB-436 cells. Protein spots 1–5 identified by mass spectrometry as Mage-A4 are indicated by black arrows (left hand panel). 2D Western blot of MDA-MB-436 cell line developed with the anti-Mage-A4 mAb 57B antibody (right-hand panel). C, 2D Western blot of purified recombinant full length Mage-A4 protein developed with the anti-Mage-A4 mAb 57B antibody.
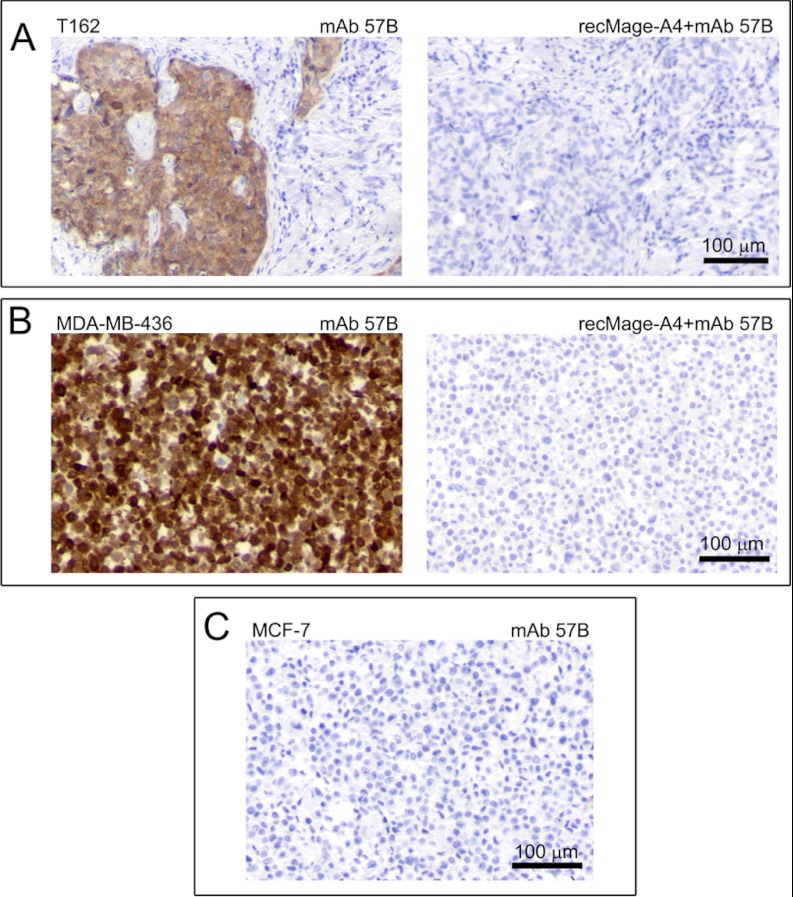
The specificity of mAb 57B for IHC analysis of breast tissue was also examined by staining representative tissue specimens with antibody pre-incubated with recombinant Mage-A4 protein (Fig. 4). A number of Mage-A4 positive tumor biopsies were selected for this experiment and one representative example is shown in (Fig. 4A, T162). The pre-incubation of the antibody with the recombinant Mage-A4 protein before IHC effectively blocked immunostaining of T162, confirming the specificity of the antibody toward Mage-A4 in mammary tissue in IHC (Fig. 4A, compare mAb 57B with recMage-A4+mAb 57B panel). These results were also confirmed with the MDA-MB-436 cell line (Fig. 4B). MCF7, a breast tumor cell line that does not express Mage-A4 at detectable levels, did not display any immunoreactivity with mAb 57B (Fig. 4C) as expected. Taken together, these data demonstrate that the mAb 57B antibody is highly specific toward the Mage-A4 antigen in breast tumor samples ruling out a potential tissue-specific cross-reactivity confounder other than Mage-A protein family. Given the extensive homology among the various Mage-A protein family members, it is possible that samples with highly deregulated expression of a Mage-A protein other than -A4, could be immunopositive in IHC analysis with mAb 57B, and consequently incorrectly supposed to be positive for Mage-A4. However, the combination of orthogonal technology approaches to the analysis of the same sample we used, with IHC analysis by three different antibodies complemented with by silver stained 2D-PAGE coupled with MS-based identification of spots and 2D Western blot, was designed to address such a scenario, making it highly unlikely.
Fig. 4.
Immunohistochemical expression analysis of Mage-A4 in (A) a representative FFPE primary breast tumor sample (T162), (B) MDA-MB-436, and (C) MCF7 cell lines. No immunostaining was observed neither in a breast tumor section (A, compare left and right hand panels) nor in MDA-MB-436 cell line (B, compare left and right hand panels) reacted with mAb 57B antibody pre-incubated with full length recombinant Mage-A4 polypeptide. No immunostaining was observed in MCF7 cell line (negative control) reacted with the anti-Mage-A4 mAb 57B antibody. Scale bar, 100 μm.
Mage-A4 Expression Pattern in Breast Carcinomas as Evaluated by IHC
We then proceeded to examine our cohort of 78 TNBCs for expression of Mage-A4 by IHC. We found that 26 out of the 78 cases (33%) expressed Mage-A4 (defined by positive reaction in more than 10% of tumor cells, see also Supplemental Fig. S4 illustrating the spectrum of Mage-A4 staining patterns). The immunoreactivity that was observed was in all cases moderate to strong. Concordance among the different orthogonal assays ensured the factual expression of Mage-A4, establishing that a subset of TNBCs, corresponding to roughly one third of cases, displayed high-level expression of this protein.
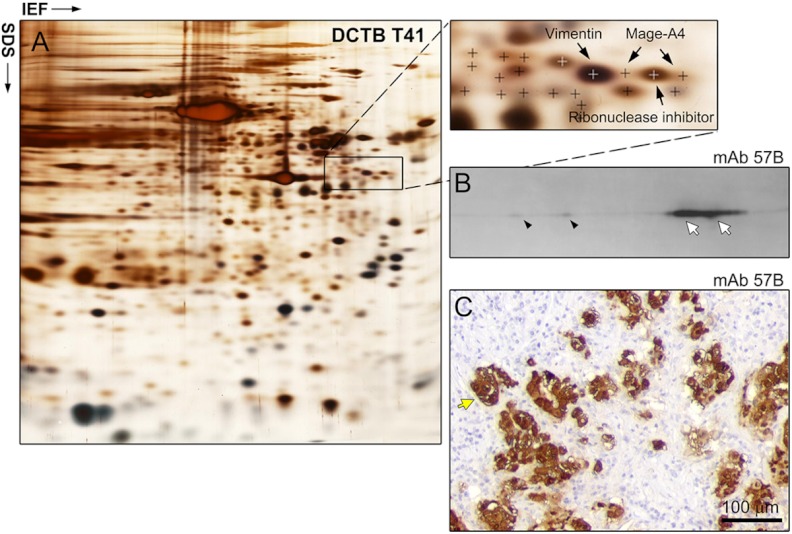
To determine if the expression of Mage-A4 was restricted to TNBCs or if it was a more general event in breast cancer, we performed IHC analysis of Mage-A4 expression using mAb 57B in an independent cohort of 228 carcinoma samples (including 22 TNBC and 18 ER− PgR− Her2+ samples) collected from mastectomies of high-risk breast cancer patients enrolled as part of a long-term translational study at DCTB (23, 25, 60). The results showed that 7 out of the 228 tumors expressed Mage-A4 (3.1% of all samples). The tumors expressing this protein belonged to two hormone receptor negative subtypes. Indeed, among the seven tumors expressing Mage-A4, four, DCTB T41, T58, T87, and T113 were of the ER− PgR− Her2+ subtype (corresponding to 1.8% of all samples and 39% of the ER− PgR− Her2+ cases), and the remaining three, DCTB T22, T89 and T162 belonged to the triple negative subtype (corresponding to 1.3% of all samples and 13.6% of the TNBC cases). These data showed that in the DCTB 228 breast carcinoma sample cohort, expression of Mage-A4 was restricted to hormone-receptor negative tumors. As before, immunoreactivity for Mage-A4 (Fig. 5C, yellow arrow), was supported by MS-based identification of the protein in 2D gels (Fig. 5A, arrows indicate Mage-A4 spots) and 2D Western blot detection (Fig. 5B), verifying the specific expression of Mage-A4 in the ER− PgR− Her2+ tumors. Notably, we were able to identify two Mage-A4 spots in tumor DCTB 41 (Fig. 5A) confirming the existence of multiple post-translational modifications of the protein we had observed by 2D Western blots.
Fig. 5.
Three-tier orthogonal technology approach to the analysis of Mage-A4 expression in ER− PgR− Her2+ breast cancer tissue. A, Silver stained IEF 2D-PAGE analysis of DCTB patient 41 tumor sample (ER− PgR− Her2+), with MS identification of the Mage-A4 protein. Two spots were identified in this case. B, 2D Western blot analyses of Mage-A4 expression confirmed the presence of Mage-A4 (white arrows correspond to the MS-identified spots; antibody mAb 57B) and, as it was the case for TNBCs, revealed the existence of multiple forms of the protein (black arrowheads). C, Expression of the protein by tumor cells was confirmed by IHC (antibody mAb 57B; yellow arrow). Scale bar, 100 μm.
To confirm the high prevalence of Mage-A4 expression in ER− PgR− Her2+ breast tumors, we analyzed by IHC a panel of 30 additional retrospective ER− PgR− Her2+ breast tumor samples. We found that six samples (20%) expressed Mage-A4, showing that although the exact number of Mage-A4 expressing tumors may vary depending on the cohort, the prevalence of Mage-A4 in hormone- receptor negative disease is quite significant.
Because of inclusion criteria (see Experimental Procedures section), the DCTB patient cohort is biased toward high-grade tumors, a potential confounding factor of our analysis, particularly because previous studies showed that CTAs, in general, and Mage-A4, in particular, are associated with poorly differentiated histological phenotypes (45, 61). With this in mind and to validate our observations in an independent sample data set, we used a set of three commercial tissue microarrays (TMAs) comprising 210 cases (BRC1501, 1502, and 1503; Pantomics Inc., USA) for which hormonal and HER2 status is known. IHC analysis of the TMAs showed that seven cases expressed Mage-A4 (3.3%). Of these, five tumors were of the TNBC subtype, one was ER− PgR− Her2+, and the remaining case was a micropapillary carcinoma (ER+ PgR− Her2−) confirming that Mage-A4 was preferentially expressed in hormone-receptor negative breast cancer (six out of the seven positive cases; p < 0.001).
In brief, we found expression of Mage-A4 in roughly 3% of the cases present in the two all-inclusive sets of breast cancer samples examined, with 228 (DCTB cohort) and 210 cases (BRCA1501, 1502, and 1503 TMAs), respectively. Of these, 7 out of 7 cases, and 6 out of 7 cases, respectively, were hormone receptor negative (ER− PgR−). Conversely, when we examined two sets of hormone receptor negative subtypes, using TNBC (78 cases) and ER− PgR− Her2+ (30 cases) breast cancer cohorts, we found that 33 and 20% of cases, respectively, expressed Mage-A4. We concluded that expression of Mage-A4 protein was predominantly associated with hormone receptor negative breast cancers.
Several studies have previously reported an association between expression of CTAs and ER status in breast cancer (43–47). However, in the specific case of Mage-A4, no significant relationships with ER status were found in the two studies that explicitly addressed this question (46, 47). The study by Grigoriadis and colleagues refers to the combined analysis of nine publicly available gene expression datasets (47). ER status-specific MAGE-A4 gene expression for these breast tumor data sets showed a nonsignificant, median adjusted, p value of 0.314, indicating that MAGE-A4 gene expression is independent of ER-status. However, in one of the data sets underlying this study, the Desmedt dataset (62), MAGE-A4 did show estrogen specific expression (p = 0.00235). Moreover, in another data set underlying this study, the van de Vijver dataset, MAGE-A4 expression showed a significant correlation with the basal subgroup (p < 0.001) (47). Basal-like tumors are of a higher grade and predominantly ER-negative. In brief, although the combined analysis of nine publicly available gene expression datasets by Grigoriadis and colleagues found no association between MAGE-A4 expression and ER- status, two of the constituent datasets did display this association.
In the case of the work by Bandić et al. (46) expression of Mage-A4 was studied by IHC using the 57B mAb antibody undiluted, and as such the results are more relatable to our own. In this study, the authors examined the expression of Mage-A4 by IHC on archival paraffin-embedded samples of breast cancer tissue from 81 patients and found that Mage-A4 immunoreactivity was not significantly associated with ER status (46). However, because the patients included in the study had to comply with a set of selection criteria (all patients had T1 to T3, N0 to N1, M0 tumors and underwent postoperative adjuvant radiotherapy), there is an inherent bias in the cohort. Moreover, Mage-A4 expression did show a trend, albeit nonsignificant, toward association with ER status (p = 0.082). In conclusion, the results we present here and the fact that several studies have reported an association between expression of CTAs and ER status in breast cancer (43–47) indicate that it is likely that expression of Mage-A4 protein is also associated with hormone receptor negative breast cancer.
Detection of Mage-A4 Protein in Interstitial Fluid and Patient Sera
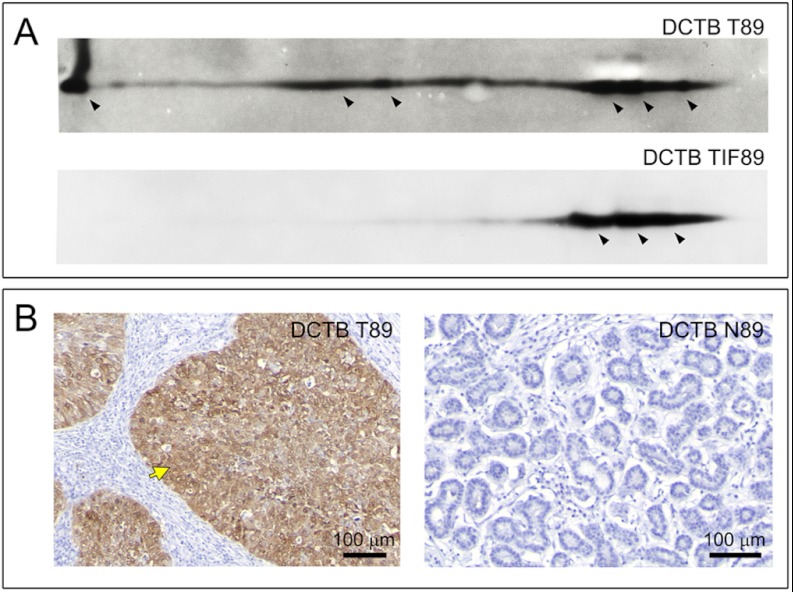
As part of the breast cancer biomarker early detection program within DCTB we have systematically collected tumor interstitial fluid (TIF) from portions of freshly dissected DCTB tumor samples (22, 63). TIFs collected from the seven DCTB cases that expressed Mage-A4 were analyzed by 2D Western blotting. We found Mage-A4 was present in TIFs derived from tumors bearing a high percentage of Mage-A4 positive cells. This is illustrated in Fig. 6 for DCTB 89, a triple negative tumor that exhibited high-level expression of Mage-A4 as determined by 2D Western immunoblotting (Fig. 6A, DCTB T89 panel) and generalized tumoral cell expression evaluated by IHC (Fig. 6B, DCTB T89 panel). The externalization of Mage-A4 to the interstitial fluid is illustrated in Fig. 6A (DCTB TIF89 panel). As expected, no immunoreactivity for Mage-A4 was observed in normal tissue (Fig. 6B; DCTB N89 panel). Externalization of Mage-A4 was coupled with cellular expression of the protein, and not solely specific for TNBCs, as we could also detect Mage-A4 in interstitial fluids from ER− PgR− Her2+ tumors that expressed Mage-A4 (data not shown).
Fig. 6.
Identification of Mage-A4 protein in tissue interstitial fluid of TNBC samples. A, 2D Western blot analyses of Mage-A4 confirmed externalization of Mage-A4 protein to the interstitial fluid of triple-negative tumor samples (TIF89). The pattern of expression was not completely identical to that of cellular lysates (compare DCTB T89 with TIF89, respectively), suggesting that only some of the forms of this protein are externalized. B, Differential expression of Mage-A4 was confirmed by IHC (antibody mAb 57B), showing that only tumor (DCTB T89; yellow arrow) but not normal cells express the protein. Scale bar, 100 μm. Additionally, full section image of low/high power IHC staining of TNBC tissue sample is presented in Supplemental Fig. S3.
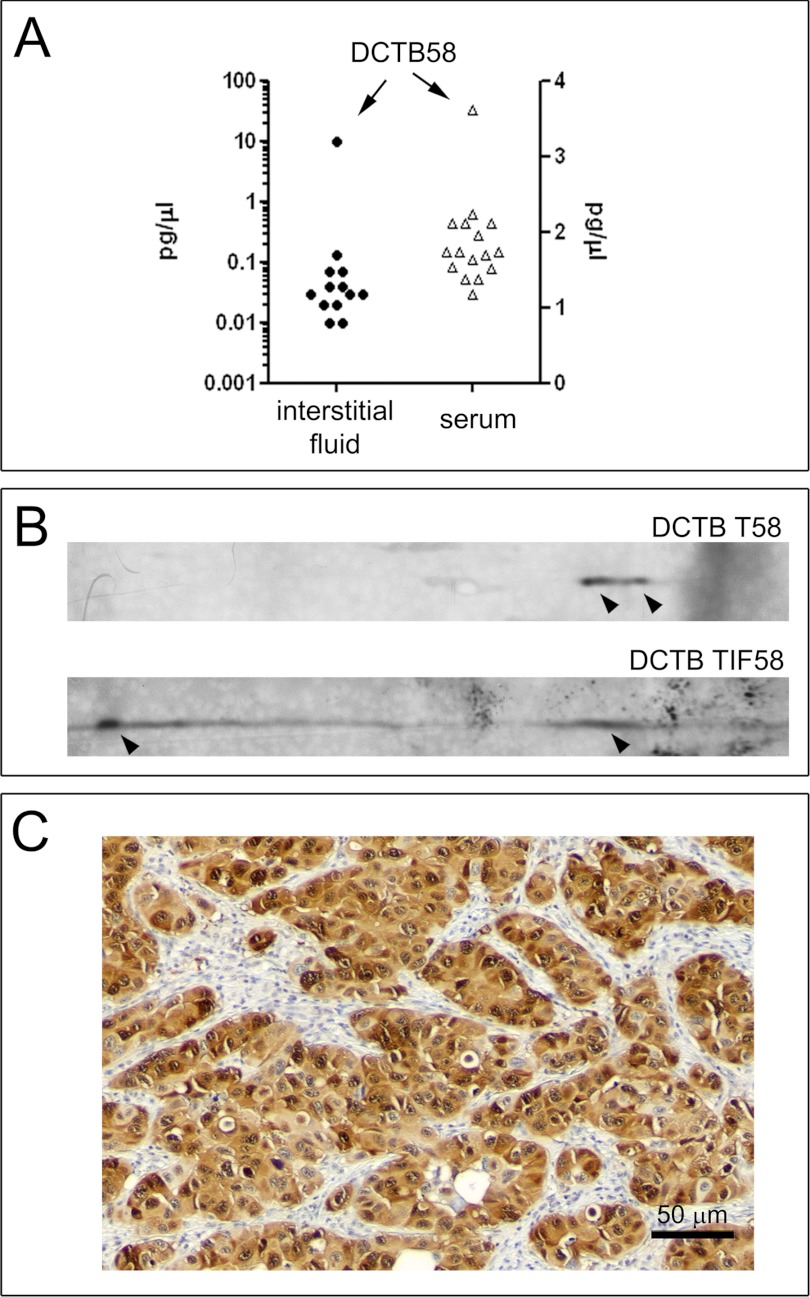
In view of these results, we decided to develop an ELISA assay for detection of Mage-A4 protein to address the question of whether Mage-A4 protein could be detected in human sera of patients bearing breast tumors. Given that we had extensively established the specificity of a set of three different antibodies recognizing Mage-A4, we decided to establish a sandwich ELISA assay for detecting Mage-A4 using a combination of these antibodies. The rab Ab 1982 and CPTC-MageA4–1 antibody combination showed the best performance in ELISA assays in exploratory studies and was chosen for further validation. The minimum reproducibly detectable level of Mage-4A protein by this assay was determined to be 0.5 pg/μl. We performed spike and recovery experiments using low (10 pg/μl), medium (25 pg/μl), and high (40 pg/μl) spike concentrations. We applied the ELISA assay to the analysis of a set of 12 interstitial fluid samples collected from patients within the DCTB cohort of 228 carcinoma samples, and for which we had sufficient biomaterial available. Of these, two samples, TIF58 and TIF113, both of the ER− PgR− Her2+ subtype, were obtained from tumors that expressed Mage-A4. The remaining TIFs were collected from tumors that did not express Mage-A4 (DCTB 46, 51, 52, 56, 57, 74, and 78). As an additional control for possible sample heterogeneity we used interstitial fluid from three matched normal samples (NIFs 46, 51, 56) because normal glandular cells do not express Mage-A4 (illustrated in Fig. 6B, DCTB N89 panel). We found that one single sample (TIF from DCTB 58) presented increased levels of Mage-A4 protein (10.04 pg/μl; Fig. 7A, left hand axis). The presence of Mage-A4 in this TIF was confirmed by 2D Western blot (Figs. 7B DCTB TIF58 panel), as well its expression in tumor cells both by 2D Western (Fig. 7B, DCTB T58 panel) and IHC (Fig. 7C). We were unable to detect Mage-A4 in TIF collected from DCTB 113, even though immunostaining of the tumor showed that it was positive for Mage-A4. Because Mage-A4 immunostaining was quite heterogenous in this tumor (data not shown), it is possible that the tissue piece we extracted TIF from did not express this protein at sufficient levels to allow detection.
Fig. 7.
Quantitative analysis of Mage-A4 protein by sandwich ELISA. A, Mage-A4 protein levels were determined in interstitial fluids from tumor samples, as well as available serum samples from our DCTB cohort by ELISA. One patient, DCTB 58, showed detectable levels of Mage-A4 protein in serum, as well as in TIF. Measurements were performed in triplicate and are expressed as mean values. Data shown are from a representative experiment out of three independent experiments. (B and C) Confirmation of Mage-A4 protein expression in DCTB patient 58. We confirmed the presence of Mage-A4 in tumor tissue and externalization of the protein by (B) 2D Western blots of tissue lysates (DCTB T58) and interstitial fluid (DCTB TIF58) of DCTB 58 tumor samples and (C) IHC (antibody mAb 57B). Scale bar, 50 μm.
Although we had access to a very limited number of sera from DCTB patients, we used our sandwich-ELISA assay to analyze the 16 sera available. Of the sera examined (DCTB 15, 19, 20, 26, 31, 40, 42, 43, 46, 47, 51, 53, 55, 55, 58, and 59) only one case was from a patient bearing a Mage-A4 positive tumor (DCTB 58), and this was also the only sample showing detectable levels of serum Mage-A4 (3.62 pg/μl) (Fig. 7A, right hand axis). These results indicate that Mage-4A protein is detectable in sera of breast cancer patients. Unfortunately, the limited number of samples available to us did not allow us to address critical issues, such as the influence of tumor size and intrinsic biological factors affecting the ability of Mage-A4 of being detected in blood (for example Mage-A4 shedding rates from tumor cells) (64). Given the potential high specificity of any well-validated detection assay based on Mage-A4, because no normal cells express Mage-A4 other than testis and placenta so that background levels of the protein are minimal, this protein has been suggested as a useful biomarker for early detection of cancer, and in some cases such as head-and-neck squamous cell carcinoma, hepatocellular carcinoma, and ovarian carcinoma, ELISA assays for detection of elevated serum Mage-A4 were successfully established (65–69) and harbor some promise. In the case of breast cancer, however, the low frequency of Mage-A4 positive tumors (3–3.3%) we showed here makes its use as screening biomarker for early detection of limited clinical potential. However, serum Mage-A4 protein might be a useful tumor marker to monitor course of treatment and the recurrence of Mage-A4-positive TNBCs and ER− PgR− Her2+ tumors. This is of particular importance as these two subtypes are associated with increased risk of recurrence (70–72). However, additional studies in well-defined case/control cohorts using validated high sensitivity assays will be required to provide level 1 evidence for the feasibility of using Mage-A4 as monitoring marker. The detection of Mage-A4 in the sera of patients would allow the implementation of a valuable clinical blood assay to monitor treatment response and recurrence of Mage-A4-positive TNBCs and ER− PgR− Her2+ tumors.
CONCLUSIONS
We have made a detailed proteomic analysis of triple-negative breast carcinomas and established an extensive database of proteins expressed in TNBCs. This allowed us to identify a number of markers that could be targeted by currently available therapeutic approaches; these will be reported elsewhere. One marker in particular, Mage-A4, was expressed in a substantial proportion of cases. Expression of MAGE-A4 was restricted to receptor negative tumors: the ER− PgR− Her2+ and TNBC subtypes.
It has been found that Mage-A4 and other members of the Mage-A protein family can elicit immune responses and consequently constitute an attractive target for vaccine-based immunotherapy. Several broad-spectrum vaccination strategies using cross-recognized epitopes, thus targeting various Mage protein family members, have been described (51, 52). But other more specific approaches targeting Mage-A4 also exist (53, 54). Given the spectrum of expression of Mage-A proteins in the basal subgroup of breast cancers (47), where CTAs, including Mage-A3 (that was not even in the list of the CTAs most prevalently expressed), were expressed at much lower levels as compared with Mage-A4, and the fact that we could unequivocally identify the Mage-A4 protein in a significant proportion of TNBCs and ER− PgR− Her2+ tumors, using a three-tier orthogonal technology approach, we would suggest that targeting of Mage-A4 protein would constitute a promising therapeutic approach to the management of patients bearing Mage-A4 positive tumors.
Supplementary Material
Acknowledgments
We thank Ewa Rajpert-De Meyts for kindly providing samples. We would also like to thank Kitt Christensen, Sofia Svensson, Dorte Holm, Lene Jørgensen, and Anni Handesten for expert technical assistance.
Footnotes
* This work was supported by the Danish Cancer Society through the budget of the Research Center, and by grants from the “Race against Breast Cancer” foundation, the John and Birthe Meyer Foundation, Novo Nordisk Foundation, and the Lisa and Gudmund Jørgensen Foundation.
 This article contains supplemental Fig. S1 to S4 and Tables S1 to S4.
This article contains supplemental Fig. S1 to S4 and Tables S1 to S4.
In memoriam: Dr. med. Fritz Rank - Consultant of Pathology at Copenhagen University Hospital.
1 The abbreviations used are:
- Her2
- human epidermal growth factor receptor 2
- IHC
- immunohistochemistry
- 2D PAGE
- two-dimensional polyacrylamide gel electrophoresis
- ER
- estrogen receptor
- PgR
- progesterone receptor.
REFERENCES
- 1. Anderson W. F., Chu K. C., Chatterjee N., Brawley O., Brinton L. A. (2011) Tumor variants by hormone receptor expression in white patients with node-negative breast cancer from the surveillance, epidemiology, and end results database. J. Clin. Oncol. 19, 18–27 [DOI] [PubMed] [Google Scholar]
- 2. Baselga J., Perez E. A., Pienkowski T., Bell R. Adjuvant trastuzumab: a milestone in the treatment of HER-2-positive early breast cancer Oncologist 11 Suppl 1, 4–12, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Chu K. C., Anderson W. F. (2002) Rates for breast cancer characteristics by estrogen and progesterone receptor status in the major racial/ethnic groups. Breast Cancer Res. Treat. 74, 199–211 [DOI] [PubMed] [Google Scholar]
- 4. Pohlmann P. R., Mayer I. A., Mernaugh R. (2009) Resistance to trastuzumab in breast cancer. Clin. Cancer Res. 15, 7479–7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Romond E. H., Perez E. A., Bryant J., Suman V. J., Geyer C. E., Jr., Davidson N. E., Tan-Chiu E., Martino S., Paik S., Kaufman P. A., Swain S. M., Pisansky T. M., Fehrenbacher L., Kutteh L. A., Vogel V. G., Visscher D. W., Yothers G., Jenkins R. B., Brown A. M., Dakhil S. R., Mamounas E. P., Lingle W. L., Klein P. M., Ingle J. N., Wolmark N. (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 353, 1673–1684 [DOI] [PubMed] [Google Scholar]
- 6. Badve S., Dabbs D. J., Schnitt S. J., Baehner F. L., Decker T., Eusebi V., Fox S. B., Ichihara S., Jacquemier J., Lakhani S. R., Palacios J., Rakha E. A., Richardson A. L., Schmitt F. C., Tan P. H., Tse G. M., Weigelt B., Ellis I. O., Reis-Filho J. S. (2011) Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod. Pathol. 24, 157–167 [DOI] [PubMed] [Google Scholar]
- 7. Anders C. K., Carey L. A. (2009) Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin. Breast Cancer 9, S73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamamoto Y., Iwase H. (2010) Clinicopathological features and treatment strategy for triple-negative breast cancer. Int. J. Clin. Oncol. 15, 341–351 [DOI] [PubMed] [Google Scholar]
- 9. Scanlan M. J., Simpson A. J., Old L. J. (2004) The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 4, 1–15 [PubMed] [Google Scholar]
- 10. Parmigiani R. B., Bettoni F., Vibranovski M. D., Lopes M. H., Martins W. K., Cunha I. W., Soares F. A., Simpson A. J., de Souza S. J., Camargo A. A. (2006) Characterization of a cancer/testis (CT) antigen gene family capable of eliciting humoral response in cancer patients. Proc. Natl. Acad. Sci. U.S.A. 103, 18066–18071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., Van den Eynde B., Knuth A., Boon T. (1991) A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254, 1643–1647 [DOI] [PubMed] [Google Scholar]
- 12. Van den Eynde B., Peeters O., De Backer O., Gaugler B., Lucas S., Boon T. (1995) A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J. Exp. Med. 182, 689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tyagi P., Mirakhur B. (2009) MAGRIT: the largest-ever phase III lung cancer trial aims to establish a novel tumor-specific approach to therapy. Clin. Lung Cancer 10, 371–374 [DOI] [PubMed] [Google Scholar]
- 14. Fratta E., Coral S., Covre A., Parisi G., Colizzi F., Danielli R., Nicolay H. J., Sigalotti L., Maio M. (2011) The biology of cancer testis antigens: putative function, regulation and therapeutic potential. Mol. Oncol. 5, 164–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der B. P., Zhang Y., Chaux P., Stroobant V., Panichelli C., Schultz E. S., Chapiro J., Van Den Eynde B. J., Brasseur F., Boon T. (2002) Tumor-specific shared antigenic peptides recognized by human T cells. Immunol. Rev. 188, 51–64 [DOI] [PubMed] [Google Scholar]
- 16. Busam K. J., Iversen K., Berwick M., Spagnoli G. C., Old L. J., Jungbluth A. A. (2000) Immunoreactivity with the anti-Mage antibody 57B in malignant melanoma: frequency of expression and correlation with prognostic parameters. Mod. Pathol. 13, 459–465 [DOI] [PubMed] [Google Scholar]
- 17. Bandić D., Juretić A., Sarcević B., Separović V., Kujundzić-Tiljak M., Hudolin T., Spagnoli G. C., Cović D., Samija M. (2006) Expression and possible prognostic role of Mage-A4, NY-ESO-1, and HER-2 antigens in women with relapsing invasive ductal breast cancer: retrospective immunohistochemical study. Croat. Med. J. 47, 32–41 [PMC free article] [PubMed] [Google Scholar]
- 18. O'Farrell P. H. (1975) High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250, 4007–4021 [PMC free article] [PubMed] [Google Scholar]
- 19. Gromov P., Celis J. E., Gromova I., Rank F., Timmermans-Wielenga V., Moreira J. M. (2008) A single lysis solution for the analysis of tissue samples by different proteomic technologies. Mol. Oncol. 2, 368–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malzahn K., Mitze M., Thoenes M., Moll R. (1998) Biological and prognostic significance of stratified epithelial cytokeratins in infiltrating ductal breast carcinomas. Virchows Arch. 433, 119–129 [DOI] [PubMed] [Google Scholar]
- 21. Celis J. E., Trentemølle S., Gromov P. (2004) Gel-based proteomics: High-resolution two-dimensional gel electrophoresis of proteins. Isoelectric focusing (IEF) and nonequilibrium pH gradient electrophoresis (NEPHGE), in Cell Biology. A Laboratory Handbook (Celis J. E., Carter N., Hunter T., Shotton D., Simons K., Small J. V., eds) Vol. 4, Academic Press, San Diego [Google Scholar]
- 22. Gromov P., Gromova I., Bunkenborg J., Cabezon T., Moreira J. M., Timmermans-Wielenga V., Roepstorff P., Rank F., Celis J. E. (2010) Up-regulated proteins in the fluid bathing the tumour cell microenvironment as potential serological markers for early detection of cancer of the breast. Mol. Oncol. 4, 65–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Celis J. E., Gromov P., Moreira J. M., Cabezón T., Friis E., Vejborg I. M., Proess G., Rank F., Gromova I. (2006) Apocrine cysts of the breast: biomarkers, origin, enlargement, and relation with cancer phenotype. Mol. Cell. Proteomics 5, 462–483 [DOI] [PubMed] [Google Scholar]
- 24. Moreira J. M., Cabezón T., Gromova I., Gromov P., Timmermans-Wielenga V., Machado I., Llombart-Bosch A., Kroman N., Rank F., Celis J. E. (2010) Tissue proteomics of the human mammary gland: towards an abridged definition of the molecular phenotypes underlying epithelial normalcy. Mol. Oncol. 4, 539–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Celis J. E., Cabezón T., Moreira J. M., Gromov P., Gromova I., Timmermans-Wielenga V., Iwase T., Akiyama F., Honma N., Rank F. (2009) Molecular characterization of apocrine carcinoma of the breast: Validation of an apocrine protein signature in a well-defined cohort. Mol. Oncol. 3, 220–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dunstan R. W., Wharton K. A., Jr., Quigley C., Lowe A. (2011) The use of immunohistochemistry for biomarker assessment–can it compete with other technologies? Toxicol. Pathol. 39, 988–1002 [DOI] [PubMed] [Google Scholar]
- 27. Taylor C. R., Levenson R. M. (2006) Quantification of immunohistochemistry–issues concerning methods, utility and semiquantitative assessment II. Histopathology 49, 411–424 [DOI] [PubMed] [Google Scholar]
- 28. Walker R. A. (2006) Quantification of immunohistochemistry–issues concerning methods, utility and semiquantitative assessment I. Histopathology 49, 406–410 [DOI] [PubMed] [Google Scholar]
- 29. Irshad S., Ellis P., Tutt A. (2011) Molecular heterogeneity of triple-negative breast cancer and its clinical implications. Curr. Opin. Oncol. 23, 566–577 [DOI] [PubMed] [Google Scholar]
- 30. Celis J. E., Gromov P., Cabezón T., Moreira J. M., Friis E., Jirström K., Llombart-Bosch A., Timmermans-Wielenga V., Rank F., vGromova I. (2008) 15-prostaglandin dehydrogenase expression alone or in combination with ACSM1 defines a subgroup of the apocrine molecular subtype of breast carcinoma. Mol Cell Proteomics 7, 1795–1809 [DOI] [PubMed] [Google Scholar]
- 31. Balleine R., Schoenberg Fejzo M., Sathasivam P., Basset P., Clarke C. L., Byrne J. A. (2000) The hD52 (TPD52) gene is a candidate target gene for events resulting in increased 8q21 copy number in human breast carcinoma. Genes Chromosomes Cancer 29, 48–57 [DOI] [PubMed] [Google Scholar]
- 32. Mirshahidi S., Kramer V. G., Whitney J. B., Essono S., Lee S., Dranoff G., Anderson K. S., Ruprecht R. M. (2009) Overlapping synthetic peptides encoding TPD52 as breast cancer vaccine in mice: prolonged survival. Vaccine 27, 1825–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Payton L. A., Lewis J. D., Byrne J. A., Bright R. K. (2008) Vaccination with metastasis-related tumor associated antigen TPD52 and CpG/ODN induces protective tumor immunity. Cancer Immunol. Immunother. 57, 799–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shehata M., Bièche I., Boutros R., Weidenhofer J., Fanayan S., Spalding L., Zeps N., Byth K., Bright R. K., Lidereau R., Byrne J. A. (2008) Nonredundant functions for tumor protein D52-like proteins support specific targeting of TPD52. Clin. Cancer Res. 14, 5050–5060 [DOI] [PubMed] [Google Scholar]
- 35. Possemato R., Marks K. M., Shaul Y. D., Pacold M. E., Kim D., Birsoy K., Sethumadhavan S., Woo H. K., Jang H. G., Jha A. K., Chen W. W., Barrett F. G., Stransky N., Tsun Z. Y., Cowley G. S., Barretina J., Kalaany N. Y., Hsu P. P., Ottina K., Chan A. M., Yuan B., Garraway L. A., Root D. E., Mino-Kenudson M., Brachtel E. F., Driggers E. M., Sabatini D. M. (2011) Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Locasale J. W., Grassian A. R., Melman T., Lyssiotis C. A., Mattaini K. R., Bass A. J., Heffron G., Metallo C. M., Muranen T., Sharfi H., Sasaki A. T., Anastasiou D., Mullarky E., Vokes N. I., Sasaki M., Beroukhim R., Stephanopoulos G., Ligon A. H., Meyerson M., Richardson A. L., Chin L., Wagner G., Asara J. M., Brugge J. S., Cantley L. C., Vander Heiden M. G. (2011) Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 43, 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rastelli F., Biancanelli S., Falzetta A., Martignetti A., Casi C., Bascioni R., Giustini L., Crispino S. (2010) Triple-negative breast cancer: current state of the art. Tumori 96, 875–888 [PubMed] [Google Scholar]
- 38. Sharma P., Shen Y., Wen S., Bajorin D. F., Reuter V. E., Old L. J., Jungbluth A. A. (2006) Cancer-testis antigens: expression and correlation with survival in human urothelial carcinoma. Clin. Cancer Res. 12, 5442–5447 [DOI] [PubMed] [Google Scholar]
- 39. Peikert T., Specks U., Farver C., Erzurum S. C., Comhair S. A. (2006) Melanoma antigen A4 is expressed in nonsmall cell lung cancers and promotes apoptosis. Cancer Res. 66, 4693–4700 [DOI] [PubMed] [Google Scholar]
- 40. Richie J. P. (2002) Mage-A4, a germ cell specific marker, is expressed differentially in testicular tumors. J. Urol. 168, 1287–1288 [PubMed] [Google Scholar]
- 41. Otte M., Zafrakas M., Riethdorf L., Pichlmeier U., Löning T., Jänicke F., Pantel K. (2001) Mage-A gene expression pattern in primary breast cancer. Cancer Res. 61, 6682–6687 [PubMed] [Google Scholar]
- 42. Chitale D. A., Jungbluth A. A., Marshall D. S., Leitao M. M., Hedvat C. V., Kolb D., Spagnoli G. C., Iversen K., Soslow R. A. (2005) Expression of cancer-testis antigens in endometrial carcinomas using a tissue microarray. Mod. Pathol. 18, 119–126 [DOI] [PubMed] [Google Scholar]
- 43. Curigliano G., Viale G., Ghioni M., Jungbluth A. A., Bagnardi V., Spagnoli G. C., Neville A. M., Nolè F., Rotmensz N., Goldhirsch A. (2011) Cancer-testis antigen expression in triple-negative breast cancer. Ann. Oncol. 22, 98–103 [DOI] [PubMed] [Google Scholar]
- 44. Karn T., Pusztai L., Ruckhäberle E., Liedtke C., Müller V., Schmidt M., Metzler D., Wang J., Coombes K. R., Gätje R., Hanker L., Solbach C., Ahr A., Holtrich U., Rody A., Kaufmann M. (2011) Melanoma antigen family A identified by the bimodality index defines a subset of triple negative breast cancers as candidates for immune response augmentation. Eur. J. Cancer doi:10.1016/j.ejca.2011.06.025 [DOI] [PubMed] [Google Scholar]
- 45. Chen Y. T., Ross D. S., Chiu R., Zhou X. K., Chen Y. Y., Lee P., Hoda S. A., Simpson A. J., Old L. J., Caballero O., Neville A. M. (2011) Multiple cancer/testis antigens are preferentially expressed in hormone-receptor negative and high-grade breast cancers. PLoS One 18, e17876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bandić D., Juretić A., Sarcević B., Separović V., Kujundzić-Tiljak M., Hudolin T., Spagnoli G. C., Cović D., Samija M. (2006) Expression and possible prognostic role of Mage-A4, NY-ESO-1, and HER-2 antigens in women with relapsing invasive ductal breast cancer: retrospective immunohistochemical study. Croat. Med. J. 47, 32–41 [PMC free article] [PubMed] [Google Scholar]
- 47. Grigoriadis A., Caballero O. L., Hoek K. S., da Silva L., Chen Y. T., Shin S. J., Jungbluth A. A., Miller L. D., Clouston D., Cebon J., Old L. J., Lakhani S. R., Simpson A. J., Neville A. M. (2009) CT-X antigen expression in human breast cancer. Proc. Natl. Acad. Sci. U.S.A. 106, 13493–13498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen Y. T., Hsu M., Lee P., Shin S. J., Mhawech-Fauceglia P., Odunsi K., Altorki N. K., Song C. J., Jin B. Q., Simpson A. J., Old L. J. (2009) Cancer/testis antigen CT45: analysis of mRNA and protein expression in human cancer. Int. J. Cancer 124, 2893–2898 [DOI] [PubMed] [Google Scholar]
- 49. Adams S., Greeder L., Reich E., Shao Y., Fosina D., Hanson N., Tassello J., Singh B., Spagnoli G. C., Demaria S., Jungbluth A. A. (2011) Expression of cancer testis antigens in human BRCA-associated breast cancers: potential targets for immunoprevention? Cancer Immunol. Immunother. 60, 999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rimoldi D., Salvi S., Schultz-Thater E., Spagnoli G. C., Cerottini J. C. (2000) Anti-Mage-3antibody 57B and anti-Mage-1 antibody 6C1 can be used to study different proteins of the Mage-A family. Int. J. Cancer 86, 749–751 [DOI] [PubMed] [Google Scholar]
- 51. Graff-Dubois S., Faure O., Gross D. A., Alves P., Scardino A., Chouaib S., Lemonnier F. A., Kosmatopoulos K. (2002) Generation of CTL recognizing an HLA-A*0201-restricted epitope shared by Mage-A1, -A2, -A3, -A4, -A6, -A10, and -A12 tumor antigens: implication in a broad-spectrum tumor immunotherapy. J. Immunol. 169, 575–580 [DOI] [PubMed] [Google Scholar]
- 52. Kawada J., Wada H., Isobe M., Gnjatic S., Nishikawa H., Jungbluth A. A., Okazaki N., Uenaka A., Nakamura Y., Fujiwara S., Mizuno N., Saika T., Ritter E., Yamasaki M., Miyata H., Ritter G., Murphy R., Venhaus R., Pan L., Old L. J., Doki Y., Nakayama E. (2012) Heteroclitic serological response in esophageal and prostate cancer patients after NY-ESO-1 protein vaccination. Int. J. Cancer 130, 584–592 [DOI] [PubMed] [Google Scholar]
- 53. Cruz C. R., Gerdemann U., Leen A. M., Shafer J. A., Ku S., Tzou B., Horton T. M., Sheehan A., Copeland A., Younes A., Rooney C. M., Heslop H. E., Bollard C. M. (2011) Improving T cell therapy for relapsed EBV negative Hodgkins Lymphoma by targeting upregulated Mage-A4. Clin. Cancer Res. 17, 7058–7066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shirakura Y, Mizuno Y, Wang L, Imai N, Amaike C, Sato E, Ito M, Nukaya I, Mineno J, Takesako K, Ikeda H, Shiku H. (2012) TCR gene therapy targeting Mage-A4 inhibits human tumor growth in NOD/SCID/γc(null) mice. Cancer Sci. 103, 17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Landry C., Brasseur F., Spagnoli G. C., Marbaix E., Boon T., Coulie P., Godelaine D. (2000) Monoclonal antibody 57B stains tumor tissues that express gene Mage-A4. Int. J. Cancer 86, 835–841 [DOI] [PubMed] [Google Scholar]
- 56. Tsai C. F., Wang Y. T., Chen Y. R., Lai C. Y., Lin P. Y., Pan K. T., Chen J. Y., Khoo K. H., Chen Y. J. (2008) Immobilized metal affinity chromatography revisited: pH/acid control toward high selectivity in phosphoproteomics. J. Proteome Res. 7, 4058–4069 [DOI] [PubMed] [Google Scholar]
- 57. Raijmakers R., Kraiczek K., de Jong A. P., Mohammed S., Heck A. J. (2010) Exploring the human leukocyte phosphoproteome using a microfluidic reversed-phase-TiO2-reversed-phase high-performance liquid chromatography phosphochip coupled to a quadrupole time-of-flight mass spectrometer. Anal. Chem. 82, 824–832 [DOI] [PubMed] [Google Scholar]
- 58. Kim W., Bennett E. J., Huttlin E. L., Guo A., Li J., Possemato A., Sowa M. E., Rad R., Rush J., Comb M. J., Harper J. W., Gygi S. P. (2011) Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell. 44, 325–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sakurai T., Itoh K., Higashitsuji H., Nagao T., Nonoguchi K., Chiba T., Fujita J. (2004) A cleaved form of Mage-A4 binds to Miz-1 and induces apoptosis in human cells. J. Biol. Chem. 279, 15505–15514 [DOI] [PubMed] [Google Scholar]
- 60. Celis J. E., Gromov P., Gromova I., Moreira J. M., Cabezón T., Ambartsumian N., Grigorian M., Lukanidin E., Thor Straten P., Guldberg P., Bartkova J., Bartek J., Lukas J., Lukas C., Lykkesfeldt A., Jäättelä M., Roepstorff P., Bolund L., Ørntoft T., Brünner N., Overgaard J., Sandelin K., Blichert-Toft M., Mouridsen H., Rank F. E. (2003) Integrating proteomic and functional genomic technologies in discovery-driven translational breast cancer research. Mol. Cell. Proteomics 2, 369–377 [DOI] [PubMed] [Google Scholar]
- 61. Kavalar R., Sarcevic B., Spagnoli G.C., Separovic V., Samija M., Terracciano L., Heberer M., Juretic A. (2001) Expression of Mage tumour-associated antigens is inversely correlated with tumour differentiation in invasive ductal breast cancers: an immunohistochemical study. Virchows Arch. 439, 127–131 [DOI] [PubMed] [Google Scholar]
- 62. Desmedt C., Piette F., Loi S., Wang Y., Lallemand F., Haibe-Kains B., Viale G., Delorenzi M., Zhang Y., Saghatchian d'Assignies M., Bergh J., Lidereau R., Ellis P., Harris A., L., Klijn J. G. M., Foekens J. A., Cardoso F., Piccart M. J., Buyse M., Sotiriou C., on behalf of the TRANSBIG Consortium (2007) Strong time dependence of the 76-gene prognostic signature for node-negative breast cancer patients in the TRANSBIG multicenter independent validation series. Clin. Cancer Res. 13, 3207–3214 [DOI] [PubMed] [Google Scholar]
- 63. Celis J. E., Gromov P., Cabezón T., Moreira J. M., Ambartsumian N., Sandelin K., Rank F., Gromova I. (2004) Proteomic characterization of the interstitial fluid perfusing the breast tumor microenvironment: a novel resource for biomarker and therapeutic target discovery. Mol. Cell. Proteomics 3, 327–344 [DOI] [PubMed] [Google Scholar]
- 64. Hori S. S., Gambhir S. S. (2011) Mathematical model identifies blood biomarker-based early cancer detection strategies and limitations. Sci. Transl. Med. 3, 109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Iwamoto O., Nagao Y., Shichijo S., Eura M., Kameyama T., Itoh K. (1997) Detection of Mage-4 protein in sera of patients with head-and-neck squamous-cell carcinoma. Int. J. Cancer 70, 287–290 [DOI] [PubMed] [Google Scholar]
- 66. Kawagoe H., Yamada A., Matsumoto H., Ito M., Ushijima K., Nishida T., Yakushiji M., Itoh K. (2000) Serum Mage-4 protein in ovarian cancer patients. Gynecol. Oncol. 76, 336–339 [DOI] [PubMed] [Google Scholar]
- 67. Shichijo S., Tsunosue R., Kubo K., Kuramoto T., Tanaka Y., Hayashi A., Itoh K. (1995) Establishment of an enzyme-linked immunosorbent assay (ELISA) for measuring cellular Mage-4 protein on human cancers. J. Immunol. Methods 186, 137–149 [DOI] [PubMed] [Google Scholar]
- 68. Tsuzurahara S., Sata M., Iwamoto O., Shichijo S., Kojiro M., Tanikawa K., Itoh K. (1997) Detection of Mage-4 protein in the sera of patients with hepatitis-C virus-associated hepatocellular carcinoma and liver cirrhosis. Jpn. J. Cancer Res. 88, 915–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shichijo S., Hayashi A., Takamori S., Tsunosue R., Hoshino T., Sakata M., Kuramoto T., Oizumi K., Itoh K. (1995) Detection of MAGE-4 protein in lung cancers. Int. J. Cancer 64, 158–165 [DOI] [PubMed] [Google Scholar]
- 70. Gabos Z., Thoms J., Ghosh S., Hanson J., Deschênes J., Sabri S., Abdulkarim B. (2010) The association between biological subtype and locoregional recurrence in newly diagnosed breast cancer. Breast Cancer Res. Treat. 124, 187–194 [DOI] [PubMed] [Google Scholar]
- 71. Kyndi M., Sørensen F. B., Knudsen H., Overgaard M., Nielsen H. M., Overgaard J.; Danish Breast Cancer Cooperative Group (2008) Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J. Clin. Oncol. 26, 1419–1426 [DOI] [PubMed] [Google Scholar]
- 72. Zaky S. S., Lund M., May K. A., Godette K. D., Beitler J. J., Holmes L. R., O'Regan R. M., Yu E. S., Yu D. S., Landry J. C. (2011) The negative effect of triple-negative breast cancer on outcome after breast-conserving therapy. Ann. Surg. Oncol. 18, 2858–2865 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.