Abstract
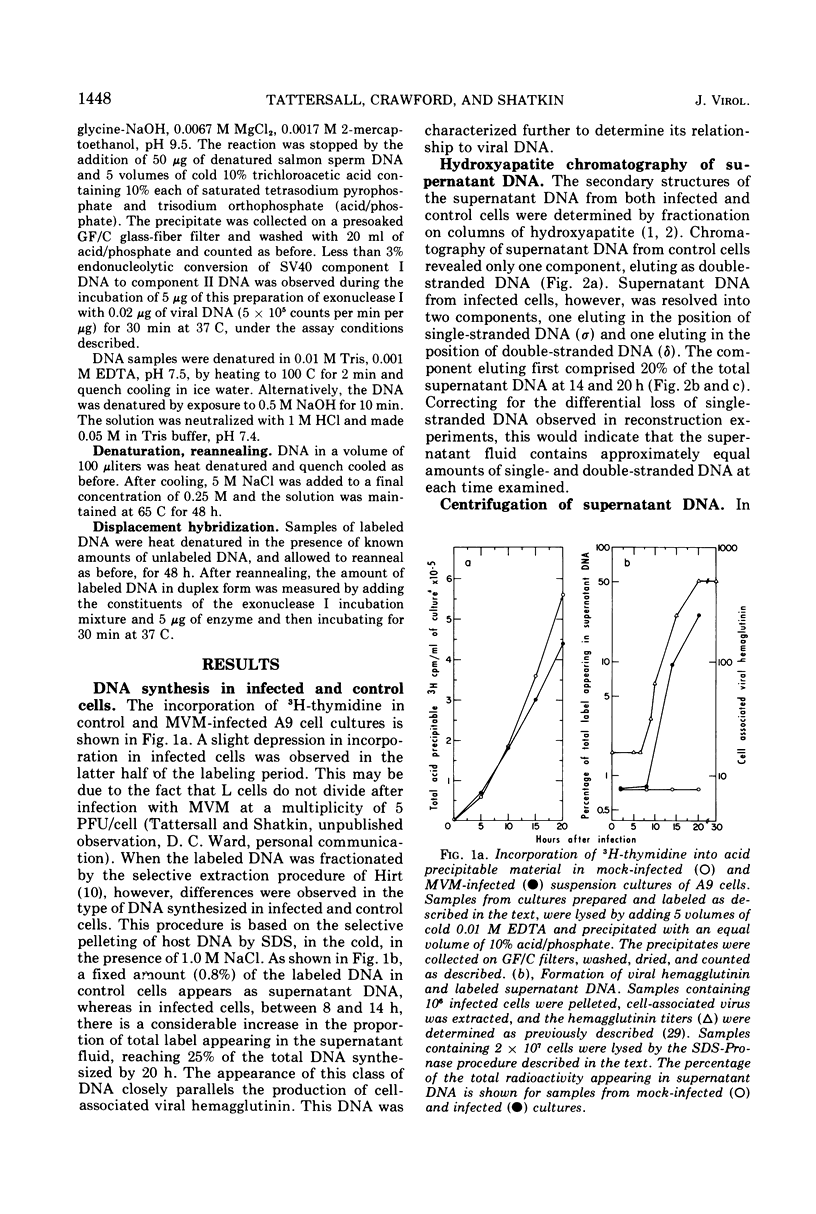
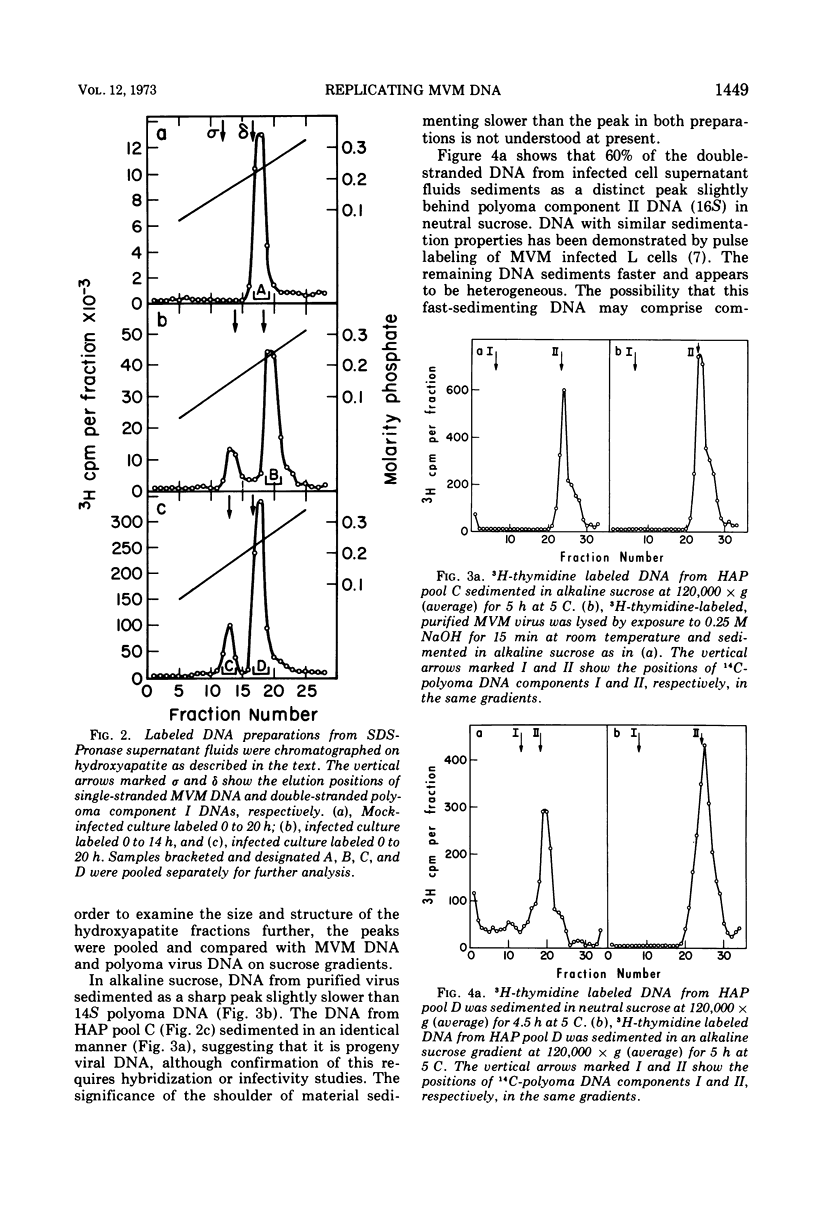
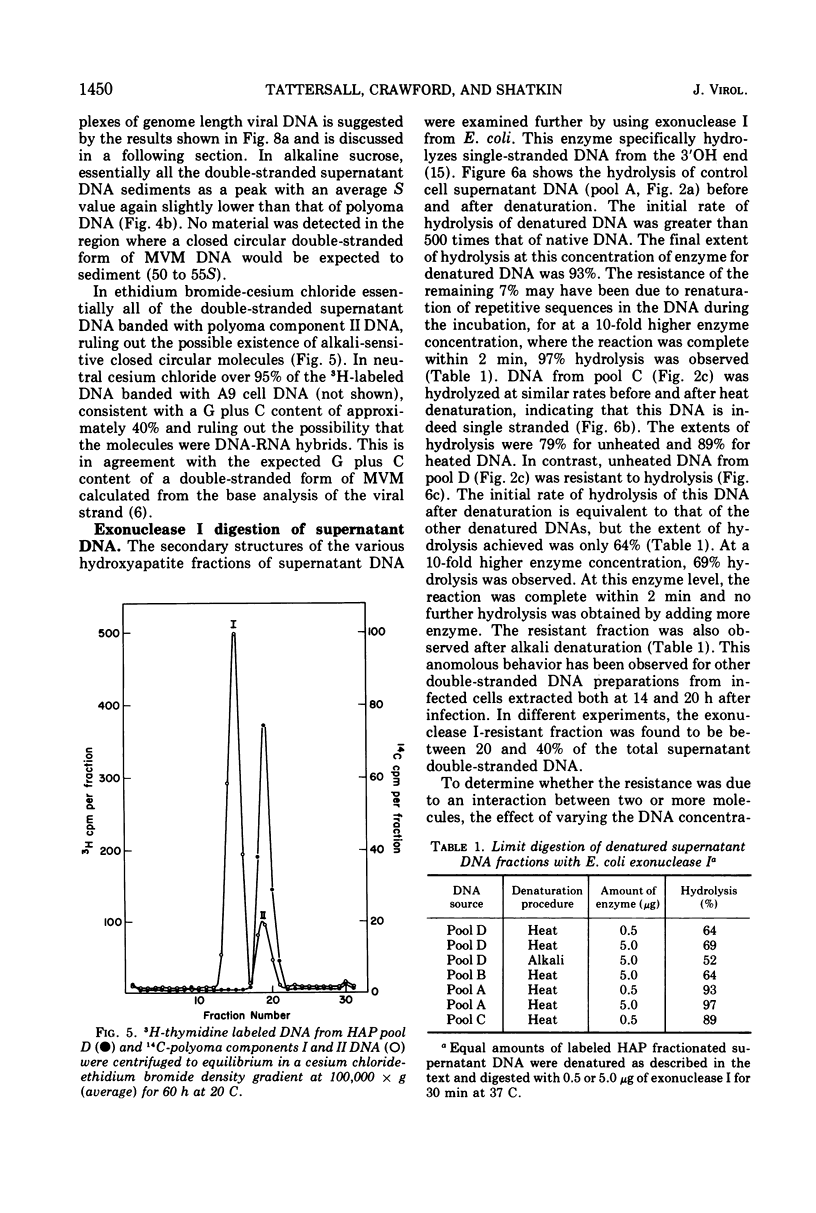
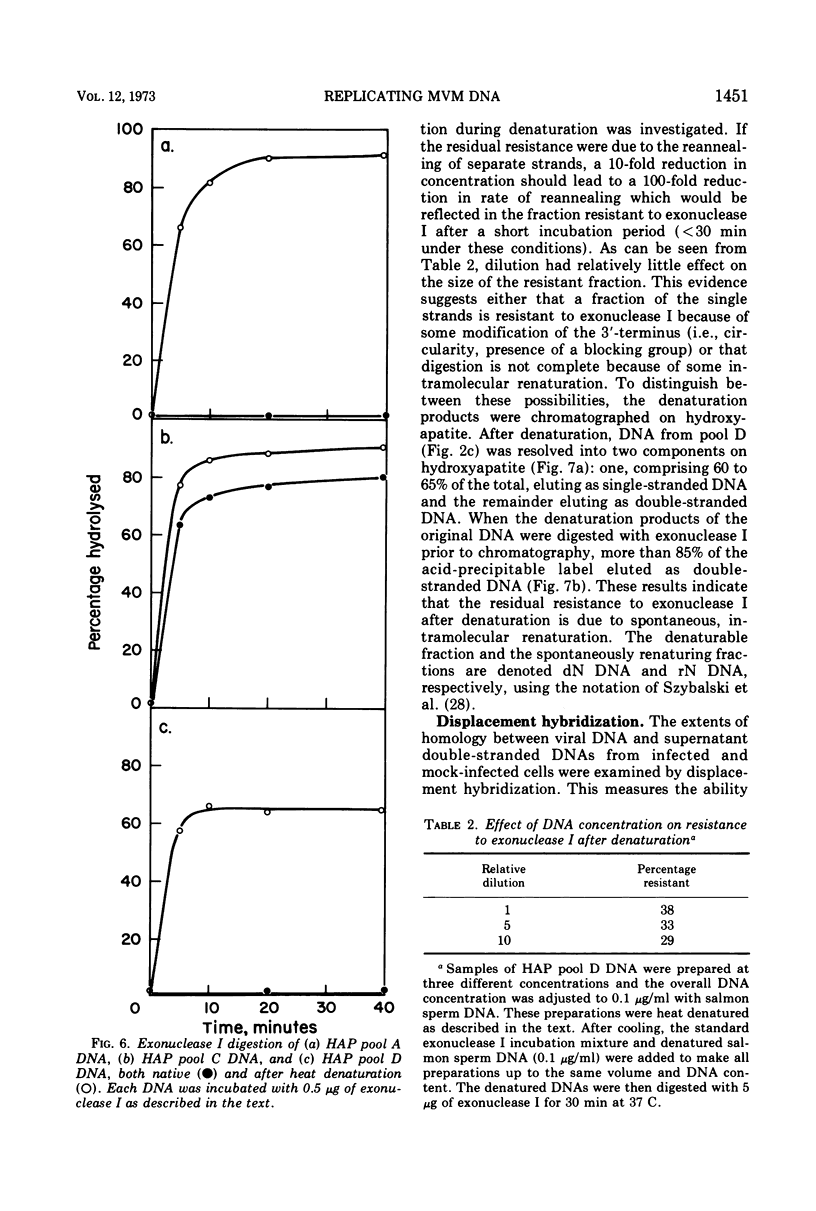
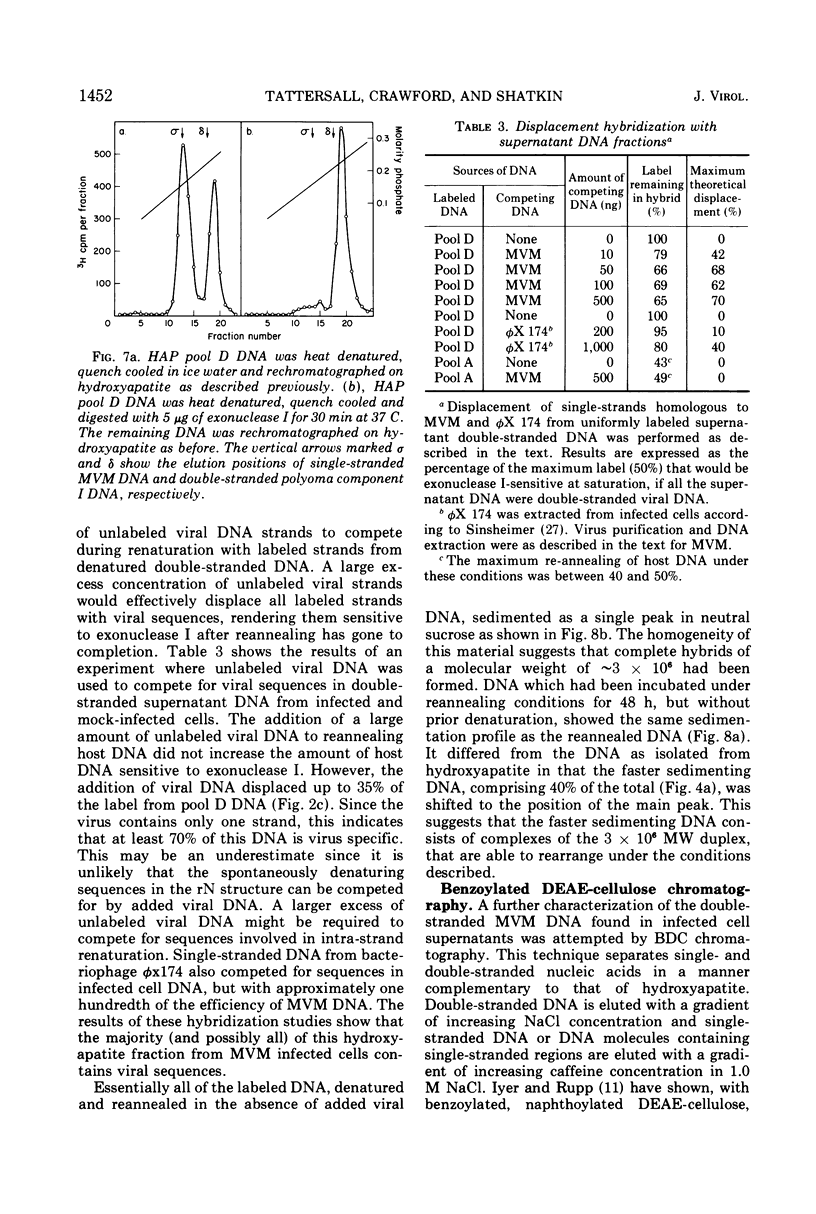
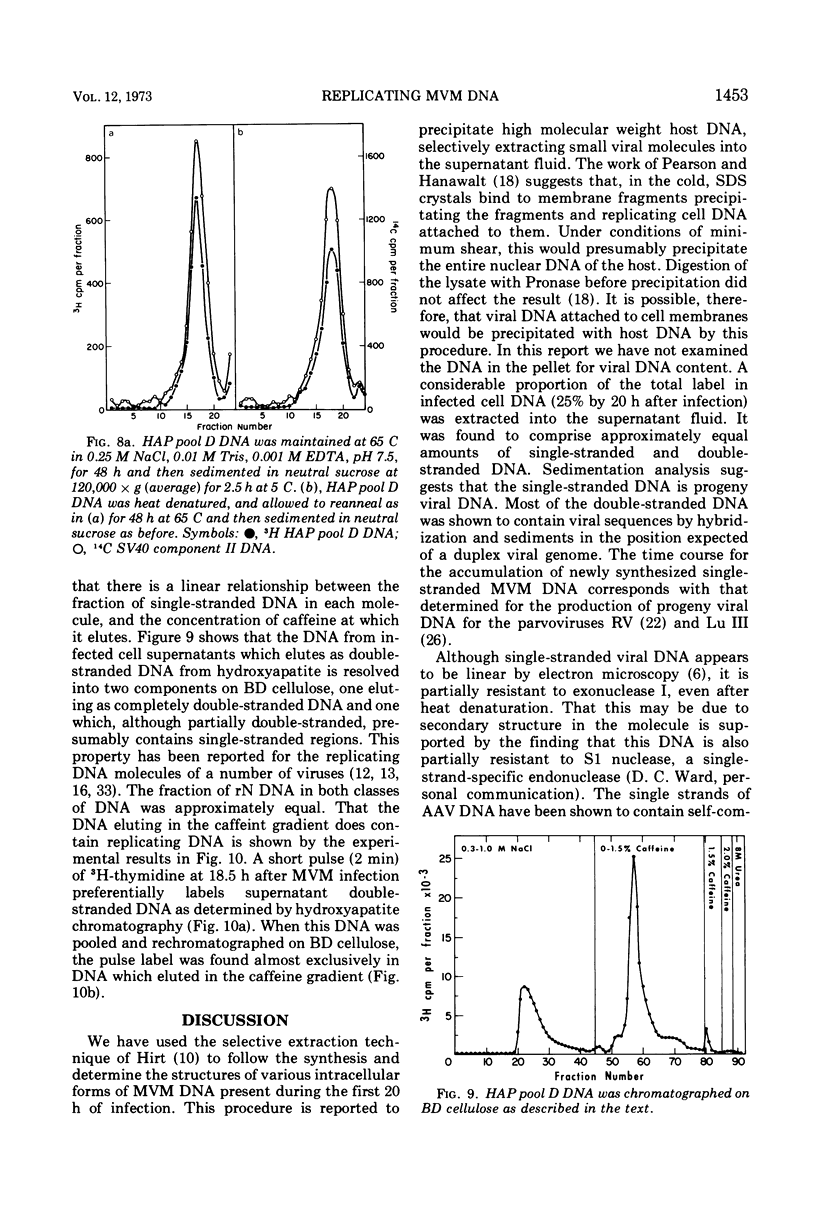
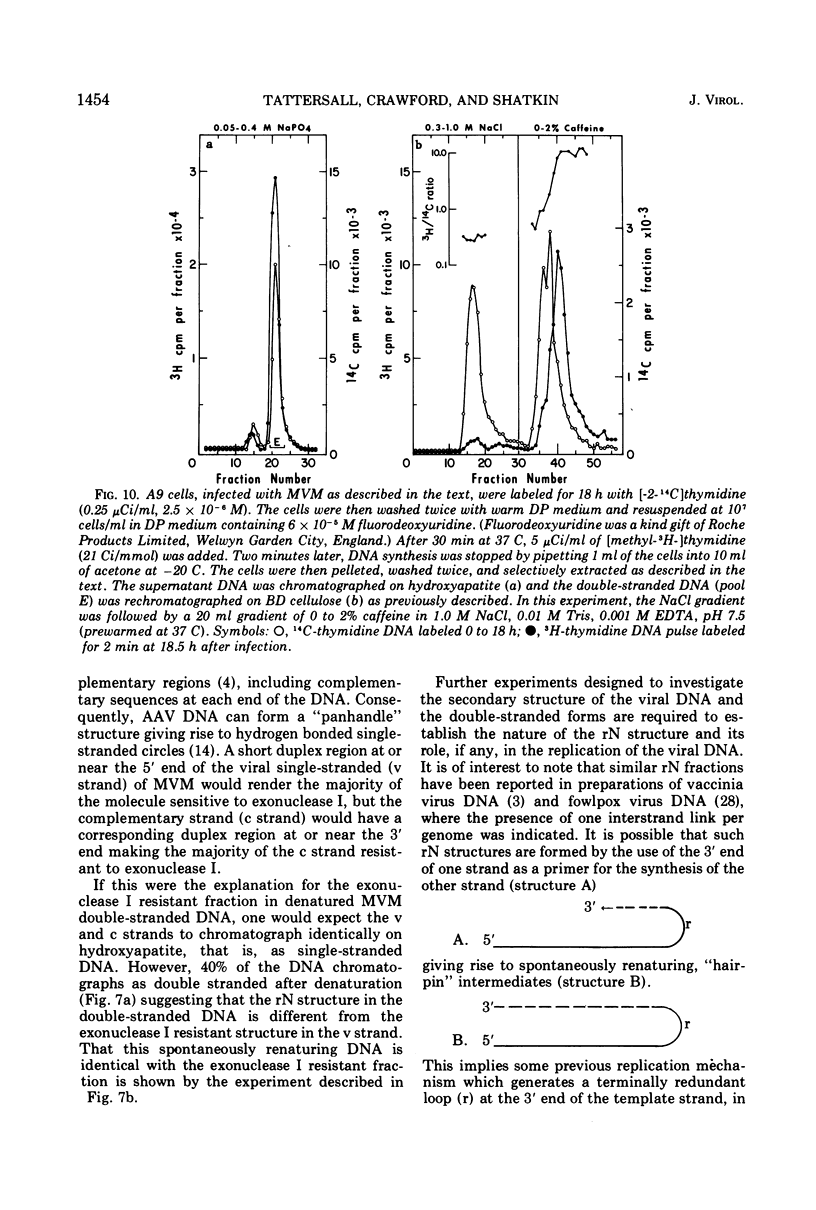
The time course of the appearance of intracellular viral DNA has been studied in mouse L cells infected with the single-stranded DNA virus MVM (minute virus of mice) by using a selective extraction procedure. Approximately half of this DNA elutes from hydroxyapatite as single-stranded DNA. It is sensitive to Escherichia coli exonuclease I and shows a sedimentation profile similar to DNA from the virus, suggesting that it is progeny viral DNA. The remainder of the selectively extracted DNA elutes from hydroxyapatite in the position of double-stranded DNA and is resistant to exonuclease I. Most of this DNA has a sedimentation coefficient of 14 to 16S, indicating that its molecular weight is twice that of the viral DNA. Denaturation renders the majority of the double-stranded DNA sensitive to exonuclease I, but a significant fraction renatures spontaneously in a monomolecular fashion, indicating that it has a cross-linked or hairpin structure. Chromatography of the double-stranded DNA on benzoylated diethylaminoethyl cellulose resolves two components, one with duplex structure and one which contains single-stranded regions. A short pulse label late in infection predominantly labels the latter class of DNA, suggesting that it contains replicating intermediates. The possible roles of these various forms of DNA in the replication of the viral genome are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardi G. Chromatography of nucleic acids on hydroxyapatite. I. Chromatography of native DNA. Biochim Biophys Acta. 1969 Feb 18;174(2):423–434. doi: 10.1016/0005-2787(69)90273-1. [DOI] [PubMed] [Google Scholar]
- Bernardi G. Chromatography of nucleic acids on hydroxyapatite. II. Chromatography of denatured DNA. Biochim Biophys Acta. 1969 Feb 18;174(2):435–448. doi: 10.1016/0005-2787(69)90274-3. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Silverman C. Natural occurrence of cross-linked vaccinia virus deoxyribonucleic acid. J Virol. 1970 Mar;5(3):299–304. doi: 10.1128/jvi.5.3.299-304.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J., Khoury G., Rose J. A. Adenovirus-associated virus multiplication. IX. Extent of transcription of the viral genome in vivo. J Virol. 1972 Dec;10(6):1118–1125. doi: 10.1128/jvi.10.6.1118-1125.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V. A minute virus of mice. Virology. 1966 Aug;29(4):605–612. doi: 10.1016/0042-6822(66)90284-4. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Follett E. A., Burdon M. G., McGeoch D. J. The DNA of a minute virus of mice. J Gen Virol. 1969 Jan;4(1):37–46. doi: 10.1099/0022-1317-4-1-37. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson P. R., Helleiner C. W. A replicative form of the DNA of minute virus of mice. Can J Microbiol. 1973 Jan;19(1):35–41. doi: 10.1139/m73-005. [DOI] [PubMed] [Google Scholar]
- Hampton E. G. H-1 virus growth in synchronized rat embryo cells. Can J Microbiol. 1970 Apr;16(4):266–268. doi: 10.1139/m70-049. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Iyer V. N., Rupp W. D. Usefulness of benzoylated naphthoylated DEAE-cellulose to distinguish and fractionate double-stranded DNA bearing different extents of single-stranded regions. Biochim Biophys Acta. 1971 Jan 1;228(1):117–126. doi: 10.1016/0005-2787(71)90551-x. [DOI] [PubMed] [Google Scholar]
- Kiger J. A., Jr, Sinsheimer R. L. Vegetative lambda DNA. IV. Fractionation of replicating lambda DNA on benzoylated-naphthoylated DEAE cellulose. J Mol Biol. 1969 Mar 28;40(3):467–490. doi: 10.1016/0022-2836(69)90166-1. [DOI] [PubMed] [Google Scholar]
- Knippers R., Whalley J. M., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXX. Replication of double-stranded phiX DNA. Proc Natl Acad Sci U S A. 1969 Sep;64(1):275–282. doi: 10.1073/pnas.64.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koczot F. J., Carter B. J., Garon C. F., Rose J. A. Self-complementarity of terminal sequences within plus or minus strands of adenovirus-associated virus DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):215–219. doi: 10.1073/pnas.70.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHMAN I. R., NUSSBAUM A. L. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI. V. ON THE SPECIFICITY OF EXONUCLEASE I (PHOSPHODIESTERASE). J Biol Chem. 1964 Aug;239:2628–2636. [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Crawford L. V., Follett E. A. The DNAs of three parvoviruses. J Gen Virol. 1970 Jan;6(1):33–40. doi: 10.1099/0022-1317-6-1-33. [DOI] [PubMed] [Google Scholar]
- Pearson G. D., Hanawalt P. C. Isolation of DNA replication complexes from uninfected and adenovirus-infected HeLa cells. J Mol Biol. 1971 Nov 28;62(1):65–80. doi: 10.1016/0022-2836(71)90131-8. [DOI] [PubMed] [Google Scholar]
- Robinson D. M., Hetrick F. M. Single-stranded DNA from the Kilham rat virus. J Gen Virol. 1969 Mar;4(2):269–281. doi: 10.1099/0022-1317-4-2-269. [DOI] [PubMed] [Google Scholar]
- Rose J. A., Berns K. I., Hoggan M. D., Koczot F. J. Evidence for a single-stranded adenovirus-associated virus genome: formation of a DNA density hybrid on release of viral DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):863–869. doi: 10.1073/pnas.64.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZYBALSKI W., ERIKSON R. L., GENTRY G. A., GAFFORD L. G., RANDALL C. C. Unusual properties of fowlpox virus DNA. Virology. 1963 Apr;19:586–589. doi: 10.1016/0042-6822(63)90056-4. [DOI] [PubMed] [Google Scholar]
- Salzman L. A., White W. L., Kakefuda T. Linear, single-stranded deoxyribonucleic acid isolated from Kilham rat virus. J Virol. 1971 Jun;7(6):830–835. doi: 10.1128/jvi.7.6.830-835.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. L., McKerlie L. Growth characteristics of Kilham rat virus and its effect on cellular cellular macromolecular synthesis. J Virol. 1972 Oct;10(4):573–577. doi: 10.1128/jvi.10.4.573-577.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. In vivo conversion of the single-stranded DNA of the kilham rat virus to a double-stranded form. J Virol. 1973 Feb;11(2):299–305. doi: 10.1128/jvi.11.2.299-305.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Gautschi M. The multiplication of parvovirus Lu3 in a synchronized culture system. I. Optimum conditions for virus replication. Arch Gesamte Virusforsch. 1973;40(1):105–118. doi: 10.1007/BF01242642. [DOI] [PubMed] [Google Scholar]
- Siegl G., Gautschi M. The multiplication of parvovirus Lu3 in a synchronized culture system. II. Biochemical characteristics of virus replication. Arch Gesamte Virusforsch. 1973;40(1):119–127. doi: 10.1007/BF01242643. [DOI] [PubMed] [Google Scholar]
- Siegl G. Parvoviruses as contaminants of permanent human cell lines. V. The nucleic acid of KBSH-virus. Arch Gesamte Virusforsch. 1972;37(2):267–274. doi: 10.1007/BF01268010. [DOI] [PubMed] [Google Scholar]
- Tattersall P. Replication of the parvovirus MVM. I. Dependence of virus multiplication and plaque formation on cell growth. J Virol. 1972 Oct;10(4):586–590. doi: 10.1128/jvi.10.4.586-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Hand R. E., Jr Requirement of cellular synthesis for Kilham rat virus replication. Virology. 1970 Dec;42(4):1054–1063. doi: 10.1016/0042-6822(70)90353-3. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Layman K. R., Hand R. E. Effect of cell physiological state on infection by rat virus. J Virol. 1969 Dec;4(6):872–878. doi: 10.1128/jvi.4.6.872-878.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usategui-Gomez M., Toolan H. W., Ledinko N., al-Lami F., Hopkins M. S. Single-stranded DNA from the Parvovirus, H-1. Virology. 1969 Nov;39(3):617–621. doi: 10.1016/0042-6822(69)90117-2. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L., Magnusson G., Reichard P. Replication of polyoma DNA in isolated nuclei. I. Characterization of the system from mouse fibroblast 3T6 cells. J Mol Biol. 1972 Dec 30;72(3):523–537. doi: 10.1016/0022-2836(72)90172-6. [DOI] [PubMed] [Google Scholar]



