Abstract
Background
The association of mortality and poor outcome with reduced levels of hemoglobin (Hb) and hematocrit (Hct) in patients admitted for ischemic stroke was recently demonstrated. The mechanisms behind this have remained unclear.
Aims
Here, we aimed to investigate a putative association between low Hb and Hct levels and infarct growth.
Methods
All consecutive patients who received intravenous thrombolysis based on multimodal magnetic resonance imaging during the years 1998–2009 were screened. Laboratory data as well as admission magnetic resonance images and follow-up computed tomography scans of 257 patients were assessed. Overall, data of 100 patients were of sufficient quality and further analyzed.
Results
Decrease in Hb and Hct as well as perfusion-weighted imaging volume, mismatch volume, and final infarct size on follow-up computed tomography were associated with infarct growth. A linear regression model revealed Hb decrease (β = 0.23, p = 0.02) to be a predictor of infarct growth, independent of mismatch volume (β = 0.27, p = 0.004) and minimum sodium (β = -0.21, p = 0.03), and adjusted to the non-predicting variables age, National Institute of Health Stroke Scale score, maximum leucocytes and C-reactive protein, blood glucose, and Hct decrease.
Conclusion
Hb levels that decrease after admission independently predict infarct growth in thrombolyzed stroke patients. The clinical implications of this relationship remain to be investigated.
Key Words: Stroke, Acute stroke, Thrombolysis, Hemoglobin, Anemia, Magnetic resonance imaging, Computed tomography
Introduction
Tissue fate after ischemic stroke depends upon timely reperfusion and thus re-establishment of oxygen supply to the penumbra. This is the rationale for thrombolysis in acute stroke, and the significant impact of recanalization on infarct growth and functional outcome has been demonstrated [1,2,3,4]. Additionally, other factors might influence infarct growth and functional outcome, such as age [5], admission National Institute of Health Stroke Scale (NIHSS) [5], treatment with r-tPA [6], time to treatment [7], elevated hematocrit (Hct) [8], and hyperglycemia [9].
Anemia is a common condition in elderly patients and nearly every fifth patient with acute ischemic stroke presents with anemia on admission [10]. However, an optimal oxygen-carrying capacity in the blood, i.e. level of hemoglobin (Hb), would appear to be decisive for the penumbral salvage. There are only few studies on the role of Hb in ischemic stroke and their results are heterogeneous. Hct, in contrast, has been addressed in some studies with regard to the effect of viscosity and cerebral blood flow in acute ischemic stroke, but again with controversial results. Hemodilution, once considered a potential treatment principle in stroke, has not proved successful. Thus, optimal Hb and Hct levels in acute ischemic stroke are still unknown and guideline recommendations are vague or lacking [11].
We have recently demonstrated that low and further decreasing levels of Hb and Hct after admission in patients thrombolyzed for ischemic stroke were associated with poor outcome and mortality [12]. Although the results of our analysis hinted at anemia being an independent factor rather than an epiphenomenon merely reflecting illness severity, the question whether this is an association or a pathophysiological causative relation remained unanswered. In particular, it is unclear whether anemia is indeed involved in loss of the penumbra, i.e. infarct growth.
Here, we have analyzed infarct development between admission and follow-up imaging in patients who received intravenous thrombolysis (IVT) based on magnetic resonance imaging (MRI) and the associations with clinical and laboratory parameters.
Methods
Consecutive patients with acute ischemic stroke treated with IVT based on MRI in the years 1998–2009 were retrospectively analyzed. Baseline and demographic characteristics, cardiovascular risk factors, treatment time intervals, and stroke severity upon admission as reflected by the NIHSS score were prospectively collected. All Hb and Hct concentrations during hospital stay were extracted from the laboratory registry of the hospital. All patients had their first blood sample including a blood count taken in our emergency room. IVT was started in our emergency room and continued on our certified stroke unit, where patients stayed for at least 3 days. We assessed the levels of Hb and Hct on admission (baseline) and minimum levels until the follow-up computed tomography (CT) scan. Anemia was defined by WHO criteria as Hb <12 g/dl in women and Hb <13 g/dl in men. Other relevant laboratory parameters such as blood glucose, leukocytes, platelets, C-reactive protein (CRP), creatinine, blood urea nitrogen (BUN), sodium and potassium were analyzed as well.
MR and CT images were available either as hard copies or in digital format. The imaging investigator (P.G.) was blinded to the laboratory and outcome data of the patients. Images from a dedicated multimodal stroke MR examination on admission were used to outline and quantify the initial volume of diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI). The volume of the latter was calculated as area under the curve of a time/bolus graph. The DWI volume was regarded as the initial infarct size. CT images from the latest follow-up examination were used to determine the final infarct size. Infarct size was determined using the ABC/2 method as follows: the slice with the largest lesion extension (hyperintense on DW and PW MRI, hypodense on CT images) diameter was chosen. This measurement is referred to as A. The second distance (B) was the largest lesion diameter perpendicular to A on the same slice. Multiplying the number of slices showing the lesion by the slice thickness yielded the third distance (C). After multiplication of the 3 distances, the product was divided by 2, resulting in the final volume in cubic millimeters (mm3). Infarct growth was defined as the difference between the ABC/2 result of the initial DWI lesion and the final follow-up CT lesion. The in-plane resolution of both the DW and PW MR images was 96 × 96 with a slice thickness of 5 mm; CT images had a resolution of 512 × 512 with a slice thickness of either 6 or 8 mm. Mismatch was defined as a visually detected PW MRI lesion that was at least 20% larger than the DWI lesion [13]. Most patients were routinely examined with extra- and intracranial ultrasound during the first day of hospital stay to detect recanalization.
The study was approved by our local ethics committee (application No. S324/2009).
Statistical Analysis
Normally distributed data are presented as means ± standard deviation (SD) and non-normally distributed data as medians and interquartile range or counts and percentages in parentheses. Correlation of variables with infarct growth was analyzed using the Pearson correlation coefficient for normally distributed and the Spearman correlation coefficient for non-normally distributed data. Multivariable analysis was performed using a linear regression model for calculating predictors of infarct growth to present regression coefficients B (non-standardized) and β (standardized), with the level of significance set at p < 0.05. Because of possible co-linearity of the included variables, a forward stepwise approach was chosen. All statistical analyses were performed using the SPSS software package (SPSS 19.0) for Windows.
Results
Of 257 patients treated with IVT selected by MRI criteria in the years 1998–2008, we excluded the following from further analysis: 59 patients due to incomplete imaging data, 35 patients because there was no infarction displayed in the follow-up imaging scan, 50 patients due to lack of DWI-PWI mismatch, 6 patients who received intra-arterial recanalization, and 7 patients with incomplete laboratory data.
Table 1 shows baseline characteristics, radiological and clinical outcome data of the remaining 100 patients, as well as their correlation with infarct growth. Mean age was 70 years, stroke severity was reflected by a median NIHSS of 14, and mean time to IVT treatment was 195 min. Mean initial DWI volume was 13.7 ml and mismatch volume was 121.7 ml. Median modified Rankin Scale (mRS) after 3 months was 4; 33% of the patients reached functional independence and 16% deceased. Correlations were found between infarct growth and PWI volume, mismatch volume, and infarct size on follow-up CT.
Table 1.
Correlation of baseline characteristics with infarct growth
| Parameters | p (infarct growth, ml) | |
|---|---|---|
| Baseline characteristics | ||
| Infarct growth, ml | 2.9 (15) | 1 |
| Age, yearsa | 70 ± 12.9 | 0.60 |
| Female sexb | 40 (40%) | 0.53 |
| NIHSS on admissionb | 14 (5) | 0.09 |
| Hypertensionb | 74 (74%) | 0.58 |
| Diabetes mellitusb | 23 (23%) | 0.15 |
| Hyperlipidemiab | 23 (23%) | 0.44 |
| Smokingb | 20 (20%) | 0.84 |
| Previous strokeb | 9 (9%) | 0.30 |
| Coronary heart diseaseb | 11 (11%) | 0.74 |
| Peripheral artery diseaseb | 1 (1%) | 0.12 |
| Atrial fibrillationb | 39 (39%) | 0.52 |
| Off-label treatmentb | 61 (61%) | 0.64 |
| Time to treatment, minb, d | 195 (207) | 0.80 |
| Recanalizationb, c, e | 64 (88%) | 0.39 |
| Time to follow-up imaging scan, hb | 24 (22) | 0.23 |
| Radiological data | ||
| DWI volume, mlb | 13.7 (25) | 0.09 |
| PWI volume, mlb, f | 155 (111) | 0.001 |
| Mismatch, mlb | 121.7 (126) | 0.001 |
| Infarct size on CT, mlb | 17 (42) | 0.001 |
| Outcome data | ||
| mRS at 3 monthsb | 4 (3) | 0.09 |
| mRS 0–2 at 3 monthsb | 33 (33%) | 0.07 |
| Death at 3 monthsb | 16 (16%) | 0.72 |
Values are means ± SD, median (IQR) or numbers with percentages in parentheses.
Pearson.
Spearman.
As assessed by transcranial doppler sonography at various time points.
n = 93, 7 unknown (3 vs. 4).
28 unknown.
n = 91, missing 4 vs. 5.
With regard to laboratory parameters, we found that decrease of Hb and Hct until follow-up CT was associated with infarct growth. In addition, there were correlations between infarct growth and maximum values of leucocyte count and CRP, as well as minimum values of sodium and blood glucose (table 2).
Table 2.
Correlation of laboratory data with infarct growth
| Parameters | p* (infarct growth, ml) | |
|---|---|---|
| Hb at baseline, g/dl | 13.6 (2.2) | 0.81 |
| Hbmin until CT scan, g/dl | 13.0 (2) | 0.20 |
| Hb decrease until CT scan, g/dl | 0.8 (1.4) | 0.02 |
| Anemia at baseline | 19 (19%) | 0.20 |
| Anemia until CT scan | 40 (40%) | 0.51 |
| Hct at baseline, % | 40 (6) | 0.59 |
| Hctmin until CT scan, % | 38 (5) | 0.18 |
| Hct decrease until CT scan, % | 2 (4) | 0.001 |
| Leukocytes at baseline, /nl | 8.7 (4) | 0.63 |
| Leukocytesmax until CT scan, /nl | 10.4 (4) | 0.02 |
| Platelets at baseline, /nl | 230 (90) | 0.86 |
| Plateletsmax until CT scan, /nl | 240 (90) | 0.39 |
| Plateletsmin until CT scan, /nl | 205 (85) | 0.82 |
| CRP at baseline, mg/l | 6 (15) | 0.72 |
| CRPmax until CT scan, mg/l | 13 (22) | 0.05 |
| Creatinine at baseline, mg/dl | 0.94 (0.25) | 0.12 |
| Creatininemax until CT scan, mg/dl | 0.98 (0.18) | 0.13 |
| BUN at baseline, mg/dl | 38 (21) | 0.21 |
| BUNmax until CT scan, mg/dl | 42 (21) | 0.48 |
| Sodium at baseline, mmol/l | 139 (4) | 0.11 |
| Sodiummax until CT scan, mmol/l | 141 (3) | 0.32 |
| Sodiummin until CT scan, mmol/l | 138 (4) | 0.05 |
| Potassium at baseline, mmol/l | 4.0 (0.6) | 0.96 |
| Potassiummax until CT scan, mmol/l | 4.2 (0.6) | 0.85 |
| Potassiummin until CT scan, mmol/l | 3.7 (0.4) | 0.96 |
| Blood glucose, mg/dl | 125 (40) | 0.04 |
Values are means ± SD, median (IQR) or numbers with percentages in parentheses.
Spearman correlation.
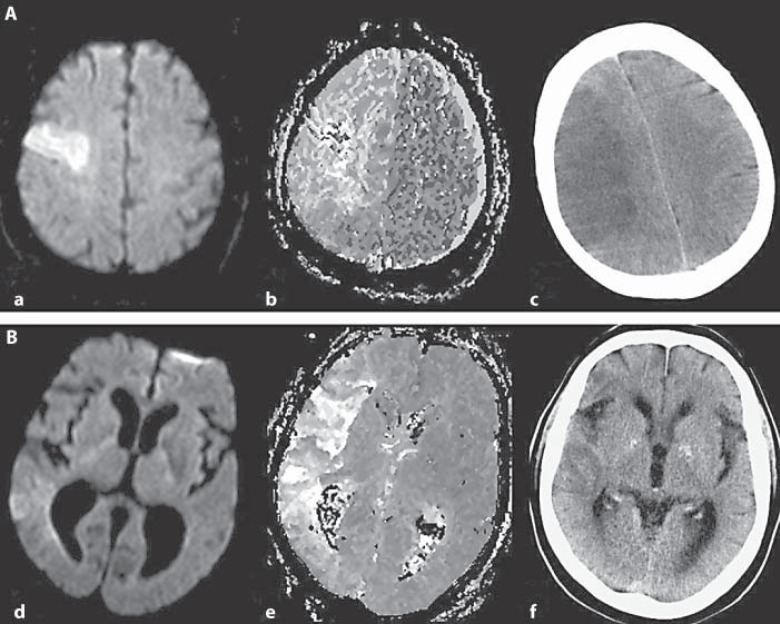
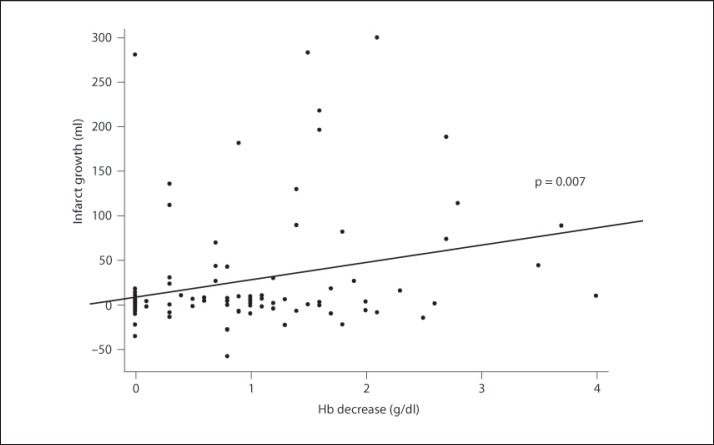
Figure 1 shows two examples with different courses of tissue at risk in relation to Hb level development after stroke onset. Figure 2 presents the correlation between infarct growth and Hb decrease.
Fig. 1.
Different courses of tissue at risk. Examples of tissue fate in a patient developing anemia between stroke onset and follow-up CT scan (patient 1; A) and in a patient without anemia (patient 2; B). Displayed are DW (a, d) and PW (b, e) MR images on admission demonstrating a substantial mismatch in both patients. In patient 1, the follow-up CT image demonstrates infarct growth in comparison to the DWI lesion (c). In patient 2, no relevant change of the initial DWI lesion size was observed (f).
Fig. 2.
Correlation between Hb decrease and infarct growth.
A linear regression model showed Hb decrease (β = 0.23, p = 0.02) to predict infarct growth independently of mismatch volume (β = 0.27, p = 0.004) and minimum sodium (β = −0.21, p = 0.03), and adjusted to the non-predicting variables age, NIHSS score, maximum leucocyte count and CRP, blood glucose, and Hct decrease (table 3).
Table 3.
Linear regression model to predict infarct growth
| Variable | B | β | p |
|---|---|---|---|
| Age | 0.98 | ||
| NIHSS on admission | 0.19 | ||
| Mismatch volume | 0.19 | 0.27 | 0.004 |
| CRPmax until CT scan | 0.84 | ||
| Leukocytesmax until CT scan | 0.13 | ||
| Sodiummin until CT scan | −4.49 | −0.21 | 0.03 |
| Blood glucose | 0.63 | ||
| Hb decrease until CT scan | 16.33 | 0.23 | 0.02 |
| Hct decrease until CT scan | 0.87 |
Discussion
Anemia on admission and thereafter is associated with worse outcome and mortality in stroke patients [12]. The pathophysiological mechanisms behind this phenomenon, however, are unknown. Here, we demonstrate that decreasing Hb levels after admission are independently associated with infarct growth in stroke patients who received MRI-based thrombolysis.
Loss of the ischemic penumbra, i.e. extension of irreversibly damaged infarct tissue, depends on mechanisms so diverse as excitotoxicity, inflammation, and apoptosis, but the most critical initial factor certainly is failure of supply of the core component of aerobic cellular metabolism, i.e. oxygen [14]. Infarct growth by penumbral transition was thought to be a rapid process during the acute stroke phase in the past, but imaging studies have revealed that this process can continue for more than 36–48 h in stroke patients [15,16] and for more than 2 weeks in animal experiments [17]. It should therefore be assumed that optimal oxygen supply to the penumbra and adjacent oligemic tissue is not only warranted in the acute phase of stroke but also during the following days. Healthy brain tissue is able to compensate falling Hb levels by increasing regional cerebral blood flow and oxygen extraction up to extremely low levels of 5–6 g/dl [18,19]. In ischemic brain tissue, these compensatory mechanisms might fail at considerably higher critical Hb levels already [20,21]. Thus, we hypothesized that infarct growth in this selected group of patients with confirmed presence of an ischemic penumbra on admission and a homogeneous recanalization regime might be associated with differences in Hb levels, as our data showed.
The main result of this study is that falling Hb levels after admission seem to be more relevant to infarct growth than levels on admission. This observation is in good agreement with our recently published data, showing outcome parameters to have a stronger association with decreasing and minimum Hb and Hct levels than with admission levels [12]. Surprisingly, this significant effect on infarct growth was observed with a relative reduction of Hb within a range of as little as 0.8 g/dl. Apparently, even little variations in oxygen-carrying capacity might contribute to loss of cerebral tissue. The negative association of good functional outcome with infarct growth was only a non-significant trend in this study, although this is an obvious clinical observation. Probably, the study population was too small for this demonstration due to our strict selection criteria.
With regard to Hct, the linear regression model failed to indicate an independent association with infarct growth, although in the univariate analysis, there was a highly significant correlation. The most probable explanation is the strong interaction of Hct with the statistically similarly effective minimum sodium levels, as both reflect fluid overload. Additionally, this discrepancy might point to a relatively higher relevance of oxygen-carrying capacity, i.e. Hb level, in comparison to the amount of blood cells, i.e. Hct, which on its own has to be balanced against flow-impairing increase in viscosity. However, our study was not designed to allow for further differentiation of these effects, and clearly, Hb and Hct can hardly be looked at separately from a clinical point of view.
Not only low, but also extremely high Hb levels have shown to be deleterious in stroke patients by other authors, who have described a U-shaped relationship between Hb and outcome or Hct and cerebral reperfusion deficits [8,10]. This constellation might be equally fatal for the penumbral tissue, probably through viscosity-related impairment of microcirculation. We do not consider our findings in disagreement with this observation. However, we cannot confirm such a relationship by our analysis, as almost none of our patients had elevated Hb or Hct levels.
Our study has several limitations. As a retrospective analysis, it is naturally prone to diverse biases and can only detect an association but not demonstrate a causal relationship between decreasing Hb and infarct growth. Our findings should therefore certainly not be interpreted as support of a more active transfusion practice. Data on recanalization based on the initial MRI angiography and follow-up transcranial ultrasound suggested a rate of >80% of recanalization, which is considerably higher than usually reported after IVT and even after intra-arterial thrombolysis [22,23]. However, in 28% of our patients, the retrospective analysis of the available recanalization data did not allow for confident determination of the recanalization status, and there was no systematic timing of transcranial doppler sonography either. This might have contributed to the appearance of a falsely high recanalization rate. Of note, however, when we dichotomized the population according to high and low infarct growth in relation to the median infarct growth and compared recanalization rates, these were evenly distributed between the groups, so we do not assume recanalization to have relevantly affected our findings (data not shown). The application of quite strict selection criteria aiming at homogeneity and sufficient quality of imaging data had led to a study population of only 100 patients, in which some effects might have been statistically undetected. However, one reason why these criteria were chosen was to overcome another limitation, i.e. the difficulty to perform an intermodal comparison of infarct sizes between MRI and CT. This latter problem, together with the application of the ABC/2 method to assess infarct growth (the method is more established for assessing ellipsoid hemorrhages) and the fact that we did not use an up-to-date software to analyze images are also methodological shortcomings of our study. Finally, we have made our observations in IVT stroke patients only and they might thus not apply to other stroke patients, or at least not to the same extent. Possibly, patients are more prone to anemia after thrombolysis, e.g. by a higher incidence of undetected gastrointestinal bleeding.
Despite these limitations, this is the first study showing the independent association of decreasing levels of Hb with infarct growth in MRI-based thrombolyzed stroke patients, independent of mismatch volume and recanalization rate.
Conclusion
Decreasing Hb levels are associated with further increase in infarct size after acute ischemic stroke. This might point to insufficient oxygen supply to the ischemic penumbra. The more detailed pathophysiology behind this observation and, more importantly, the clinical implications for hematologic management of stroke patients remain to be investigated prospectively.
Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- 1.Alexandrov AV, Burgin WS, Demchuk AM, El-Mitwalli A, Grotta JC. Speed of intracranial clot lysis with intravenous tissue plasminogen activator therapy: sonographic classification and short-term improvement. Circulation. 2001;103:2897–2902. doi: 10.1161/01.cir.103.24.2897. [DOI] [PubMed] [Google Scholar]
- 2.Molina CA, Alexandrov AV, Demchuk AM, Saqqur M, Uchino K, Alvarez-Sabin J, CLOTBUST Investigators. Improving the predictive accuracy of recanalization on stroke outcome in patients treated with tissue plasminogen activator. Stroke. 2004;35:151–156. doi: 10.1161/01.STR.0000106485.04500.4A. [DOI] [PubMed] [Google Scholar]
- 3.Mazighi M, Serfaty JM, Labreuche J, Laissy JP, Meseguer E, Lavallée PC, et al. RECANALISE investigators: Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol. 2009;8:802–809. doi: 10.1016/S1474-4422(09)70182-6. [DOI] [PubMed] [Google Scholar]
- 4.Becktepe JS, You SJ, Berkefeld J, Neumann-Haefelin T, Singer OC. Clinical outcome after mechanical recanalization as mono- or adjunctive therapy in acute stroke: importance of time to recanalization. Cerebrovasc Dis. 2011;32:211–218. doi: 10.1159/000328814. [DOI] [PubMed] [Google Scholar]
- 5.Wahlgren N, Ahmed N, Eriksson N, Aichner F, Bluhmki E, Davalos A, et al. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy (SITS-MOST) Stroke. 2008;39:3316–3322. doi: 10.1161/STROKEAHA.107.510768. [DOI] [PubMed] [Google Scholar]
- 6.The National Institute of Neurological Disorders and Stroke rt-PA Study Group Tissue plasminogen activator for acute ischaemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 7.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 8.Allport LE, Parsons MW, Butcher KS, MacGregor L, Desmond PM, Tress BM, et al. Elevated haematocrit is associated with reduced reperfusion and tissue survival in acute stroke. Neurology. 2005;65:1382–1387. doi: 10.1212/01.wnl.0000183057.96792.a8. [DOI] [PubMed] [Google Scholar]
- 9.Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208–2214. doi: 10.1161/01.STR.0000085087.41330.FF. [DOI] [PubMed] [Google Scholar]
- 10.Tanne D, Molshatzki N, Merzeliak O, Tsabari R, Toashi M, Schwannenthal Y. Anaemia status, haemoglobin concentration and outcome after acute stroke: a cohort study. BMC Neurol. 2010;10:22. doi: 10.1186/1471-2377-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams HP, Jr, Del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. American Heart Association, American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 12.Kellert L, Martin E, Sykora M, Bauer H, Gussmann P, Diedler J, et al. Cerebral oxygen transport failure?: decreasing haemoglobin and haematocrit levels after ischaemic stroke predict poor outcome and mortality: STroke: RelevAnt Impact of haemoGlobin, Haematocrit and Transfusion (STRAIGHT) – an observational study. Stroke. 2011;42:2832–2837. doi: 10.1161/STROKEAHA.110.606665. [DOI] [PubMed] [Google Scholar]
- 13.Butcher KS, Parsons M, MacGregor L, Barber PA, Chalk J, Bladin C, EPITHET Investigators. Refining the perfusion-diffusion mismatch hypothesis. Stroke. 2005;36:1153–1159. doi: 10.1161/01.str.0000166181.86928.8b. [DOI] [PubMed] [Google Scholar]
- 14.Pestalozza IF, Di Legge S, Calabresi M, Lenzi GL. Ischaemic penumbra: highlights. Clin Exp Hypertens. 2002;24:517–529. doi: 10.1081/ceh-120015328. [DOI] [PubMed] [Google Scholar]
- 15.Pantano P, Caramia F, Bozzao L, Dieler C, von Kummer R. Delayed increase in infarct volume after cerebral ischemia: correlations with thrombolytic treatment and clinical outcome. Stroke. 1999;30:502–507. doi: 10.1161/01.str.30.3.502. [DOI] [PubMed] [Google Scholar]
- 16.Baird AE, Benfield A, Schlaug G, SIewert B, Lövblad KO, Edelman RR, et al. Enlargement of human cerebral ischaemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;41:581–589. doi: 10.1002/ana.410410506. [DOI] [PubMed] [Google Scholar]
- 17.Dereski MO, Chopp M, Knight RA, Rodolosi LC, Garcia JH. The heterogeneous temporal evolution of focal ischaemic neuronal damage in the rat. Acta Neuropathol. 1993;85:327–333. doi: 10.1007/BF00227730. [DOI] [PubMed] [Google Scholar]
- 18.Weiskopf RB, Toy P, Hopf HW, Feiner J, Finlay HE, Takahashi M, et al. Acute isovolemic anaemia impairs central processing as determined by P300 latency. Clin Neurophysiol. 2005;116:1028–1032. doi: 10.1016/j.clinph.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Baron JC. Perfusion thresholds in human cerebral ischemia: historical perspective and the therapeutic implications. Cerebrovasc Dis. 2001;11:2–8. doi: 10.1159/000049119. [DOI] [PubMed] [Google Scholar]
- 20.Reasoner DK, Ryu KH, Hindman BJ, Cutkom J, Smith T. Marked hemodilution increases neurologic injury after focal cerebral ischemia in rabbits. Anesth Analg. 1996;82:61–67. doi: 10.1097/00000539-199601000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Dexter F, Hindman BJ. Effect of haemoglobin concentration on brain oxygenation in focal stroke: a mathematical modelling study. Br J Anaesth. 1997;79:346–351. doi: 10.1093/bja/79.3.346. [DOI] [PubMed] [Google Scholar]
- 22.Del Zoppo GJ, Poeck K, Pessin MS, Wolpert SM, Furlan AJ, Ferbert A, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32:78–86. doi: 10.1002/ana.410320113. [DOI] [PubMed] [Google Scholar]
- 23.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]