Abstract
Background/Aims
Low vitamin D status is highly prevalent worldwide, and the major determinants are sun exposure and vitamin D intake. We aimed to measure vitamin D status in a sample of overweight/obese adults in Puerto Rico, an area with plenty of sun exposure, and relate it to vitamin D intake, sun exposure and body composition.
Methods
Serum 25(OH)D levels (liquid chromatography-tandem mass spectrometry), body weight and fat (bioimpedance), vitamin D intake and sun exposure (questionnaires) were assessed. Analysis included age-adjusted correlations and multivariate regression.
Results
In 98 subjects (66% females; 40–65 years), median serum 25(OH)D levels were 30.7 ng/ml (25–75th percentile 25.0–37.3); 55% had levels >30 ng/ml, 31% had levels between 20 and 30 ng/ml and 14% had levels <20 ng/ml. Total vitamin D intake was 180 IU/day (45–615), and the sun exposure score was 22 (17–27). After adjusting for gender, 25(OH)D levels were significantly correlated with vitamin D intake (r = 0.24, p = 0.018), the sum of sun exposure and vitamin D intake indices (r = 0.34, p = 0.001) and percent body fat (r = −0.25, p = 0.01). After adjusting for age, gender and percent body fat, the sum of sun exposure and vitamin D intake indices remained statistically associated with 25(OH)D levels (β = 1.5, p < 0.01).
Conclusions
In this group of overweight and obese individuals, 25(OH)D was significantly related to vitamin D intake, sun exposure and vitamin D intake indices and percent body fat.
Key Words: Vitamin D status, Overweight, Obesity, Puerto Ricans, Hispanics
Introduction
Vitamin D deficiency is found worldwide, even in low-latitude countries, where it was generally assumed that UV radiation was adequate to prevent vitamin D deficiency in industrialized countries, where vitamin D fortification has been implemented now for years [1,2]. Although vitamin D is a liposoluble vitamin obtained through exposure to sunlight and intake of foods and supplements [3], there are several factors that limit the synthesis and bioavailability of vitamin D. Such factors include age, skin pigmentation, obesity, sunscreen use, clothing, season, geographic latitude, time of day of sun exposure, cloudiness and smog [4]. People with the greatest amount of melanin (dark skin) have a reduced ability to synthesize vitamin D from sun exposure [5]. In fact, several studies have reported high vitamin D deficiency prevalence in Hispanics [6,7,8,9]. In addition, obese individuals usually have a low concentration of 25(OH)D in plasma [10], and this level decreases with increasing obesity and percent body fat [11].
Currently, there are no published studies that have assessed vitamin D status in overweight or obese Puerto Ricans. Therefore, the objective of this study was to determine the nutritional status of vitamin D in a group of overweight and obese Puerto Rican individuals living at latitude 18° and to understand the association of serum 25(OH)D levels with vitamin D intake, sun exposure and body composition. Serum 25(OH)D level is the most widely accepted biomarker to estimate short-term vitamin D status, since it reflects both the dermal vitamin D synthesis and vitamin D obtained from foods and supplements [3] and has a half-life in the circulation of 15 days [12]. However, serum 25(OH)D does not indicate the amount of vitamin D stored in body tissues; therefore, the long-term indicators of lifetime exposure to vitamin D in population-based studies include dietary and supplemental intakes of vitamin D and sunlight exposure [13].
Methods
This study is a secondary analysis of a cross-sectional study on periodontal disease and type 2 diabetes [14].
Subjects
A convenience sample of 100 overweight and obese adult residents of the municipality of San Juan, who responded to flyers posted on the Medical Sciences Campus of the University of Puerto Rico or to media advertisements, were recruited. Study participants provided written informed consent, and the study was approved by the institutional review board of the University of Puerto Rico.
Inclusion criteria were as follows: resident of the San Juan municipality, 40–65 years old, overweight [body mass index (BMI) 25.0–29.9] or obese (BMI ≥30.0) and free of self-reported diabetes diagnosed by a physician prior to the screening. This age group was chosen because their risk of developing type 2 diabetes and periodontal disease is higher than that in younger populations, which was important for the main study. The exclusion criteria were dental conditions that prevented adequate periodontal examination (fewer than 4 teeth or having braces) and the following medical conditions: hypoglycemia, heart conditions (i.e. coronary heart disease, congenital heart murmurs, valve problems, congenital heart disease or endocarditis) or stroke and rheumatic fever, dialysis, pacemaker, automatic defibrillator, artificial material in the heart or vessels, anticoagulant medication, hemophilia or bleeding disorders, hip bone or joint replacement, pregnant women and individuals not mentally capable of participating in the study or understanding the informed consent. The medical exclusions were made due to potential systemic complications from the main study procedures.
Data Collection
Participants who qualified were invited to come to the Medical Sciences Campus of the University of Puerto Rico in a fasting state. A fasting blood sample was taken for the determination of serum 25(OH)D and other biochemical parameters. Participants then underwent several anthropometric measurements, a dental exam and an interviewer-administered questionnaire that collected data on sociodemographic characteristics, lifestyle and general health. The data were collected between November and December; therefore, minimal seasonal variation was expected.
Serum 25(OH)D
The serum 25(OH)D levels (D2 and D3) were measured by liquid chromatography-tandem mass spectrometry in duplicate (interassay coefficient of variation 9–15%, intra-assay coefficient of variation <10%). Although there is debate about which level of serum 25(OH)D is associated with deficiency (rickets), suitability for bone health and in general for optimal health, a concentration of less than 20 ng/ml (<50 nmol/l) is considered inadequate by the Institute of Medicine [3]. Some authors have suggested that the goal should be to maintain levels above 30 ng/ml (75 nmol/l) to take full advantage of all the health benefits that vitamin D provides [15], including optimal fracture reduction [16,17]. Therefore, in the present study vitamin D status was divided as follows: (1) 25(OH)D levels above 30 ng/ml; (2) 25(OH)D levels between 20 and 30 ng/ml, and (3) 25(OH)D levels below 20 ng/ml.
Anthropometric Measurements
Anthropometric measurements (weight, height, percent body fat and waist and hip circumference) were taken in duplicate following the National Health and Nutrition Examination Survey III Anthropometric Video Procedures, and the average of the two measurements was used. Weight was measured using an electronic digital scale and recorded in kilograms from the automated system on the body measurement exam space. Standing height was measured with a stadiometer and recorded to the nearest 0.1 cm. Percent of fat mass was measured using special bioelectrical impedance scales (Tanita Scale, TCA Inc.). Since these measurements could lead to complications among people with automatic implantable cardiodefibrillators, such patients were excluded from the study [18]. Waist circumference was measured at the umbilical level at minimal respiration and recorded to the nearest 0.1 cm. Hip circumference was measured at the maximum extension of the buttocks and recorded to the nearest 0.1 cm. BMI was calculated using the weight and height of the participants, using the standard formula (weight in kilograms/height in meters squared) and waist to hip ratio (WHR; waist circumference in centimeters/hip circumference in centimeters). Subjects were classified by percent body fat according to age and sex as follows: high risk (women: >39% if 20–39 years, >40% if 40–59 years and >42% if 60–79; men: >25% if 20–39 years, >27% if 40–59 years and >30% if 60–79 years) or low risk (if below these percentages) [19].
Food Frequency Questionnaire
A semiquantitative food frequency questionnaire (FFQ) focusing on foods and supplements rich in vitamin D was prepared. The semiquantitative FFQ was composed of 22 items which are considered potential sources of vitamin D in the population of interest [20]. Not all foods listed in the questionnaire were a significant source of vitamin D. For the foods considered major sources of vitamin D, an open-ended question regarding the type of such products consumed was included. For the frequency of the use of vitamin and mineral supplements, herbs, tea and other supplemental foods, open-ended questions regarding the type and amount of the supplements consumed were also included. Each food item included a fixed commonly used portion size. The frequency of each food item was assessed for the previous month and included 8 frequency responses ranging from ‘3 or more servings per day’ to ‘rarely or never’. The participants were informed how to complete the questionnaire with the help of the investigators at the beginning of the study. To estimate vitamin D consumption from the FFQ, the vitamin D content of a serving of each food was multiplied by its frequency. The vitamin D content of each food was obtained from the Nutritionist Pro Nutrient Analysis Software (2007, Axxya Systems, Stafford, Tex., USA) and the US Department of Agriculture National Nutrient Database for Standard Reference.
Sun Exposure Questionnaire
A sun exposure questionnaire was designed to record the amount of sun exposure for each participant. Those questions included the frequency at which the participants were outside more than 15 min, the time of day they were outdoors, type of clothing worn when outdoors, frequency of sunscreen use and level of sun protection factor (SPF) used, anatomical sites protected with sunscreen and ability to tan and tendency to burn after sun exposure. These types of questions have been used in other studies [21,22]. A sun exposure index was defined using the following variables: (1) frequency of outdoor activity for more than 15 min: daily = 7, 4–6 times a week = 5, 2–3 times a week = 2.5, once a week = 1, less than once a week/never = 0 [20,23]; (2) usual time of day of outdoor activities: between 7 and 11 a.m. = 1, between 11 a.m. and 3 p.m. = 2, between 3 and 5 p.m. = 1 [21]; (3) type of clothing worn outdoors: long pants, long sleeves, closed shoes, socks and hat = 0 each, short sleeves, short pants or skirts and open shoes = 1 each, bathing suit = 2 [24,25,26]; (4) frequency of sunscreen use: never = 3, less than 3 times per week = 2, 3–6 times per week = 1, daily = 0 [27]; (5) level of sunscreen protection: no use = 3, SPF <15 = 2, SPF 15–30 = 1, SPF >30 = 0 [22,28]. The ability to tan and tendency to burn were also included: never burns or tans (deeply pigmented) = 0; never burns, tans deeply brown or black = 1; rarely burns and tans brown = 2; burns minimally and tans easily = 3; burns moderately, tans moderately and uniformly = 4; burns easily, tans minimally = 5; burns easily, never tans = 6 [29]. This skin classification system is based on the amount of skin melanin and responses to sun exposure and ranges from very fair (skin type I) to very dark (skin type VI) [29]. Type I is a highly sensitive skin that always burns and never tans; type II is a very sun-sensitive skin that burns easily and tans minimally; type III is a sun-sensitive skin that sometimes burns and slowly tans to light brown; type IV is a minimally sun sensitive skin that burns minimally and always tans to moderate brown; type V is a sun-insensitive skin that rarely burns and tans well (Hispanics), and type VI is a sun-insensitive skin that never burns and is deeply pigmented (blacks). The total sun exposure index ranged from 0 [no sun exposure, high use of clothing and sunscreen when outdoors and never burns or tans (deeply pigmented)] to 38 (high sun exposure, no sunscreen and light clothing when outdoors and burns easily, never tans).
Physical Activity
A short questionnaire on physical activity included the following items: participation in physical activity during the previous month (yes/no), participation in vigorous physical activity or structured exercise (yes/no) and time usually dedicated to that activity (hours per week).
Statistical Analysis
Normality assumptions for the sun exposure index, dietary intake of vitamin D and serum levels of 25(OH)D were assessed using the Shapiro-Wilk statistic. Serum 25(OH)D was normally distributed, but vitamin D intake and sun exposure indices were not; therefore, nonparametric tests were used. Medians and 25th and 75th percentiles were computed for all the continuous variables. The Mann-Whitney-Wilcoxon test was used to compare the distribution of sociodemographic characteristics, anthropometric measurements, vitamin D intake, sun exposure index, physical activity and serum 25(OH)D levels by gender. The χ2 test was used to compare vitamin D status (normal, insufficient and deficient) between obese and overweight individuals and between gender, since there were gender differences in the general characteristics. The associations between serum 25(OH)D and total vitamin D intake, the sun exposure index, the sum of the sun exposure and vitamin D intake indices and body composition were assessed using partial Spearman's correlation coefficients adjusted by age. In addition, age-adjusted partial correlations were performed between sun exposure and body composition. A multivariable linear regression model was used to examine the relationship between serum 25(OH)D and the sum of sun exposure and vitamin D intake indices. Age (continuous), gender (categorical) and percent body fat (continuous) were adjusted in the model. All statistical analyses were performed using SAS statistical software (version 9.1, SAS Institute Inc., Cary, N.C., USA).
Results
Table 1 shows the general characteristics of the sample. From the 100 subjects recruited, 98 had complete data collection (66% were females and 34% were males). Female subjects had significantly more years of education (p = 0.002), higher percent body fat (p < 0.0001), smaller waist circumference (p = 0.014) and smaller WHR (p < 0.0001) compared to male subjects. Participation in any physical activity was low but similar among females and males. Less than 30% of the sample did vigorous work or participated in a structured physical activity weekly, with participation of less than 1 h per day on average (data not shown).
Table 1.
Characteristics of the sample
| Variable | Females (n = 65) | Males (n = 33) | Total (n = 98) |
|---|---|---|---|
| Age, years | 52 (44–57) | 51 (45–59) | 52 (45–58) |
| Years of education | 16 (15–18) | 15 (12–16)* | 15 (15–18) |
| Physical activity in the previous month, % | 37.8 | 21.4 | 59.2 |
| Vigorous or structured physical activity, % | 18.4 | 10.2 | 28.6 |
| BMI | 31.7 (28.4–36.4) | 32.4 (28.9–37.7) | 32.2 (28.4–36.8) |
| Percent body fat | 43.0 (40.2–46.6) | 33.4 (27.2–38.7)* | 41.0 (35.0–45.0) |
| Waist circumference, cm | 100.0 (93.3–110.0) | 109.1 (101.5–121.5)* | 104.2 (96.3–116.5) |
| Hip circumference, cm | 113.6 (105.3–121.5) | 112.9 (107.3–123.2) | 113.3 (106.5–121.6) |
| WHR | 0.88 (0.83–0.92) | 0.97 (0.95–1.00)* | 0.91 (0.85–0.97) |
Values are shown as medians (25th and 75th percentiles), where appropriate.
p < 0.01 compared to females (Mann-Whitney-Wilcoxon test).
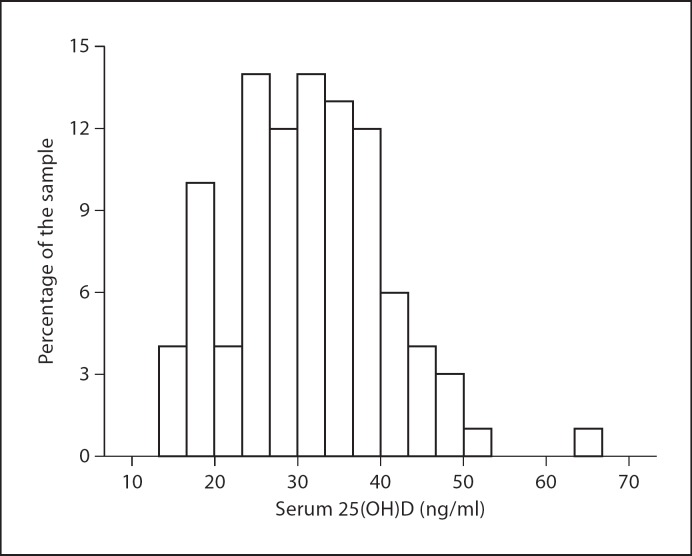
Table 2 shows vitamin D intake, sun exposure index and serum 25(OH)D levels by gender. Vitamin D intake from foods and supplements and total vitamin D intake were similar between females and males. Median total vitamin D intake was 179.7 IU/day (44.7–615.2); 72% came from supplements, while only 28% came from foods. The foods contributing the most to vitamin D intake in this group were fish (31%), margarine (22%) and milk (20%; data not shown). In terms of sun exposure, the median sun exposure index was 22 (17.0–27.0). Female subjects had a significantly lower sun exposure index compared to male subjects (p < 0.05). In general, about 56% of the subjects reported being outdoors more than 15 min every day with similar frequencies across the different times of the day; most subjects usually wore short sleeves (90%), long pants (75%) and closed shoes (77%) when outdoors; 47% reported the use of sun block, with most of them (26%) using it less than 3 times per week, mainly on the face (45%) and arms (30%); 43% reported minimally, rarely or never burning or tanning when exposed to sunlight (they could be considered individuals with medium to very dark skin), while 57% reported burning and tanning moderately and easily (they could be considered individuals with very fair to light skin) [29] (data not shown). Serum 25(OH)D levels were similar in female and male subjects (table 2). In general, 55% of the subjects had vitamin D levels above 30 ng/ml; 31% had levels between 20 and 30 ng/ml, and 14% had levels below 20 ng/ml (fig. 1). Additionally, we stratified serum 25(OH)D levels by BMI classification and ability to tan and tendency to burn. Although the differences were not significant (Kruskal-Wallis test), the levels were lower among dark-skinned people, as expected, but the difference between overweight and obese individuals was contrary to expectations. In overweight subjects with light skin, median 25(OH)D was 34.2 ng/ml (25th–75th percentile 24–34 ng/ml); in overweight subjects with dark skin it was 31.1 ng/ml (25th–75th percentile 25–37 ng/ml); in obese subjects with light skin it was 39.0 ng/ml (25th–75th percentile 29–40 ng/ml), and in obese subjects with dark skin it was 30.9 ng/ml (25th–75th percentile 24–39 ng/ml).
Table 2.
Vitamin D intake, sun exposure index and serum 25(OH)D levels
| Variable | Females (n = 65) | Males (n = 33) | Total (n = 98) |
|---|---|---|---|
| Vitamin D intake, IU/day | |||
| Food | 76 (32.3–148.5) | 64.5 (9.0–91.1) | 73.5 (23.1–142.2) |
| Supplements | 0 (0–600) | 0 (0–600) | 0 (0–600) |
| Total vitamin D | 166.6 (64.5–616.9) | 210.3 (33.2–603.8) | 179.7 (44.7–615.2) |
| Sun exposure index1 | 21.0 (14.0–25.0) | 26.5 (23.0–28.0)* | 22.0 (17.0–27.0) |
| Serum 25(OH)D level, ng/ml2 | 30.4 (24.7–34.7) | 34.0 (25.0–39.0) | 30.7 (25.0–37.3) |
Values are shown as medians (25th and 75th percentiles).
p < 0.01 compared to females (Mann-Whitney-Wilcoxon test).
Index calculated from sun exposure questionnaire, which included frequency, usual time and type of clothing used in outdoor activities >15 min, frequency and level of sunscreen use, and ability to tan and tendency to burn, with a possible range of 0–38.
To convert to nanomoles per liter multiply by 2.5.
Fig. 1.
Distribution of serum 25(OH)D levels in the total sample (n = 98).
Age-adjusted partial correlations between serum 25(OH)D levels with different variables according to gender are shown in table 3. Serum 25(OH)D levels were negatively correlated with percent body fat (r = −0.24, p = 0.02), and positively associated with total vitamin D intake (r = 0.23, p = 0.03) and the sum of sun exposure and vitamin D intake indices (r = 0.32, p < 0.01) in the total sample. However, these significant correlations were observed mainly in females.
Table 3.
Partial correlations between serum 25(OH)D levels and different variables adjusted by age
| Variable | Females (n = 65) |
Males (n = 33) |
Total (n = 98) |
|||
|---|---|---|---|---|---|---|
| Spearman's rho | p value | Spearman's rho | p value | Spearman's rho | p value | |
| BMI | −0.12 | 0.32 | 0.04 | 0.81 | −0.08 | 0.45 |
| Percent body fat | −0.21 | 0.09 | −0.17 | 0.35 | −0.24 | 0.02 |
| WHR | −0.10 | 0.45 | 0.02 | 0.09 | 0.01 | 0.90 |
| Total vitamin D intake | 0.35 | 0.047 | 0.07 | 0.70 | 0.22 | 0.03 |
| Sun exposure index | 0.18 | 0.16 | 0.03 | 0.98 | 0.16 | 0.10 |
| Sun exposure + vitamin D intake index | 0.31 | 0.01 | 0.19 | 0.30 | 0.32 | <0.01 |
Stratification by BMI category did not change the direction or significance of the correlations between 25(OH)D levels and sun exposure index (r = 0.12, p = 0.12 in overweight individuals; r = 0.16, p = 0.22 in obese individuals) or the sum of the sun exposure and vitamin D intake indices (r = 0.36, p < 0.05 in overweight individuals; r = 0.30, p < 0.05 in obese individuals), but it did for vitamin D intake, which lost statistical significance (r = 0.27, p = 0.14 in overweight individuals; r = 0.20, p = 0.10 in obese individuals).
In addition, the sun exposure index was inversely correlated with percent body fat (r = −0.22, p < 0.05) and WHR (r = −0.23, p < 0.05; data not shown). Although the ability to tan and tendency to burn were not related to serum 25(OH)D levels in the total sample, when stratified by high and low ability to tan and tendency to burn, those with a high ability/tendency had a significant correlation between 25(OH)D levels and the sum of sun exposure and vitamin D intake indices (r = 0.33, p < 0.05) compared to those with a low ability/tendency (r = 0.23, p = 0.16; data not shown). Those individuals who consumed the recommended dietary allowance (RDA) of vitamin D of ≥600 IU/day (Institute of Medicine, 2010) had significantly greater 25(OH)D serum levels compared to those who did not meet this recommendation (p < 0.01). Those who reported being outdoors for at least 15 min 2–7 times per week also had significantly greater 25(OH)D levels (p < 0.05).
After multivariate adjustment for age, gender and percent body fat, the sum of sun exposure and vitamin D intake indices remained statistically associated with serum 25(OH)D levels, where a higher score for sun exposure and vitamin D intake was associated with higher serum levels (β = 1.5, p < 0.01). When stratified by the ability to tan and tendency to burn, the multivariate analysis adjusting for age, gender and percent body fat showed that the sum of the sun exposure and vitamin D intake indices was statistically associated with serum 25(OH)D levels in those with a high ability/tendency (β = 2.06, p < 0.05) but not in those with a low ability/tendency (β = 0.94, p = 0.33).
Discussion
In the present study, 14% of our subjects had an inadequate vitamin D status, while 31% had levels between 20 and 30 ng/ml. Some authors consider these levels as not optimal for bone [16,17] and general health [15,30]. Serum 25(OH)D levels were significantly correlated with percent body fat, total vitamin D intake and the combined sun and vitamin D intake index. A higher score of sun exposure and vitamin D intake was significantly associated with higher serum 25(OH)D levels, even after adjustment for age, sex and percent body fat.
A study in 93 individuals from Hawaii [31], with a latitude similar to Puerto Rico (latitude 21°), found similar 25(OH)D levels measured by HPLC (31.6 ng/ml in Hawaii; 30.7 ng/ml in the present study), and 51% of their subjects had levels below 30 ng/ml, similar to the rate in our study (45%). Several studies have reported a high prevalence of vitamin D deficiency in Hispanics [6,7,8,9]. In a study conducted in South Florida, vitamin D deficiency was more prevalent in Hispanics [32], although 25(OH)D levels were measured using a DiaSorin radioimmunoassay method. Recently, a study in a group of 358 Hispanic American men found the highest prevalence of vitamin D deficiency (<20 ng/ml or <50 nmol/l) among Puerto Ricans (26%), compared to those from the Dominican Republic (21%), Central America (11%) and South America (9%) [33]. Furthermore, recent data from the National Health and Nutrition Examination Survey, which measured 25(OH)D levels by a competitive binding protein assay, found vitamin D insufficiency (<30 ng/ml or <75 nmol/l) in 97% of non-Hispanic Blacks and 90% of Mexican Americans [34]. However, there are significant variations in 25(OH)D levels between the methods used [35]; therefore, these comparisons should be viewed with caution.
Vitamin D status is influenced by several factors, including vitamin D intake, sun exposure, skin pigmentation and obesity. In the present study, median vitamin D intake was considerably lower than the RDA for vitamin D (600 IU/day) [3]. Only 69.4% of our subjects met this recommendation. Although vitamin D is high in certain fish, and several foods are fortified with vitamin D, such as milk, margarine and some brands of orange juice and cereals, these foods only contributed to a small amount of total vitamin D in this group. Most vitamin D was obtained from supplements. Furthermore, only those using vitamin D supplements met the RDA. Serum 25(OH)D levels were significantly correlated with total vitamin D intake in women but not in men. Those consuming ≥600 IU/day had significantly higher 25(OH)D levels compared to those consuming <600 IU/day, and only 8% compared to 37% had low vitamin D status. Other studies estimating vitamin D intake from FFQs have also found significant correlations between vitamin D intake and serum 25(OH)D levels in older US women (r = 0.52, p < 0.01 during summer and r = 0.63, p < 0.01 during winter) [20], in the Framingham Heart Study cohort (r = 0.24, p < 0.001) [36] and in young Finnish girls (r = 0.28, p < 0.01) [37], while others have not in older Spanish women [21].
Limited sun exposure also affects 25(OH)D levels. Although clothing in Puerto Rico commonly leaves the arms and legs uncovered, the sun exposure index level found in the present study was in the middle of the possible range for this index (0–38) but it was not correlated with 25(OH)D levels in the total sample (r = 0.16, p = 0.11). Similar low correlations were found between overweight and obese individuals. However, being outdoors for at least 15 min 2–7 times per week led to significantly greater 25(OH)D levels (p < 0.05) compared to being outdoors less frequently. Other authors, using a similar categorization of sun exposure, have found sun exposure levels to be significantly correlated with serum 25(OH)D levels in normal-weight older US women [20], normal-weight older adults in the UK during the summer (r = 0.62, p < 0.01), but not during winter (r = 0.23, p < 0.01) [38], normal-weight Danish women (r = 0.24, p < 0.001) [23] and older Spanish individuals (r = 0.377, p < 0.05) [27]. Since most of these studies were performed in normal-weight individuals, further studies are needed to understand the mechanisms of vitamin D photosynthesis in overweight and obese individuals. In addition, most of the published studies have measured 25(OH)D levels by a competitive binding protein assay, which, as discussed above, differs significantly from liquid chromatography-tandem mass spectrometry, the method used in the present study.
The actual amount of sun exposure that is needed to maintain adequate levels of vitamin D is difficult to ascertain. It has been suggested that approximately 5–30 min of sun exposure between 10 a.m. and 3 p.m. at least twice a week on the face, arms or legs without sunscreen usually leads to sufficient vitamin D synthesis [39,40]. However, these estimates are for individuals with light skin. People with the greatest amount of melanin (dark skin) have a reduced ability to produce vitamin D from exposure to sunlight [5] and therefore may need longer times in the sun. This may be the case for many of the individuals in our study, as 43% reported minimally, rarely or never burning or tanning when exposed to sunlight, which could be considered as having a skin phototype IV–VI, or medium to very dark skin. Individuals with dark skin (type IV and higher) have lower vitamin D photosynthesis compared to individuals with light skin (type I–III) with the same UVB exposure and latitude and may need 6 times more sun exposure to increase their 25(OH)D levels to the levels obtained by light-skinned individuals [41]. In the present study, we found that, although the ability to tan and tendency to burn were not related to 25(OH)D levels in the total sample, when stratified by the ability to tan and tendency to burn, those with a high ability/tendency had a significant correlation between 25(OH)D levels and the sum of sun exposure and vitamin D intake indices compared to those with a low ability/tendency. A study in 1,191 French adults found self-reported skin phototypes to be related to 25(OH)D levels during the winter [42].
Fat accumulation also influences 25(OH)D levels. In our study, we found an inverse and significant correlation between percent body fat and serum 25(OH)D levels (r = −0.24, p = 0.02), similar to the results of a study in 410 healthy non-Hispanic Black and White women [43]. Others have found lower serum 25(OH)D levels in obese individuals [44,45,46]. The mechanism underlying the increased risk of vitamin D deficiency in obesity is uncertain. It has been postulated that obese individuals may avoid exposure to solar UV radiation [47]. This explanation was supported by our results; we found a significant inverse correlation between the sun exposure index and percent body fat and WHR. Alternatively, it has been proposed that the production of 1,25(OH)2D is enhanced in obese individuals and, thus, its higher concentrations exert negative feedback control on the hepatic synthesis of serum levels of 25(OH)D [48]. There is evidence that alteration of the vitamin D endocrine system in obese subjects is characterized by secondary hyperparathyroidism, which is associated with enhanced renal tubular reabsorption of calcium and increased circulating 1,25(OH)2D. It has also been suggested that the metabolic clearance of vitamin D may increase in obesity, possibly through enhanced uptake by adipose tissue [49], thus decreasing the bioavailability of vitamin D3 from cutaneous and dietary sources because of its deposition in body fat compartments [10]. Recently, a study in 3,890 subjects found an inverse association between serum 25(OH)D levels and waist circumference (p < 0.005), subcutaneous adipose tissue (p = 0.016) and visceral adipose tissue (p < 0.0001) [44]. Animal studies also show that 25(OH)D may suppress uncoupling protein 2 expression, which could increase energy efficiency and may also increase glucocorticoid, which regulates adipose tissue [50]. These effects are mediated by the vitamin D receptor, whereby vitamin D may decrease peroxisome proliferator-activated receptor-γ availability, which leads to other metabolic processes in the preadipocyte [51].
Serum 25(OH)D levels are the most valid estimate for determining vitamin D status in humans, but this measurement, when used in population-based research, has some limitations [52]. One limitation is that the baseline measurement of serum 25(OH)D level may not accurately reflect a person's vitamin D status over the course of a year, since serum 25(OH)D levels vary by season, whereby concentrations are highest in the summer and fall and are lowest in the spring [53]. In addition, it is an expensive measurement, not often available for population studies or in the clinical setting. Therefore, vitamin D intake estimates using FFQs and sun exposure assessment could be used as indicators of vitamin D status throughout a person's lifetime in a population.
Several limitations need to be considered when interpreting our results. Sun exposure was self-reported by the subjects. Within our sun exposure questionnaire, we included the tendency to burn and ability to tan after sun exposure as an indirect measure of skin phototypes [29]. Although this measure was not related to 25(OH)D levels in the total sample, it was related in those with light skin. We did not have direct measures of skin color, which would be more accurate than self-reports (such as using a reflectance spectrophotometer and colorimeter). Also, it is important to distinguish between constitutive or unexposed skin and facultative or exposed skin. As constitutive pigmentation gets lighter and facultative pigmentation gets darker, 25(OH)D levels should increase [54,55]. A study using a portable reflectance spectrophotometer found a high reliability for measuring skin phototypes, with less variation in constitutive than facultative skin color [56]. Another study using reflectance colorimetry showed a significant correlation between skin color and 25(OH)D levels, whereby facultative skin color appeared to be a stronger predictor of sun exposure and 25(OH)D levels [57]. Therefore, these should be included in future studies. Also, another potential limitation of the present study is the inherent measurement error due to misreporting of food intake and the low number of male subjects. In addition, the use of bioimpedance for measuring fat mass in obese individuals may be inaccurate, underestimating fat mass in some studies [58], while overestimating fat mass in others [59,60], compared to gold standard methods. However, others have found good agreement between methods for obese population studies [61]. Furthermore, we had limited data on physical activity and sedentary lifestyles, and these may be indirect measures of sun exposure. Finally, the sample size was small, which may have affected our ability to detect associations between some of the study variables and 25(OH)D levels.
In conclusion, we found that although only 14% of our subjects had deficient 25(OH)D levels (<20 ng/ml), 31% had vitamin D levels considered by some authors as not optimal (20–30 ng/ml). This suggests that even people living in high sun exposure areas all year round like Puerto Rico are not protected against poor vitamin D status. This study also showed that serum 25(OH)D levels were significantly and independently associated with vitamin D intake, but also with the sum of the vitamin D intake and sun exposure indices and percent body fat. Therefore, vitamin D intake, extent of sun exposure, skin pigmentation and obesity are important determinants of vitamin D status in this sample.
Disclosure Statement
This investigation was supported in part by grants G12RR03051 and 1U54RR026139-01A1 from the National Center for Research Resources and K24DE016884 from the National Institutes of Health awards. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
Acknowledgements
We thank the Vitamin D, Skin and Bone Research Laboratory at the Boston University Medical Center for performing the vitamin D measurements.
References
- 1.Bandeira F, Griz L, Dreyer P, Eufrazino C, Bandeira C, Freese E. Vitamin D deficiency: a global perspective. Arq Bras Endocrinol Metabol. 2006;50:640–646. doi: 10.1590/s0004-27302006000400009. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine . Dietary Reference Intakes for Calcium and Vitamin D. Washington: The National Academy Press; 2011. [Google Scholar]
- 4.Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, Ragi-Eis S, Chandler J. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260:245–254. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 5.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 6.Fradinger EE, Zanchetta JR. Vitamin D and bone mineral density in ambulatory women living in Buenos Aires, Argentina. Osteoporos Int. 2001;12:24–27. doi: 10.1007/s001980170153. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs ET, Alberts DS, Foote JA, Green SB, Hollis BW, Yu Z, Martinez ME. Vitamin D insufficiency in southern Arizona. Am J Clin Nutr. 2008;87:608–613. doi: 10.1093/ajcn/87.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 9.Oliveri B, Plantalech L, Bagur A, Wittich AC, Rovai G, Pusiol E, Lopez Giovanelli J, Ponce G, Nieva A, Chaperon A, Ladizesky M, Somoza J, Casco C, Zeni S, Parisi MS, Mautalen CA. High prevalence of vitamin D insufficiency in healthy elderly people living at home in Argentina. Eur J Clin Nutr. 2004;58:337–342. doi: 10.1038/sj.ejcn.1601786. [DOI] [PubMed] [Google Scholar]
- 10.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 11.Vilarrasa N, Maravall J, Estepa A, Sanchez R, Masdevall C, Navarro MA, Alia P, Soler J, Gomez JM. Low 25-hydroxyvitamin D concentrations in obese women: their clinical significance and relationship with anthropometric and body composition variables. J Endocrinol Invest. 2007;30:653–658. doi: 10.1007/BF03347445. [DOI] [PubMed] [Google Scholar]
- 12.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–586S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 13.Millen AE, Bodnar LM. Vitamin D assessment in population-based studies: a review of the issues. Am J Clin Nutr. 2008;87:1102S–1105S. doi: 10.1093/ajcn/87.4.1102S. [DOI] [PubMed] [Google Scholar]
- 14.Joshipura KJ, Andriankaja MO, Hu FB, Ritchie CS. Relative utility of 1-hour oral glucose tolerance test as a measure of abnormal glucose homeostasis. Diabetes Res Clin Pract. 2011;93:268–275. doi: 10.1016/j.diabres.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, Josse RG, Lips P, Morales-Torres J, Yoshimura N. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151–1154. doi: 10.1007/s00198-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 18.Fuller NJ, Elia M. Potential use of bioelectrical impedance of the ‘whole body’ and of body segments for the assessment of body composition: comparison with densitometry and anthropometry. Eur J Clin Nutr. 1989;43:779–791. [PubMed] [Google Scholar]
- 19.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 20.Salamone LM, Dallal GE, Zantos D, Makrauer F, Dawson-Hughes B. Contributions of vitamin D intake and seasonal sunlight exposure to plasma 25-hydroxyvitamin D concentration in elderly women. Am J Clin Nutr. 1994;59:80–86. doi: 10.1093/ajcn/59.1.80. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez M, Beltrán de Miguel B, Cuadrado C, Moreiras O. Influence of sun exposure and diet to the nutritional status of vitamin D in adolescent Spanish women: the five countries study (OPTIFORD Project) (in Spanish) Nutr Hosp. 2010;25:755–762. [PubMed] [Google Scholar]
- 22.Pasco JA, Henry MJ, Nicholson GC, Sanders KM, Kotowicz MA. Vitamin D status of women in the Geelong Osteoporosis Study: association with diet and casual exposure to sunlight. Med J Aust. 2001;175:401–405. doi: 10.5694/j.1326-5377.2001.tb143643.x. [DOI] [PubMed] [Google Scholar]
- 23.Brot C, Vestergaard P, Kolthoff N, Gram J, Hermann AP, Sorensen OH. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr. 2001;86(suppl 1):S97–S103. doi: 10.1079/bjn2001345. [DOI] [PubMed] [Google Scholar]
- 24.Barger-Lux MJ, Heaney RP. Effects of above average summer sun exposure on serum 25-hydroxyvitamin D and calcium absorption. J Clin Endocrinol Metab. 2002;87:4952–4956. doi: 10.1210/jc.2002-020636. [DOI] [PubMed] [Google Scholar]
- 25.Atli T, Gullu S, Uysal AR, Erdogan G. The prevalence of Vitamin D deficiency and effects of ultraviolet light on Vitamin D levels in elderly Turkish population. Arch Gerontol Geriatr. 2005;40:53–60. doi: 10.1016/j.archger.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 26.van der Mei IA, Blizzard L, Ponsonby AL, Dwyer T. Validity and reliability of adult recall of past sun exposure in a case-control study of multiple sclerosis. Cancer Epidemiol Biomarkers Prev. 2006;15:1538–1544. doi: 10.1158/1055-9965.EPI-05-0969. [DOI] [PubMed] [Google Scholar]
- 27.Moreiras O, Carbajal A, Perea I, Varela-Moreiras V. The influence of dietary intake and sunlight exposure on the vitamin D status in an elderly Spanish group. Int J Vitam Nutr Res. 1992;62:303–307. [PubMed] [Google Scholar]
- 28.Sowers MR, Wallace RB, Hollis BW, Lemke JH. Parameters related to 25-OH-D levels in a population-based study of women. Am J Clin Nutr. 1986;43:621–628. doi: 10.1093/ajcn/43.4.621. [DOI] [PubMed] [Google Scholar]
- 29.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 30.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 31.Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, Lensmeyer G, Hollis BW, Drezner MK. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92:2130–2135. doi: 10.1210/jc.2006-2250. [DOI] [PubMed] [Google Scholar]
- 32.Levis S, Gomez A, Jimenez C, Veras L, Ma F, Lai S, Hollis B, Roos BA. Vitamin D deficiency and seasonal variation in an adult South Florida population. J Clin Endocrinol Metab. 2005;90:1557–1562. doi: 10.1210/jc.2004-0746. [DOI] [PubMed] [Google Scholar]
- 33.Araujo AB, Travison TG, Esche GR, Holick MF, Chen TC, McKinlay JB. Serum 25-hydroxyvitamin D and bone mineral density among Hispanic men. Osteoporos Int. 2009;20:245–255. doi: 10.1007/s00198-008-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth HJ, Schmidt-Gayk H, Weber H, Niederau C. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem. 2008;45:153–159. doi: 10.1258/acb.2007.007091. [DOI] [PubMed] [Google Scholar]
- 36.McAlindon TE, Felson DT, Zhang Y, Hannan MT, Aliabadi P, Weissman B, Rush D, Wilson PW, Jacques P. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125:353–359. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 37.Lehtonen-Veromaa M, Mottonen T, Irjala K, Karkkainen M, Lamberg-Allardt C, Hakola P, Viikari J. Vitamin D intake is low and hypovitaminosis D common in healthy 9- to 15-year-old Finnish girls. Eur J Clin Nutr. 1999;53:746–751. doi: 10.1038/sj.ejcn.1600844. [DOI] [PubMed] [Google Scholar]
- 38.Lawson DE, Paul AA, Black AE, Cole TJ, Mandal AR, Davie M. Relative contributions of diet and sunlight to vitamin D state in the elderly. Br Med J. 1979;2:303–205. doi: 10.1136/bmj.2.6185.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007;22(suppl 2):V28–V33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 40.Holick MF. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes. 2002;9:87–98. [Google Scholar]
- 41.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 42.Malvy DJ, Guinot C, Preziosi P, Galan P, Chapuy MC, Maamer M, Arnaud S, Meunier PJ, Hercberg S, Tschachler E. Relationship between vitamin D status and skin phototype in general adult population. Photochem Photobiol. 2000;71:466–469. doi: 10.1562/0031-8655(2000)071<0466:rbvdsa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 43.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88:157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 44.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, Robins SJ, O'Donnell CJ, Hoffmann U, Jacques PF, Booth SL, Vasan RS, Wolf M, Wang TJ. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59:242–248. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 46.Rockell JE, Skeaff CM, Williams SM, Green TJ. Serum 25-hydroxyvitamin D concentrations of New Zealanders aged 15 years and older. Osteoporos Int. 2006;17:1382–1389. doi: 10.1007/s00198-006-0118-x. [DOI] [PubMed] [Google Scholar]
- 47.Compston JE, Vedi S, Ledger JE, Webb A, Gazet JC, Pilkington TR. Vitamin D status and bone histomorphometry in gross obesity. Am J Clin Nutr. 1981;34:2359–2363. doi: 10.1093/ajcn/34.11.2359. [DOI] [PubMed] [Google Scholar]
- 48.Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76:370–373. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liel Y, Ulmer E, Shary J, Hollis BW, Bell NH. Low circulating vitamin D in obesity. Calcif Tissue Int. 1988;43:199–201. doi: 10.1007/BF02555135. [DOI] [PubMed] [Google Scholar]
- 50.Shi H, Norman AW, Okamura WH, Sen A, Zemel MB. 1alpha,25-dihydroxyvitamin D3 inhibits uncoupling protein 2 expression in human adipocytes. FASEB J. 2002;16:1808–1810. doi: 10.1096/fj.02-0255fje. [DOI] [PubMed] [Google Scholar]
- 51.Wood RJ. Vitamin D and adipogenesis: new molecular insights. Nutr Rev. 2008;66:40–46. doi: 10.1111/j.1753-4887.2007.00004.x. [DOI] [PubMed] [Google Scholar]
- 52.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87:1087S–1091S. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- 53.Maxwell JD. Seasonal variation in vitamin D. Proc Nutr Soc. 1994;53:533–543. doi: 10.1079/pns19940063. [DOI] [PubMed] [Google Scholar]
- 54.Taylor S, Westerhof W, Im S, Lim J. Noninvasive techniques for the evaluation of skin color. J Am Acad Dermatol. 2006;54:S282–S290. doi: 10.1016/j.jaad.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 55.Tangpricha V, Turner A, Spina C, Decastro S, Chen TC, Holick MF. Tanning is associated with optimal vitamin D status (serum 25-hydroxyvitamin D concentration) and higher bone mineral density. Am J Clin Nutr. 2004;80:1645–1649. doi: 10.1093/ajcn/80.6.1645. [DOI] [PubMed] [Google Scholar]
- 56.Pershing LK, Tirumala VP, Nelson JL, Corlett JL, Lin AG, Meyer LJ, Leachman SA. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633–1640. doi: 10.1038/sj.jid.5701238. [DOI] [PubMed] [Google Scholar]
- 57.Rockell JE, Skeaff CM, Williams SM, Green TJ. Association between quantitative measures of skin color and plasma 25-hydroxyvitamin D. Osteoporos Int. 2008;19:1639–1642. doi: 10.1007/s00198-008-0620-4. [DOI] [PubMed] [Google Scholar]
- 58.Verdich C, Barbe P, Petersen M, Grau K, Ward L, Macdonald I, Sorensen TI, Oppert JM. Changes in body composition during weight loss in obese subjects in the NUGENOB study: comparison of bioelectrical impedance vs. dual-energy X-ray absorptiometry. Diabetes Metab. 2011;37:222–229. doi: 10.1016/j.diabet.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Shafer KJ, Siders WA, Johnson LK, Lukaski HC. Validity of segmental multiple-frequency bioelectrical impedance analysis to estimate body composition of adults across a range of body mass indexes. Nutrition. 2009;25:25–32. doi: 10.1016/j.nut.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Linares CL, Ciangura C, Bouillot JL, Coupaye M, Decleves X, Poitou C, Basdevant A, Oppert JM. Validity of leg-to-leg bioelectrical impedance analysis to estimate body fat in obesity. Obes Surg. 2011;21:917–923. doi: 10.1007/s11695-010-0296-7. [DOI] [PubMed] [Google Scholar]
- 61.Pateyjohns IR, Brinkworth GD, Buckley JD, Noakes M, Clifton PM. Comparison of three bioelectrical impedance methods with DXA in overweight and obese men. Obesity (Silver Spring) 2006;14:2064–2070. doi: 10.1038/oby.2006.241. [DOI] [PubMed] [Google Scholar]