Abstract
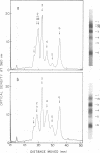
Polypeptides of egg-borne Sendai virus (egg Sendai), which is biologically active on the basis of criteria of the infectivity for L cells and of hemolytic and cell fusion activities, were compared by polyacrylamide gel electrophoresis with those of L cell-borne (L Sendai) and HeLa cell-borne Sendai (HeLa Sendai) viruses, which are judged biologically inactive by the above criteria. Densitometer profiles on the stained gels of egg Sendai resolved six polypeptides (virion protein [VP] 1 to VP6), in which VP2 and VP4 were identified as glycoproteins by PAS stain. Comparative electropherograms of both L Sendai and HeLa Sendai revealed that there were significantly larger amounts in the VP2 region of these viruses but VP4 was present only in greatly reduced amounts as compared to egg Sendai. It was also found that VP2 of L Sendai and HeLa Sendai consisted of two components, VP2a and VP2b, but the one of egg Sendai consisted of only VP2a. A mild trypsin treatment which converts both L Sendai and HeLa Sendai to a biologically active form selectively removed VP2b from these viruses and increased concomitantly the amounts of materials in the VP4 region. The same treatment of egg Sendai affected neither its biological activities nor its electropherogram. Consequently, gross polypeptide profiles on the stained gels of L Sendai and HeLa Sendai after trypsin treatment became favorably comparable to that of egg Sendai. Electrophoresis of labeled L Sendai and HeLa Sendai with a 3H-amino acids mixture and 14C-glucosamine resolved at least three glycoproteins, GP1, GP2, and GP3, each corresponding to VP2a, VP2b, and VP4, respectively. The trypsin treatment of these viruses removed almost all the radioactivity of GP2 and simultaneously increased the radioactive counts of GP3 and raised small amounts of rapidly moving heterogeneous glycoprotein, GP4. A possible relationship between the biological modification and the above characteristic polypeptide patterns of Sendai virus was discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bikel I., Duesberg P. H. Proteins of Newcastle disease virus and of the viral nucleocapsid. J Virol. 1969 Oct;4(4):388–393. doi: 10.1128/jvi.4.4.388-393.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliguiri L. A., Klenk H. D., Choppin P. W. The proteins of the parainfluenza virus SV5. 1. Separation of virion polypeptides by polyacrylamide gel electrophoresis. Virology. 1969 Nov;39(3):460–466. doi: 10.1016/0042-6822(69)90094-4. [DOI] [PubMed] [Google Scholar]
- Content J., Duesberg P. H. Electrophoretic distribution of the proteins and glycoproteins of influenza virus and Sendai virus. J Virol. 1970 Dec;6(6):707–716. doi: 10.1128/jvi.6.6.707-716.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAKE J. W., LAY P. A. Host-controlled variation in NDV. Virology. 1962 May;17:56–64. doi: 10.1016/0042-6822(62)90081-8. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Kingsbury D. W. Separation of Newcastle disease virus proteins by polyacrylamide gel electrophoresis. Virology. 1969 Apr;37(4):597–604. doi: 10.1016/0042-6822(69)90277-3. [DOI] [PubMed] [Google Scholar]
- HOMMA M. A particular binding of L cell-grown Sendal virus by host L cells. (Growth characteristics of myxoviruses in tissue culture. 5th). Tohoku J Exp Med. 1961 Feb 25;73:215–229. doi: 10.1620/tjem.73.215. [DOI] [PubMed] [Google Scholar]
- HOSAKA Y. Characteristics of growth of HVJ in PS cells. Biken J. 1962 Jun;5:121–126. [PubMed] [Google Scholar]
- Haslam E. A., Cheyne I. M., White D. O. The structural proteins of Newcastle disease virus. Virology. 1969 Sep;39(1):118–129. doi: 10.1016/0042-6822(69)90353-5. [DOI] [PubMed] [Google Scholar]
- Homma M., Tamagawa S. Restoration of the fusion activity of L cell-borne Sendai virus by trypsin. J Gen Virol. 1973 Jun;19(3):423–426. doi: 10.1099/0022-1317-19-3-423. [DOI] [PubMed] [Google Scholar]
- Homma M. Trypsin action on the growth of Sendai virus in tissue culture cells. I. Restoration of the infectivity for L cells by direct action of tyrpsin on L cell-borne Sendai virus. J Virol. 1971 Nov;8(5):619–629. doi: 10.1128/jvi.8.5.619-629.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M. Trypsin action on the growth of Sendai virus in tissue culture cells. II. Restoration of the hemolytic activity if L cell-borne Sendai virus by trypsin. J Virol. 1972 May;9(5):829–835. doi: 10.1128/jvi.9.5.829-835.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka Y., Shimizu Y. K. Artificial assembly of envelope particles of HVJ (Sendai virus). I. Assembly of hemolytic and fusion factors from envelopes solubilized by Nonidet P40. Virology. 1972 Sep;49(3):627–639. doi: 10.1016/0042-6822(72)90519-3. [DOI] [PubMed] [Google Scholar]
- ISHIDA N., HOMMA M. Host-controlled variation observed with Sendai virus grown in mouse fibroblast (L) cells. Virology. 1961 Aug;14:486–488. doi: 10.1016/0042-6822(61)90342-7. [DOI] [PubMed] [Google Scholar]
- Iinuma M., Yoshida T., Nagai Y., Maeno K., Matsumoto T. Subunits of NDV. Hemagglutinin and neuraminidase subunits of Newcastle disease virus. Virology. 1971 Dec;46(3):663–677. doi: 10.1016/0042-6822(71)90069-9. [DOI] [PubMed] [Google Scholar]
- Ito Y., Okazaki H., Sakuma S., Homma M., Ishida N. Specific requirement of serine for the growth of Newcastle disease virus. Virology. 1969 Oct;39(2):277–285. doi: 10.1016/0042-6822(69)90048-8. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Glycosphingolipids of plasma membranes of cultured cells and an enveloped virus (SV5) grown in these cells. Proc Natl Acad Sci U S A. 1970 May;66(1):57–64. doi: 10.1073/pnas.66.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Lipids of plasma membranes of monkey and hamster kidney cells and of parainfluenza virions grown in these cells. Virology. 1969 Jun;38(2):255–268. doi: 10.1016/0042-6822(69)90367-5. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO T., MAENO K. A host-induced modification of hemagglutinating virus of Japan (HVJ, Sendai virus) in its hemolytic and cytopathic activity. Virology. 1962 Aug;17:563–570. doi: 10.1016/0042-6822(62)90156-3. [DOI] [PubMed] [Google Scholar]
- Maeno K., Yoshida T., Iinuma M., Nagai Y., Matsumoto T. Isolation of hemagglutinin and neuraminidase subunits of hemagglutinating virus of Japan. J Virol. 1970 Oct;6(4):492–499. doi: 10.1128/jvi.6.4.492-499.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Caliguiri L. A., Choppin P. W. Nucleocapsid protein subunits of simian virus 5, Newcastle disease virus, and Sendai virus. J Virol. 1970 Nov;6(5):677–684. doi: 10.1128/jvi.6.5.677-684.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Choppin P. W. Proteins and glycoproteins of paramyxoviruses: a comparison of simian virus 5, Newcastle disease virus, and Sendai virus. J Virol. 1971 Jan;7(1):47–52. doi: 10.1128/jvi.7.1.47-52.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENBACK W. A., DURAND D. P. HOST INFLUENCE ON THE DENSITY OF NEWCASTLE DISEASE VIRUS (NDV). Virology. 1963 Aug;20:545–551. doi: 10.1016/0042-6822(63)90278-2. [DOI] [PubMed] [Google Scholar]
- Scheid A., Caliguiri L. A., Compans R. W., Choppin P. W. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972 Dec;50(3):640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Isolation and purification of the envelope proteins of Newcastle disease virus. J Virol. 1973 Feb;11(2):263–271. doi: 10.1128/jvi.11.2.263-271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Hosaka Y., Shimizu Y. K. Solubilization of envelopes of HVJ (Sendai virus) with alkali-emasol treatment and reassembly of envelope particles with removal of the detergent. J Virol. 1972 May;9(5):842–850. doi: 10.1128/jvi.9.5.842-850.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N. P., Ash R. J. Polykaryocyte induction by Newcastle disease virus propagated on different hosts. J Gen Virol. 1970 Apr;7(1):81–82. doi: 10.1099/0022-1317-7-1-81. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]