Abstract
Oral mucositis affects more than three-fourths of patients undergoing chemotherapy and represents a significant burden to patients and caregivers. Lesions develop as a result of chemotherapeutic agents attacking the rapidly dividing cells of the gastrointestinal tract. Severity can range from mild, painless tissue changes to bleeding ulcerations that prevent oral intake and require narcotic pain relievers. Oral mucositis also leads to an increased risk of infection and can often delay further chemotherapy treatment. A number of assessment scales have been developed to better qualify the symptoms associated with this condition. Few pharmacologic agents have been approved to either prevent the development or alleviate the symptoms of oral mucositis. Current options include the use of antimicrobial mouthwashes, amino acid rinses, and topical healing agents. Palifermin, a keratinocyte growth factor, may be a future option after its use in children is explored. With achievements in other areas of supportive care in patients undergoing chemotherapy, oral mucositis should represent the forefront of new research. This review will provide a comprehensive examination of available options for children who have oral mucositis.
INDEX TERMS: cancer, child, mucositis, stomatitis
INTRODUCTION
One of the often overlooked and under-researched complications of cancer treatment is oral mucositis. This condition can range from mild to severe and represents a common cause of dose reduction and treatment delays. Development of oral mucositis can also increase mortality by nearly 40% in severe cases.1 Oral mucositis can become a serious problem for any cancer patient, as it may necessitate the use of parenteral nutrition, can lead to infection, and results in pain and discomfort for the patient. With improvements in pharmaceutical products used to combat other side effects such as nausea and vomiting, oral mucositis is now receiving more attention and focus.
Up to 80% of children undergoing chemotherapy will experience some degree of mucositis, although the incidence of oral mucositis differs according to the type of cancer and treatment regimen.2 Children with hematologic malignancies experience mucositis more frequently than those with solid tumors. Furthermore, this group of patients is also more likely to have severe mucositis compared with patients suffering other malignancies.3 Up to 99% of patients undergoing bone marrow transplantation with myeloablative regimens will experience mucositis.4 It appears that the prevalence of mucositis in pediatric patients is even greater than in adults; this may be secondary to the more rapid cell division in this patient population.5 Inflammatory lesions associated with mucositis pose serious risks to pharmacologic treatment and care if chemotherapy protocols need to be altered or delayed. This review will include the majority of clinical trials carried out in the pediatric population, as well as some that are felt to be more significant in the adult population and that may offer additional treatment options for children.
PATHOPHYSIOLOGY
Chemotherapy and radiation-induced toxicity selectively affect rapidly dividing cells; therefore, the oral mucosa is very susceptible to damage by chemotherapy. Inflammation of the oral and gastrointestinal mucosa can lead to painful ulcerations, infections, and difficulty or inability to eat, drink, or swallow. Patients undergoing these types of treatments are more prone to cuts or scratches caused by chewing. Disruption of this mucosa breaks down one of the body's defenses against microbial invasion. The open sores in the mouth and weakened defenses against foreign invaders of chemotherapy-induced mucositis create an environment in which bacteria thrive. Chemotherapeutic medications that have been shown to lead to mucositis include fluorouracil (5- FU), doxorubicin, etoposide, and methotrexate.3,5
Recent research has shown that mucositis may not begin with direct epithelial cell damage but may be a complex process beginning in the submucosal endothelium and fibroblasts, with influence by local cytokines. Using electron microscopy, researchers have demonstrated that this damage occurs days before epithelial cell breakdown after acute radiation challenge in animals.6 Through a better understanding of the common intracellular pathway of endothelial cell apoptosis and the interactions between the apoptotic and inflammatory pathways, newer treatments are being developed.
Oral mucositis begins 3 to 10 days after chemotherapy is initiated and can persist for 3 weeks. It has been shown to peak at around 7 to 14 days, at which time it slowly resolves unless complicated by infection.7,8 Clinical improvement of mucositis seems to correlate with neutrophil recovery. While several regimens have been used in the pediatric population for prevention and treatment of this condition, none have emerged as the treatment of choice.
ASSESSMENT SCALES
Multiple assessment scales have been used to evaluate the extent of oral mucositis. Each takes similar criteria into account; however, grading between them varies greatly, making it difficult to accurately compare study results. Some of the more broad scales include the World Health Organization (WHO) Recommendations for Grading of Acute and Subacute Toxic Effects9 and the National Cancer Institute's Common Terminology Criteria for Adverse Events.10 The first considers the degree of soreness, erythema, and ability to eat, while the latter evaluates the severity of pain, change in oral intake, and death related to mucositis (Tables 1 and 2).9,10 The Oral Assessment Guide, developed by a group of nurses, rates the mucosa in 6 different areas (Table 3).11 It reviews the extent of mucositis in greater depth than other scales, and researchers have praised its ease of use, especially in the pediatric population. 2 Finally, many researchers choose to create their own scales to assess their patients. Toth et al12 developed a scale examining the degree of tissue changes, sensitivity, pain, and ability to eat (Table 4). This scale has been adopted by other researchers as well.12,13 Despite the number of scales available, however, some published studies do not specifically address the origin or describe the scale used. Individual institutions are encouraged to evaluate the scales and adopt one for consistent use.
Table 1.
Recommendations for Grading of Acute and Subacute Toxic Effects (World Health Organization)9
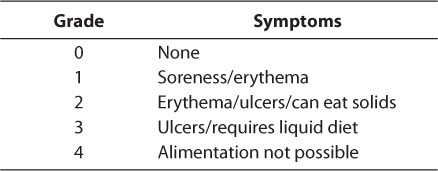
Table 2.
Common Terminology Criteria for Adverse Events, version 4.0 (National Cancer Institute)10
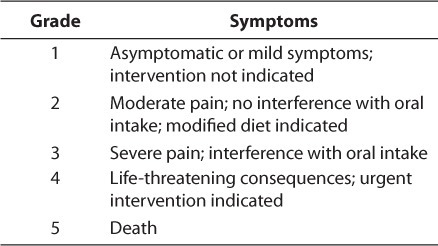
Table 3.
Oral Assessment Guide11
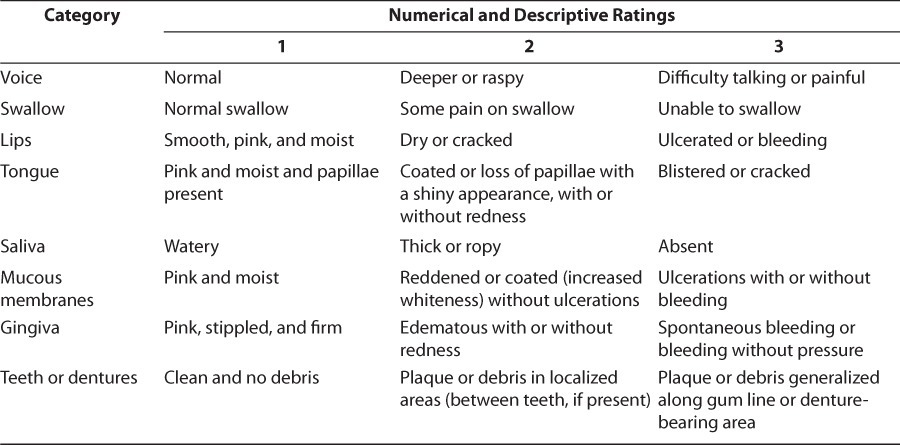
Table 4.
Mucositis Grading Developed by Toth et al12

PREVENTATIVE AND TREATMENT OPTIONS
A variety of non-pharmacological and pharmacological agents have been used in practice for the prevention and management of oral mucositis in children. There is no gold standard at this time, as there is a lack of evidence-based recommendations. A review of the literature identified the following approaches as credible options for the management of pediatric oral mucositis.
Oral Care Protocols
A standard first step to preventing mucosal injury is the implementation of good oral hygiene and the use of a standardized oral care protocol for all children undergoing chemotherapy. A dental consult is recommended prior to induction therapy if possible. A number of oral care protocols have been studied in the literature.
Cheng et al8 reported on the effectiveness of an oral care protocol for the prevention of chemotherapy-induced oral mucositis; the protocol was evaluated over an 8-month period in 42 pediatric cancer patients who ranged in age from 6 to 17 years. The experimental group consisted of 21 children who were instructed in the proper technique of toothbrushing; they were also given a 0.2% chlorhexidine mouth rinse, which was used twice a day, and a 0.9% saline rinse, which was used in the morning, after each meal, and before going to bed. Another 21 patients made up the control group and did not receive the oral care protocol intervention or information concerning the importance of oral care. If oral lesions developed, these patients were treated with symptomatic measures, including the use of 0.9% sodium chloride solution and benzydamine hydrochloride rinse to control the pain. The results obtained from this study were significant and demonstrated a 38% reduction in the incidence of ulcerative mucositis in the experimental group (p=0.01). Furthermore, the severity and related pain were considerably reduced in the experimental group throughout the study. Of note, in the United States, chlorhexidine oral rinse is available only as a 0.12% solution. Normally used for gingival infections, this formulation has also demonstrated success when used with oral care protocols.13,14
De Brito Costa et al14 evaluated 14 children, whose ages ranged from 2 to 10 years, who were receiving intensive chemotherapy for treatment of acute lymphoblastic leukemia. They received a chemotherapy regimen consisting of 6-mercaptopurine (oral dose of 50 mg/m2/day over 6 weeks); methotrexate (intravenous dose of 2 mg/ m2 as a continuous infusion for 24 hours on days 1, 15, 30, and 45); leucovorin (oral dose of 15 mg/ m2 4 times per day, on days 2, 3, 16, 17, 31, 32, 46, and 47); and MADIT intrathecal (a combination of methotrexate 12 mg, cytosine arabinoside 70 mg, and dexamethasone 2 mg/m2) on days 1, 15, 30, and 45. None of the children had any clinical signs of oral or esophageal candidiasis at the start of the study, and none had any other complications of the oral mucosa before beginning chemotherapy or the study's oral protocol. The oral protocol was used during the intensification period of the chemotherapy. It began at least 1 day before initiating the chemotherapy and ended 10 days after the end of this period. The average length of treatment was 8 weeks. The treatment group consisted of 7 patients who received a mouth rinse with a non-alcoholic solution of 0.12% chlorhexidine and oral hygiene care, including twice-daily toothbrushing (in the morning and evening) supervised by guardians and/or dentists. The control group followed the same protocol for brushing their teeth but was given a placebo mouth rinse. The results were considered statistically significant. Only 1 child from the treatment group, compared to 5 children in the control group, developed oral mucositis. The oral lesions were less severe and of a shorter duration in the children who received chlorhexidine mouth rinses compared to the control group, although the authors did not give specific values. The authors cited limited numbers of participants as a weakness; the results are nevertheless promising.
Levy-Polack et al13 evaluated a preventive protocol for oral complications associated with acute leukemia. A control group of 60 Caucasian children already undergoing chemotherapy who received only palliative treatment for complications was compared with a treatment group of 36 Caucasian children (ages 1 to 16) with newly diagnosed acute lymphoblastic leukemia receiving a daily mouth care protocol for a 13-month period. The protocol consisted of the following: a mouthwash with sodium bicarbonate and water after every meal; a mouthwash with a nonalcoholic solution of chlorhexidine (0.12%) twice a day; cleaning of mucosa with gauze soaked in iodopovidone 4 times a day prior to the use of nystatin; “swishing and swallowing” with nystatin (500,000 units oral suspension prepared with sorbitol) 4 times a day; and a daily rinse with sodium fluoride 0.05%. Postintervention, the control group showed a 68.2% incidence of poor oral hygiene versus 51.6% in the experimental group (p<0.001), a 28.2% incidence of candidasis versus 16.1% in the experimental group (p<0.009), and a 10.75% incidence of oral bleeding versus 5.1% in the experimental group (p=0.08), respectively. Furthermore, the rate and severity of mucositis were higher in the control group, with 30.2% of the control patients versus 21.9% of the experimental patients exhibiting oral mucositis (p=0.1). The severity of the disease was graded on the basis of the clinical appearance of the mucosa according to a scale similar to the WHO scale.12 In the control group, 58.7% of the patients were grade I, 33.3% were grade II, and 8% were grade III. Those figures—compared to the experimental group of 85% grade I, 6% grade II, and 9% grade III—shows that this protocol, while time consuming, may have been useful in limiting the incidence and severity of oral mucositis.
Topical Agents
Chlorhexidine and Benzydamine
Two specific oral rinse agents have been compared in clinical trials: chlorhexidine and benzydamine. Chlorhexidine mouthwash has been widely used as an antimicrobial compound and topical prophylactic agent against oral mucositis and candidiasis.14 The bactericidal effect of this mouthwash is attributed to the binding of the cationic molecule to negatively charged bacterial cell walls and extramicrobial complexes. It is active against Gram-positive and Gram-negative organisms, anaerobes, facultative anaerobes, and yeast.15 Chlorhexidine plays an integral role in many of the oral care protocols used for the prevention of oral mucositis. Benzydamine is commonly used outside the United States for the treatment of oral mucositis and pharyngitis. It acts as a local anesthetic and anti-inflammatory agent that possesses no antimicrobial activity.15
Cheng and Chang16 conducted a randomized 2-period crossover study that compared the efficacy of 0.15% benzydamine and 0.2% chlorhexidine mouthwashes in alleviating the symptoms of oral mucositis in children undergoing chemotherapy. Forty pediatric patients ages 6 to 17 years were randomized into groups receiving 1 of the 2 mouthwashes. Each protocol was started on the first day of chemotherapy and continued for 21 days. Each subject was evaluated at intervals of 3 to 4 days using the WHO scale (Table 1) for mucositis and a 10-cm visual analog scale to evaluate oral symptoms. Of the 34 patients who were evaluated, 26% of the chlorhexidine group compared to 48% of the benzydamine group showed WHO grade II mucositis (p<0.05). The results revealed a significant difference in mouth pain (p<0.05) and a trend of decreased difficulty in eating/chewing and swallowing in favor of chlorhexidine.
Cheng et al2 performed a prospective, randomized, and non-blinded 2-period crossover study with continuous sequential analysis; they included 40 children between the ages of 6 and 17 who had received 2 cycles of combination or high-dose chemotherapy. While the dosing and treatment regimens of chemotherapy used for hematological malignancies varied, they included methotrexate, cisplatin, 5-FU, cytarabine, doxorubicin, vincristine, daunorubicin, cyclophosphamide, etoposide, and combinations of these agents. The patients were randomly assigned to receive either benzydamine or chlorhexidine mouth rinses upon their first chemotherapy cycle. The researchers were unable to blind the patients, as the oral rinses had distinct colors and tastes. After 1 to 2 weeks, the patients were switched to the other protocol for their next cycle of chemotherapy. Also included in the study was a “wash-out” period of 1 to 2 weeks between chemotherapy cycles. All patients were put on a protocol that consisted of brushing their teeth using the Bass method (a method that concentrates on cleaning the gum margins), mouth rinsing using one of the 2 rinses in the morning and at bedtime, and normal saline rinsing within 30 minutes of meals and every 4 hours in the first and third weeks and every 2 hours in the second week after chemotherapy. Of the 34 children who completed the study, fewer chlorhexidine patients developed ulcerative lesions compared with those using benzydamine (27% and 59%, respectively). Similarly, when the 2 mouthwashes were compared patient by patient, the chlorhexidine significantly reduced the severity of mucositis (p<0.05).
GLUTAMINE
The amino acid glutamine serves a variety of purposes throughout the body. During times of stress, including cancer, glutamine stores can decrease by over 50%, contributing to the development of oral mucositis.17 Use of supplemental glutamine has been said to regulate gastrointestinal cell growth, function, and regeneration.15 Few studies exist regarding potential drug interactions. Animal studies and 1 small study in humans demonstrated an increase in tumoricidal activity when glutamine was given with methotrexate; the associated toxicities were actually decreased versus subjects who received methotrexate alone.18,19 Potential interactions between glutamine and chemotherapeutic agents should be monitored closely.
A randomized, double-blinded crossover trial in cancer patients with previous mouth pain was performed20 following a pilot study21 that displayed a reduction in the severity and duration of oral mucositis. Thirteen patients completed the study; they had to receive 2 or more identical chemotherapy courses. The ages of the 10 pediatric patients ranged from 4 to 17 years; the other 3 patients were adults. Patients received either glutamine or glycine placebo suspension; they swished and swallowed twice daily beginning on the day chemotherapy was started and continued this regimen for at least 14 days. Using a questionnaire and calendar, patients reported their symptoms daily, including the ability to eat or drink. Researchers found that glutamine decreased the duration of mucositis by 4.5 days compared with placebo (p=0.005). Additionally, glutamine lessened the pain associated with mucositis, specifically pain that altered eating habits (p=0.02). Investigators described glutamine as “nearly without taste” and suspended it in a sucrose vehicle. Children found this easy to use, while adolescents and adults described it as too sweet. A second study evaluated mucositis occurring during bone marrow transplantation.22 A similar symptom scale was used; in addition, opiate use was used as an objective measurement of effectiveness. In patients undergoing autologous transplants, mouth pain and difficulty in eating were decreased in those who received glutamate compared with those who received placebo (p=0.05). However, in patients receiving allogeneic transplants, no difference was noted between the 2 groups. When looking at the use of opiates, the patients undergoing autologous transplants and receiving glutamine were less likely to require opiates than those receiving placebo (p=0.04).
ORAL SUCRALFATE SUSPENSION
Sucralfate, commonly used for gastric ulcer prophylaxis and treatment, has been used off-label for treatment of oral mucositis following chemotherapy. Its mechanism of action is not clear, but it is believed that sucralfate binds to ulcerations, creating a protective barrier to allow healing, similar to the action displayed in the stomach.15
Shenep and colleagues23 sought to determine the efficacy of orally administered sucralfate suspension in preventing and treating chemotherapy- induced mucositis. They conducted a double-blinded, placebo-controlled trial that included 48 children and adolescents with newly diagnosed acute non-lymphocytic leukemia. Patients were randomized to receive suspensions of either sucralfate or a similar oral placebo given once every 6 hours during the first 10 weeks of chemotherapy. When the groups were compared, a significant difference was found in the percent colonization with potentially pathogenic microorganisms. The treatment group had a 58% colonization rate, versus the control group's 92% (p=0.008); however, no effect on baseline colonization was observed. No oral pain was reported in 58% of patients receiving sucralfate, compared to 25% receiving placebo. Mucositis was graded using a scale ranging from normal mucosa to severe ulceration. The investigators used this scale, subjective reporting of discomfort, and the maximal percent of body weight lost during therapy; they found that all measures were similar between groups. While the researchers concluded that sucralfate suspension was of limited efficacy in the treatment of chemotherapy-induced mucositis, they did deduce that its administration could decrease the colonization of pathogens, possibly by interfering with their attachment to mucosal membranes.
SUPERSATURATED CALCIUM PHOSPHATE RINSE
One option specifically studied in patients undergoing stem cell transplants is supersaturated calcium phosphate rinse (Caphosol, EUSAPharma [USA] Inc, Langhorne, PA). This vanilla-flavored product consists of 1 ampule containing dibasic sodium phosphate and monobasic sodium phosphate and a second ampule with calcium chloride and sodium chloride. The 2 compounds are mixed, and the patient swishes and expectorates 4 times daily to prevent the development of mucositis.24 Categorized as a saliva substitute, Caphosol is thought to maintain a moist and clean environment.25 At this time, no study has evaluated the use of supersaturated calcium phosphate in children. A randomized, placebo-controlled trial is underway following children ages 4 through 12 years.26
Researchers assessed the effectiveness of Caphosol in 32 adult patients scheduled to undergo hematopoietic stem cell transplant by comparison with 24 control subjects transplanted prior to Caphosol's availability.25 Each patient in the treatment group received Caphosol 4 times daily from the day before chemotherapy started to the end of hospitalization or death. The groups were stratified by chemotherapy regimen and received either BEAM (carmustine, etoposide, cytarabine, and melphalan) or melphalan only (200 mg/m2). Severity was graded with the WHO scale. No patient in the BEAM group who received Caphosol experienced greater than grade II mucositis. Control patients were more likely to experience grade III or grade IV mucositis (p<0.05) and have a longer duration of symptoms (8.6 ± 2.45 vs 2.25 ± 2.7 days in control and Caphosol groups, respectively; p<0.001). However, in the patients treated with melphalan only, no difference was seen in the severity or duration of oral mucositis between groups. No adverse events were noted, although 1 patient did not like the taste of Caphosol. Researchers concluded that Caphosol should be an option for patients receiving high-dose chemotherapy, but in high-dose melphalan regimens, another preventative measure should be used.
TOBRAMYCIN, POLYMYXIN E, AND AMPHOTERICIN
A study performed by Spijkervet et al used a combination of tobramycin, polymyxin E, and amphotericin in an attempt to eradicate the Gram-negative organisms that are present in normal oral flora.27 Elimination of Gram-negative bacteria is thought to decrease the incidence and severity of oral mucositis, as this bacteria may release endotoxins causing inflammation. Amphotericin was added to prevent yeast growth. Fifteen adults with head and neck cancers received lozenges containing 2 mg polymyxin E, 1.8 mg tobramycin, and 10 mg amphotericin B 4 times daily during their 5-week course of radiation. The outcomes were compared with the results of a previous double-blinded, placebocontrolled trial evaluating the effectiveness of chlorhexidine 0.1% rinse to prevent mucositis. Patients receiving the lozenges experienced erythema but no ulcerations. No patients required nasogastric feeding or experienced candidiasis. Patients receiving placebo or chlorhexidine had significantly higher mucositis scores, determined by the type and extent of mucositis symptoms (p<0.05). This study indicates that oral decontamination could potentially be used to prevent the development of oral ulcerations.
PALIFERMIN
Palifermin is the newest agent to be approved to prevent the development of mucositis. As a recombinant keratinocyte growth factor, palifermin stimulates the proliferation, differentiation, and migration of epithelial cells throughout the gastrointestinal tract. Currently, palifermin is approved by the U.S. Food and Drug Administration only for patients with hematologic malignancies receiving myeloablation in preparation for stem cell transplants. The dosing regimen consists of 60 mcg/kg/day for 3 consecutive days before and after the myeloablative therapy. 15 Studies performed in pediatric patients are sparse, although a dose-escalation study is currently being performed in those undergoing hematopoietic stem cell transplants.28
Blazar et al29 examined the utility of palifermin in preventing graft-versus-host disease in 100 patients ages 7 to 65 years (median age, 46). Further investigations regarding safety and efficacy among pediatric patients specifically were not performed. Several dosing strategies were explored. Doses of either 40 or 60 mcg/kg/day were given for 3 days prior to myeloablative regimens. Following myeloablation, palifermin was given for 3, 6, or 9 days, depending upon treatment arm. While palifermin did not demonstrate clear benefits in these patients, the safety profile in palifermin-treated patients was somewhat favorable. Skin rash was the only adverse event that occurred more frequently in the palifermin group than in the placebo group (94% vs 68%; p<0.01). Of note, 11 dose-limiting toxicities occurred among 6 patients, 4 of whom were in the treatment group. Each incidence of toxicity occurred after 8 days of treatment, possibly indicating that extended duration of palifermin treatment should be studied more extensively before implemented.
Vadhan-Raj et al30 evaluated adult and pediatric patients diagnosed with sarcoma who received multiple-cycle doxorubicin-based chemotherapy. Patient ages ranged from 15 to 64 years old (median ages, 39 and 42 years in the treatment and placebo groups, respectively); again, researchers did not report separate findings for pediatric patients. Forty-eight patients were randomized in a 2:1 fashion to receive 180 mcg/kg palifermin or placebo once daily for 3 days prior to each chemotherapy cycle. Patients were instructed to rinse their mouths 5 times daily with a salt and soda solution but were not permitted to use any other preventative therapies. Eighty-eight percent of the patients receiving placebo experienced grade II or higher mucositis, compared to only 44% of the palifermin-treated patients (p<0.001). Opioid use was also decreased in the palifermintreated group, who received an average of 28 mg of morphine equivalents per cycle, compared to 161 mg in the placebo group (p=0.013). Thickening of the tongue and oral mucosa was seen more commonly in the treatment group, although it was transient (72% vs 23% in the placebo and palifermin groups, respectively; p=0.007).
Researchers randomized 212 adult patients undergoing total-body irradiation and high-dose chemotherapy in preparation for an autologous stem cell transplant.31 Chemotherapy included etoposide or cyclophosphamide. Patients received either palifermin 60 mcg/kg/day or placebo for 3 days before and 3 days after treatment. The primary endpoint, duration of grade III or IV oral mucositis (primarily assessed by the WHO scale), decreased with palifermin treatment: 9 days in the placebo group vs 6 days in the palifermin group (p<0.001). Additionally, only 63% of treated patients experienced grade III or IV oral mucositis, compared with 98% of the control group patients (p<0.001). Palifermin-treated patients required less parenteral or transdermal analgesia, developed febrile neutropenia less frequently, and required total parenteral nutrition less often (p<0.001). The more common adverse drug reactions in the palifermin group included rash, pruritis, erythema, paresthesia, and taste alterations; however, each was transient and not severe enough to prompt discontinuation of treatment.
DISCUSSION
Oral mucositis is a common condition associated with cancer treatment that does not have definitive guidelines for treatment in the pediatric population. It is an especially important issue, as it has been shown that children may be at higher risk for mucositis than adults.16 Poor control and prevention of mucositis in pediatric patients may have a detrimental outcome on growth and nutrition and may necessitate delays in chemotherapy treatment. While there are many published reports on the prevention and treatment of this condition, none have shown results that have designated them as the treatment of choice. Large, well-controlled studies in pediatric patients are lacking.
Prevention of mucositis should be a goal for all health care providers. Before initiating chemotherapy, all newly diagnosed children with cancer should receive an evaluation of their oral cavity, including the baseline condition of their teeth and gums. Ideally, a pediatric dental team should be included for oral examinations with the patient. These examinations should become routine, with the frequency determined by the toxicity of the treatment and the patient's baseline oral status.4 Because children with poor oral hygiene have been shown to be at increased risk of oral mucositis, brushing and flossing techniques should be evaluated and a complete dental history should be recorded.4 Children should be instructed to brush their teeth with fluoride toothpaste after each meal and at bedtime, along with flossing once a day. The tongue should be gently cleaned with a toothbrush or tongue scraper. For those on intensive chemotherapy, chlorhexidine gluconate mouthwash (0.12% or 0.2%) has been shown to decrease the severity and duration of oral mucositis. While there is variability in study findings of the significance of chlorhexidine mouthwash in the adult population, the lack of availability of product and pediatric literature on benzydamine in the United States makes chlorhexidine the more logical addition to an oral prevention protocol. Most trials have used chlorhexidine mouth rinse twice a day along with a 0.9% saline rinse in the morning, before going to bed, and after each meal. These studies are aimed at children 6 years of age and older who are able to swish, swallow, and spit. Younger children unable to perform these tasks should have these solutions applied to the oral mucosa with a soft cloth.
Not all mucositis can be prevented. Once mucositis has developed, therapy should focus on supportive care. Goals are to maintain hydration, provide appropriate caloric intake through enteral or parenteral nutrition support, and relieve pain and prevent infection. Mucositis severity should be graded using a validated scale, such as the WHO scale, prior to initiating supportive therapy, to establish a baseline. Patients with grade I and grade II mucositis should brush their teeth as described earlier with a soft toothbrush and fluoride toothpaste and rinse with a salt and bicarbonate solution. With grade III mucositis, children should be advised to clean the oral cavity 4 times a day as described earlier or with gauze dipped in a salt and bicarbonate solution. The salt and bicarbonate solutions should be used every 4 to 6 hours if possible. Grade IV mucositis requires the patient to cleanse the oral cavity 4 times a day with either a soft toothbrush or gauze and to use a salt and bicarbonate solution every 4 hours.5
When treating pain associated with oral mucositis, the health care provider should use a stepwise approach similar to the WHO analgesic ladder. Recently published practice guidelines recommend patient-controlled analgesia with morphine for treatment of the pain associated with oral mucositis in patients undergoing hematopoietic stem cell transplantation.32 This technique should be explored in children of an appropriate age and knowledge base.
When approaching analgesic therapy, clinicians should focus on providing adequate pain control to the patient. The amount of pain medication may not correlate with the grade of mucositis. The stepwise approach should begin with oral rinses (saline solution, sodium bicarbonate rinses, etc); topical anesthetics (lidocaine, benzocaine); combination mouthwashes (“magic mouth rinse” containing diphenhydramine, lidocaine, and combinations of aluminum hydroxide, magnesium hydroxide, and simethicone); and possibly mucosal surface protectants such as hydroxypropyl cellulose gels or sucralfate solutions. When these medications do not provide adequate relief, a step-up approach to systemic analgesics is warranted.33 The use of opioids should parallel the WHO analgesic ladder, with those opioids used for mild to moderate pain being used first and then switching to the medications for moderate to severe pain, if needed (Table 5)34. The route of administration, dosage, and treatment of side effects associated with opioids should be done on a patient-specific basis.
Table 5.
WHO 3-Step Analgesic Ladder34

Adequate hydration and nutrition should be maintained in every pediatric patient. The oral route should be used whenever possible, although this becomes difficult with progressing mucositis. Because children have fewer caloric stores and a higher metabolic rate than adults, they are unable to tolerate inadequate nutrition for long. Practitioners should be aware of patients' nutritional status and may initiate total parenteral nutrition sooner than in those who are not experiencing mucositis. Guidelines published by the American Society for Parenteral and Enteral Nutrition35 state that parenteral nutrition should be considered in children who cannot maintain adequate nutritional intake orally or enterally for 5 to 7 days. However, the potential risks of parenteral nutrition, including increased risks of infection, electrolyte abnormalities, and cholestatic liver disease, must be taken into account.35
Prevention of secondary infections is a key issue in severe mucositis treatment with neutropenic pediatric patients. The oral cavity should be kept as clean as possible. When a bacterial infection is suspected, the patient should be treated empirically with broad-spectrum systemic antibiotic medication. In patients with a history of recurrent oral candidal lesions, nystatin suspension or fluconazole may be used prophylactically.4
CONCLUSION
Evidence of effective pharmacotherapy for the treatment of oral mucositis in pediatrics is lacking. While it appears that there are a few new medications that could have a positive role, there are limited clinical trials in adults and even fewer in children. Some medications, such as palifermin, a recombinant human keratinocyte growth factor, have been shown to be of benefit in adults, but studies in children are ongoing. The superiority of any specific agent or treatment protocol for prevention and/or treatment of oral mucositis in children has not been established. A multimodal approach of appropriate oral cavity care, topical rinses, and rapid identification and supportive care of mucositis may help to decrease the duration and severity of serious oral mucositis.
ACKNOWLEDGMENT
At the time of manuscript preparation, Dr Miller was a PGY2 Pediatric Pharmacy Resident at the University of Oklahoma College of Pharmacy, Oklahoma City, Oklahoma.
ABBREVIATIONS
- BEAM
carmustine, cytarabine, etoposide, melphalan
- 5-FU
5-fluorouracil
- WHO
World Health Organization
Footnotes
DISCLOSURES The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Sonis ST. Oral mucositis in cancer therapy. J Support Oncol. 2004;2(6 suppl 3):3–8. [PubMed] [Google Scholar]
- 2.Cheng KK, Chang AM, Yuen MP. Prevention of oral mucositis in paediatric patients treated with chemotherapy; a randomised crossover trial comparing two protocols of oral care. Eur J Cancer. 2004;40(8):1208–1216. doi: 10.1016/j.ejca.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Otmani N, Alami R, Hessissen L. Determinants of severe oral mucositis in paediatric cancer patients: a prospective study. Int J Paediatr Dent. 2011;21(3):210–216. doi: 10.1111/j.1365-263X.2011.01113.x. et al. [DOI] [PubMed] [Google Scholar]
- 4.Cheng KK. Oral mucositis, dysfunction, and distress in patients undergoing cancer therapy. J Clin Nurs. 2007;16(11):2114–2121. doi: 10.1111/j.1365-2702.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy L, Diamond J. Assessment and management of chemotherapy-induced mucositis in children. J Pediatr Oncol Nurs. 1997;14(3):164–174. doi: 10.1016/s1043-4542(97)90052-7. [DOI] [PubMed] [Google Scholar]
- 6.Hofmeister CC, Stiff PJ. Mucosal protection by cytokines. Curr Hematol Rep. 2005;4(6):446–453. [PubMed] [Google Scholar]
- 7.Medina PJ, Shord SS. Cancer treatment and chemotherapy. In: Talbert RL, DiPiro JT, Matzke GR, editors. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York: McGraw-Hill; 2011. et al, eds. [Google Scholar]
- 8.Cheng KK, Molassiotis A, Chang AM. Evaluation of an oral care protocol intervention in the prevention of chemotherapyinduced oral mucositis in paediatric cancer patients. Eur J Cancer. 2001;37(16):2056–2063. doi: 10.1016/s0959-8049(01)00098-3. et al. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Handbook for Reporting Results of Cancer Treatment. Geneva, Switzerland: World Health Organization; 1979. pp. 15–22. [Google Scholar]
- 10.Common Terminology Criteria for Adverse Events, version 4.0. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2010. Cancer Therapy Evaluation Program. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. [Google Scholar]
- 11.Eilers J, Berger AM, Peterson MC. Development, testing, and application of the Oral Assessment Guide. Oncol Nurs Forum. 1998;15(3):325–330. [PubMed] [Google Scholar]
- 12.Toth BB, Martin JW, Fleming TJ. Oral complications associated with cancer therapy. An M.D. Anderson Cancer Center Experience. J Clin Periodontol. 1990;17(7, part 2):508–515. doi: 10.1111/j.1365-2710.1992.tb01225.x. [DOI] [PubMed] [Google Scholar]
- 13.Levy-Polack MP, Sebelli P, Polack NL. Incidence of oral complications and application of a preventive protocol in children with acute leukemia. Spec Care Dent. 1998;18(5):189–193. doi: 10.1111/j.1754-4505.1998.tb01738.x. [DOI] [PubMed] [Google Scholar]
- 14.de Brito Costa EM, Fernandes MZ, Quinder LB. Evaluation of an oral preventive protocol in children with acute lymphoblastic leukemia. Pesqui Odontol Bras. 2003;17(2):147–150. doi: 10.1590/s1517-74912003000200009. et al. [DOI] [PubMed] [Google Scholar]
- 15.Lexi-Comp Online. Pediatric Lexi-Drugs Online. Hudson, Ohio: Lexi-Comp, Inc; 2011, August 12. [Google Scholar]
- 16.Cheng KK, Chang AM. Palliation of oral mucositis symptoms in pediatric patients treated with cancer chemotherapy. Cancer Nurs. 2003;26(6):476–484. doi: 10.1097/00002820-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Storey B. The role of oral glutamine in pediatric bone marrow transplant. J Pediatr Oncol Nurs. 2007;24(1):41–45. doi: 10.1177/1043454206296032. [DOI] [PubMed] [Google Scholar]
- 18.Rubio IT, Cao Y, Hutchins LF. Effect of glutamine on methotrexate efficacy and toxicity. Ann Surg. 1998;227(5):772–780. doi: 10.1097/00000658-199805000-00018. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klimberg SV, Nwokedi E, Hutchins LF. Glutamine facilitates chemotherapy while reducing toxicity. J Parenter Enteral Nutr. 1992;16(6S):83–87. doi: 10.1177/014860719201600609. et al. [DOI] [PubMed] [Google Scholar]
- 20.Anderson PM, Schroeder G, Skubitz KM. Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer. 1998;83(7):1433–1499. doi: 10.1002/(sici)1097-0142(19981001)83:7<1433::aid-cncr22>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Skubitz KM, Anderson PM. Oral glutamine to prevent chemotherapy induced stomatitis: a pilot study. J Lab Clin Med. 1996;127(2):223–228. doi: 10.1016/s0022-2143(96)90082-7. [DOI] [PubMed] [Google Scholar]
- 22.Anderson PM, Ramsay NKC, Shu XO. Effect of low-dose oral glutamine on painful stomatitis during bone marrow transplantation. Bone Marrow Transplant. 1998;22(4):339–344. doi: 10.1038/sj.bmt.1701317. et al. [DOI] [PubMed] [Google Scholar]
- 23.Shenep JL, Kalwinsky DK, Hutson PR. Efficacy of oral sucralfate suspension in prevention and treatment of chemotherapy- induced mucositis. J Pediatr. 1988;113(4):758–763. doi: 10.1016/s0022-3476(88)80397-4. et al. [DOI] [PubMed] [Google Scholar]
- 24.Caphosol. Drug Facts and Comparisons [online] 2012. Available from Wolters Kluwer Health, Inc. Accessed August 5, 2012. http://online.factsandcomparisons.com/MonoDisp.aspx?monoID=fandc-hcp14030&quick=244598%7c5&search=244598%7c5&isstemmed=True&fromTop=true#firstMatch.
- 25.Wasko-Grabowska A, Rzepecki P, Oborska S. Efficiency of supersaturated calcium phosphate mouth rinse treatment in patients receiving high-dose melphalan or BEAM prior to autologous blood stem cell transplantation: a single-center experience. Transplant Proc. 2011;43(8):3111–3113. doi: 10.1016/j.transproceed.2011.08.053. et al. [DOI] [PubMed] [Google Scholar]
- 26.Children's Oncology Group. Supersaturated calcium phosphate rinse in preventing oral mucositis in young patients undergoing autologous or donor stem cell transplant. http://clinicaltrials.gov/ct2/show/NCT01305200. Updated 9/12/2012. Accessed 9/26/2012.
- 27.Spijkervet FKL, van Saene HKF, van Saene JJM. Mucositis prevention by selective elimination of oral flora in irradiated head and neck cancer patients. J Oral Pathol Med. 1990;19(10):486–489. doi: 10.1111/j.1600-0714.1990.tb00792.x. et al. [DOI] [PubMed] [Google Scholar]
- 28.St. Jude Children's Research Hospital. Dose escalation study of palifermin in pediatric research participants undergoing allogeneic hematopoietic stem cell transplantation. http://clinicaltrials.gov/ct2/show/results/NCT00701688. Updated 8/14/2011. Accessed 8/15/2011.
- 29.Blazar BR, Weisdorf DJ, DeFor T. Phase 1/2 randomized, placebo-control trial of palifermin to prevent graft-versus-hostdisease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT) Blood. 2006;108(9):3216–3222. doi: 10.1182/blood-2006-04-017780. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vadhan-Raj S, Trent J, Patel S. Singledose palifermin prevents severe oral mucositis during multicycle chemotherapy in patients with cancer. Ann Intern Med. 2010;153(6):358–367. doi: 10.7326/0003-4819-153-6-201009210-00003. et al. [DOI] [PubMed] [Google Scholar]
- 31.Spielberger R, Stiff P, Bensinger W. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351(25):2590–2598. doi: 10.1056/NEJMoa040125. et al. [DOI] [PubMed] [Google Scholar]
- 32.Keefe DM, Schubert MM, Elting LS. Mucositis Study Section of the Multinational Association of Supportive Care in Cancer, International Society for Oral Oncology. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109(5):820–831. doi: 10.1002/cncr.22484. et al. [DOI] [PubMed] [Google Scholar]
- 33.Epstein JB, Schubert MM. Managing pain in mucositis. Semin Oncol Nurs. 2004;20(1):30–37. doi: 10.1053/j.soncn.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Cancer Pain Relief with a Guide to Opioid Availability. 2nd ed. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 35.A.S.P.E.N. Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nut. 2002;26(1)(suppl):1SA–138SA. [PubMed] [Google Scholar]


