Abstract
Attention to comfort and pain control are essential components of neonatal intensive care. Preterm neonates are uniquely susceptible to pain and agitation, and these exposures have a negative impact on brain development. In preterm neonates, chronic pain and agitation are common adverse effects of mechanical ventilation, and opiates or benzodiazepines are the pharmacologic agents most often used for treatment. Questions remain regarding the efficacy, safety, and neurodevelopmental impact of these therapies. Both preclinical and clinical data suggest troubling adverse drug reactions and the potential for adverse longterm neurodevelopmental impact. The negative impacts of standard pharmacologic agents suggest that alternative agents should be investigated. Dexmedetomidine is a promising alternative therapy that requires further interprofessional and multidisciplinary research in this population.
INDEX TERMS: mechanical ventilation, neonate, neurodevelopment, preterm, sedation
INTRODUCTION
Prior to the 1980s, neonatal pain was poorly understood and often unrecognized. Throughout the latter half of the 20th century, research emerged describing the developmental physiology of nociception and adverse responses of neonates to noxious stimuli.1,2 This work clearly shows that nociception occurs in neonates, even those at the lower limit of viability. However, despite early maturation of the ascending neural pathways responsible for nociception, the descending inhibitory pathways, which localize and mitigate pain, do not form until later in maturation.3 In addition, rapid and critical development of the nervous system occurs during the period of gestation interrupted by preterm birth.4 This results in a unique susceptibility to neurologic remodeling after repetitive noxious stimuli.5
Despite significant advances in neonatal intensive care, poor neurodevelopmental outcomes in former preterm neonates remain common.6 Preclinical studies have extensively demonstrated the negative consequences of untreated pain and stress on neurologic development. For both practical and ethical reasons, human data are less robust. However, numerous retrospective studies7 suggest negative consequences of pain and stress during the newborn period on long-term neurodevelopment and neurobehavior.
Despite increased understanding of the developmental physiology and long-term consequences of neonatal pain and agitation, there is no consensus regarding a safe and effective strategy for controlling these complications in many routine clinical situations. Mechanical ventilation is a common stressful experience in preterm neonates.8 Non-pharmacologic therapies, including non-nutritive sucking and swaddling, form the foundation of neonatal pain and agitation relief, but they are likely inadequate alone to provide comfort during invasive ventilation.9 Routine administration of pharmacologic sedation or analgesia during mechanical ventilation in preterm neonates is not recommended due to concerns regarding the safety and efficacy of pharmacotherapy observed in clinical trials.10 However, the use of benzodiazepines and opiates in clinical practice remains common due to the lack of available alternative therapies.11,12 This review summarizes the available data regarding the clinical and neurodevelopmental impact of these agents in preterm neonates (Tables 1 and 2, respectively). Dexmedetomidine, one potential therapeutic alternative, is explored.
Table 1.
Advantages and Disadvantages of Available Agents in Preterm Neonates
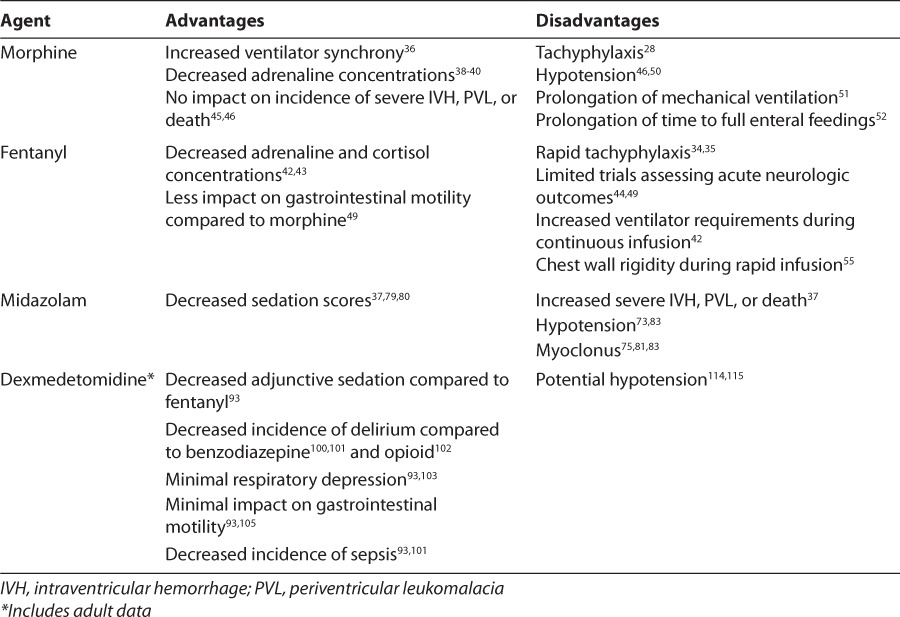
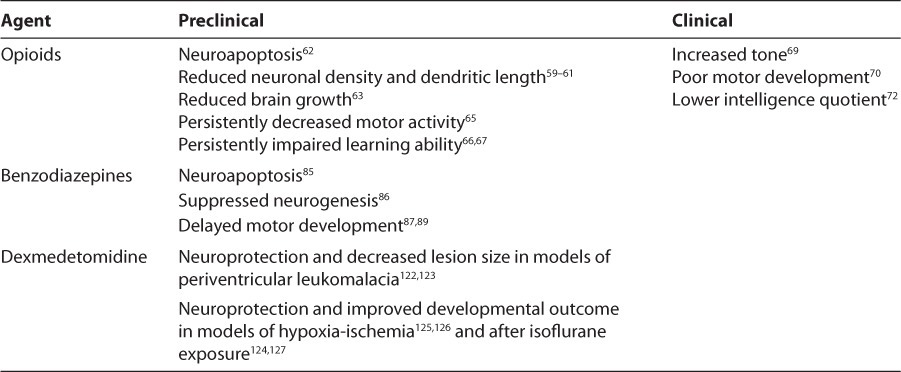
Table 2.
Neurodevelopmental Impact of Available Agents
BACKGROUND
Assessment of the literature regarding pharmacologic analgesics and sedatives in preterm neonates presents unique challenges. Despite extensive research, questions linger regarding the pharmacokinetics, efficacy, safety, and neurodevelopmental impact of these therapies. Pharmacokinetic studies in preterm neonates are confined by concerns about excessive invasive sampling and phlebotomy losses, limiting the robustness of data regarding the metabolism and elimination of commonly used agents. Pharmacodynamic assessment provides an even greater challenge for researchers and clinicians. Several tools have been used to assess the efficacy of analgesia and sedation in preterm neonates and are therefore referenced in this review.13 Clinical trials routinely report stress hormone levels, including adrenaline and cortisol, but serial assessment of these concentrations is not feasible in clinical practice. For this reason, subjective scoring systems must be utilized to assess pain and agitation. The COMFORT score, a behavioral and physiologic scale of distress developed for pediatric patients, is routinely used to assess agitation during stable mechanical ventilation.14 Acute pain occurs during procedures such as endotracheal suctioning. More than 40 tools have been developed to assess acute pain in the newborn, including the Premature Infant Pain Profile (PIPP) and Neonatal Infant Pain Scale (NIPS).15,16 However, to date, no tools have been developed and validated for the assessment of prolonged or recurrent pain in preterm neonates.
Determination of the neurologic impact of pharmacologic analgesics and sedatives in preterm neonates presents further complexity. Large randomized controlled trials focus on acute brain injury (i.e., intraventricular hemorrhage [IVH] and periventricular leukomalacia [PVL]) based on the theory that these complications may result from acute and chronic pain and stress.17 However, the pathophysiology of these injuries is multifactorial.18,19 In addition, these pathologies do not solely predict neurodevelopmental outcome. Limited data exist regarding the long-term neurodevelopmental impact of analgesics and sedatives in preterm neonates, likely a result of the challenging logistics of longterm developmental follow-up. Although direct extrapolation of animal data to humans is not appropriate, preclinical evidence from animal models that elucidates the potential impact of pharmacotherapy on the developing brain forms an essential bridge to both human studies and informed clinical practice.20,21 Therefore, this review incorporates preclinical data pertaining to the impact of analgesics and sedatives on the developing brain.
OPIATES
Pharmacokinetics and Pharmacodynamics
Morphine and fentanyl are the most commonly utilized opiates in neonates.11,12 Morphine is an agonist of the μ-opioid receptor that produces analgesia and sedation through inhibition of ascending pathways in the brain stem, inhibition of neuronal firing in the dorsal horn of the spinal cord, and depression of both presynaptic and postsynaptic neuronal membrane potentials peripherally. The pharmacokinetics of morphine have been described extensively in preterm neonates.22–27 Morphine is active as a parent compound. Adults rapidly metabolize morphine hepatically to morphine-3-glucuronide, an opioid antagonist, and morphine-6-glucuronide, a potent analgesic. In preterm neonates, metabolite formation is slow and incomplete, and morphine- 3-glucoronide formation predominates.28 This results in decreased analgesia and the development of tachyphylaxis within several days in morphine-treated neonates. Elimination half-life in neonates is approximately 6 to 12 hours and increases significantly with decreasing gestational age. This results in significant accumulation of parent drug and metabolites when morphine is administered as a continuous infusion. In preterm neonates, no relationship has been observed between morphine concentrations and clinical efficacy.25–27
Fentanyl, a synthetic μ-opioid receptor agonist, has been examined as an alternative to morphine. Interest in fentanyl was initially driven by its faster onset and shorter duration of action in adults compared to morphine, which make it ideal for procedural sedation.29,30 Fentanyl is active as a parent drug and is hepatically metabolized to inactive metabolites prior to renal elimination. Immaturity of hepatic enzymes in neonates suggests that delayed metabolism is likely in this population. Small pharmacokinetic descriptions of fentanyl in preterm neonates support this hypothesis, demonstrating an elimination half-life similar to that of morphine and suggesting accumulation during continuous infusion.31–33 Tachyphylaxis develops more rapidly with continuous infusion of fentanyl compared to morphine.34,35 There appears to be a direct correlation between fentanyl concentrations and clinical effect.33
Efficacy
Morphine has been demonstrated to increase ventilator synchrony in preterm neonates.36 Although treatment during mechanical ventilation does not impact COMFORT scores, morphine-treated neonates exhibit significantly decreased adrenaline concentrations compared to placebo-treated neonates.37–40 Similarly, fentanyl reduces behavioral state scores as well as adrenaline and cortisol levels in mechanically ventilated preterm neonates.41–44
Two large studies37,45 demonstrated a significant impact of morphine compared to placebo on PIPP scores during endotracheal suctioning, although 1 large trial46 showed no impact on this scale. Despite the statistical significance of the findings, the clinical relevance of the differences detected may be questioned. For example, the largest trial found a 1-point reduction in the PIPP scores of morphine-treated neonates compared to controls46; however, an approximately 6-point difference is required to differentiate a painful event from baseline on this 21-point scale.47 Additionally, data from the same cohort48 demonstrated no difference in PIPP scores between morphine- and placebo-treated neonates after a heel stick. Preterm neonates treated with fentanyl and morphine displayed similar NIPS scores during endotracheal suctioning.49
Adverse Drug Reactions
The adverse drug reaction profiles of morphine and fentanyl may significantly impact clinical outcomes in mechanically ventilated preterm neonates. Clinically significant hypotension (requiring intravenous vasopressors or fluid boluses) occurs in a significant proportion of morphine-treated neonates, most often following a bolus dose.46,50 Morphine depresses respiratory drive, resulting in a prolonged duration of mechanical ventilation.51 Morphine also decreases gastrointestinal motility, resulting in a prolonged time required to reach full enteral feedings.52
Differences between the adverse drug reaction profiles of fentanyl and morphine may exist due to the lack of histamine release with synthetic fentanyl. 53 Observational studies54 show no cardiovascular impact of fentanyl in preterm neonates. However, direct comparisons of fentanyl and morphine infusions revealed a similar need for vasopressors.49 Fentanyl likely produces similar respiratory depression to morphine at equianalgesic doses, although the impact of fentanyl on the duration of mechanical ventilation has not been examined in a controlled trial. Of note, increased ventilator pressures have been observed during continuous infusion of fentanyl, suggesting the potential for adverse pulmonary impact independent of respiratory depression.42 This impact is corroborated by the frequent occurrence of chest wall rigidity during rapid fentanyl infusion.55 Fentanyl may have less impact on gastrointestinal motility than morphine; nevertheless, severe gastrointestinal adverse drug reactions have been reported with fentanyl.49,56
Impact on Acute Brain Injury
Three large randomized controlled trials37,45,46 examined the impact of morphine on acute brain injury in mechanically ventilated preterm neonates. All trials randomized neonates in the first 72 hours of life who were mechanically ventilated for less than 8 hours and allowed open-label morphine boluses at the clinician's discretion for episodes of acute pain. The Neonatal Outcome and Prolonged Analgesia in Neonates (NOPAIN) trial37 randomized 67 preterm neonates to receive midazolam, morphine, or placebo. Morphine group neonates received a 100 mcg/kg loading dose followed by a continuous infusion of 10, 20, or 30 mcg/kg/hr for gestational ages of 24 to 26 weeks, 27 to 29 weeks, and 30 to 32 weeks, respectively. Study drug infusion was continued for a duration determined by the treating clinician. The incidence of the composite outcome of severe IVH, PVL, or death was decreased in the morphine group (4%) compared to the midazolam (32%) and placebo (24%) groups (p=0.03).
The promising results of the NOPAIN trial with regard to morphine influenced the design and undertaking of 2 larger randomized controlled trials.45,46 Simons and colleagues45 randomized 150 preterm neonates to morphine or placebo. Treatment group neonates received a 100 mcg/kg loading dose followed by 10 mcg/kg/hr, regardless of gestational age at birth. Study drug infusion was continued for a maximum of 7 days. The incidence of any IVH was significantly decreased in the morphine group (23% versus 40%; p=0.04). However, no difference was detected in the composite outcome of severe IVH, PVL, or death (10% versus 16%; p=0.66).
The largest trial to date, the NEurologic Outcomes and Preemptive Analgesia in Neonates (NEOPAIN) trial,46 randomized 898 preterm neonates to morphine or placebo. Morphine was dosed as described in the NOPAIN trial. No difference was detected in the composite outcome of severe IVH, PVL, or death (27% versus 26%; p=0.5777). Infants who received open-label morphine at the clinician's discretion were excluded from a subgroup analysis. In this analysis, the rate of the composite outcome was increased in neonates randomized to morphine (24% versus 15% in the placebo group; p=0.0338). In a separate subgroup analysis, placebo group neonates who received open-label morphine for episodes of acute pain had a higher rate of the composite outcome (34% versus 15%; p<0.0001).
The impact of fentanyl on acute brain injury was examined in a randomized placebo-controlled trial of mechanically ventilated preterm neonates (n=27).44 Fentanyl infusion was dosed at 0.5 to 2 mcg/kg/hr and adjusted to achieve adequate sedation. Duration of therapy was at the discretion of the treating clinician. No impact was detected on the incidence of the composite outcome of severe IVH, PVL, or death (21% versus 15%; p>0.05).
Finally, acute brain injury was assessed in a randomized trial of fentanyl versus morphine in 163 mechanically ventilated preterm neonates.49 Fentanyl group neonates received a 10 mcg/kg loading dose followed by 1.5 mcg/kg/hr. Morphine group neonates received a 140 mcg/kg loading dose followed by 20 mcg/kg/hr. Study infusion was continued for a minimum of 24 hours. No difference was detected in the combined incidence of mortality and severe IVH between groups (15% versus 14%; p>0.05).
Impact on Long-Term Neurodevelopment
Preclinical data suggest that opiates may negatively impact the developing brain. There are a number of opiate-mediated systems that influence neural cell differentiation, proliferation, and apoptosis.57,58 Reductions of neuronal density and dendritic length as well as apoptosis have been observed in rodent models of early opiate exposure.59–62 This exposure reduces brain growth and results in lower levels of brain-derived neurotrophic factor, a marker of synaptic plasticity and modulator of learning and memory.63,64 Further, evidence suggests that these effects on central nervous system development translate into abnormalities in later cognitive function and behavior. Rodents exposed to postnatal morphine exhibit persistently decreased motor activity and impaired learning ability.65–67
Conflicting results exist in human neonates with regard to the long-term neurodevelopmental impact of early morphine exposure. A 5- to 6-year follow-up of neonates enrolled in 2 previously published randomized controlled trials38,39,68 (n=87) showed no impact of morphine on motor function, intelligence, or behavior. However, developmental follow-up of NEOPAIN infants69 (n=572) at 36 weeks postmenstrual age found higher popliteal angle cluster scores, indicative of increased tone, in neonates randomized to morphine. This finding is corroborated by a retrospective analysis70 indicating an association between greater intravenous morphine exposure and poor motor development. A 5- to 7-year pilot follow-up of a small subset of NEOPAIN infants71 (n=19) found no difference in overall intelligence quotient. However, morphine-treated children had smaller head circumference, impaired short-term memory, and more social problems compared to placebo-treated children. Additionally, a 5-year follow-up of participants in the randomized trial of Simons and colleagues45,72 (n= 90) found lower overall intelligence quotient scores in neonates randomized to morphine (94 versus 100; p=0.049). Human data regarding the long-term neurodevelopmental impact of fentanyl are not currently available.
BENZODIAZEPINES
Pharmacokinetics and Pharmacodynamics
Midazolam binds to the benzodiazepine site on the γ-aminobutyric acidA (GABAA) receptor complex, increasing the action of this inhibitory neurotransmitter and resulting in sedation and anxiolysis. In neonatal intensive care, midazolam is preferred over lorazapem and diazepam due to its shorter half-life and the availability of a preservative-free intravenous preparation. The pharmacokinetics of midazolam in preterm neonates have been described extensively.73–76 Midazolam is active as a parent drug, with an equipotent metabolite of 1-hydroxymidazolam formed through hepatic hydroxylation, which undergoes glucuronide conjugation before renal excretion. Its elimination half-life is approximately 6 to 12 hours, with the rates of metabolism and excretion inversely proportional to gestational age, resulting in significant drug and metabolite accumulation during continuous infusion. The pharmacodynamics of midazolam have not been studied extensively in preterm neonates, although there is no known relationship between midazolam concentration and clinical effect in term neonates or children.77,78
Efficacy
The efficacy of midazolam in preterm neonates has been examined with various subjective assessment scales. Significantly lower sedation scores compared to placebo were observed during continuous infusion.37,79,80 Additionally, midazolam infusion reduced PIPP scores during endotracheal suctioning compared to placebo infusion (9 versus 13; p<0.001).37 Of concern, the risk of myoclonus from midazolam is greater in neonates compared to older populations, due to neonates' decreased number of GABAA receptors.75,81–83 This adverse drug reaction may complicate the assessment of comfort in the neonatal population.
Adverse Drug Reactions
Significant safety concerns exist regarding midazolam use in preterm neonates. Midazolam boluses of 200 mcg/kg produce clinically significant hypotension in a large proportion of preterm neonates (27%–45%), resulting in decreases in oxygen saturation, cerebral oxygenation index, and cerebral blood flow velocity.73,83 Conflicting data exist regarding the impact of lower bolus doses (100 mcg/kg) on blood flow and oxygenation; however, this dose is likely clinically ineffective.75,81,84
Impact on Acute Brain Injury
Two randomized controlled trials37,79 have examined the impact of midazolam on acute brain injury in mechanically ventilated preterm neonates. Jacqz-Aigrain and colleagues79 randomized 46 neonates with respiratory distress syndrome at less than 48 hours of life to midazolam or placebo. Midazolam group infants received 30 or 60 mcg/kg/hr for gestational ages of ≤32 weeks or >32 weeks, respectively. Twenty-five neonates were ≤32 weeks gestation. No difference was detected in the incidence of intracranial hemorrhage or death.
The NOPAIN trial37 randomized 69 preterm neonates to receive midazolam, morphine, or placebo. Midazolam group neonates received a 200 mcg/kg loading dose followed by a continuous infusion of 20, 40, or 60 mcg/kg/hr for gestational ages of 24 to 26 weeks, 27 to 29 weeks, and 30 to 32 weeks, respectively. Neonates in the midazolam group had a trend toward a higher rate of the composite outcome of severe IVH, PVL, or death compared to neonates receiving placebo (32% versus 24%), although the difference was not statistically significant. The difference in composite outcome was statistically significant compared to morphine (32% versus 4%; p=0.03).
Impact on Long-Term Neurodevelopment
Troubling preclinical data have been gathered regarding the impact of benzodiazepines on the developing brain. Rodent models have described widespread neuroapoptosis and suppressed neurogenesis elicited by early benzodiazepine exposure.85,86 Additionally, prenatal benzodiazepine exposure produces lasting changes in hypothalamic neuron expression and delayed motor development.87–89 Despite efficacy in mechanically ventilated preterm neonates, clinical use of midazolam has declined due to the finding of increased acute adverse neurologic events in the NOPAIN trial.37 Therefore, no data exist in humans regarding the long-term neurodevelopmental impact of benzodiazepine therapy in newborns.
DEXMEDETOMIDINE: A THERAPEUTIC ALTERNATIVE?
Dexmedetomidine is a highly selective α2-adrenergic receptor agonist that provides analgesia, anxiolysis, and sedation via reduction in sympathetic outflow from the locus coeruleus and release of substance P from the dorsal horn of the spinal cord.90 Dexmedetomidine was approved by the United States Food and Drug Administration in 1999 for short-term sedation in adults. Currently, dexmedetomidine is not approved for pediatric use. However, more than 200 studies and reports have been published regarding the use of dexmedetomidine in infants and children, several of which described prolonged use.91 With the exception of a single case report,92 the previous issue of The Journal of Pediatric Pharmacology and Therapeutics includes the first description of the efficacy and safety of dexmedetomidine infusion in mechanically ventilated preterm neonates.93
Pharmacokinetics and Pharmacodynamics
The pharmacokinetics and pharmacodynamics of dexmedetomidine have not been described in preterm neonates, and limited descriptions exist for pediatric patients.94 Pharmacokinetic studies in preterm neonates will be challenging due to limitations on invasive blood sampling. Development of dexmedetomidine assays requiring minimal blood volume for determination of concentration will be vital. Pharmacodynamic studies in preterm neonates will be challenging due to the inherent difficulties of pain and comfort assessment in this population. Development of new assessment strategies or validation of currently available tools in the chronic setting may assist in accomplishing this goal.95
Efficacy
Mechanically ventilated preterm neonates treated with dexmedetomidine infusion require less adjunctive sedation compared to historical controls treated with fentanyl infusion.93 These data support the findings of randomized controlled trials in adult patients. Evidence in mechanically ventilated adult intensive care unit patients96–99 suggests that standard sedation (i.e., benzodiazepines and/or opiates) contributes to the development of delirium. Clinical comparisons of dexmedetomidine with benzodiazepine infusion100,101 have shown similar or superior sedation and a lower incidence of delirium. Additionally, a single randomized trial102 that compared dexmedetomidine to morphine infusion in postoperative adult patients found similar time at target sedation level and a trend toward a reduction in delirium.
Adverse Drug Reactions
Dexmedetomidine has many theoretical advantages over standard sedative regimens with regard to adverse drug reactions. Dexmedetomidine does not affect respiratory drive.103 Neonates treated with dexmedetomidine have a shorter duration of mechanical ventilation compared to fentanyl-treated controls.93 These data correlate with the findings of randomized controlled trials in adults100–102 showing decreased duration of mechanical ventilation compared to benzodiazepines and morphine. In addition, preclinical studies104 show decreased levels of chemokines and cytokines in dexmedetomidine-treated rodents exposed to high-tidal volume ventilation. Thus, theoretically, high-dose dexmedetomidine may decrease the risk of bronchopulmonary dysplasia in preterm neonates by minimizing the duration of mechanical ventilation and attenuating ventilator-induced lung injury.
Dexmedetomidine has minimal impact on gastric motility.105 Neonates treated with dexmedetomidine require a shorter time to reach full enteral feeds compared to neonates treated with fentanyl. 93 In neonates, delayed enteral feeding may lead to numerous short-term complications, including an increased risk of sepsis.106 Additionally, dexmedetomidine preserves neutrophil function and inhibits cytokine response in animal models of endotoxic shock, which may be of benefit in preterm neonates with numerous additional risk factors for sepsis.107–109 This contrasts sharply with the inhibitory effects of opioids and benzodiazepines on neutrophil function.110,111 These differences may contribute to the mortality benefit with dexmedetomidine and detriment with benzodiazepine and opioid sedation in animal models of septic shock.112 Of note, the impact of dexmedetomidine on cytokine levels has been reproduced in septic adult humans.113 All of the aforementioned factors may contribute to the reduction in the incidence of culture-positive sepsis observed in neonates treated with dexmedetomidine over fentanyl, as well as adults randomized to dexmedetomidine versus midazolam.93,101
Unfortunately, dexmedetomidine has the potential for significant adverse drug reactions. The most concerning is hypotension, which is common with bolus doses of dexmedetomidine in both adult and pediatric patients.114,115 The incidence and degree of hypotension after bolus dosing appears to be similar to that typical of fentanyl and midazolam.116,117 Avoidance of bolus doses or rapid titration of dexmedetomidine attenuates this effect in adults.118 Further studies to define the incidence and clinical impact of this effect in preterm neonates are necessary. Prospective studies of dexmedetomidine in preterm neonates must include continuous assessment of blood pressure and heart rate as well as utilize available technologies to assess perfusion.119,120
Impact on Acute Brain Injury
The case-control study presented in the previous issue of The Journal of Pediatric Pharmacology and Therapeutics provides the only data regarding the impact of dexmedetomidine on the development of acute brain injury in preterm neonates.93 No difference was observed in the incidence of severe IVH or PVL between dexmedetomidine-treated cases and fentanyl-treated controls. Prospective randomized controlled trials are needed to further investigate this outcome.
Impact on Long-Term Neurodevelopment
Clinical data regarding dexmedetomidine are complemented by preclinical data suggesting neuroprotection of the developing brain by multiple potential mechanisms.121 Initial models in newborn rodents examined the impact of a single bolus dose of dexmedetomidine after ibotenate-induced brain lesions (designed to mirror the pathology of PVL in the preterm human neonate). Dexmedetomidine reduced the number of damaged neurons in vitro and reduced the size of the lesions in vivo.122,123 Subsequent experiments confirming both in vitro and in vivo neuroprotection, demonstrating clinical efficacy, and showing improved neurologic function were conducted in rodents exposed to a hypoxic-ischemic insult or isoflurane anesthesia.124–127 Preclinical experiments assessing the neurodevelopmental impact of prolonged dexmedetomidine infusion in both developing rodent and non-human primate models are necessary. Additionally, the long-term neurodevelopmental impact of dexmedetomidine must be assessed in all trials involving preterm neonates. Advanced magnetic resonance imaging will provide early insight regarding potential brain injury as well as assess brain growth and development.128 Long-term developmental follow-up will be necessary to confirm the prognosis of early magnetic resonance imaging findings and assess cognitive, motor, behavioral, and psychosocial domains.129
CONCLUSIONS
Research describing the developmental physiology of nociception clearly demonstrates the ability of preterm neonates to feel pain. Numerous preclinical and clinical studies have confirmed the adverse consequences of untreated pain and stress on brain development. Based on the available evidence, pharmacotherapy is likely indicated for sedation of preterm neonates during mechanical ventilation. However, routine administration of analgesia or sedation in this population is not recommended, mostly due to concerns regarding the safety and efficacy of these interventions.
Based on the available data from both preclinical and clinical studies, non-pharmacologic therapies should continue to form the foundation of neonatal pain and agitation relief. As stated, morphine is not recommended for routine use in mechanically ventilated preterm neonates due to the lack of benefit on the incidence of brain injury and concerns regarding safety. However, morphine is likely the most appropriate first-line therapy for preterm neonates requiring sedation on the basis of available data. Morphine should be avoided in neonates with pre-existing hypotension. There is a paucity of data regarding the developmental impact of other opiates, including fentanyl. Midazolam should be avoided in preterm neonates, due to the concerning incidence of brain injury in randomized trial. Patients requiring midazolam should receive a continuous infusion at a dose appropriate for gestational age, and bolus dosing should be avoided. Concerning data exist from both animal and human studies regarding the long-term neurodevelopmental impact of both opioids and benzodiazepines. Dexmedetomidine is a promising alternative, with data from both animal models and adult humans suggesting potential utility. Extensive multidisciplinary research must be completed before widespread use in preterm neonates is considered.
ABBREVIATIONS
- GABAA
γ-aminobutyric acidA
- IVH
intraventricular hemorrhage
- NEOPAIN
NEurologic Outcomes and Preemptive Analgesia in Neonates
- NIPS
Neonatal Infant Pain Scale
- NOPAIN
Neonatal Outcome and Prolonged Analgesia in Neonates
- PIPP
Premature Infant Pain Profile
- PVL
periventricular leukomalacia
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med. 1987;317(21):1321–1329. doi: 10.1056/NEJM198711193172105. [DOI] [PubMed] [Google Scholar]
- 2.Anand KJ, Sippell WG, Aynsley-Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: effects on the stress response. Lancet. 1987;1(8524):62–66. doi: 10.1016/s0140-6736(87)91907-6. [DOI] [PubMed] [Google Scholar]
- 3.Fitzgerald M, Koltzenburg M. The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord. Brain Res. 1986;389(1–2):261–270. doi: 10.1016/0165-3806(86)90194-x. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald M, Beggs S. The neurobiology of pain: developmental aspects. Neuroscientist. 2001;7(3):246–257. doi: 10.1177/107385840100700309. [DOI] [PubMed] [Google Scholar]
- 5.Taddio A, Shah V, Atenafu E, Katz J. Influence of repeated painful procedures and sucrose analgesia on the development of hyperalgesia in newborn infants. Pain. 2009;144(1–2):43–48. doi: 10.1016/j.pain.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Hintz SR, Kendrick DE, Wilson-Costello DE. Early-childhood neurodevelopmental outcomes are not improving for infants born at <25 weeks' gestational age. Pediatrics. 2011;127(1):62–70. doi: 10.1542/peds.2010-1150. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouza H. The impact of pain in the immature brain. J Matern Fetal Neonatal Med. 2009;22(9):722–732. doi: 10.3109/14767050902926962. [DOI] [PubMed] [Google Scholar]
- 8.Hall RW, Boyle E, Young T. Do ventilated neonates require pain management? Semin Perinatol. 2007;31(5):289–297. doi: 10.1053/j.semperi.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Golianu B, Krane E, Seybold J. Nonpharmacological techniques for pain management in neonates. Semin Perinatol. 2007;31(5):318–322. doi: 10.1053/j.semperi.2007.07.007. et al. [DOI] [PubMed] [Google Scholar]
- 10.Batton DG, Barrington KJ, Wallman C. Prevention and management of pain in the neonate: an update. Pediatrics. 2006;118(5):2231–2241. doi: 10.1542/peds.2006-2277. [DOI] [PubMed] [Google Scholar]
- 11.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117(6):1979–1987. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- 12.Kumar P, Walker JK, Hurt KM. Medication use in the neonatal intensive care unit: current patterns and off-label use of parenteral medications. J Pediatr. 2008;152(3):412–415. doi: 10.1016/j.jpeds.2007.07.050. et al. [DOI] [PubMed] [Google Scholar]
- 13.Ranger M, Johnston CC, Anand KJ. Current controversies regarding pain assessment in neonates. Semin Perinatol. 2007;31(5):283–288. doi: 10.1053/j.semperi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Marx CM, Smith PG, Lowrie LH. Optimal sedation of mechanically ventilated pediatric critical care patients. Crit Care Med. 1994;22(1):163–170. doi: 10.1097/00003246-199401000-00029. et al. [DOI] [PubMed] [Google Scholar]
- 15.Stevens B, Johnston C, Petryshen P, Taddio A. Premature Infant Pain Profile: development and initial validation. Clin J Pain. 1996;12(1):13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence J, Alcock D, McGrath P. The development of a tool to assess neonatal pain. Neonatal Netw. 1993;12(6):59–66. et al. [PubMed] [Google Scholar]
- 17.Anand KJ. Clinical importance of pain and stress in preterm neonates. Biol Neonate. 1998;73(1):1–9. doi: 10.1159/000013953. [DOI] [PubMed] [Google Scholar]
- 18.Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67(1):1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50(5):553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Jevtovic-Todorovic V, Olney JW. PRO: anesthesia-induced developmental neuroapoptosis: status of the evidence. Anesth Analg. 2008;106(6):1659–1663. doi: 10.1213/ane.0b013e3181731ff2. [DOI] [PubMed] [Google Scholar]
- 21.Loepke AW, McGowan FX, Jr, Soriano SG. CON: the toxic effects of anesthetics in the developing brain: the clinical perspective. Anesth Analg. 2008;106(6):1664–1669. doi: 10.1213/ane.0b013e3181733ef8. [DOI] [PubMed] [Google Scholar]
- 22.Bhat R, Chari G, Gulati A. Pharmacokinetics of a single dose of morphine in preterm infants during the first week of life. J Pediatr. 1990;117(3):477–481. doi: 10.1016/s0022-3476(05)81102-3. et al. [DOI] [PubMed] [Google Scholar]
- 23.Chay PC, Duffy BJ, Walker JS. Pharmacokinetic-pharmacodynamic relationships of morphine in neonates. Clin Pharmacol Ther. 1992;51(3):334–342. doi: 10.1038/clpt.1992.30. [DOI] [PubMed] [Google Scholar]
- 24.Hartley R, Green M, Quinn M, Levene MI. Pharmacokinetics of morphine infusion in premature neonates. Arch Dis Child. 1993;69(1 spec no):55–58. doi: 10.1136/adc.69.1_spec_no.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott CS, Riggs KW, Ling EW. Morphine pharmacokinetics and pain assessment in premature newborns. J Pediatr. 1999;135(4):423–429. doi: 10.1016/s0022-3476(99)70163-0. et al. [DOI] [PubMed] [Google Scholar]
- 26.Saarenmaa E, Neuvonen PJ, Rosenberg P, Fellman V. Morphine clearance and effects in newborn infants in relation to gestational age. Clin Pharmacol Ther. 2000;68(2):160–166. doi: 10.1067/mcp.2000.108947. [DOI] [PubMed] [Google Scholar]
- 27.Anand KJ, Anderson BJ, Holford NH. Morphine pharmacokinetics and pharmacodynamics in preterm and term neonates: secondary results from the NEOPAIN trial. Br J Anaesth. 2008;101(5):680–689. doi: 10.1093/bja/aen248. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhat R, Abu-Harb M, Chari G, Gulati A. Morphine metabolism in acutely ill preterm newborn infants. J Pediatr. 1992;120(5):795–799. doi: 10.1016/s0022-3476(05)80251-3. [DOI] [PubMed] [Google Scholar]
- 29.Koehntop DE, Rodman JH, Brundage DM. Pharmacokinetics of fentanyl in neonates. Anesth Analg. 1986;65(3):227–232. et al. [PubMed] [Google Scholar]
- 30.Yaster M. The dose response of fentanyl in neonatal anesthesia. Anesthesiology. 1987;66(3):433–435. doi: 10.1097/00000542-198703000-00035. [DOI] [PubMed] [Google Scholar]
- 31.Collins C, Koren G, Crean P. Fentanyl pharmacokinetics and hemodynamic effects in preterm infants during ligation of patent ductus arteriosus. Anesth Analg. 1985;64(11):1078–1080. et al. [PubMed] [Google Scholar]
- 32.Santeiro ML, Christie J, Stromquist C. Pharmacokinetics of continuous infusion fentanyl in newborns. J Perinatol. 1997;17(2):135–139. et al. [PubMed] [Google Scholar]
- 33.Saarenmaa E, Neuvonen PJ, Fellman V. Gestational age and birth weight effects on plasma clearance of fentanyl in newborn infants. J Pediatr. 2000;136(6):767–770. [PubMed] [Google Scholar]
- 34.Arnold JH, Truog RD, Scavone JM, Fenton T. Changes in the pharmacodynamic response to fentanyl in neonates during continuous infusion. J Pediatr. 1991;119(4):639–643. doi: 10.1016/s0022-3476(05)82419-9. [DOI] [PubMed] [Google Scholar]
- 35.Franck LS, Vilardi J, Durand D, Powers R. Opioid withdrawal in neonates after continuous infusions of morphine or fentanyl during extracorporeal membrane oxygenation. Am J Crit Care. 1998;7(5):364–369. [PubMed] [Google Scholar]
- 36.Dyke MP, Kohan R, Evans S. Morphine increases synchronous ventilation in preterm infants. J Paediatr Child Health. 1995;31(3):176–179. doi: 10.1111/j.1440-1754.1995.tb00780.x. [DOI] [PubMed] [Google Scholar]
- 37.Anand KJ, Barton BA, McIntosh N. Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Neonatal Outcome and Prolonged Analgesia in Neonates. Arch Pediatr Adolesc Med. 1999;153(4):331–338. doi: 10.1001/archpedi.153.4.331. et al. [DOI] [PubMed] [Google Scholar]
- 38.Quinn MW, Otoo F, Rushforth JA. Effect of morphine and pancuronium on the stress response in ventilated preterm infants. Early Hum Dev. 1992;30(3):241–248. doi: 10.1016/0378-3782(92)90073-p. et al. [DOI] [PubMed] [Google Scholar]
- 39.Quinn MW, Wild J, Dean HG. Randomised double-blind controlled trial of effect of morphine on catecholamine concentrations in ventilated pre-term babies. Lancet. 1993;342(8867):324–327. doi: 10.1016/0140-6736(93)91472-x. et al. [DOI] [PubMed] [Google Scholar]
- 40.Simons SH, van Dijk M, van Lingen RA. Randomised controlled trial evaluating effects of morphine on plasma adrenaline/noradrenaline concentrations in newborns. Arch Dis Child Fetal Neonatal Ed. 2005;90(1):F36–40. doi: 10.1136/adc.2003.046425. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roth B, Schlunder C, Houben F. Analgesia and sedation in neonatal intensive care using fentanyl by continuous infusion. Dev Pharmacol Ther. 1991;17(3–4):121–127. doi: 10.1159/000457510. et al. [DOI] [PubMed] [Google Scholar]
- 42.Orsini AJ, Leef KH, Costarino A. Routine use of fentanyl infusions for pain and stress reduction in infants with respiratory distress syndrome. J Pediatr. 1996;129(1):140–145. doi: 10.1016/s0022-3476(96)70201-9. et al. [DOI] [PubMed] [Google Scholar]
- 43.Guinsburg R, Kopelman BI, Anand KJ. Physiological, hormonal, and behavioral responses to a single fentanyl dose in intubated and ventilated preterm neonates. J Pediatr. 1998;132(6):954–959. doi: 10.1016/s0022-3476(98)70390-7. et al. [DOI] [PubMed] [Google Scholar]
- 44.Lago P, Benini F, Agosto C, Zacchello F. Randomised controlled trial of low dose fentanyl infusion in preterm infants with hyaline membrane disease. Arch Dis Child Fetal Neonatal Ed. 1998;79(3):F194–197. doi: 10.1136/fn.79.3.f194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simons SH, van Dijk M, van Lingen RA. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA. 2003;290(18):2419–2427. doi: 10.1001/jama.290.18.2419. et al. [DOI] [PubMed] [Google Scholar]
- 46.Anand KJ, Hall RW, Desai N. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363(9422):1673–1682. doi: 10.1016/S0140-6736(04)16251-X. et al. [DOI] [PubMed] [Google Scholar]
- 47.Ballantyne M, Stevens B, McAllister M. Validation of the premature infant pain profile in the clinical setting. Clin J Pain. 1999;15(4):297–303. doi: 10.1097/00002508-199912000-00006. et al. [DOI] [PubMed] [Google Scholar]
- 48.Carbajal R, Lenclen R, Jugie M. Morphine does not provide adequate analgesia for acute procedural pain among preterm neonates. Pediatrics. 2005;115(6):1494–1500. doi: 10.1542/peds.2004-1425. et al. [DOI] [PubMed] [Google Scholar]
- 49.Saarenmaa E, Huttunen P, Leppaluoto J. Advantages of fentanyl over morphine in analgesia for ventilated newborn infants after birth: a randomized trial. J Pediatr. 1999;134(2):144–150. doi: 10.1016/s0022-3476(99)70407-5. et al. [DOI] [PubMed] [Google Scholar]
- 50.Hall RW, Kronsberg SS, Barton BA. Morphine, hypotension, and adverse outcomes among preterm neonates: who's to blame? Secondary results from the NEOPAIN trial. Pediatrics. 2005;115(5):1351–1359. doi: 10.1542/peds.2004-1398. et al. [DOI] [PubMed] [Google Scholar]
- 51.Bhandari V, Bergqvist LL, Kronsberg SS. Morphine administration and short-term pulmonary outcomes among ventilated preterm infants. Pediatrics. 2005;116(2):352–359. doi: 10.1542/peds.2004-2123. et al. [DOI] [PubMed] [Google Scholar]
- 52.Menon G, Boyle EM, Bergqvist LL. Morphine analgesia and gastrointestinal morbidity in preterm infants: secondary results from the NEOPAIN trial. Arch Dis Child Fetal Neonatal Ed. 2008;93(5):F362–367. doi: 10.1136/adc.2007.119297. et al. [DOI] [PubMed] [Google Scholar]
- 53.Rosow CE, Moss J, Philbin DM, Savarese JJ. Histamine release during morphine and fentanyl anesthesia. Anesthesiology. 1982;56(2):93–96. doi: 10.1097/00000542-198202000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Hamon I, Hascoet JM, Debbiche A, Vert P. Effects of fentanyl administration on general and cerebral haemodynamics in sick newborn infants. Acta Paediatr. 1996;85(3):361–365. doi: 10.1111/j.1651-2227.1996.tb14033.x. [DOI] [PubMed] [Google Scholar]
- 55.Fahnenstich H, Steffan J, Kau N, Bartmann P. Fentanyl-induced chest wall rigidity and laryngospasm in preterm and term infants. Crit Care Med. 2000;28(3):836–839. doi: 10.1097/00003246-200003000-00037. [DOI] [PubMed] [Google Scholar]
- 56.Pezzati M, Bertini G, Chiti G. Paralytic ileus in a mechanically ventilated preterm infant treated with fentanyl. Pediatr Med Chir. 2001;23(3–4):201–202. et al. [PubMed] [Google Scholar]
- 57.Sargeant TJ, Miller JH, Day DJ. Opioidergic regulation of astroglial/neuronal proliferation: where are we now? J Neurochem. 2008;107(4):883–897. doi: 10.1111/j.1471-4159.2008.05671.x. [DOI] [PubMed] [Google Scholar]
- 58.Chen YL, Law PY, Loh HH. The other side of the opioid story: modulation of cell growth and survival signaling. Curr Med Chem. 2008;15(8):772–778. doi: 10.2174/092986708783955518. [DOI] [PubMed] [Google Scholar]
- 59.Hammer RP, Jr, Ricalde AA, Seatriz JV. Effects of opiates on brain development. Neurotoxicology. 1989;10(3):475–483. [PubMed] [Google Scholar]
- 60.Ricalde AA, Hammer RP., Jr. Perinatal opiate treatment delays growth of cortical dendrites. Neurosci Lett. 1990;115(2–3):137–143. doi: 10.1016/0304-3940(90)90444-e. [DOI] [PubMed] [Google Scholar]
- 61.Seatriz JV, Hammer RP., Jr. Effects of opiates on neuronal development in the rat cerebral cortex. Brain Res Bull. 1993;30(5–6):523–527. doi: 10.1016/0361-9230(93)90078-p. [DOI] [PubMed] [Google Scholar]
- 62.Atici S, Cinel L, Cinel I. Opioid neurotoxicity: comparison of morphine and tramadol in an experimental rat model. Int J Neurosci. 2004;114(8):1001–1011. doi: 10.1080/00207450490461314. et al. [DOI] [PubMed] [Google Scholar]
- 63.Zagon IS, McLaughlin PJ. Morphine and brain growth retardation in the rat. Pharmacology. 1977;15(3):276–282. doi: 10.1159/000136699. [DOI] [PubMed] [Google Scholar]
- 64.Schrott LM, Franklin LM, Serrano PA. Prenatal opiate exposure impairs radial arm maze performance and reduces levels of BDNF precursor following training. Brain Res. 2008;1198:132–140. doi: 10.1016/j.brainres.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Handelmann GE, Dow-Edwards D. Modulation of brain development by morphine: effects on central motor systems and behavior. Peptides. 1985;6(suppl 2):29–34. doi: 10.1016/0196-9781(85)90131-7. [DOI] [PubMed] [Google Scholar]
- 66.McPherson RJ, Gleason C, Mascher-Denen M. A new model of neonatal stress which produces lasting neurobehavioral effects in adult rats. Neonatology. 2007;92(1):33–41. doi: 10.1159/000100084. et al. [DOI] [PubMed] [Google Scholar]
- 67.Ma MX, Chen YM, He J. Effects of morphine and its withdrawal on Y-maze spatial recognition memory in mice. Neuroscience. 2007;147(4):1059–1065. doi: 10.1016/j.neuroscience.2007.05.020. et al. [DOI] [PubMed] [Google Scholar]
- 68.MacGregor R, Evans D, Sugden D. Outcome at 5-6 years of prematurely born children who received morphine as neonates. Arch Dis Child Fetal Neonatal Ed. 1998;79(1):F40–43. doi: 10.1136/fn.79.1.f40. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rao R, Sampers JS, Kronsberg SS. Neurobehavior of preterm infants at 36 weeks postconception as a function of morphine analgesia. Am J Perinatol. 2007;24(9):511–517. doi: 10.1055/s-2007-986675. et al. [DOI] [PubMed] [Google Scholar]
- 70.Grunau RE, Whitfield MF, Petrie-Thomas J. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143(1–2):138–146. doi: 10.1016/j.pain.2009.02.014. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferguson SA, Ward WL, Paule MG. A pilot study of preemptive morphine analgesia in preterm neonates: effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicol Teratol. 2012;34(1):47–55. doi: 10.1016/j.ntt.2011.10.008. et al. [DOI] [PubMed] [Google Scholar]
- 72.de Graaf J, van Lingen RA, Simons SH. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children's functioning: five-year follow-up of a randomized controlled trial. Pain. 2011;152(6):1391–1397. doi: 10.1016/j.pain.2011.02.017. et al. [DOI] [PubMed] [Google Scholar]
- 73.Jacqz-Aigrain E, Daoud P, Burtin P. Pharmacokinetics of midazolam during continuous infusion in critically ill neonates. Eur J Clin Pharmacol. 1992;42(3):329–332. doi: 10.1007/BF00266357. et al. [DOI] [PubMed] [Google Scholar]
- 74.Burtin P, Jacqz-Aigrain E, Girard P. Population pharmacokinetics of midazolam in neonates. Clin Pharmacol Ther. 1994;56(6 pt 1):615–625. doi: 10.1038/clpt.1994.186. et al. [DOI] [PubMed] [Google Scholar]
- 75.Harte GJ, Gray PH, Lee TC. Haemodynamic responses and population pharmacokinetics of midazolam following administration to ventilated, preterm neonates. J Paediatr Child Health. 1997;33(4):335–338. doi: 10.1111/j.1440-1754.1997.tb01611.x. et al. [DOI] [PubMed] [Google Scholar]
- 76.Lee TC, Charles BG, Harte GJ. Population pharmacokinetic modeling in very premature infants receiving midazolam during mechanical ventilation: midazolam neonatal pharmacokinetics. Anesthesiology. 1999;90(2):451–457. doi: 10.1097/00000542-199902000-00020. et al. [DOI] [PubMed] [Google Scholar]
- 77.Jacqz-Aigrain E, Wood C, Robieux I. Pharmacokinetics of midazolam in critically ill neonates. Eur J Clin Pharmacol. 1990;39(2):191–192. doi: 10.1007/BF00280059. [DOI] [PubMed] [Google Scholar]
- 78.de Wildt SN, de Hoog M, Vinks AA. Pharmacodynamics of midazolam in pediatric intensive care patients. Ther Drug Monit. 2005;27(1):98–102. doi: 10.1097/00007691-200502000-00018. et al. [DOI] [PubMed] [Google Scholar]
- 79.Jacqz-Aigrain E, Daoud P, Burtin P. Placebo-controlled trial of midazolam sedation in mechanically ventilated newborn babies. Lancet. 1994;344(8923):646–650. doi: 10.1016/s0140-6736(94)92085-0. et al. [DOI] [PubMed] [Google Scholar]
- 80.Arya V, Ramji S. Midazolam sedation in mechanically ventilated newborns: a double blind randomized placebo controlled trial. Indian Pediatr. 2001;38(9):967–972. [PubMed] [Google Scholar]
- 81.van Straaten HL, Rademaker CM, de Vries LS. Comparison of the effect of midazolam or vecuronium on blood pressure and cerebral blood flow velocity in the premature newborn. Dev Pharmacol Ther. 1992;19(4):191–195. doi: 10.1159/000457484. [DOI] [PubMed] [Google Scholar]
- 82.Brooks-Kayal AR, Pritchett DB. Developmental changes in human gamma-aminobutyric acidA receptor subunit composition. Ann Neurol. 1993;34(5):687–693. doi: 10.1002/ana.410340511. [DOI] [PubMed] [Google Scholar]
- 83.van Alfen-van der Velden AA, Hopman JC, Klaessens JH. Effects of midazolam and morphine on cerebral oxygenation and hemodynamics in ventilated premature infants. Biol Neonate. 2006;90(3):197–202. doi: 10.1159/000093489. et al. [DOI] [PubMed] [Google Scholar]
- 84.Treluyer JM, Zohar S, Rey E. Minimum effective dose of midazolam for sedation of mechanically ventilated neonates. J Clin Pharm Ther. 2005;30(5):479–485. doi: 10.1111/j.1365-2710.2005.00678.x. et al. [DOI] [PubMed] [Google Scholar]
- 85.Young C, Jevtovic-Todorovic V, Qin YQ. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146(2):189–197. doi: 10.1038/sj.bjp.0706301. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stefovska VG, Uckermann O, Czuczwar M. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol. 2008;64(4):434–445. doi: 10.1002/ana.21463. et al. [DOI] [PubMed] [Google Scholar]
- 87.Kellogg C, Tervo D, Ison J. Prenatal exposure to diazepam alters behavioral development in rats. Science. 1980;207(4427):205–207. doi: 10.1126/science.7350658. et al. [DOI] [PubMed] [Google Scholar]
- 88.Simmons RD, Miller RK, Kellogg CK. Prenatal exposure to diazepam alters central and peripheral responses to stress in adult rat offspring. Brain Res. 1984;307(1–2):39–46. doi: 10.1016/0006-8993(84)90457-8. [DOI] [PubMed] [Google Scholar]
- 89.Kellogg CK, Simmons RD, Miller RK, Ison JR. Prenatal diazepam exposure in rats: long-lasting functional changes in the offspring. Neurobehav Toxicol Teratol. 1985;7(5):483–488. [PubMed] [Google Scholar]
- 90.Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur J Pharmacol. 1988;150(1–2):9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- 91.Mason KP, Lerman J. Dexmedetomidine in children: current knowledge and future applications. Anesth Analg. 2011;113(5):1129–1142. doi: 10.1213/ANE.0b013e31822b8629. [DOI] [PubMed] [Google Scholar]
- 92.O'Mara K, Gal P, Ransom JL. Successful use of dexmedetomidine for sedation in a 24-week gestational age neonate. Ann Pharmacother. 2009;43(10):1707–1713. doi: 10.1345/aph.1M245. et al. [DOI] [PubMed] [Google Scholar]
- 93.O'Mara K, Gal P, Wimmer J. Dexmedetomidine versus standard therapy with fentanyl for sedation in mechanicallyventilated premature neonates. J Pediatr Pharmacol Ther. 2012;17(3):252–262. doi: 10.5863/1551-6776-17.3.252. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Potts AL, Anderson BJ, Warman GR. Dexmedetomidine pharmacokinetics in pediatric intensive care—a pooled analysis. Paediatr Anaesth. 2009;19(11):1119–1129. doi: 10.1111/j.1460-9592.2009.03133.x. et al. [DOI] [PubMed] [Google Scholar]
- 95.Boyle EM, Freer Y, Wong CM. Assessment of persistent pain or distress and adequacy of analgesia in preterm ventilated infants. Pain. 2006;124(1–2):87–91. doi: 10.1016/j.pain.2006.03.019. et al. [DOI] [PubMed] [Google Scholar]
- 96.Marcantonio ER, Juarez G, Goldman L. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272(19):1518–1522. et al. [PubMed] [Google Scholar]
- 97.Dubois MJ, Bergeron N, Dumont M. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27(8):1297–1304. doi: 10.1007/s001340101017. et al. [DOI] [PubMed] [Google Scholar]
- 98.Pandharipande P, Shintani A, Peterson J. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26. doi: 10.1097/00000542-200601000-00005. et al. [DOI] [PubMed] [Google Scholar]
- 99.Pandharipande P, Cotton BA, Shintani A. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34–41. doi: 10.1097/TA.0b013e31814b2c4d. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pandharipande PP, Pun BT, Herr DL. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. et al. [DOI] [PubMed] [Google Scholar]
- 101.Riker RR, Shehabi Y, Bokesch PM. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499. doi: 10.1001/jama.2009.56. et al. [DOI] [PubMed] [Google Scholar]
- 102.Shehabi Y, Grant P, Wolfenden H. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study) Anesthesiology. 2009;111(5):1075–1084. doi: 10.1097/ALN.0b013e3181b6a783. et al. [DOI] [PubMed] [Google Scholar]
- 103.Hoy SM, Keating GM. Dexmedetomidine: a review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs. 2011;71(11):1481–1501. doi: 10.2165/11207190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 104.Yang CL, Tsai PS, Huang CJ. Effects of dexmedetomidine on regulating pulmonary inflammation in a rat model of ventilatorinduced lung injury. Acta Anaesthesiol Taiwan. 2008;46(4):151–159. doi: 10.1016/S1875-4597(09)60002-3. [DOI] [PubMed] [Google Scholar]
- 105.Memis D, Dokmeci D, Karamanlioglu B. A comparison of the effect on gastric emptying of propofol or dexmedetomidine in critically ill patients: preliminary study. Eur J Anaesthesiol. 2006;23(8):700–704. doi: 10.1017/S0265021506000512. et al. [DOI] [PubMed] [Google Scholar]
- 106.Hartel C, Haase B, Browning-Carmo K. Does the enteral feeding advancement affect short-term outcomes in very low birth weight infants? J Pediatr Gastroenterol Nutr. 2009;48(4):464–470. doi: 10.1097/MPG.0b013e31818c5fc3. et al. [DOI] [PubMed] [Google Scholar]
- 107.Taniguchi T, Kidani Y, Kanakura H. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2004;32(6):1322–1326. doi: 10.1097/01.ccm.0000128579.84228.2a. et al. [DOI] [PubMed] [Google Scholar]
- 108.Taniguchi T, Kurita A, Kobayashi K. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. J Anesth. 2008;22(3):221–228. doi: 10.1007/s00540-008-0611-9. et al. [DOI] [PubMed] [Google Scholar]
- 109.Nishina K, Akamatsu H, Mikawa K. The effects of clonidine and dexmedetomidine on human neutrophil functions. Anesth Analg. 1999;88(2):452–458. doi: 10.1097/00000539-199902000-00042. et al. [DOI] [PubMed] [Google Scholar]
- 110.Tubaro E, Borelli G, Croce C. Effect of morphine on resistance to infection. J Infect Dis. 1983;148(4):656–666. doi: 10.1093/infdis/148.4.656. et al. [DOI] [PubMed] [Google Scholar]
- 111.Nishina K, Akamatsu H, Mikawa K. The inhibitory effects of thiopental, midazolam, and ketamine on human neutrophil functions. Anesth Analg. 1998;86(1):159–165. doi: 10.1097/00000539-199801000-00032. et al. [DOI] [PubMed] [Google Scholar]
- 112.Sanders RD, Hussell T, Maze M. Sedation and immunomodulation. Crit Care Clin. 2009;25(3):551–570. ix. doi: 10.1016/j.ccc.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 113.Tasdogan M, Memis D, Sut N, Yuksel M. Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis. J Clin Anesth. 2009;21(6):394–400. doi: 10.1016/j.jclinane.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 114.Venn RM, Bradshaw CJ, Spencer R. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54(12):1136–1142. doi: 10.1046/j.1365-2044.1999.01114.x. et al. [DOI] [PubMed] [Google Scholar]
- 115.Petroz GC, Sikich N, James M. A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology. 2006;105(6):1098–1110. doi: 10.1097/00000542-200612000-00009. et al. [DOI] [PubMed] [Google Scholar]
- 116.Erdil F, Demirbilek S, Begec Z. The effects of dexmedetomidine and fentanyl on emergence characteristics after adenoidectomy in children. Anaesth Intensive Care. 2009;37(4):571–576. doi: 10.1177/0310057X0903700405. et al. [DOI] [PubMed] [Google Scholar]
- 117.Koroglu A, Demirbilek S, Teksan H. Sedative, haemodynamic and respiratory effects of dexmedetomidine in children undergoing magnetic resonance imaging examination: preliminary results. Br J Anaesth. 2005;94(6):821–824. doi: 10.1093/bja/aei119. et al. [DOI] [PubMed] [Google Scholar]
- 118.Gerlach AT, Dasta JF, Steinberg S. A new dosing protocol reduces dexmedetomidine-associated hypotension in critically ill surgical patients. J Crit Care. 2009;24(4):568–574. doi: 10.1016/j.jcrc.2009.05.015. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roche-Labarbe N, Fenoglio A, Aggarwal A. Near-infrared spectroscopy assessment of cerebral oxygen metabolism in the developing premature brain. J Cereb Blood Flow Metab. 2012;32(3):481–488. doi: 10.1038/jcbfm.2011.145. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kluckow M, Seri I, Evans N. Functional echocardiography: an emerging clinical tool for the neonatologist. J Pediatr. 2007;150(2):125–130. doi: 10.1016/j.jpeds.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 121.Ma D, Rajakumaraswamy N, Maze M. Alpha2-adrenoceptor agonists: shedding light on neuroprotection? Br Med Bull. 2004;71:77–92. doi: 10.1093/bmb/ldh036. [DOI] [PubMed] [Google Scholar]
- 122.Laudenbach V, Mantz J, Lagercrantz H. Effects of alpha(2)-adrenoceptor agonists on perinatal excitotoxic brain injury: comparison of clonidine and dexmedetomidine. Anesthesiology. 2002;96(1):134–141. doi: 10.1097/00000542-200201000-00026. et al. [DOI] [PubMed] [Google Scholar]
- 123.Paris A, Mantz J, Tonner PH. The effects of dexmedetomidine on perinatal excitotoxic brain injury are mediated by the alpha2A-adrenoceptor subtype. Anesth Analg. 2006;102(2):456–461. doi: 10.1213/01.ane.0000194301.79118.e9. et al. [DOI] [PubMed] [Google Scholar]
- 124.Sanders RD, Sun P, Patel S. Dexmedetomidine provides cortical neuroprotection: impact on anaesthetic-induced neuroapoptosis in the rat developing brain. Acta Anaesthesiol Scand. 2010;54(6):710–716. doi: 10.1111/j.1399-6576.2009.02177.x. et al. [DOI] [PubMed] [Google Scholar]
- 125.Ma D, Hossain M, Rajakumaraswamy N. Dexmedetomidine produces its neuroprotective effect via the alpha 2Aadrenoceptor subtype. Eur J Pharmacol. 2004;502(1–2):87–97. doi: 10.1016/j.ejphar.2004.08.044. et al. [DOI] [PubMed] [Google Scholar]
- 126.Sanders RD, Giombini M, Ma D. Dexmedetomidine exerts dose-dependent age-independent antinociception but age-dependent hypnosis in Fischer rats. Anesth Analg. 2005;100(5):1295–1302. doi: 10.1213/01.ANE.0000149595.41576.B3. et al. table of contents. [DOI] [PubMed] [Google Scholar]
- 127.Sanders RD, Xu J, Shu Y. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110(5):1077–1085. doi: 10.1097/ALN.0b013e31819daedd. et al. [DOI] [PubMed] [Google Scholar]
- 128.Mathur AM, Neil JJ, Inder TE. Understanding brain injury and neurodevelopmental disabilities in the preterm infant: the evolving role of advanced magnetic resonance imaging. Semin Perinatol. 2010;34(1):57–66. doi: 10.1053/j.semperi.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang CJ, McGlynn EA, Brook RH. Quality-of-care indicators for the neurodevelopmental follow-up of very low birth weight children: results of an expert panel process. Pediatrics. 2006;117(6):2080–2092. doi: 10.1542/peds.2005-1904. et al. [DOI] [PubMed] [Google Scholar]


