Abstract
OBJECTIVES
Adverse events during antiretroviral treatment are frequent and various. Their diagnosis incurs some various difficulties according to the geographic context. Our aim was to describe the frequency, nature, and preventability of adverse drug reactions (ADRs) due to antiretroviral treatment in Malian outpatient children.
METHODS
The study was a 6-month (June 1 to November 30, 2010) prospective, observational study of 92 children admitted to a pediatric hospital in Sikasso, Mali. The patients were treated with a generic drug and/or drug combinations. Prior to treatment initiation, demographic characteristics, clinical history, and biologic parameters, including CD4 cell counts, were collected for each patient. The World Health Organization's adverse drug reactions classification was used to characterize the side effects. Adverse effects and toxicities were graded 1, 2, and 3. Analysis of data was performed using SPSS Version 17.0 software.
RESULTS
Ninety-two human immunodeficiency virus–infected children met the criteria of inclusion. After 24 weeks of treatment, we observed that 14.1% of children had at least one side effect during our study. Side effects were many and varied, with the most frequent being cutaneous rash, nausea, vomiting, and diarrhea (38.5%, 23.1%, 15.4%, and 15.4%, respectively). Side effects were grade 1 in most cases. One case of grade 2 and one case of grade 3 were observed with rash. We observed one case of grade 3 side effects during our study. The treatment regimen was changed in 15.2% of cases, including one case because of side effects.
CONCLUSION
ADRs are not rare in Mali, particularly in children. These ADRs have an impact on quality of life for patients. We recommend a pharmacovigilance system for sustainable management of side effects in patients infected with human immunodeficiency virus in Mali.
INDEX TERMS: adverse drug reactions, antiretroviral therapy, children, Mali
INTRODUCTION
An estimated 33 million people are living with human immunodeficiency virus (HIV) infection, and around 3 million people have access to antiretroviral therapy (ART) worldwide.1,2 Antiretrovirals have brought a ray of hope to people living with HIV. Unfortunately, the adverse effects of these drugs are of serious concern. Adverse reaction to antiretrovirals in HIV patients is a major cause of medication non-adherence, leading to treatment failure.3 The Malian government has exerted a continuous effort to expand access to antiretrovirals. The High Committee National AIDS Control Organization has established ART centers that offer free treatment for HIV and related opportunistic infections.4 It is estimated that across Mali, free ART will be provided to 37,000 patients in 2011.5 The Malian National Pharmacovigilance Programme, however, lacks continuity. There is insufficient awareness and inadequate training about drug safety monitoring among health care professionals in Mali. Often, adverse drug reactions (ADRs) go unnoticed or are not reported. Monitoring and reporting of ADRs to ART in the Malian population are very important. To our knowledge, there are no systematic studies conducted in Mali concerning ADRs in HIV patients receiving ART. This study was conducted to assess the nature, severity, predictability, and preventability of ADRs to ART, and to identify risk factors for ADRs in HIV-positive patients receiving ART in Sikasso.
MATERIALS AND METHODS
The study was conducted at the pediatric department of the hospital of Sikasso, Mali. The study was approved by the Institutional Human Ethical Committee of Faculty of Medicine, Pharmacy and Odonto-stomatology in Bamako. The active (intensive monitoring) pharmacovigilance methodology was adopted. HIV-positive patients receiving fixed-dose drug combinations of ART were included. Written informed consent was obtained from these patients.
Included in the study were children on ART for a period of 3 months or longer who had a manifestation of ADR and their parents or guardians informed consent. This inclusion criterion was chosen to avoid confusion between adverse events associated with antiretroviral drugs in actual use, and adverse events associated with antiretrovirals given in a previous round of therapy but interrupted more than 3 months (change of regimen). We did not include patients naive to ART, patients receiving the same ART for less than 3 months, patients with acute concomitant illness, and children whose parental consent was not obtained.
Between June and November 2010, these patients were intensively monitored by a pharmacist for any ADRs during follow-up visits to the ART center: (an initial visit after a 2-week period, followed by monthly visits). ADRs were identified by an interview with the patient and/ or the patient's attendants, as well as a review of outpatient case records, laboratory reports, clinicians' notes, and prescriptions at each follow-up visit. Suspected ADRs documented with necessary information were reviewed and assessed by a senior academic clinical pharmacist. Wherever appropriate, suspected ADRs were discussed with the clinicians. The World Health Organization (WHO) ADR probability scale and Naranjo algorithm were used for causality assessment.6,7 Severity of ADRs was assessed using the Modified Hartwig and Siegel scale.8 If the drug had previously been well tolerated by the patient at the same dose and route of administration, the ADR was considered “not predictable.” If there was a history of allergy or reactions to the drug during previous exposure, the ADR was considered “predictable.” In patients who had never received the drug previously, any ADR with a literature incidence of 1 in 100 was considered “predictable.” Modified Shumock and Thornton criteria were used to assess the preventability of ADRs.9 Reactions were coded using WHO Adverse Reaction Terminologies.10 Data were collected through questionnaires and chart reviews. The interview of the child or person having custody of the child was performed at each follow-up.
After inclusion, children were seen every month. Day 1 of inclusion is denoted T0; the first month of follow-up is M1, the second month is M2, the third month is M3, and the sixth month is M6. Adherence was measured by the statement by the parent or guardian to 4 days preceding the appointment.11 ADRs were graded on a 4-point scale using the WHO severity grading.12 Grade 1 was classified as “mild,” with no limitation of daily activities; grade 2 was classified as “moderate,” with mild to moderate limitation of activities; grade 3 was classified as “severe,” with marked limitation of activities; and grade 4 was classified as “life threatening,” with extreme limitation of activities and significant medical intervention. This study was approved by the ethics committee of the Faculty of Medicine, Pharmacy, and Odonto-stomatology in Bamako. All statistical calculations were performed using the Statistical Package for Social Science (SPSS) Version 17.0 software (Chicago, IL, USA). A p value of <0.05 was considered as statistically significant.
RESULTS
Of 121 infected children, the study covered 92. The 29 children not included did not meet the inclusion criteria for immunologic and clinical therapy. The average age of the sample was 6 ± 1.8 years and ranged from 5 months to 14 years. Patients with ages between 5 and 9 years and between 10 and 14 years were the most represented, with 40.2% and 38%, respectively.
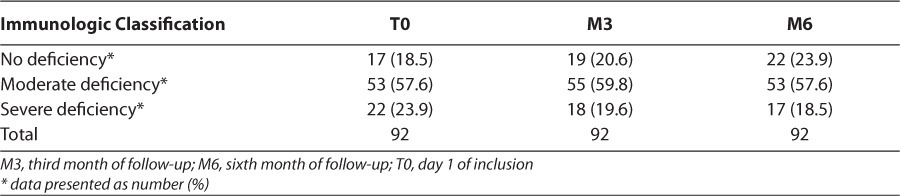
The sex ratio was 1.24 in favor of boys. A total of 58.7% of children were orphaned (Table 1). Children who had moderate immune deficiency were predominant at T0 and M3, with 57.6% and 59.8%, respectively (Table 2). For most preventable adverse events, preventive measures for adverse events were given as hints or administered to patients: for example, it was commonly stated that patients should avoid fatty foods and dairy products for the prevention of nausea and vomiting in patients receiving zidovudine or efavirenz. It was recommended that patients should take the drugs before or after meals. It was recommended that patients drink plenty of water with the regimen containing lopinavir/ritonavir in order to prevent gastrointestinal upset. In severe cases, nausea and vomiting were treated with an antiemetic; diarrhea with an antidiarrheal drug; and rash, according to the nature of the offending molecule, by the prescription of an antihistaminic and/or an antipyretic drug. We observed 14.1% of children had developed adverse events during the study compared with 85.9% of children who did not have adverse events.
Table 1.
Sociodemographic Characteristics of Children in the Cohort
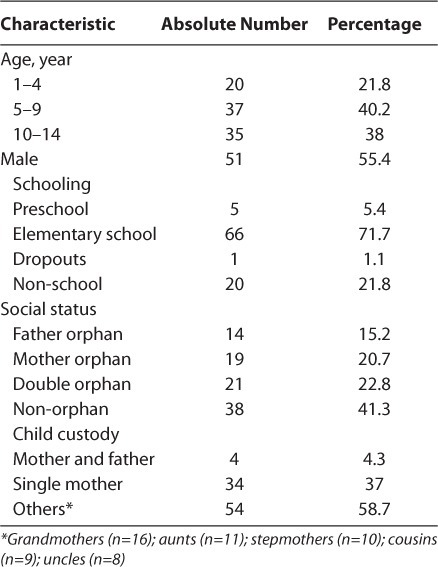
Table 2.
Distribution of Patients According to the Immunological Classification During Follow-Up
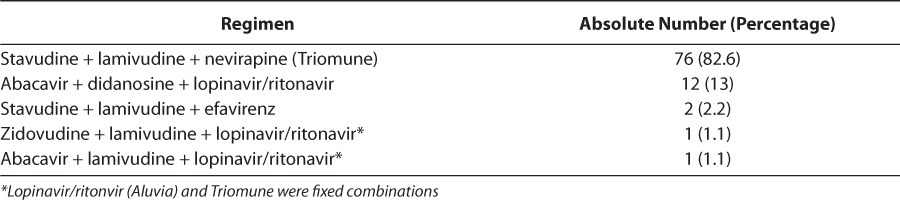
Adverse reactions were varied, with the most reported being rash and nausea (38.5% and 23.1%, respectively). Adverse events were grade 1 in most cases. One case of grade 2 and one case of grade 3 were observed (Table 3). Therapy was changed for 15.2% of patients and unchanged for 84.8% of patients. Treatment failure was the major reason for change, accounting for 78.6% of instances, followed by medications being out of stock and adverse reactions. Children who had no side effects during the study were less compliant with treatment than those who had at least one side effect, with no significant difference seen between ADR and treatment compliance (p=1; Table 4). The regimens that showed the most side effects were stavudine + lamivudine + nevirapine, and zidovudine + lamivudine + lopinavir/ ritonavir (Table 5). In most ADRs, causality was “probable” (53.9%) and “possible” (46.1%) according to the WHO probability scale. Using the Naranjo algorithm, causality was “possible” and “probable” in 76.9% and 23.1% of cases, respectively (Table 6). The regimens used in the cohort are described (Table 7).
Table 3.
Distribution of Adverse Events by Grade
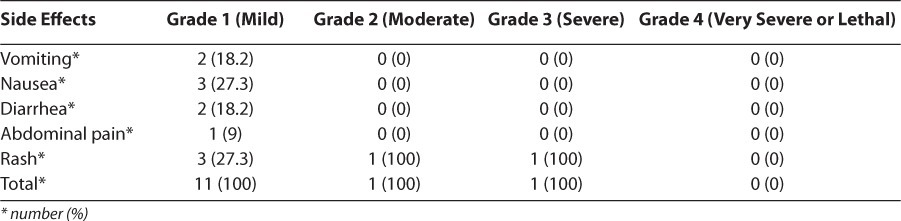
Table 4.
Distribution of Patients According to the Presence of Side Effects and Treatment Adherence
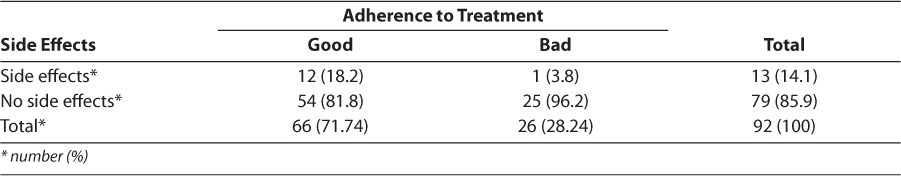
Table 5.
Distribution of Side Effects Depending on the Regimen
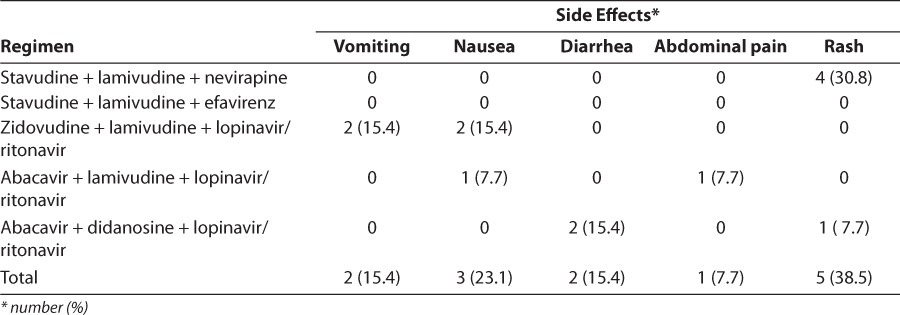
Table 6.
Causality of Adverse Drug Reactions
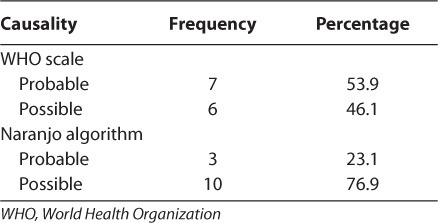
Table 7.
Describing the Regimens Used Would Be Helpful
DISCUSSION
This is the first study in Mali assessing the nature and severity of ADRs to ARTs in HIV-positive Malian children. An active surveillance method was adopted. The study observed no significant morbidity associated with the use of ART in the local population. Sex-wise prevalence of ADRs observed in intensive monitoring was similar to that observed in other studies.13–16 Most ADRs observed in children were similar to those in another study.17 However, a previous study has reported a larger percentage of ADRs in geriatric and pediatric populations.18 Most of the ADRs were predictable because they were common (incidence 1 in 100 and <1 in 10) or very common (incidence 1 in 10). Findings of preventability were substantially higher than those observed in a study conducted by Mehta et al17 (46.2%). In most of the preventable ADRs, preventive measures for ADRs were not prescribed or administered to patients: for example, often no instructions were given to patients to avoid fatty foods and dairy products for the prevention of nausea and vomiting in patients receiving zidovudine. Vomiting was a common ADR observed among patients who were on regimens containing zidovudine. It was noted that most of these patients experienced vomiting half an hour after ingestion of the drug. Most of the gastrointestinal ADRs were observed in the first few weeks of therapy, and symptoms were self-limiting. Gastrointestinal disorders are one cause of medication non-adherence.3
Patients receiving a zidovudine-containing regimen had a greater risk of vomiting, similar to that observed in an Iranian study.19 Anemia occurred in patients receiving zidovudine-containing regimens (hemoglobin [(Hb] <70 g/L) within the first few weeks to few months after initiation of therapy. In most cases, severe anemia (Hb <40 g/L) was not observed in the study. In almost all cases, an improvement in Hb concentration was observed on discontinuation of zidovudine, similar to the findings reported by Koduri and Parekh.20 Results from the Treat Asia HIV Observational Database study found that anemia (Hb <100 g/L) with zidovudine therapy was associated with low baseline Hb concentration, young age, and female sex.21 In our study, patients were initiated on a zidovudine-containing regimen only if Hb concentration was more than 80 g/L at baseline, thereby avoiding the occurrence of anemia. Young age and female sex were not significantly associated with anemia. However, we observed a highly significant association between the use of zidovudine and anemia that was similar to other studies.22,23
All skin reactions occurred in patients receiving a nevirapine-containing regimen. In most patients, a definite improvement in skin reaction was observed after nevirapine was discontinued. Nevirapine use and female sex were identified as risk factors for the development of skin reactions in the population, similar to findings of a study conducted by De Lazzari et al.24 However, these reactions did not meet all of the qualitative criteria for an ADR signal. The routine collection of ADR-related treatment modification/interruptions is feasible, but improvements concerning how information on drug-related adverse events is collected are required to respond to some of the key research questions related to known and suspected drug toxicities in the context of ART scale-up in Africa.25 Poor compliance in our study could explain the low number of adverse events reported during clinical monitoring. Indeed, poor compliance would result in lower global drug dosage. However, this also results in a difficulty in maintaining an undetectable viral load and being one of the main reasons for poor virologic response.
CONCLUSION
ART with zidovudine, lamivudine, plus nevirapine/ efavirenz is a predictor of ADRs. HIVinfected children who are of female sex, with a CD4 count of <200 cells per milliliter, need intensive monitoring for ADRs. Attention needs to be drawn to how to monitor ADRs with antiretrovirals while simultaneously improving access to ART for the Malian population, particularly among HIV- infected children. Some preventive measures could reduce the morbidity of ADRs. We recommend a pharmacovigilance system for sustainable management of side effects in patients infected with HIV in Mali.
ACKNOWLEDGMENTS
This work has been presented in Istanbul (Turkey) as a poster presentation at the 11th annual meeting of the International Society of Pharmaco-vigilance (ISOP), 2011, abstract number PP-165. We thank the staff of the Pediatric Department of the Hospital of Sikasso and the University of Bamako for their support. We are also indebted to Dr Almoustapha Maiga and Guida Landoure for their help.
ABBREVIATIONS
- ADR
adverse drug reactions
- ART
antiretroviral therapy
- Hb
hemoglobin
- HIV
human immunodeficiency virus
- WHO
World Health Organization
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.UNAIDS. UNAIDS Report on the Global AIDS Epidemic. http://www.unaids.org/globalreport/global_report.htm. Accessed October 15, 2011.
- 2.AVERT. Universal Access to AIDS Treatment: Targets and Challenges. http://www.avert.org/universal-access.htm. Accessed April 15, 2011.
- 3.Cooper CL, Breau C, Laroche A. Clinical outcomes of first antiretroviral regimen in HIV/hepatitis C virus co-infection. HIV Med. 2006;7(1):32–37. doi: 10.1111/j.1468-1293.2005.00340.x. et al. [DOI] [PubMed] [Google Scholar]
- 4.Cellule de Coordination du Comité Sectoriel de Lutte contre le SIDA du Ministère de la santé/Mali. 2010. Politique et protocole de prise en charge antirétrovirale du VIH et du Sida. 2nd Edition. Bamako, Mali.
- 5.Sylla A. Epidemiology/universal access to care, treatment and prevention of HIV in Mali [in French] Paper presented at: 6th Annual Meeting AIDS in Mali; March 15, 2011; Bamako, Mali.
- 6.Mayboom RH, Hekster YA, Egberts AC. Causal or casual? The role of causality assessment in pharmacovigilance. Drug Saf. 1997;17(6):374–389. doi: 10.2165/00002018-199717060-00004. et al. [DOI] [PubMed] [Google Scholar]
- 7.Naranjo CA, Busto U, Sellers EM. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. et al. [DOI] [PubMed] [Google Scholar]
- 8.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229–2232. [PubMed] [Google Scholar]
- 9.Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27(6):538. [PubMed] [Google Scholar]
- 10.A practical handbook on the pharmacovigilance of antiretroviral medicines. http://www.who.int/hiv/pub/pharmacovigilance/handbook/en/index.html. Accessed June 30, 2011.
- 11.Aboubacrine SA, Niamba P, Boileau C. Inadequate adherence to antiretroviral treatment and prevention in hospital and community sites in Burkina Faso and Mali: a study by the ATARAO Group. Int J STD AIDS. 2007;18(11):741–747. doi: 10.1258/095646207782212243. et al. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. Accessed June 30, 2011. [PubMed]
- 13.Gonzalez-Martin G, Caroca CM, Paris E. Adverse drug reactions (ADRs) in hospitalized pediatric patients. A prospective study. Int J Clin Pharmacol Ther. 1998;36(10):530–533. [PubMed] [Google Scholar]
- 14.Cooper JW. Adverse drug reaction-related hospitalizations of nursing facility patients: a 4 -year study. South Med J. 1999;92(5):485–490. doi: 10.1097/00007611-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 15.May FW, Rowett DS, Gilbert AL. Outcomes of an educational-outreach service for community medical practitioners: nonsteroidal anti-inflammatory drugs. Med J Aust. 1999;170(10):471–474. et al. [PubMed] [Google Scholar]
- 16.Moore N, Lecointre D, Noblet C, Mabill M. Frequency and cost of serious adverse drug reactions in a department of general medicine. Br J Clin Pharmacol. 1998;45(3):301–308. doi: 10.1046/j.1365-2125.1998.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta U, Durrheim DN, Blockman M. Adverse drug reactions in adult medical inpatients in a South African hospital serving a community with a high HIV/AIDS prevalence: prospective observational study. Br J Clin Pharmacol. 2008;65(3):396–406. doi: 10.1111/j.1365-2125.2007.03034.x. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melmon KL. Preventable drug reactions-causes and cures. N Engl J Med. 1971;284(24):1361–1368. doi: 10.1056/NEJM197106172842408. [DOI] [PubMed] [Google Scholar]
- 19.Khalili H, Dashti-Khavidaki S, Mohraz M. Antiretroviral induced adverse drug reactions in Iranian human immunodeficiency virus positive patients. Pharmacoepidemiol Drug Saf. 2009;18(9):848–857. doi: 10.1002/pds.1793. et al. [DOI] [PubMed] [Google Scholar]
- 20.Koduri PR, Parekh S. Zidovudine-related anemia with recticulocytosis. Ann Hematol. 2003;82(3):184–185. doi: 10.1007/s00277-002-0587-8. [DOI] [PubMed] [Google Scholar]
- 21.Huffam SE, Srasuebkul P, Zhou J. Prior antiretroviral therapy experience protects against zidovudine-related anaemia. HIV Med. 2007;8(7):465–471. doi: 10.1111/j.1468-1293.2007.00498.x. et al. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan PS, Hanson DL, Chu SY. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood. 1998;91(1):301–308. et al. [PubMed] [Google Scholar]
- 23.Curkendall SM, Richardson JT, Emons MF. Incidence of anaemia among HIV-infected patients treated with highly active antiretroviral therapy. HIV Med. 2007;8(8):483–490. doi: 10.1111/j.1468-1293.2007.00500.x. et al. [DOI] [PubMed] [Google Scholar]
- 24.De Lazzari E, León A, Arnaiz JA. Hepatotoxicity of nevirapine in virologically suppressed patients according to gender and CD4 cell counts. HIV Med. 2008;9(4):221–226. doi: 10.1111/j.1468-1293.2008.00552.x. et al. [DOI] [PubMed] [Google Scholar]
- 25.Jaquet A, Djima MM, Coffie P. Pharmacovigilance for antiretroviral drugs in Africa: lessons from a study in Abidjan, Cote d'Ivoire. Pharmacoepidemiol Drug Saf. 2011;20(12):1303–1310. doi: 10.1002/pds.2182. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]


