Abstract
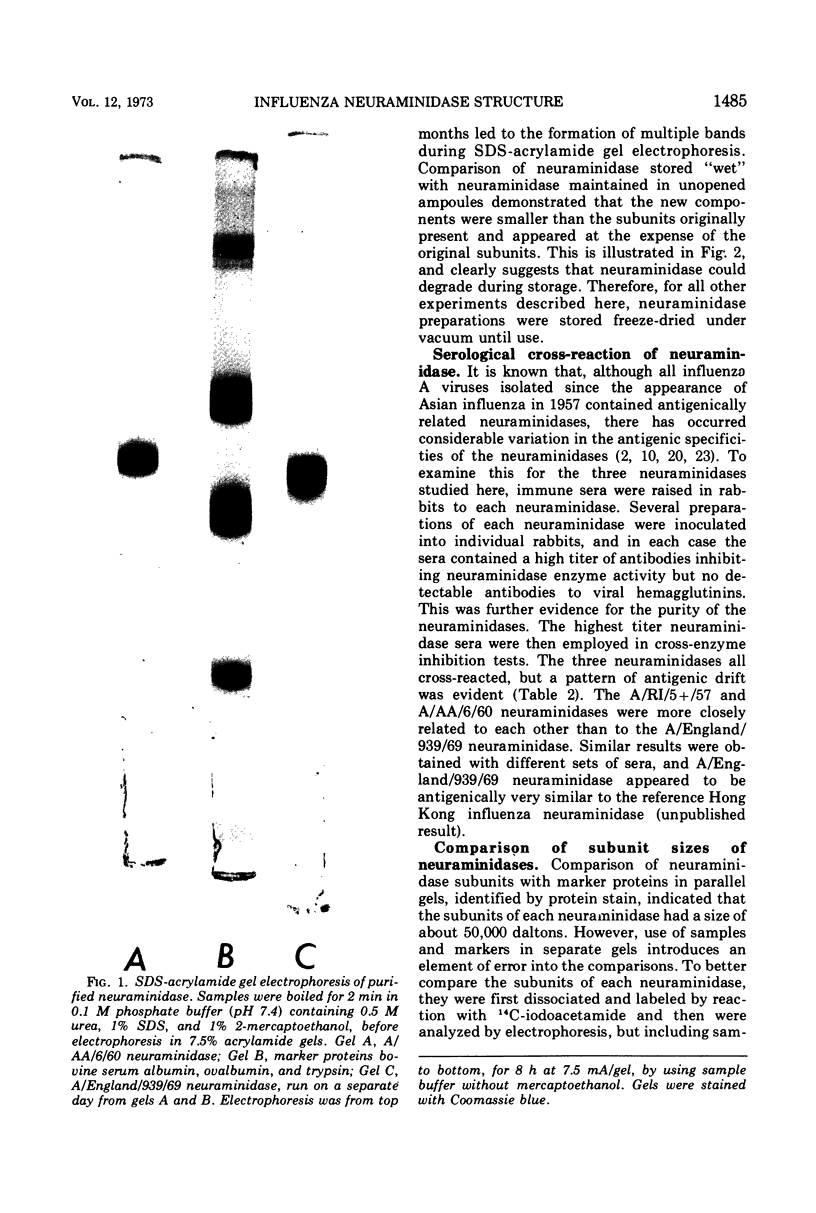
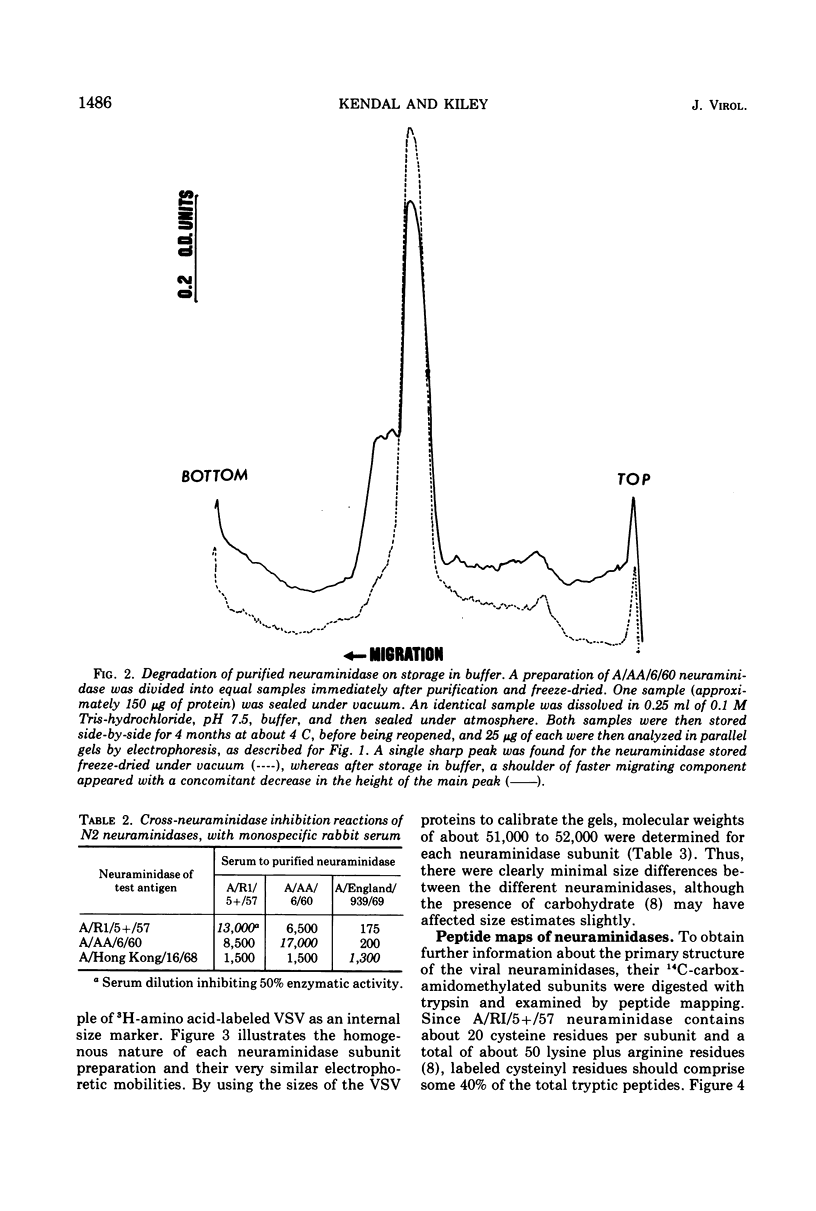
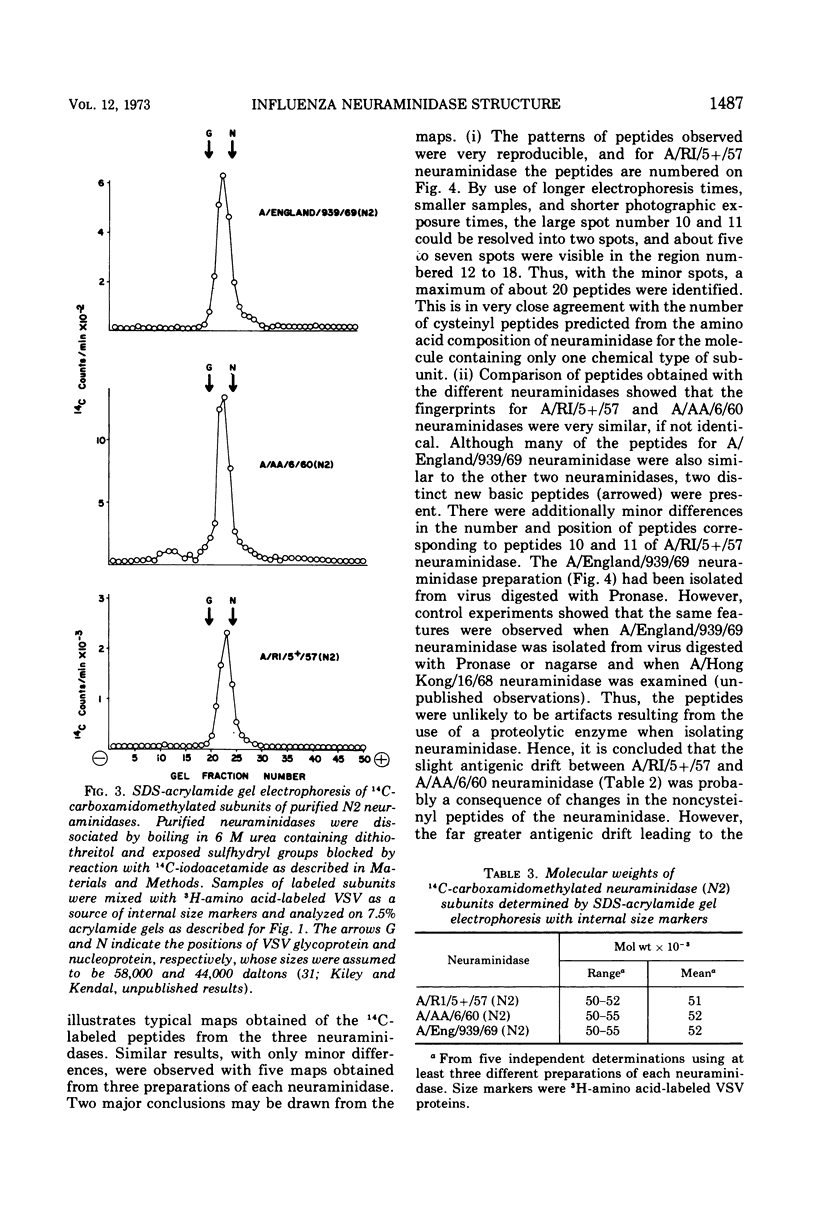
Neuraminidases (EC 3.2.1.18) of 1957, 1960, and 1969 influenza virus strains were isolated after proteolytic digestion of viral hemagglutinin. Each neuraminidase was recovered with a final yield of about 15% and had similar specific activities. Immunization of rabbits with the neuraminidases elicited monospecific neuraminidase antibodies, with no antibodies to viral hemagglutinin. Further evidence of purity was the existence of only a single component, about 50,000 daltons in size, when reduced neuraminidase preparations were examined by sodium dodecyl sulfate acrylamide gel electrophoresis. However, storage of neuraminidase in solution resulted in the appearance of slightly smaller degradation products. Preparations of each neuraminidase were denatured under reducing conditions, and exposed sulfhydryl residues were blocked by reaction with 14C-iodoacetamide. After tryptic digestion, peptide maps were prepared for the neuraminidases, and the 14C-labeled cysteinyl peptides were then identified by autoradiography. About 20 peptides were present, in agreement with the number predicted from amino acid analysis for neuraminidase subunits of only one type. The 1957 and 1960 neuraminidases exhibited a small antigenic divergence from each other, and maps of their cysteinyl peptides appeared to be identical. The 1969 neuraminidase exhibited considerable antigenic divergence from the other two neuraminidases, and maps of 1969 neuraminidase peptides revealed two major and several minor differences from the other maps. Thus, antigenic divergence between the neuraminidases of Asian and Hong Kong influenza viruses is associated with a small number of changes in the primary structure of the neuraminidase subunit.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bucher D. J., Kilbourne E. D. A 2 (N2) neuraminidase of the X-7 influenza virus recombinant: determination of molecular size and subunit composition of the active unit. J Virol. 1972 Jul;10(1):60–66. doi: 10.1128/jvi.10.1.60-66.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle W. R., Coleman M. T., Hall E. C., Knez V. Properties of the Hong Kong influenza virus. Antigenic relationship of the Hong Kong virus haemagglutinin to that of other human influenza A viruses. Bull World Health Organ. 1969;41(3):419–424. [PMC free article] [PubMed] [Google Scholar]
- Gregoriades A. Isolation of neuraminidase from the WSN strain of influenza virus. Virology. 1972 Jul;49(1):333–336. doi: 10.1016/s0042-6822(72)80039-4. [DOI] [PubMed] [Google Scholar]
- Hoyle L., Almeida J. D. The chemical reactions of the haemagglutinins and neuraminidases of different strains of influenza viruses. 3. Effects of proteolytic enzymes. J Hyg (Lond) 1971 Sep;69(3):461–469. doi: 10.1017/s0022172400021719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle L. The chemical reactions of the haemagglutinins and neuraminidases of different strains of influenza viruses. II. Effects of reagents modifying the higher order structure of the protein molecule. J Hyg (Lond) 1969 Jun;67(2):301–310. doi: 10.1017/s002217240004170x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal A. P., Biddle F., Belyavin G. Influenza virus neuraminidase and the viral surface. Biochim Biophys Acta. 1968 Oct 15;165(3):419–431. doi: 10.1016/0304-4165(68)90221-3. [DOI] [PubMed] [Google Scholar]
- Kendal A. P., Eckert E. A. The preparation and properties of 14 C-carboxamidomethylated subunits from A 2 -1957 influenza neuraminidase. Biochim Biophys Acta. 1972 Feb 28;258(2):484–495. doi: 10.1016/0005-2744(72)90240-9. [DOI] [PubMed] [Google Scholar]
- Kendal A. P., Kiley M. P., Eckert E. A. Isoelectric focusing studies of A2-1957 influenza neuraminidase and its subunits. Biochim Biophys Acta. 1973 Jul 12;317(1):28–33. doi: 10.1016/0005-2795(73)90196-7. [DOI] [PubMed] [Google Scholar]
- Kendal A. P., Madeley C. R. A comparative study of influenza virus neuraminidases, using automated techniques. Biochim Biophys Acta. 1969 Jul 8;185(1):163–177. doi: 10.1016/0005-2744(69)90292-7. [DOI] [PubMed] [Google Scholar]
- Kendal A. P., Minuse E., Maassab H. F., Hennessy A. V., Davenport F. M. Influenza neuraminidase antibody patterns of man. Am J Epidemiol. 1973 Aug;98(2):96–103. doi: 10.1093/oxfordjournals.aje.a121543. [DOI] [PubMed] [Google Scholar]
- Kiley M. P., Wagner R. R. Ribonucleic acid species of intracellular nucleocapsids and released virions of vesicular stomatitis virus. J Virol. 1972 Aug;10(2):244–255. doi: 10.1128/jvi.10.2.244-255.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Kilbourne E. D. Identification in a recombinant influenza virus of structural proteins derived from both parents. Virology. 1966 Nov;30(3):493–501. doi: 10.1016/0042-6822(66)90125-5. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Valentine R. C. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969 May;38(1):105–119. doi: 10.1016/0042-6822(69)90132-9. [DOI] [PubMed] [Google Scholar]
- Lazdins I., Haslam E. A., White D. O. The polypeptides of influenza virus. VI. Composition of the neuraminidase. Virology. 1972 Sep;49(3):758–765. doi: 10.1016/0042-6822(72)90532-6. [DOI] [PubMed] [Google Scholar]
- Madeley C. R., Allan W. H., Kendal A. P. Studies with avian influenza A viruses: serological relations of the haemagglutinin and neuraminidase antigens of ten virus isolates. J Gen Virol. 1971 Aug;12(2):69–78. doi: 10.1099/0022-1317-12-2-69. [DOI] [PubMed] [Google Scholar]
- McCahon D., Schild G. C. Segregation of antigenic and biological characteristics during influenza virus recombination. J Gen Virol. 1972 Apr;15(1):73–77. doi: 10.1099/0022-1317-15-1-73. [DOI] [PubMed] [Google Scholar]
- Paniker C. K. Serological relationships between the neuraminidases in influenza viruses. J Gen Virol. 1968 May;2(3):385–394. doi: 10.1099/0022-1317-2-3-385. [DOI] [PubMed] [Google Scholar]
- RAFELSON M. E., Jr, SCHNEIR M., WILSON V. W., Jr STUDIES ON THE NEURAMINIDASE OF INFLUENZA VIRUS. II. ADDITIONAL PROPERTIES OF THE ENZYMES FROM THE ASIAN AND PR 8 STRAINS. Arch Biochem Biophys. 1963 Dec;103:424–430. doi: 10.1016/0003-9861(63)90432-6. [DOI] [PubMed] [Google Scholar]
- Rott R., Becht H., Klenk H. D., Scholtissek C. Interactions of concanavalin A with the membrane of infleunza virus infected cells and with envelope components of the virus particle. Z Naturforsch B. 1972 Mar;27(3):227–233. doi: 10.1515/znb-1972-0303. [DOI] [PubMed] [Google Scholar]
- Schulman J. L., Kilbourne E. D. Independent variation in nature of hemagglutinin and neuraminidase antigens of influenza virus: distinctiveness of hemagglutinin antigen of Hong Kong-68 virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):326–333. doi: 10.1073/pnas.63.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Schild G. C. The polypeptide composition of influenza A viruses. Virology. 1971 May;44(2):396–408. doi: 10.1016/0042-6822(71)90270-4. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. A., Snyder R. M. Structural proteins of vesicular stomatitis viruses. J Virol. 1969 Apr;3(4):395–403. doi: 10.1128/jvi.3.4.395-403.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G. Estimation of the molecular weights of the polypeptide chains from the isolated hemagglutinin and neuraminidase subunits of influenza viruses. Virology. 1970 Mar;40(3):643–654. doi: 10.1016/0042-6822(70)90209-6. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Antigenic variation in influenza virus. Biology and chemistry. Prog Med Virol. 1971;13:271–338. [PubMed] [Google Scholar]
- Wrigley N. G., Skehel J. J., Charlwood P. A., Brand C. M. The size and shape of influenza virus neuraminidase. Virology. 1973 Feb;51(2):525–529. doi: 10.1016/0042-6822(73)90457-1. [DOI] [PubMed] [Google Scholar]
- Wunner W. H., Pringle C. R. Comparison of structural polypeptides from vesicular stomatitis virus (Indiana and New Jersey serotypes) and Cocal virus. J Gen Virol. 1972 Jul;16(1):1–10. doi: 10.1099/0022-1317-16-1-1. [DOI] [PubMed] [Google Scholar]