Abstract
Background and purpose
Studies have linked elevated homocysteine (tHcy) levels to atherosclerotic carotid plaque development, but data are limited to predominantly white populations. We examined the association between tHcy and carotid plaque burden and morphology in a multi-ethnic cohort.
Methods
In the Northern Manhattan Study, we conducted a cross–sectional analysis among 1327 stroke-free subjects (mean age 66±9, 41% men, 19% black, 62% Hispanic, 17% white) with serum tHcy and ultrasonographic assessment of plaque morphology measured by Gray-Scale Median (GSM) and total plaque area (TPA). GSM and TPA were examined in 4 categories. High and low GSM categories were considered echodense and echolucent plaque respectively and compared to no plaque. Logistic regression models were used to assess the associations of tHcy with GSM and TPA adjusting for demographics, vascular risk factors, renal insufficiency, and B12 deficiency.
Results
The mean tHcy was 9.4±4.8μmol/L (median=8.6). The prevalence of carotid plaque was 57% (52% among Hispanics, 58% black, 70% white). Among those with plaque, the mean TPA was 20.3±20.6 mm2(median=13.6) and mean GSM 90.9±28.5 (median=93.0). The top two tHcy quartiles (vs. quartile 1) had an elevated risk of having either echolucent plaque (tHcy Q3: OR=1.8 (95%CI 1.2–2.8); tHcy Q4: OR=1.9(95% CI 1.2–3.1)) or echodense plaque (tHcy Q3: OR=1.7 (95%CI 1.1–2.7); tHcy Q4 OR=1.9 (95% CI1.2–3.2)). The top two tHcy quartiles were also more likely to be in the highest TPA category (tHcy Q3: OR=1.8 (95%CI 1.1–3.0); tHcy Q4: OR=2.2 (95%CI 1.3–3.7)).
Conclusions
In this population-based multi-ethnic cohort, elevated tHcy was independently associated with plaque morphology and increased plaque area, subclinical markers of stroke risk.
Keywords: Atherosclerosis, carotid arteries, ultrasonography, plaque area, Gray Scale Median (GSM), echodense plaque, echolucent plaque, homocysteine
Introduction
Several epidemiological studies have shown that elevated total homocysteine (tHcy) is strongly related to atherosclerosis (1–10), a leading cause of stroke. (11) However, most of the available data are derived from case-control studies with a small number of cases or from cross-sectional studies limited to predominantly white populations. The increased risk of atherosclerosis and stroke among blacks and Hispanics underscores the importance of examining the role of modifiable risk factors in racially and ethnically diverse populations. (12) Therefore, in the multi-ethnic population-based Northern Manhattan Study (NOMAS), we investigated how elevated homocysteine levels are related to carotid plaque area and plaque morphology, two novel, distinct, and reliable measures of subclinical atherosclerosis. (13–15)
Total plaque area and plaque echogenicity, which correspond to the ‘vulnerable’ plaque histology, may be useful subclinical measurements to assess the effects of anti-atherosclerotic treatments. (16–19) The gray-scale median (GSM), an ultrasonographic measure of plaque echogenicity, represents a novel and promising marker of plaque stabilization of potential clinical utility because of simplicity of assessment, reliability, and ability to be measured from plaque images collected during a standard clinical B-mode ultrasonography. (20) Data on risk factors for these plaque phenotypes in general and multi-ethnic populations are limited. We hypothesized that tHcy would be associated with high total plaque area (TPA), as well as an increased risk of having either echolucent or echodense carotid plaque.
Methods
Study population
The Northern Manhattan Study (NOMAS) is a prospective, population-based cohort study with a unique race–ethnic distribution of community residents. The study was designed to study the incidence and risk factors for stroke in a multiethnic urban community. A total of 3298 subjects, identified by random digit dialing utilizing dual frame sampling as previously described, were enrolled between 1993 and 2001. (15) Inclusion criteria were: 1) age ≥ 39 years old, 2) no prior history of stroke, and 3) had resided in the Northern Manhattan area for at least three months with a telephone. The overall response rate was approximately 68%. This study was approved by the Columbia University Medical Center and the University of Miami Institutional Review Boards.
Using a primarily cross-sectional design, the current study is an analysis of a sample of NOMAS participants who had baseline tHcy measured and a carotid ultrasound evaluation of carotid plaque area and morphology. Among 3298 NOMAS subjects, carotid ultrasound with GSM and TPA measurements was available for 1590, of which 1327 had baseline tHcy measured.
Baseline Evaluation
Data regarding baseline functional status, vascular risk factors and medical conditions were collected through in-person interviews conducted by trained bilingual research assistants. Physical examinations were conducted by study physicians. Race-ethnicity was based upon self-identification using questions modeled after the US census and conforming to standard definitions outlined by Directive 15. (21) Standardized questions were adapted from the Behavioral Risk Factor Surveillance System by the Centers for Disease Control regarding hypertension, diabetes, smoking, and cardiac conditions. Methods regarding the measurement of blood pressure (BP), collection of fasting blood specimens for glucose, lipids, creatinine, and vitamin B12, and the definitions of vascular risk factor covariates in NOMAS have been described previously. (22–25)
Assessment of Homocysteine
Blood samples were drawn from the participants after an overnight fast by trained phlebotomists. After venipuncture, the blood samples were immediately put on crushed ice. In the following hour samples were centrifuged at 3000 g for 20 minutes at 4°C and immediately frozen at −70°C until analysis. At the University of Colorado Health Science Center’s research laboratory, tHcy levels were assayed by stable isotope dilution gas chromatography-mass spectrometry (GS-MS), which is reported to be a highly sensitive and accurate method for determination of moderate hyperhomocysteinemia in human plasma. (26) Homocysteine was examined as a continuous variable and in quartiles.
Assessment of Carotid Atherosclerosis
High-resolution B-mode ultrasounds (GE LogIQ 700, 9- to 13-MHz linear-array transducer) were performed by trained and certified sonographers as described previously. (27) Presence of plaque is defined as a focal wall thickening or protrusion in the lumen more than 50% greater than the surrounding thickness (28). Carotid plaque area (mm2), and plaque echodensity expressed as the gray scale median (GSM) index were measured using an automated computerized edge tracking software M’Ath (Paris, France). (29) Among those with plaque, low GSM (the first tertile of the GSM distribution) is considered echolucent plaque and high GSM (the top tertile) is considered echodense plaque. Total plaque area (TPA) was defined as the sum of all plaque areas measured in any of the carotid artery segments within an individual. (28) Both TPA and GSM were treated as separate dependent variables and each variable was categorized into four categories: For TPA, there were 4 categories: no plaque (reference), mild plaque (tertile 1 TPA), moderate plaque (tertile 2 TPA), and severe plaque (tertile 3 TPA). For GSM, there were 4 categories: no plaque (reference), echolucent plaque (tertile 1 GSM), intermediate density (tertile 2 GSM), and echodense (tertile 3 GSM).
Statistical Analysis
Multinomial logistic regression models with no plaque as the reference were constructed to examine the association between tHcy (continuous and in quartiles) and each plaque phenotype after adjusting for demographics (age, sex and race/ethnicity) in model 1, demographics and vascular risk factors (diabetes, hypertension, HDL, LDL, BMI, smoking, and alcohol use) in model 2, and demographics, vascular risk factors, renal insufficiency, and vitamin B12 deficiency in model 3.
Results
Cohort characteristics
Baseline cohort characteristics are shown in table 1. Among the 1327 subjects, the mean age was 66±9 years, 41% were men, 19% non-Hispanic black, 62% Hispanic, and 17% non-Hispanic white. Renal insufficiency (serum creatinine>1.5 mg/dL) was observed in only 38 participants (3%), while vitamin B12 deficiency (methylmalonic acid>271 nmol/L) was observed in 177 participants (13%).
Table 1.
Demographic and vascular risk factors overall and in relation to mean plaque density.
| Variable | All N=1327 | No plaque N=575 | Echolucent Plaque (GSM 17.0–80.7) N=250 | Intermediate Density Plaque (GSM 81.0–103.3) N=249 | Echodense Plaque (GSM 103.5–180.0) N=250 |
|---|---|---|---|---|---|
|
| |||||
| Age, mean (SD)* | 66 (9) | 63(8) | 66(8) | 69(9) | 68(8) |
|
| |||||
| sex, % | |||||
| Male | 41 | 37 | 51 | 39 | 39 |
| Female | 59 | 63 | 45 | 61 | 61 |
|
| |||||
| Race/ethnicity, %* | |||||
| Black | 19 | 18 | 19 | 19 | 20 |
| White | 17 | 12 | 20 | 24 | 23 |
| Hispanic | 62 | 68 | 59 | 56 | 57 |
|
| |||||
| Diabetes, %* | 20 | 15 | 22 | 22 | 24 |
|
| |||||
| Hypertension, %* | 71 | 67 | 72 | 75 | 78 |
|
| |||||
| HDL, mean (SD) | 46 (14) | 46 (14) | 45 (14) | 46(14) | 47(15) |
|
| |||||
| LDL, mean (SD) | 129 (34) | 127 (33) | 132 (35) | 131(35) | 127(36) |
|
| |||||
| BMI, mean (SD)* | 28 (5) | 29 (5) | 28 (5) | 28(5) | 28(5) |
|
| |||||
| Smoking, %* | |||||
| Never | 48 | 56 | 43 | 44 | 40 |
| Former | 37 | 31 | 35 | 44 | 44 |
| Current | 15 | 13 | 22 | 12 | 17 |
|
| |||||
| Moderate alcohol use†, % | 38 | 36 | 43 | 39 | 38 |
|
| |||||
| Renal insufficiency†, % | 3 | 2 | 2 | 4 | 4 |
|
| |||||
| B12 Deficiency†, % | 13 | 10 | 15 | 17 | 15 |
P<0.05 across categories of plaque density
Moderate alcohol: current drinking of >1 drink per month and ≤2 drinks per day, Renal insufficiency: serum creatinine>1.5 mg/dL, B12 deficiency: methylmalonic acid>271 nmol/L
The mean tHcy was 9.4±4.8μmol/L and the median was 8.6μmol/L. Figure 1 shows the distribution of tHcy in the study population. tHcy quartiles were 3.0–7.0μmol/L, 7.1–8.5μmol/L, 8.6–10.4μmol/L, and 10.5–86.3μmol/L. In regards to clinically utilized cut points, 24% had tHcy 10–15μmol/L and 6%had tHcy≥ 15 μmol/L. Elevated tHcy was greater among men, non-Hispanic blacks, and those with renal insufficiency and B12 deficiency (not shown)
Figure 1.
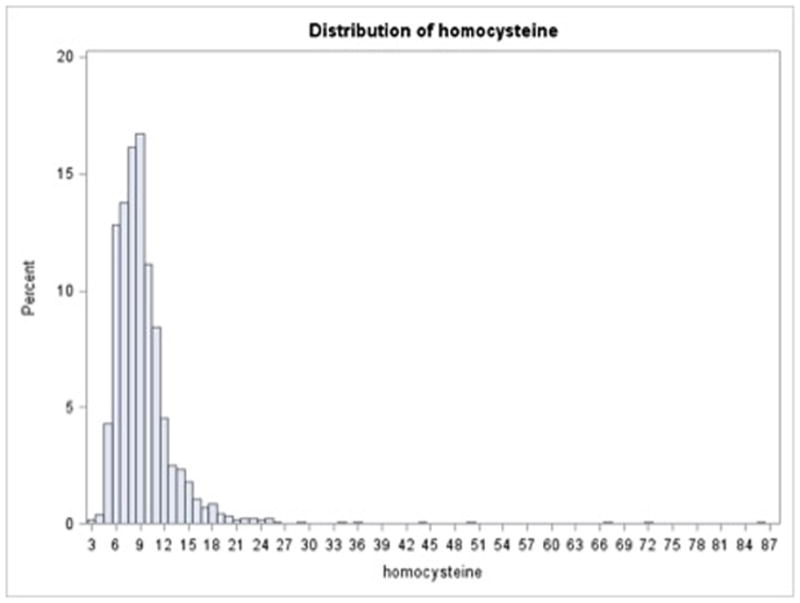
Distribution of homocysteine in the study population
For 40% of the study population, carotid ultrasound was performed at baseline when the homocysteine levels were measured. The other 60% of the study population had their carotid ultrasound after baseline. The mean and median time span between baseline homocysteine measurement and carotid ultrasound was 3 years (range=0–13 years). Carotid plaque was detected in 752(57%) participants (70% in whites, 52% in Hispanics, and 58% in blacks). Among those with carotid plaque, the mean plaque area was 20.3±20.6 mm2 and the median was 13.6 mm2 (Tertile 1/mild TPA: N=257, mean area=5.2, range=1.3–9.1; Tertile 2/moderate TPA: N=255, mean area=14.2, range=9.1–21.5; Tertile 3/severe TPA: N=237, mean area=43.2, range=21.5- 155.9). Among those with carotid plaque, the mean plaque echodensity was 90.9±28.5, and the median was 93.0 (Tertile 1/echolucent plaque: N=250, mean density=58.7, range=17.0–80.7; Tertile 2/intermediate density plaque: N=249, mean density =92.7, range=81.0–103.3; Tertile 3/echodense plaque: N=250, mean density =121.4, range=103.5–180.0). Among non-Hispanic blacks with plaque, the mean plaque area was 22.7±23.1, median=13.8 mm2, and the mean density was 91.8±27.6, median=95.6. Among Hispanics with plaque, the mean plaque area was 18.1±19.5, median=12.0 mm2, and the mean density was 90.7±29.1, median=92.0. Among non-Hispanic whites with plaque, the mean plaque area was 23.6±20.4, median=17.4 mm2, and the mean density was 90.8±28.0, median=94.8. Table 1 shows the characteristics of the study population stratified by category of GSM.
Association between homocysteine and plaque
Table 2 shows the relationship between homocysteine quartiles and carotid plaque density and plaque area in model 3 and the appendix table provides the results from models 1 and 2. When tHcy was examined as a continuous variable, an association between increased levels of tHcy and having echodense plaque was suggested, although a clear dose-response relationship was not observed. In all three multivariable-adjusted models, tHcy quartiles 3 and 4 were significantly associated with a greater prevalence of both echolucent plaque and echodense plaque. No significant association was observed for the second quartile of tHcy with GSM.
Table 2.
Adjusted* Odds ratio (95% CI) for homocysteine and plaque echodensity and area
| Outcome | Homocysteine£ | ||||
|---|---|---|---|---|---|
| Plaque echodensity | Quartile 1 (3.0–7.0 μmol/L) | Quartile 2 (7.1–8.5 μmol/L) | Quartile 3 (8.6–10.4 μmol/L) | Quartile 4 (10.5–86.3 μmol/L) | Continuous |
| Echolucent Plaque vs. no plaque | Ref | 0.9(0.6–1.5) | 1.8(1.2–2.8) | 1.9(1.2–3.1) | 1.0 (1.0–1.1) |
| Intermediate density plaque vs. no plaque | Ref | 1.0(0.7–1.6) | 1.0(0.7–1.7) | 1.3(0.8–2.1) | 1.0(1.0–1.1) |
| Echodense Plaque vs. no plaque | Ref | 1.1(0.7–1.7) | 1.7(1.1–2.7) | 1.9(1.2–3.2) | 1.0 (1.0–1.1) |
| Plaque area | |||||
| Mild TPA vs. no plaque | Ref | 1.0 (0.7–1.6) | 1.3 (0.9–2.0) | 1.7 (1.0–2.7) | 1.0 (1.0–1.1) |
| Moderate TPA vs. no plaque | Ref | 1.0 (0.7–1.6) | 1.5 (1.0–2.4) | 1.4 (0.9–2.3) | 1.0 (1.0–1.1) |
| Severe TPA vs. no plaque | Ref | 1.0 (0.6–1.7) | 1.8 (1.1–3.0) | 2.2 (1.3–3.7) | 1.0 (1.0–1.1) |
Adjusted for demographics, vascular risk factors, renal insufficiency, vitamin B12 deficiency
The cutoff thresholds for tHcy quartiles (1–4): 3–7, 7.1–8.5, 8.6–10.4, and 10.5–86.3 μmol/l
Examination of tHcy as a continuous variable suggested that increasing tHcy was associated with an increasing risk of being in the severe category of TPA vs. having no plaque. Across all three multivariable-adjusted models, those in the 3rd and 4th quartiles of tHcy were more likely to be in the severe TPA category.
No significant interactions were observed between tHcy and race/ethnicity, renal insufficiency and vitamin B12 deficiency in relation to the plaque phenotypes in model 3 (p<0.05).
Discussion
Our study is among the first to evaluate the relationship between tHcy and carotid plaque density measured by the ultrasonographic GSM (gray-scale median) index. We have observed a U-shaped relationship between tHcy and GSM, such that elevated levels of tHcy are independently associated with both echolucent, low-density plaques with low content of calcification, and echodense, high-density plaques with high content of calcification. Both echolucent plaque as vulnerable plaque prone to ulceration and echodense plaques as a marker of generalized atherosclerosis have been associated with increased risk of stroke. (18,30–33) Among patients with asymptomatic carotid stenosis, plasma tHcy was significantly higher in those with microemboli detected by transcranial Doppler. This was confirmed in a later study, which showed higher levels of tHcy among subjects with microemboli, but not with ulceration of carotid plaques. (34,35) These findings suggests that plaque density and its embolic potential may be useful markers of vascular disease and endpoints in future clinical trials examining tHcy, because previous trials using recurrent CVD as an outcome have failed to demonstrate clinical benefits of tHcy modification. (33,36)
Our results support the possibility of an atherogenic role of tHcy. Homocysteine is hypothesized to impact the etiology of atherosclerosis through its involvement in complex pathways of inflammation and calcification. (37) tHcy may promote plaque formation through various mechanisms that are still not well understood. It is has been postulated that tHcy may increase clotting factors, tissue factor expression, platelet aggregation, and inhibit the anticoagulant protein thrombomodulin. It may also cause abnormalities in the function of fibrinogen and thrombin generation. (38–43)
We have shown that elevated tHcy is an independent risk factor for greater plaque burden, as measured by total carotid plaque area, confirming the results from previous studies. (1–10,44) In the Atherosclerosis Risk in Communities Study, participants in the top homocysteine quintile were 3.16 times at risk of developing a thickened carotid wall, a distinct yet related atherosclerotic phenotype, as compared to those in the bottom quintile. (2) tHcy was also positively associated with carotid intima-media thickness (IMT) and focal plaque formation in a cohort with broad age range from 27–77 years and mutation of Methylenetetrahydrofolate Reductase.(4) The findings of our study extend this association to a race-ethnic diverse population with a large percentage of blacks and Hispanics at an increased risk of stroke. Also, we used TPA (total plaque area), which is a measure more related to atherosclerosis burden and risk of stroke than IMT. (14)
Although effect modification by race/ethnicity was not suggested for the associations between tHcy and plaque burden and echodensity, the power to detect interactions was limited. Our findings did not support a monotonic dose-response relationship, and suggested that an elevation in carotid plaque burden and echodensity was observed at tHcy levels that are not conventionally believed to be “pathological” (i.e. <15 μmol/L). Compared to other observational studies, tHcy quartiles cut points were lower (45–47), which raises an important clinical question: how much should tHcy levels be lowered since, the definition of “normal tHcy level” is based on population means. While our current and prior NOMAS results suggest a relationship between elevated tHcy and carotid atherosclerosis, as well as incidence of stroke, MI and vascular death (15), results from large clinical trials (VISP and VITATOPS) did not support the use of vitamin B supplements as a secondary preventive measure to reduce the incidence of recurrent stroke and transient ischemic attacks (33,36). However, the effect of lowering tHcy on atherosclerotic lesions in primary prevention is still unknown.
Limitations of our study include the primarily cross-sectional design, which limits inferences about temporality and causality. However, in a previous prospective study in our cohort we showed that elevated homocysteine was associated with an increased risk of vascular events, including ischemic stroke. (15) The results of the current study suggest that increased plaque burden may be an underlying mechanism through which homocysteine is associated with an elevated risk of vascular events including ischemic stroke in our cohort. We did not systematically measure folate and vitamin B6 levels, important predictors of tHcy level, which may bias the association. However these factors may not be as important as previously thought, since the era of folic acid fortification the incidence of folate deficiency has dropped from 22% to less than 2%(47), and no well-documented vascular benefits of vitamin B6 use exist. (33,36)
In conclusion, the current study builds on existing and consistent evidence that tHcy is a modifiable risk factor for carotid atherosclerosis, now associated with two novel imaging biomarkers of atherosclerosis, carotid plaque morphology (echodensity) and, total carotid plaque area, in an ethnically diverse population.
Supplementary Material
Acknowledgments
Funding:
This research was supported by the grants from the National Institutes of Health/National Institute of Neurological Diseases and Stroke (R37 NS 29993; and K24 NS 062737).
Footnotes
Conflict of interest: None.
References
- 1.Dietrich M, Jacques PF, Polak JF, Keyes MJ, Pencina MJ, Evans JC, et al. Segment-specific association between plasma homocysteine level and carotid artery intima-media thickness in the Framingham Offspring Study. J Stroke Cerebrovasc Dis. 2011;20:155–61. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McQulillan MB, Beibly JP, Nidrof M, Thompson PL, Hung J. Hyperhomocysteinemia but not the C677T mutation of methylene tetrahydrofolatereductase is an indepenedent risk determinant of wall thickening: The Perth Carotid Ultrasound Disease Assessment Study (CUDAS) Circulation. 1999;99:2383–2388. doi: 10.1161/01.cir.99.18.2383. [DOI] [PubMed] [Google Scholar]
- 3.Adachi H, Hirai Y, Fujiura Y, Matsuoka H, Satoh A, Imaizumi T. Plasma Homocysteine Levels and Atherosclerosis in Japan Epidemiological Study by Use of Carotid Ultrasonography. Stroke. 2002;33:2177–2181. doi: 10.1161/01.str.0000026861.18199.89. [DOI] [PubMed] [Google Scholar]
- 4.Malinow R, Nieto FJ, Szklo M, Chambless LE, Bond G. Carotid Artery Intimal-Medial Wall Thickening and Plasma Homocysteine in Asymptomatic Adults: The Atherosclerosis Risk in Communities Study. Circulation. 1993;87:1107–1113. doi: 10.1161/01.cir.87.4.1107. [DOI] [PubMed] [Google Scholar]
- 5.Nukata M, Taguchi A, Kitagawa K, Kinoshita N, Sasaki M, Watanabe M, et al. Association of Plasma Homocysteine Concentration With Atherosclerotic Carotid Plaques and Lacunar Infarction. Stroke. 2002;33:1493–1496. doi: 10.1161/01.str.0000016463.01398.d0. [DOI] [PubMed] [Google Scholar]
- 6.Wilcken DE, Wilcken B. The pathogenesis of coronary artery disease. A possible role for methionine metabolism. J Clin Invest. 1976;57:1079–1082. doi: 10.1172/JCI108350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. Jama. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 8.Collaboration HLT. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J ClinNutr. 2005;82:806–12. doi: 10.1093/ajcn/82.4.806. [DOI] [PubMed] [Google Scholar]
- 9.Heijer MD, Rosendaal FR, Blom HJ, Gerrits WB, Bos GM. Hyperhomocysteinemia and Venous Thrombosis: A Meta-analysis. Thromb Haemost. 1998;80:874–877. [PubMed] [Google Scholar]
- 10.Spence JD, Barnett PA, Hegele RA, Marian AJ, Freeman D, Malinow MR. Plasma Homocysteine, but not MTHFR genotype, is associated with variation in carotid plaque area. Stroke. 1999;30:969–973. doi: 10.1161/01.str.30.5.969. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics—2010 update: A report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2010;121:e1–e170. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 12.Rundek T, Sacco RL. Risk Factor Management to Prevent First Stroke. NeurolClin. 2008;26:1007–1045. doi: 10.1016/j.ncl.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roman Sztajzel R. Ultrasonographic assessment of the morphological characteristics of the carotid plaque. Swiss Med Wkly. 2005;135:635–643. doi: 10.4414/smw.2005.11038. [DOI] [PubMed] [Google Scholar]
- 14.Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid Plaque Area: A tool for targeting and evaluating vascular preventive therapy. Stroke. 2002;33:2916–2922. doi: 10.1161/01.str.0000042207.16156.b9. [DOI] [PubMed] [Google Scholar]
- 15.Sacco RL, Anand K, Seung Lee H, Boden-Albala B, Stabler S, Allen R, et al. Homocysteine and the Risk of Ischemic Stroke in a Triethnic Cohort The Northern Manhattan Study. Stroke. 2004;35:2263–2269. doi: 10.1161/01.STR.0000142374.33919.92. [DOI] [PubMed] [Google Scholar]
- 16.Spence JD, Blake C, Landry A, Fenster A. Measurement of carotid plaque and effect of vitamin therapy for total homocysteine. ClinChem Lab Med. 2003;41:1498–1504. doi: 10.1515/CCLM.2003.230. [DOI] [PubMed] [Google Scholar]
- 17.Robertson J, Iemolo F, Stabler SP, Allen RH, Spence JD. Vitamin B12, homocysteine and carotid plaque in the era of folic acid fortification of enriched cereal grain products. CMAJ. 2005;172:1569–1573. doi: 10.1503/cmaj.045055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Della-Morte D, Moussa I, Elkind MS, Sacco RL, Rundek T. The short-term effect of atorvastatin on carotid plaque morphology assessed by computer-assisted gray-scale densitometry: a pilot study. Neurol Res. 2011;33:991–994. doi: 10.1179/1743132811Y.0000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spence JD, Stampfer MJ. Understanding the complexity of homocysteine lowering with vitamins: the potential role of subgroup analyses. JAMA. 2011;306 (23):2610–2611. doi: 10.1001/jama.2011.1834. [DOI] [PubMed] [Google Scholar]
- 20.Office of Management and Budget. Race and ethnic standards for federal statistics and administrative reporting. Federal Register. 1978 May 4;43:19269. Directive No. 15. [PubMed] [Google Scholar]
- 21.Elkind MS, Cheng J, Boden-Albala B, Paik MC, Sacco RL. Elevated White Blood Cell Count and Carotid Plaque Thickness: The Northern Manhattan Stroke Study. Stroke. 2001;32:842–849. doi: 10.1161/01.str.32.4.842. [DOI] [PubMed] [Google Scholar]
- 22.Gardener H, Della Morte D, Elkind M, Sacco RL, Rundek T. Lipid and carotid plaque in the Northern Manhattan Study (NOMAS) BMC cardiovascular Disorder. 2009;9:55. doi: 10.1186/1471-2261-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatri M, Wright CB, Nickolas TL, Yoshita M, Paik MC, Kranwinkel PG, et al. Chronic Kidney Disease Is Associated With White Matter Hyperintensity Volume: The Northern Manhattan Study (NOMAS) Stroke. 2007;38:3121–3126. doi: 10.1161/STROKEAHA.107.493593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 25.Holleland G, Schneede J, Ueland PM, Lund PK, Refsum H, Sandberg S. Cobalamin deficiency in general practice. Assessment of the diagnostic utility and cost-benefit analysis of methylmalonic acid determination in relation to current diagnostic strategies. Clin Chem. 1999;45:189–198. [PubMed] [Google Scholar]
- 26.Ducros V, Schmitt D, Pernod G, Faure H, Polack B, Favier A. Gas chromatographic mass spectrometric determination of total homocysteine in human plasma by stable isotope dilution: method and clinical applications. Journal of Chromatography B Biomed Sci Appl. 1999;729:333–339. doi: 10.1016/s0378-4347(99)00171-1. [DOI] [PubMed] [Google Scholar]
- 27.Carrelli AL, Walker MD, Lowe H, McMahon DJ, Rundek T, Sacco RL, et al. Vitamin D Deficiency Is Associated With Subclinical Carotid Atherosclerosis: The Northern Manhattan Study. Stroke. 2011;42:2240–2245. doi: 10.1161/STROKEAHA.110.608539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo F, Gardener H, Dong C, Cabral D, Della-Morte D, Blanton SH, et al. Traditional cardiovascular risk factors explain the minority of the variability in carotid plaque. Stroke. 2012;43:1755–1760. doi: 10.1161/STROKEAHA.112.651059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC, et al. The North American Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke. 1999;30:1751–1758. doi: 10.1161/01.str.30.9.1751. [DOI] [PubMed] [Google Scholar]
- 30.Endarterectomy for asymptomatic carotid artery stenosis: Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 31.Biasi MG, Froio A, Diethrich EB, Deleo G, Galimberti S, Mingazzini P, et al. Carotid Plaque Echolucency Increases the Risk of Stroke in Carotid Stenting: The Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) Study. Circulation. 2004;110:756–762. doi: 10.1161/01.CIR.0000138103.91187.E3. [DOI] [PubMed] [Google Scholar]
- 32.Casscells W, Naghavi M, Willerson JT. Vulnerable atherosclerotic plaque: a multifocal disease. Circulation. 2003;107:2072–2075. doi: 10.1161/01.CIR.0000069329.70061.68. [DOI] [PubMed] [Google Scholar]
- 33.Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, et al. Lowering Homocysteine in Patients With Ischemic Stroke to Prevent Recurrent Stroke, Myocardial Infarction, and Death The Vitamin Intervention for Stroke Prevention (VISP) Randomized Controlled Trial. JAMA. 2004;291:565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 34.Spence JD, Tamayo A, Lownie S, Ng W, Ferguson GG. Absence of microemboli on transcranial Doppler identifies low-risk patients with asymptomatic carotid stenosis who do not warrant endarterectomy or stenting. Stroke. 2005;36:2373–2378. doi: 10.1161/01.STR.0000185922.49809.46. [DOI] [PubMed] [Google Scholar]
- 35.Madani A, Beletsky V, Tamayo A, Munoz C, Spence JD. High-Risk Asymptomatic Carotid Stenosis: Ulceration on 3D ultrasound versus TCD Microemboli. Neurology. 2011;77(8):744–750. doi: 10.1212/WNL.0b013e31822b0090. [DOI] [PubMed] [Google Scholar]
- 36.VITATOPS Study Trial Group. B vitamins in patients with recent transient ischemic attack or stroke in the Vitamins To Prevent Stroke (VITATOPS) trial: a randomized, double blind, parallel, placebo-controlled trial. Lancet Neurol. 2010;9:855–865. doi: 10.1016/S1474-4422(10)70187-3. [DOI] [PubMed] [Google Scholar]
- 37.Held C, Sumner G, Sheridan P, McQueen M, Smith S, Dagenais G, et al. Correlations between plasma homocysteine and folate concentrations and carotid atherosclerosis in high-risk individuals: baseline data from the Homocysteine and Atherosclerosis Reduction Trial (HART) Vascular Medicine. 2008;13:245–253. doi: 10.1177/1358863X08092102. [DOI] [PubMed] [Google Scholar]
- 38.Jones B, Rose F, Tudball N. Lipid peroxidation and homocysteine induced toxicity. Atherosclerosis. 1994;105:165–1670. doi: 10.1016/0021-9150(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 39.Upchurch G, Welch G, Fabian A, Freedman JE, Johnson JL, Keaney JF, et al. Homocysteine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J BiolChem. 1997;272:17012–17017. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- 40.Moat S, Bonham J, Cragg R, Powers H. Elevated plasma homocysteine elicits an increase in antioxidant enzyme activity. Free Rad Res. 2000;32:171–179. doi: 10.1080/10715760000300171. [DOI] [PubMed] [Google Scholar]
- 41.Chambers JC, McGregor A, Jean-Marie J, Obeid OA, Kooner JS. Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia. An effect reversible with vitamin C therapy. Circulation. 1999;99:1156–1160. doi: 10.1161/01.cir.99.9.1156. [DOI] [PubMed] [Google Scholar]
- 42.Chambers JC, Obeid OA, Kooner JS. Physiological increments in plasma homocysteine induce vascular endothelial dysfunction in normal human subjects. Arterioscler Thromb. 1999;19:2922–2927. doi: 10.1161/01.atv.19.12.2922. [DOI] [PubMed] [Google Scholar]
- 43.Selhub J, Jacques PF, Bostom AG, Wilson PW, Rosenberg IH. Relationship between plasma homocysteine and vitamin status in the Framingham study population. Impact of folic acid fortification. Public Health Rev. 2000;28:117–145. [PubMed] [Google Scholar]
- 44.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- 45.Kang SS, Wong PWK, Malinow MR. Hyperhomocysteinemia as a risk factor for occlusive vascular disease. Ann Rev Nutr. 1992;12:279–298. doi: 10.1146/annurev.nu.12.070192.001431. [DOI] [PubMed] [Google Scholar]
- 46.Selhub J, Jacques PF, Bostom AG, D’Agostino RB, Wilson PW, Belanger AJ, et al. Association between plasma homocysteine concentrations and extracranial carotid artery stenosis. N Engl J Med. 1995;332:286–291. doi: 10.1056/NEJM199502023320502. [DOI] [PubMed] [Google Scholar]
- 47.Malinow RM, Bostom AG, Krauss RM. Homocysteine, Diet, and Cardiovascular Diseases: A Statement for Healthcare Professionals From the Nutrition Committee, American Heart Association. Circulation. 1999;99:178–182. doi: 10.1161/01.cir.99.1.178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


