Abstract
N-methyl-D-aspartate receptors (NMDARs) are a subtype of ionotropic glutamate receptor, which play a central role in learning, memory, and synaptic development. NMDARs are assembled as tetramers composed of two GluN1 subunits and two GluN2 or GluN3 subunits. Although NMDARs are widely expressed throughout the central nervous system, their number, localization, and subunit composition are strictly regulated and differ in a cell- and synapse-specific manner. The brain area, developmental stage and level of synaptic activity are some of the factors that regulate NMDARs. Molecular mechanisms that control subunit-specific NMDAR function include developmental regulation of subunit transcription/translation, differential trafficking through the secretory pathway, post-transcriptional modifications such as phosphorylation, and protein-protein interactions. The GluN2A and GluN2B subunits are highly expressed in cortex and hippocampus and confer many of the distinct properties on endogenous NMDARs. Importantly, the synaptic NMDAR subunit composition changes from predominantly GluN2B-containing to GluN2A-containing NMDARs during synaptic maturation and in response to activity and experience. Some of the molecular mechanisms underlying this GluN2 subunit switch have been recently identified. In addition, the balance between synaptic and extrasynaptic NMDARs is altered in several neuronal disorders. Here, we summarize the recent advances in the identification of NMDAR subunit-specific regulatory mechanisms.
NMDA Receptors
NMDA receptors (NMDARs) are ionotropic glutamate receptors, which play a critical role in excitatory neurotransmission in the central nervous system (CNS). NMDARs are cationic channels permeable to sodium, potassium and calcium. The calcium influx through NMDARs is the critical factor that mediates many of the NMDAR-specific physiological and pathogenic conditions. NMDAR activation, and the subsequent increase in postsynaptic calcium concentration, is a trigger for synaptic plasticity, a cascade of events that dramatically modifies synaptic efficacy and neuronal morphology. In classic NMDAR-dependent synaptic plasticity, NMDAR activation can lead to either potentiation or depression depending on the amount and kinetics of the calcium influx. A rapid and robust entry of calcium leads to synaptic strengthening, best characterized in CA1 hippocampal long-term potentiation (LTP). This classic LTP is defined by an increase in the number of AMPA receptors inserted in the postsynaptic membrane and by an enlargement in the postsynaptic spine. In contrast, a smaller influx of calcium over a longer time course triggers the induction of long-term depression (LTD), which results in a decrease in synaptic efficiency via the removal of AMPARs from the postsynaptic membrane and the shrinkage of dendritic spines (Holtmaat and Svoboda 2009).
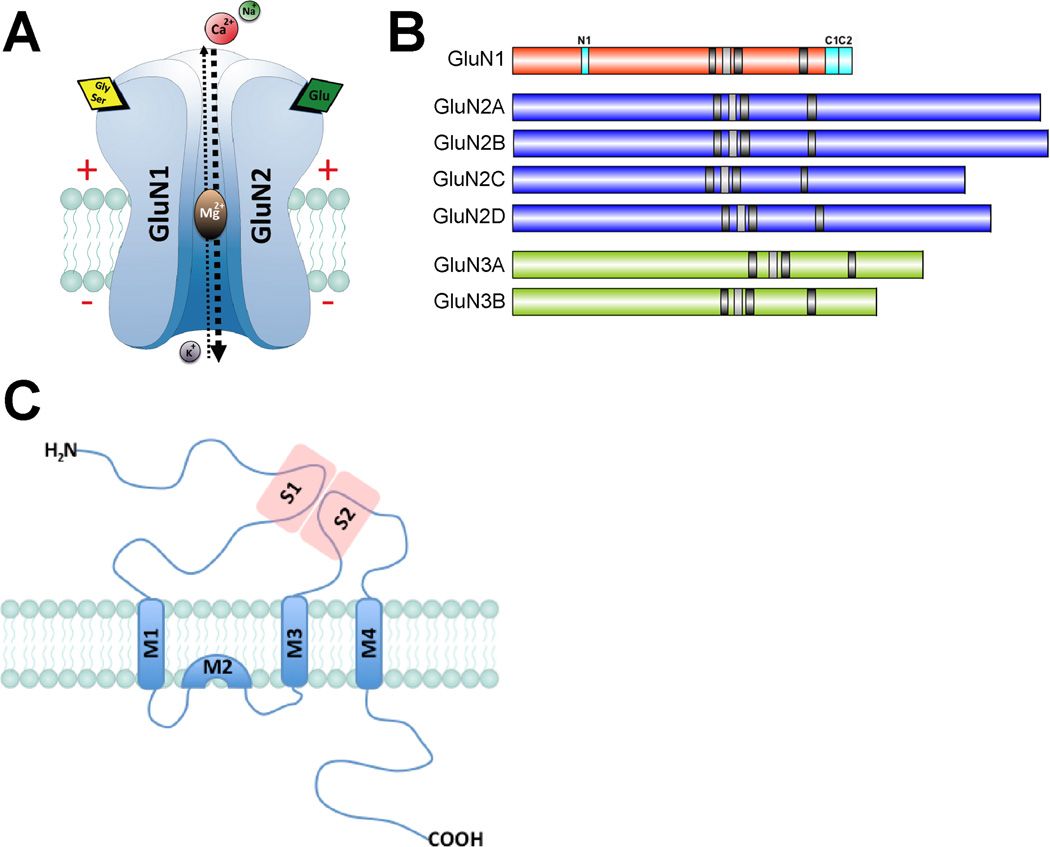
At resting membrane potential, the pore of the NMDAR channel is blocked by physiological levels of extracellular Mg2+. This blockade is voltage-dependent resulting in the unique role of NMDARs as molecular coincidence detectors. Specifically, ion influx only occurs when both presynaptic and postsynaptic neurons are stimulated at the same time. Therefore, NMDAR activation requires postsynaptic depolarization (to relieve the Mg2+ block) that coincides with presynaptic release of glutamate that binds to GluN2 subunits. A third element is required for NMDAR activation: the presence of glycine or D-serine occupying a binding site present in the GluN1 subunit (Labrie and Roder 2010). Both amino-acids are mainly derived from astrocytes and evidence indicates that D-serine is the major endogenous ligand for the glycine/serine binding site (Panatier and others 2006). Figure 1A.
FIGURE 1. NMDA receptor structure, subunits, and topology.
A. NMDA receptors are ionotropic glutamate receptors composed of two GluN1 subunits and two GluN2 or GluN3 subunits. NMDARs are permeable to Ca2+, Na+ and K+. To be activated, they need to bind to glutamate (via GluN2 subunits), glycine (via GluN1) and release the Mg2+ blockade by membrane depolarization. B. Seven different NMDAR subunits have been identified: GluN1, GluN2A-D and GluN3A-B. Three regions of GluN1 (N1, C1 and C2), which are subjected to alternative splicing allows for further heterogeneity. C. Each NMDAR subunit is composed of three transmembrane domains (M1, 3 and 4) and one re-entrant loop (M2). Glutamate binds in the pocket created by two extracellular regions (S1-2) present in the N-terminal tail and the loop between M3 and M4, respectively. The C-terminus is cytoplasmic and varies in length between subunits.
Functional NMDARs are tetramers composed of different subunits (GluN1, GluN2A-D, GluN3A-B). Typically, endogenous NMDARs are di-heteromers comprising two GluN1 subunits and two GluN2 or GluN3 subunits, which assemble as a dimer of dimers. However, NMDARs are also able to assemble as tri-heteromers. Specifically, GluN1/GluN2B/GluN3A or GluN1/GluN2B/GluN2D complexes are expressed at early stages of development and GluN1/GluN2A/GluN2B or GluN1/GluN2A/GluN2C in adulthood (Al-Hallaq and others 2007; Brothwell and others 2008). Alternative splicing of the NMDAR subunits provides additional heterogeneity. For example, GluN1 occurs as eight distinct isoforms encoded by a single gene. Alternatively spliced “cassettes” within the GluN1 C-terminus modulate NMDAR trafficking (Horak and Wenthold 2009) and the pH sensitivity of NMDARs is determined by the inclusion of exon 5 within the extracellular N-terminus of GluN1 (Traynelis and others 1995). It has been reported that GluN2 and GluN3 also exist in several alternatively spliced forms, although the functional differences between them are far from clear. Figure 1B.
NMDAR subunits
NMDAR subunit structure and properties
All of the ionotropic glutamate receptor subunits, including the seven GluNs, share a common membrane topology defined by three transmembrane segments (M1, M3 and M4) and 5 a re-entrant pore loop (M2). The long N-terminal region is extracellular, whereas the C-terminus is intracellular and interacts with multiple cytosolic proteins. For NMDARs, glutamate binds to GluN2 subunits in a binding pocket created by two regions present in the proximal N-terminal domain and the long extracellular loop between M3 and M4 (S1 and S2, respectively). The re-entrant M2 loop is part of the channel pore and it contains a critical asparagine residue that determines calcium permeability of the channel and mediates the magnesium blockade (Mayer and Armstrong 2004). Figure 1C.
Despite their structural similarity, there are dramatic functional differences between NMDAR subunits (see below for a detailed comparison between GluN2A and GluN2B). GluN1 is the product of a single gene and is an obligatory subunit of all endogenous NMDA receptors. The genetic elimination of GluN1 is lethal in neonatal stages, and studies using conditional GluN1 knock-out mice revealed an absence of functional NMDARs as the GluN2 subunits are retained in the ER (Fukaya and others 2003). In addition, GluN1 influences some characteristics of NMDARs such as their inhibition by protons or zinc and their potentiation by polyamines (Cull-Candy and Leszkiewicz 2004). Finally, the GluN1 C-terminus contains several motifs that regulate receptor trafficking and binding to many proteins, including calmodulin, CaMKII, yotiao, alpha-actinin, tubulin, neurofilaments and DREAM (Cull-Candy and Leszkiewicz 2004). GluN2C is highly expressed in the cerebellum in the adult and it shows relatively unique channel properties, including low conductance, open probability and sensitivity to magnesium (Farrant and others 1994). This latter characteristic allows GluN2C to be activated without the requirement of postsynaptic depolarization, at least in some brain areas (Binshtok and others 2006). GluN2D is characterized by its expression early in development and, overall, by its extremely slow decay time (4–5 seconds). Although GluN2D has been identified at extrasynaptic sites in some neurons, the presence of synaptic GluN2D is still controversial. Recently, however, it has been reported that synaptic tri-heteromers including GluN2D and GluN2B exist, which could explain the absence of very long lasting events expected for pure GluN2D-containing NMDARs (Brothwell and others 2008). Although considerably less attention has been dedicated to the GluN3 subunits, it is known that they also have unique properties. For example, unlike GluN2 subunits, GluN3 binds to glycine and not to glutamate. Therefore, NMDARs containing exclusively GluN1/GluN3 subunits can act as excitatory glycine receptors, which are impermeable to calcium. Tri-heteromers containing GluN2 and GluN3 subunits, however, are sensitive to glutamate, but they show a decrease in open probability, calcium permeability and magnesium sensitivity in comparison with GluN1/GluN2 NMDARs (Henson and others 2010). Therefore, the early onset of GluN3A expression and unique channel properties suggest that it may play a role during development and synaptic maturation by attenuating NMDAR function.
NMDAR subunit expression patterns
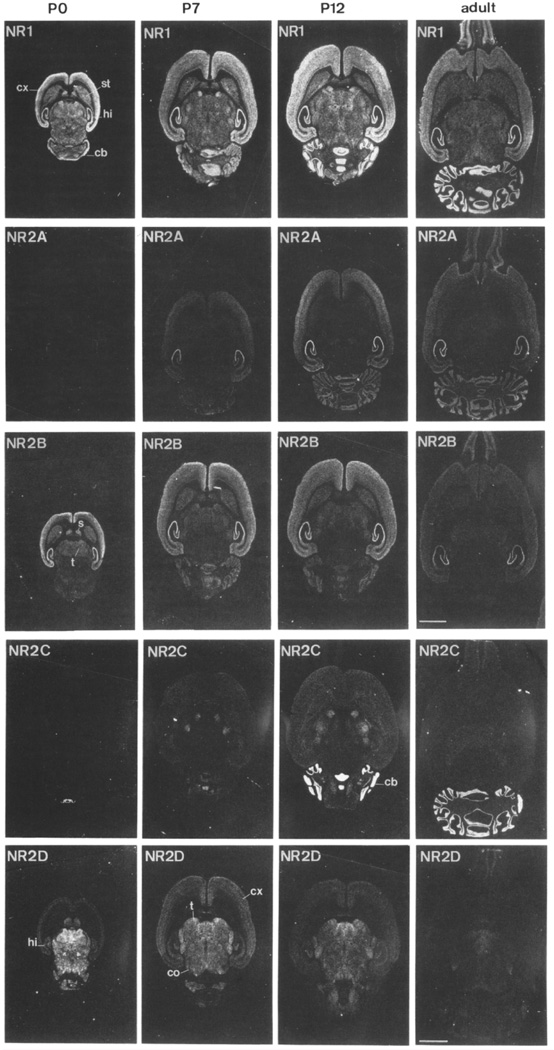
NMDARs are widely distributed throughout the CNS, although the expression of individual subunits is highly dependent on brain area and developmental stage. In fact, NMDAR subunits show distinct, yet often overlapping, expression patterns that allow for subunit-specific function and regulation of NMDARs in a region- and age-dependent manner. Figure 2. Even GluN1, which is uniformly expressed in the CNS before birth (beginning at E14), displays isoform-specific differences in expression. For example, GluN1 expressing the N-terminal cassette is restricted to the caudate, cerebellum and thalamus (Laurie and Seeburg 1994). However, the functional significance of the differential expression of GluN1 isoforms is not clear. The diversity in GluN2 subunit expression is more profound. Unlike GluN1, there are four genes encoding GluN2 subunits and each has a unique spatiotemporal profile. For example, GluN2B is widely expressed during prenatal development and, in adult brain, it is restricted to the forebrain. In sharp contrast, GluN2A expression is ubiquitous in the CNS, starts at very low levels around the time of birth, and increases dramatically during the second postnatal week. GluN2C expression is first detected postnatally (P10-11) and it is highly enriched in the adult cerebellum. GluN2D is present early in development and is strongest in the diencephalon, mesencephalon and spinal cord in adulthood (Monyer and others 1997). GluN3 subunits also display differential expression patterns, with GluN3A peaking in early postnatal life and GluN3B increasing throughout development (Henson and others 2010). Therefore, NMDAR subunit content is subject to strict spatiotemporal regulation allowing for functional differences following NMDAR activation at different synapses throughout the brain.
FIGURE 2. GluN1 and GluN2 subunits display different spatiotemporal expression.
In situ hybridization showing the developmental profile of GluN1 and GluN2 subunits in horizontal rat brain sections. “cx” denotes “cortex”, “st” striatum, “hi” hippocampus, “cb” cerebellum, “t” thalamus, “s” septum and “co” colliculi. Reprinted from Figure 2 in Monyer et al., 1994.
There are also important differences in the subcellular expression of the NMDAR subunits. For example, GluN1 exists in two pools: a population in the plasma membrane, assembled with GluN2 or GluN3 subunits, and another pool retained in the ER with a short half-life (Huh and Wenthold 1999). GluN1 retention in the ER is modulated by alternative splicing and PKC phosphorylation (Scott and others 2001). In contrast, GluN2 subunits are mainly localized at the plasma membrane. Although there are some reports of presynaptic NMDARs (Corlew and others 2008), typically NMDARs are localized at postsynaptic sites throughout the CNS. The current simplified model is that GluN2A-containing NMDARs are predominantly expressed at synaptic sites whereas GluN2B-containing NMDARs are enriched at extrasynaptic sites in the adult CNS (Groc and others 2009).
GluN2A vs GluN2B
Over the past few decades GluN2A and GluN2B have been the subject of intense investigation. Both subunits are highly expressed in cortex and hippocampus, and play a central role in synaptic function by controlling synaptic plasticity and metaplasticity. In addition, both subunits are involved in learning and memory and implicated in several neurological disorders and diseases. However, despite the intense research efforts, many open questions and controversial points about mechanisms regulating GluN2A and GluN2B subunits still remain.
Like all NMDAR subunits, GluN2A and GluN2B have unique characteristics. Table 1. First, GluN2A-containing receptors have faster kinetics than GluN2B-containing receptors. Electrophysiological studies of single channels in heterologous systems (such as HEK293 cells or Xenopus oocytes) expressing either GluN1/2A or GluN1/2B receptors showed that GluN2A-containing channels have a higher open probability (Erreger and others 2005) and a faster deactivation time (Vicini and others 1998) than GluN2B-containing ones. Although it has also been reported that exogenous GluN2A and GluN2B subunits exhibit similar open probabilities when expressed in cultured cerebellar granule cells (CGCs) (Prybylowski and others 2002), recent data examining hippocampal synapses that contain only GluN2A or GluN2B (Gray and others 2011) support the difference in the open probabilities reported previously for expressed receptors.
TABLE 1.
Comparison of GluN2A and GluN2B subunits
| GluN2A | GluN2B | References | |
|---|---|---|---|
| Kinetics | Fast | Slow |
Gray and others 2011 Vicini and others 1998 |
| Open probability | High | Low | Erreger and others 2005 |
| Deactivation time | Fast | Slow | Vicini and others 1998 |
| Regional expression pattern | Ubiquitous in CNS | Forebrain |
Monyer and others 1994 Wenzel and others 1997 |
| Developmental expression pattern | Increases during second developmental week | Slightly reduced throughout development |
Monyer and others 1994 Wenzel and others 1997 |
| Localization in mature neurons | Mainly synaptic | Synaptic and extrasynaptic | Groc and others 2009 |
| Surface expression | Relatively stable | Dynamic | Groc and others 2006 |
| Postendocytic sorting | Late endosomes | Recycling endosomes | Lavezzari and others 2004 |
| Role of PDZ ligand | Unclear | Essential for synaptic expression | Prybylowski and others 2005 |
GluN2A and GluN2B subcellular localization and regulation
The affinity of glutamate for GluN2B is higher than for GluN2A; however, synaptic glutamate release in adult neurons results in a higher activation of GluN2A- than GluN2B-containing NMDARs. This seemingly contradictory finding is likely due to the segregated subcellular localization of these subunits, which favors GluN2A activation (predominantly expressed at synaptic sites) over GluN2B (predominantly enriched at extrasynaptic sites). This model relies largely on the use of selective GluN2B-containing NMDAR inhibitors such as ifenprodil, Ro25-6981 or CP101,606. Therefore, only approximately 30 % of the NMDAR-mediated excitatory postsynaptic current (NMDAR-EPSC) can be blocked by ifenprodil in adulthood, indicating the predominant presence of GluN2A at synaptic sites. A similar approach supports the existence of GluN2B subunits at extrasynaptic sites. The irreversible blocking of synaptic NMDARs (by incubation with the open channel blocker MK-801) demonstrates that the NMDAR pool that can still be activated (i.e. extrasynaptic) is sensitive to ifenprodil (Kew and others 1998). In addition, glutamate spillover (from astrocytes or neighboring neurons) activates mainly ifenprodil-sensitive NMDARs (Scimemi and others 2004). Some evidence, however, challenges this model and, for example, experiments using uncaged glutamate suggest a similar sensitivity for ifenprodil between synaptic and extrasynaptic sites (Harris and Pettit 2007). In any case, the segregated subcellular localization is not absolute, as the presence of GluN2A in the extrasynaptic membranes of cultured neurons has been reported (Thomas and others 2006) and GluN2B is also present at the postsynaptic density (PSD).
How is the subcellular localization of GluN2 subunits regulated? It is believed that the protein-protein interactions in the cytoplasmic C-terminus and the extracellular N-terminus of the receptor determine the precise localization of GluN2 subunits. For example, the PDZ binding motif at the extreme C-terminus of both GluN2A and GluN2B subunits binds to the second PDZ domain of MAGUK proteins, which act as scaffolding proteins. Members of this family (PSD-93, PSD-95, SAP97 and SAP102) show differential subcellular localization, with PSD-95 predominantly expressed at the postsynaptic density and SAP102 being distributed more evenly between synaptic and extrasynaptic sites. In addition, a preferential association of GluN2A/PSD-95 and GluN2B/SAP102 has been reported (Sans and others 2000 although see Al-Hallaq and others 2007). Therefore, a working model proposes that binding of GluN2 subunits to different MAGUK proteins controls NMDAR localization. Current data support this scenario for GluN2B because the disruption of the GluN2B PDZ binding domain results in a lost of synaptic GluN2B as demonstrated by electrophysiological and confocal imaging approaches (Chung and others 2004; Prybylowski and others 2005). In contrast, the literature for GluN2A is less consistent because GluN2A expressing a point mutation disrupting its PDZ binding domain has similar NMDA-mEPSCs compared to GluN2A wild-type in transfected CGCs (Prybylowski and others 2005, but see Barria and Malinow 2002). However, a genetically-modified mouse line expressing GluN2A lacking the C-terminus (GluN2AΔC/ΔC) (Sprengel and others 1998) shows a reduced synaptic GluN2A expression, as revealed by biochemical subcellular fractionation and electron microscopy (Steigerwald and others 2000). Consistently, GluN2AΔC/ΔC mice display slower NMDAR kinetics, indicating a decrease in synaptic GluN2A (Steigerwald and others 2000). A possible explanation for these data is the existence of additional protein binding domains, other than the PDZ binding, in the GluN2A C-terminus that act to stabilize the receptor at synaptic sites. Recent reports identifying PDZ-independent binding sites between GluN2 and MAGUKs support this model (Chen and others 2011; Cousins and others 2009). The extracellular domain of NMDARs also plays a role in controlling their subcellular localization via interaction with postsynaptic proteins such as the activated EphB receptor (Dalva and others 2000) and with components of the extracellular matrix (ECM) such as reelin (Groc and others 2007). Other proteins are likely to modulate NMDAR localization via indirect processes, including neuroligins or integrins (Jung and others 2010).
Synaptic versus extrasynaptic NMDARs
What has become clear during the past few years is the dramatic difference between the activation of synaptic or extrasynaptic NMDARs. Whereas the activation of synaptic NMDARs triggers intracellular cascades promoting cell survival, the entry of calcium through extrasynaptic NMDARs leads to neuronal death via mitochondrial dysfunction (a process known as excitotoxicity) (Hardingham and Bading 2010). Specifically, synaptic NMDAR activation induces the expression of the cyclic-AMP response element binding protein (CREB) transcription factor that plays well-known roles in neuronal survival. In addition, it blocks the transcription of several pro-apoptotic and oxidative genes (such as Puma and APAF1 or Txnip, respectively). In contrast, activation of extrasynaptic NMDARs results in neuronal death mediated by a variety of pathways, including CREB dephosphorylation, ERK1/2 inactivation and activation of pro-apoptotic genes. The activation of extrasynaptic NMDARs has been shown in pathological circumstances such as ischemia, but it is unclear if this pool of NMDARs can be activated under physiological conditions. It is also not known if the GluN2 subunit composition of extrasynaptic NMDARs determines the precise intracellular signaling cascade triggered by NMDAR activation; more specifically, if the deleterious effect of the calcium entry through extrasynaptic NMDARs depends on its GluN2 content.
GluN2A and GluN2B trafficking and regulation
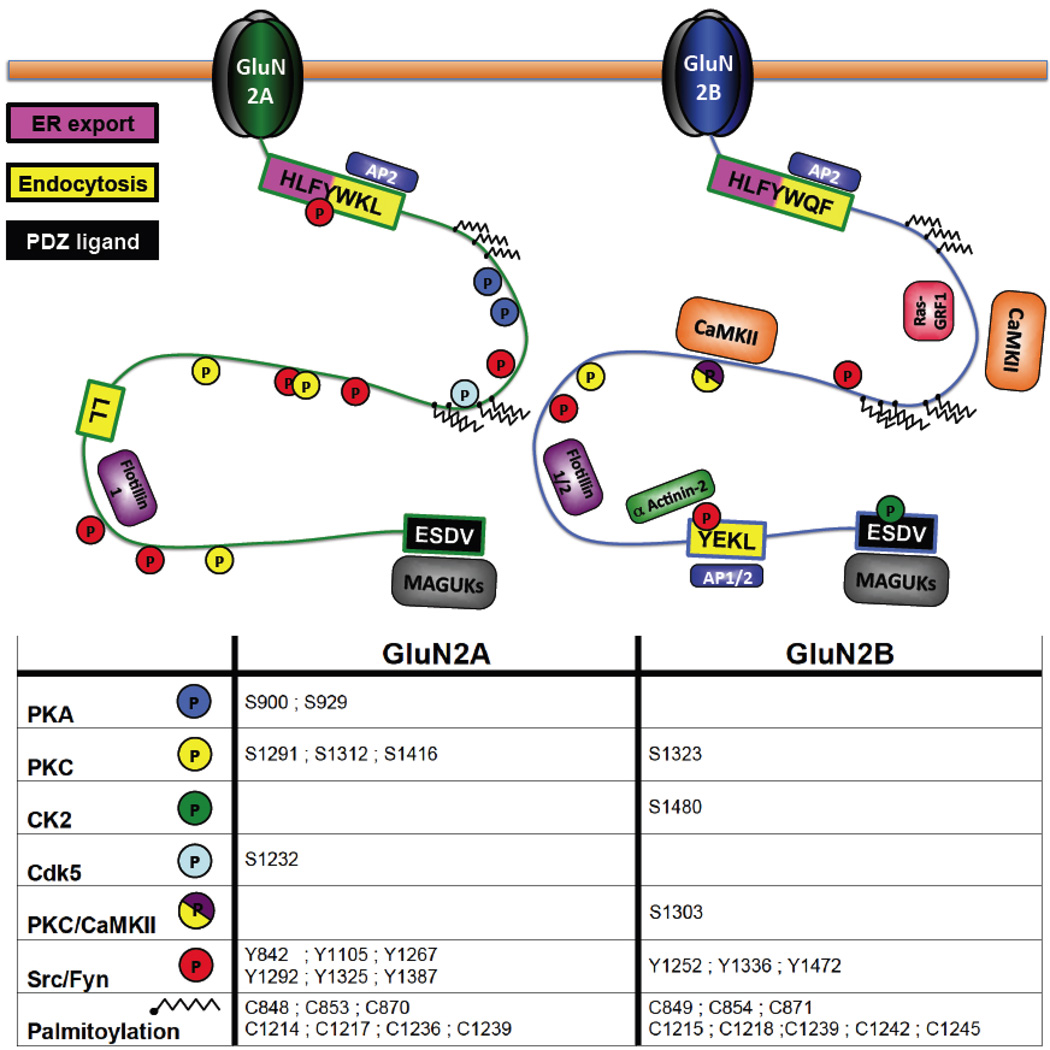
The cytoplasmic C-tails of GluN2A and GluN2B contain distinct motifs that control their trafficking Figure 3. Evidence supports a model in which GluN2B is more mobile than GluN2A and it is subject to regulated lateral diffusion, endocytosis and recycling. Using two different approaches, it has been demonstrated that NMDARs move in and out of synapses, and that GluN2B-containing NMDARs have a much higher (250-fold) surface mobility. First, Tovar and Westbrook blocked synaptic NMDARs using the irreversible inhibitor MK-801 and monitored the recovery of NMDAR-EPSCs. They found that approximately 65% of blocked (i.e. synaptic) NMDARs were replaced in less than 10 minutes, indicating a high level of mobility (Tovar and Westbrook 2002). A few years later, Groc and collaborators used the single particle detection approach (labeling an antibody against an extracellular epitope with a quantum dot) to demonstrate that GluN2B-containing NMDARs have a much higher diffusion coefficient than GluN2A at both synaptic and extrasynaptic sites (Groc and others 2006). A possible molecular explanation of these data emerges with a report showing that GluN2B is phosphorylated by casein kinase 2 (CK2) within its PDZ-binding domain, which disrupts the association with scaffolding proteins, whereas GluN2A is not (Sanz-Clemente and others 2010). The phosphorylation of the PDZ ligand domain, however, is NMDAR-activity dependent whereas the coefficient of diffusion for GluN2B is not modified by synaptic activity. Also, GluN2B shows a higher ratio of endocytosis than GluN2A in adult neurons (Lavezzari and others 2004). Another important difference in GluN2 subunit trafficking is post-endocytic sorting. Whereas GluN2B is sorted to recycling endosomes (Rab11-positive) and re-inserted into the plasma membrane, GluN2A colocalizes better with Rab9 in late endosomes following endoctyosis and is subjected to lysosomal degradation (Lavezzari and others 2004; Tang and others 2010).
FIGURE 3.
The GluN2A and GluN2B C-termini contain distinct regulatory motifs, phosphorylation sites, and protein-protein interaction domains.
GluN2A and GluN2B are subject to differential regulation by several post-translational mechanisms, including palmitoylation and nitrosylation. However, the best characterized example is the modulation of NMDARs by phosphorylation (Chen and Roche 2007) Figure 3. For example, it has been shown that the phosphorylation of the GluN2A C-terminus by cyclin-dependent kinase 5 (cdk5) increases NMDAR currents and cdk5 inhibition has protective effects against ischemic insults (Wang and others 2003). However, no cdk5-mediated phosphorylation has been reported so far for GluN2B. Conversely, CK2 and calcium- and calmodulin-dependent kinase II (CaMKII) phosphorylate GluN2B on S1480 (within its PDZ binding domain) and S1303 (within the CaMKII binding site) respectively, but not GluN2A (Omkumar and others 1996; Sanz-Clemente and others 2010). It should be noted, however, that S1291 on GluN2A and S1096 on GluN2C (analogous to S1303 on GluN2B) are phosphorylated by PKC and PKB, respectively (Chen and Roche 2009; Jones and Leonard 2005). Other kinases such as PKA, PKC and several protein tyrosine kinases (Fyn and Src) phosphorylate both GluN2A and GluN2B subunits, although the precise residues and the consequences of their phosphorylation also differ.
As discussed above, the interaction of GluN2 subunits with MAGUKs and other synaptic proteins defines their differential subcellular localization. Similarly, many other parameters are determined by their distinct protein-protein interactions, including trafficking regulation and activation of intracellular pathways. One of the classic examples is the binding of CaMKII to NMDARs. It has been reported that CaMKII binds to GluN2A in vitro but this interaction is much weaker than the well-documented association between CaMKII and GluN2B (Bayer and others 2001). Calcium entry through NMDARs activates CaMKII that can then bind to GluN2B (residues 1290–1310). This CaMKII-GluN2B association is regulated by the CaMKII/PKC phosphorylation of S1303 within the CaMKII binding site (Raveendran and others 2009). The presence of CaMKII bound to GluN2B at synaptic sites is believed to be an important requirement for the maintenance of LTP, since disrupting this interaction reverses the potentation (Sanhueza and others 2011).
The complexity of these mechanisms working in a coordinated manner to regulate GluN2A and GluN2B properties and trafficking becomes even greater when the presence of tri-heteromers (i.e. GluN1/2A/2B) is taken into account. Tri-heteromers have been identified in adult cortex and hippocampus, although the degree to which they contribute to the total NMDAR population remains unclear. In adult hippocampus, the percentage of tri-heteromeric NMDAR has been estimated to be approximately one-third of the total NMDARs based on serial immunoprecipitation experiments (Al-Hallaq and others 2007). A study using indirect electrophysiological methods to isolate the GluN1/2A/2B population estimates their contribution to more than 50 % in adult CA1 pyramidal cells (Rauner and Kohr 2011). A recent report using single-cell deletion of GluN2A or GluN2B subunits supports a more significant presence of tri-heteromers at synapses (Gray and others 2011). In heterologous cells, NMDARs containing both GluN2A and GluN2B subunits shows intermediate characteristics between pure GluN2A- or GluN2B-containing NMDARs (Hatton and Paoletti 2005; Vicini and others 1998). It has been shown that GluN2B can play a dominant role in NMDAR trafficking, with tri-heteromers showing a similar ratio of internalization and recycling compared to GluN1/GluN2B receptors (Tang and others 2010). However, specific pharmacological and biochemical tools to directly analyze endogenous tri-heteromers do not exist.
The developmental GluN2B to GluN2A switch
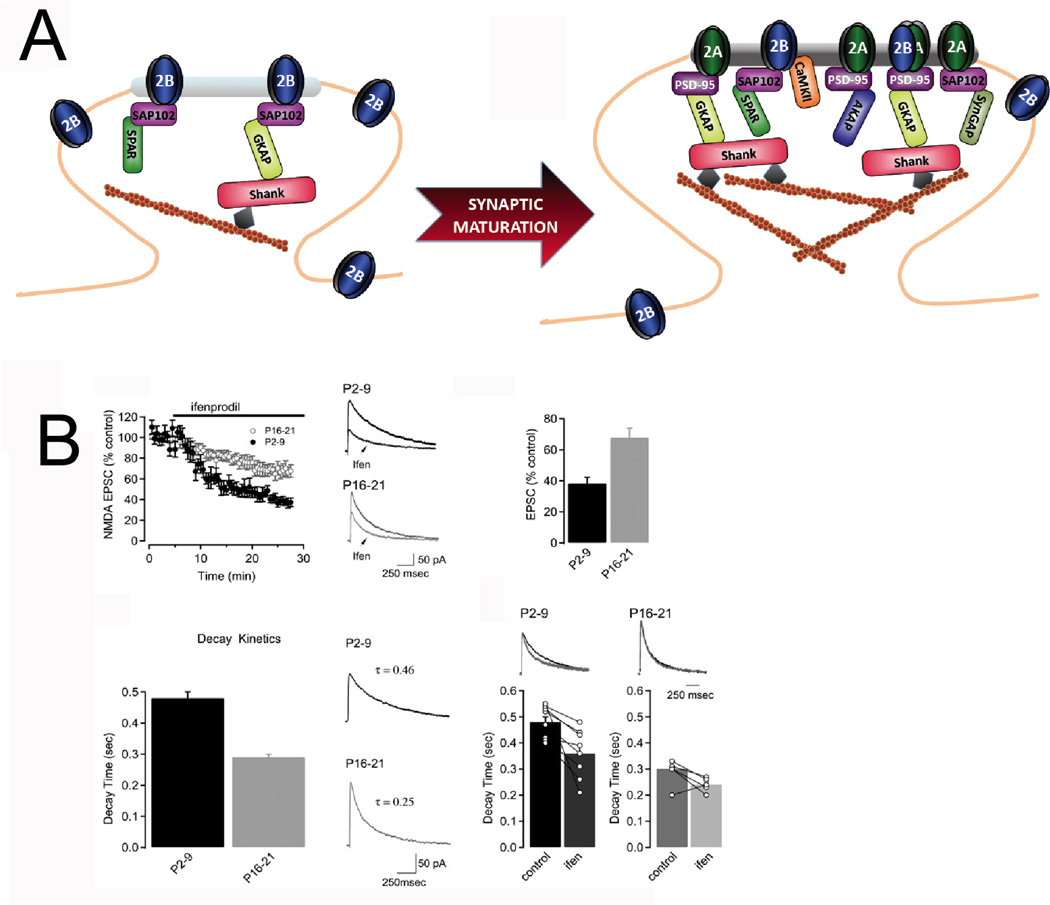
GluN2A replaces GluN2B as the primary GluN2 subunit at synaptic sites during the second postnatal week in cortex and hippocampus. At early developmental stages, GluN1/2B diheteromers are the primary type of NMDARs expressed at synapses, as indicated by the high sensitivity of EPSCs to the selective GluN2B inhibitors ifenprodil and CP101,606. At mature synapses, however, the ifenprodil sensitivity is much reduced and the kinetic decay of EPSCs is faster than at younger synapses, consistent with a switch in the synaptic content from predominantly GluN2B-containing to predominantly GluN2A-containing NMDARs (Bellone and Nicoll 2007; Rauner and Kohr 2011) Figure 4. This shift is an evolutionarily conserved process observed in frogs, birds and mammals that occurs in many brain areas, including cortex, hippocampus, amygdala and cerebellum (Dumas 2005). The timing for the switch varies for each region, but remarkably it is coincident with an increase in associative learning abilities, suggesting that this process is important for the refinement and fine tuning of neuronal circuits. This shift in synaptic GluN2 subunit composition requires synaptic activity or sensory experience to occur, as exemplified in the visual cortex. Dark reared rats show an impaired GluN2 switch, with a reduced GluN2A/2B ratio at synapses, higher sensitivity for ifenprodil, and slower kinetics than control animals. Importantly, just a few hours (< 2 hours) of light exposure is enough to drive the switch and results in a shift of the GluN2A/2B ratio to control levels. This experience-dependent process is bidirectional since returning the animals to the dark (over 72 hours) reduces the GluN2A/2B ratio to the level observed in animals that have never been exposed to the light (Philpot and others 2001).
FIGURE 4.
A. Synaptic maturation results in a switch in synaptic GluN2 subunit composition from predominantly GluN2B-containing to GluN2A-containing NMDARs. Synaptic maturation also results in changing levels of several scaffolding and signaling proteins and an increase in synaptic AMPA receptors. B. Characterization of the developmental GluN2B/A switch by electrophysiological approaches. NMDAR-EPSCs in young animals are more sensitive to ifenprodil inhibition (a selective GluN2B inhibitor) than in older animals. Consistent with slower kinetics for GluN2B vs GluN2A, the decay time in young animals is slower than in adults. Reprinted from Figure 1 in Bellone C and Nicoll RA, 2007.
Remarkably, an acute and bidirectional change in GluN2 subunit composition can be induced by synaptic activity in the hippocampus. Thus, the induction of LTP in young (P2-9) hippocampal slices results in a very rapid switch in synaptic composition (from GluN2B to GluN2A), as revealed by a speeding in the kinetics and a reduction of the ifenprodil sensitivity of NMDARs (Bellone and Nicoll 2007). A subsequent depotentiation reverses this activity-dependent switch. Interestingly, this protocol fails to induce any change (by LTP or depotentation) in older hippocampal slices, indicating that synaptic maturation occludes this kind of plasticity. The precise details about how an increase in the GluN2A/2B ratio results in the refinement of neuronal circuits are still unknown. Given the differential characteristics of GluN2 subunits, it is likely that a synapse with a high GluN2A content exhibits a reduced window for spike-timing plasticity, integrating stimuli received in a shorter period of time than a synapse with a reduced GluN2A/B ratio. This may limit the formation of inappropriate synapses by reducing synaptic response time. In addition, the threshold for LTP induction is elevated in these synapses, making their potentiation more difficult. It may be that the elevated threshold for LTP could play a role in the pruning of excess synapses formed in the initial developmental stages as it has been reported that synapses that are not activated are eliminated (Yasuda and others 2011). Similarly, the molecular mechanisms that result in the developmental GluN2 subunit switch remain uncertain. Although it is well documented that the total expression of GluN2A is dramatically increased during the critical period and that GluN2A replaces GluN2B in synaptic membranes in a NMDAR activity-dependent manner (Barria and Malinow 2002), the precise mechanisms underlying the switch are unknown.
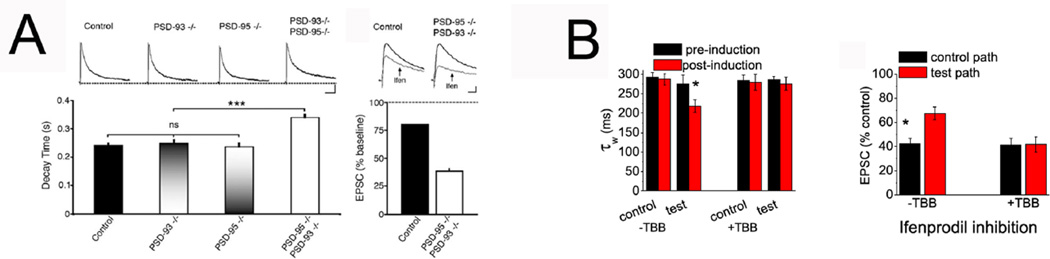
Interestingly, mouse lines lacking PSD-95 (Beique and others 2006) or both PSD-95 and PSD-93 (Elias and others 2008) have a deficit in the GluN2 subunit switch Figure 5A. Whether these scaffolding proteins play a role in the insertion of newly synthesized GluN2A into synapses, their stabilization, or in other trafficking mechanisms remains unexplored. In addition, it has recently been demonstrated that CK2 activity reduces the synaptic content of GluN2B and increases GluN2A, and is required for the acute activity-induced GluN2 subunit switch (Sanz-Clemente and others 2010). The role of CK2 in the shift is likely mediated by the phosphorylation on the PDZ binding domain of GluN2B (S1480) that decreases synaptic GluN2B and is NMDAR activity-dependent Figure 5B. In addition to NMDARs, group I metabotropic glutamate receptor (mGluR1 and mGluR5) play a role in this switch. For example, inhibitors of mGluR5 block the activity-dependent switch and mGluR5 knock-out mice have deficits in both hippocampus and visual cortex plasticity (Matta and others 2011). Activation of PKC via calcium release from intracellular stores is the final mediator of the role of mGluR5 in the GluN2 subunit switch. Finally, it has recently been reported that mGluR1 activation is also critical for the GluN2 subunit switch in the ventral tegmental area (VTA) (Bellone and others 2011). Therefore, it seems that several players and mechanisms act in a coordinated manner to replace the predominant GluN2B-containing NMDARs for GluN2A-containing ones in an synaptic activity dependent-manner.
FIGURE 5. Molecular mechanisms regulating the synaptic GluN2B/A switch.
A. Adult animals lacking PSD-95 and PSD-93 show an elevated decay time and sensitivity to ifenprodil inhibition compared to wild-type animals, indicating an impared GluN2 subunit switch. Reprinted from Figure 5 in Elias GM et al., 2008. B. The CK2 inhibitor TBB blocks the GluN2 subunit switch induced by activity in young rat slices. Reprinted from Figure 7 in Sanz-Clemente et al., 2010.
NMDAR subunits in disease
NMDA receptors are essential for neuronal development and synaptic plasticity. Therefore it is not surprising that mislocalization and abnormal trafficking of NMDAR subunits have been reported in several brain disorders and pathological conditions Table 2. We briefly summarize some of these below.
TABLE 2.
NMDAR alterations in pathological conditions
| Disorder | NMDAR alteration | References |
|---|---|---|
| Alzheimer’s disease (AD) | Reduction in GluN2B surface expression Pathological activation of extrasynaptic NMDARs |
Snyder and others 2005 Ronicke and others 2010 |
| Parkinson’s disease (PD) | GluN2B redistribution from synaptic to extrasynaptic sites Increased synaptic GluN2A |
Dunah and others 2000 |
| Huntington’s disease (HD) | Enhanced extrasynaptic NMDAR activity | Gladding and Raymond 2011 |
| Ischemia and stroke | Enhanced extrasynaptic NMDAR activity | Hardingham and Bading 2010 |
| Schizophrenia | Decreased NMDAR function Altered GluN2A trafficking via neuregulin/ErbB4 receptor |
Gaspar and others 2009 |
| Alcohol abuse | Decreased synaptic GluN2A expression following acute ethanol treatment Increase in NMDAR currents following chronic ethanol treatment |
Suvarna and others 2005 Carpenter-Hyland and others 2004 |
| Cocaine abuse | Elevated insertion of GluN2A following acute cocaine treatment Increased AMPAR:NMDAR ratio after repeated cocaine administration |
Borgland and others 2006 |
| Chronic pain | Reduction in synaptic GluN2B-containing NMDARs | Vikman and others 2008 |
Alzheimer’s disease
Alzheimer’s disease (AD) is a dementia characterized by the elevated generation of beta-amyloid peptide (Abeta) and the formation of intracellular tangles composed mainly of the protein tau in a hyperphosphorylated state. Abeta can aggregate to form insoluble amyloid plaques or assemble as soluble oligomers composed of a variable number of peptides. Both the plaques and the oligomers have been reported to have neurotoxic effects. Given the importance of NMDARs in cognitive processes, it has long been thought that NMDARs play a critical role in the effects of Abeta (Malinow 2011). For instance, pharmacological blockade of NMDARs prevents both the depression in synaptic transmission and the structural changes induced by overexpression of Abeta. Importantly, it has been described that Abeta oligomers are able to bind with specificity to excitatory synapses and alter synaptic function and synaptic plasticity perhaps by generating reactive oxygen species (ROS) resulting in spine loss (Selkoe 2002). Abeta oligomers bind to synapses and trigger a decrease in the surface expression of GluN2B, but not GluN2A. Abeta treatment increases the activity of the phosphatase STEP61 that results in a reduction in the phosphorylation level of GluN2B on Y1472 (Snyder and others 2005). This dephosphorylation allows better binding to clathrin adaptor proteins and, therefore, GluN2B is internalized more efficiently. In addition, NMDARs may be part of the protein complex that targets Abeta-oligomers to synapses, because cultures from GluN1 knock-out mice show decreased Abeta-oligomer binding. Finally, NMDAR activity, probably through extrasynaptic NMDARs, is required for Abeta-mediated spine loss and neuronal dysfunction (Ronicke and others 2010).
Parkinson’s disease
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by a dramatic loss of dopaminergic neurons in the substantia nigra and, consequently, the depletion of dopamine in the striatum. The alteration in the nigrostriatal pathway results in the motor symptoms characteristic of this disease. It is well known that dopaminergic and glutamatergic signaling interact to control motor function; therefore, it is not surprising that NMDARs are also altered in this disorder. For instance, a biochemical study analyzing NMDAR subunit composition and phosphorylation in the striatum of an animal model for PD (6-hydroxydopamine-lesioned rat) revealed a decrease in the amount of GluN1 and GluN2B, but not GluN2A, expressed in membrane fractions. Remarkably, L-DOPA treatment reversed these alterations (Dunah and others 2000). In addition, a shift in GluN2B distribution from synaptic to extrasynaptic sites and an elevated presence of synaptic GluN2A in dyskinetic animals treated with L-DOPA has been reported (Gardoni and others 2006). Interestingly, the expression of MAGUK proteins is also reduced in PD model animals and it appears that the disruption of GluN2B/MAGUK binding is involved in this disorder (Gardoni and others 2006). Finally NMDAR antagonists block the motor impairments produced by L-DOPA treatment, highlighting the importance of these receptors in PD (Wessell and others 2004).
Huntington’s disease
Huntington’s disease (HD) is a genetic neurodegenerative disorder that leads to dementia (primarily affecting striatum and cortex) and defects in muscle coordination. HD is defined by an expanded CAG repeat mutation in the huntington gene that results in the pathological presence of polyglutamine repeat in the huntington protein (htt). Mutated htt exhibits an abnormal conformation and can be cleaved in different points to generate toxic fragments with an elevated presence of β-sheet conformation. These fragments (likely associated as oligomers) can form aggregates and interfere with several physiological cellular processes including Ca2+ homeostasis, mitochondrial function and vesicular trafficking (Zuccato and others 2010). There is evidence that NMDAR activity and trafficking may be affected in HD. A transgenic mouse model for HD shows elevated NMDAR activation in striatum, specifically an increase in expression and activity of extrasynaptic NMDARs and concomitant enhanced excitotoxicity. How mutated htt can affect NMDAR trafficking is still unclear, but it is known that htt can interact with several proteins involved in vesicular trafficking and endo/exocytosis mechanisms (Gladding and Raymond 2011).
Schizophrenia
Schizophrenia is a mental disorder characterized by disintegration of thought processes and emotional responsiveness caused by altered brain connectivity. Although the classical hypothesis proposes a hyperactivity of the dopaminergic system as the culprit for this disorder, accumulating evidence suggests a role for glutamate receptors, specifically NMDARs, in this disease. The glutamatergic hypothesis suggests that the observed symptoms in schizophrenia result from the hypofunction of NMDARs in cortico-striatal projections that leads to an increase in the dopaminergic input. Importantly, it has been reported that NMDAR trafficking is altered by several proteins involved in schizophreina such as neuregulin 1, ErbB4, PPP3CC and dysbindin (Gaspar and others 2009; Gerber and others 2003; Hahn and others 2006; Tang and others 2009).
Summary
In summary, NMDARs play a central role in important processes such as learning, memory, and development. Many of the functional properties of NMDARs rely on the GluN2 subunit content. Therefore, it is not surprising that NMDAR subunits are subject to strict mechanisms of regulation. GluN2A and GluN2B are highly expressed in cortex and hippocampus and they differ in their kinetic properties, developmental expression pattern, subcellular localization and trafficking regulation. GluN2A expression begins later in development and it is mainly localized at synaptic sites. In contrast, GluN2B is expressed prenatally and is enriched at extrasynaptic sites in adult animals. Many of the molecular mechanisms regulating GluN2B trafficking have been elucidated in recent years, whereas less is known about GluN2A regulation. For example, the interaction with MAGUKs proteins is an important determinant for GluN2B, but not GluN2A, regulation. The developmental switch in GluN2 subunit composition from GluN2B to 2A occurs during development and in response to activity and experience. Recent studies provide insight into the mechanisms regulating this switch, including NMDAR and group I mGluR activity, CK2 phosphorylation, and PSD-95 MAGUK binding. Consistent with the importance of precise NMDAR regulation for proper synaptic and neuronal function, several neuronal disorders are characterized by altered NMDAR subunit expression and synaptic and extrasynaptic localization.
ACKNOWLEDGMENTS
This work was supported by the Intramural Program of the National Institute of Neurological Disorders and Stroke (A.S-C.; K.W.R.). R.A.N. is funded by grants from the National Institute of Mental Health.
References
- Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci. 2007;27(31):8334–8343. doi: 10.1523/JNEUROSCI.2155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35(2):345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411(6839):801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- Beique JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci U S A. 2006;103(51):19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Mameli M, Luscher C. In utero exposure to cocaine delays postnatal synaptic maturation of glutamatergic transmission in the VTA. Nat Neurosci. 2011;14(11):1439–1446. doi: 10.1038/nn.2930. [DOI] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Rapid Bidirectional Switching of Synaptic NMDA Receptors. Neuron. 2007;55(5):779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Fleidervish IA, Sprengel R, Gutnick MJ. NMDA receptors in layer 4 spiny stellate cells of the mouse barrel cortex contain the NR2C subunit. J Neurosci. 2006;26(2):708–715. doi: 10.1523/JNEUROSCI.4409-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49(4):589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Brothwell SL, Barber JL, Monaghan DT, Jane DE, Gibb AJ, Jones S. NR2B- and NR2Dcontaining synaptic NMDA receptors in developing rat substantia nigra pars compacta dopaminergic neurones. J Physiol. 2008;586(3):739–750. doi: 10.1113/jphysiol.2007.144618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24(36):7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53(3):362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Growth factor-dependent trafficking of cerebellar NMDA receptors via protein kinase B/Akt phosphorylation of NR2C. Neuron. 2009;62(4):471–478. doi: 10.1016/j.neuron.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BS, Thomas EV, Sanz-Clemente A, Roche KW. NMDA receptor-dependent regulation of dendritic spine morphology by SAP102 splice variants. J Neurosci. 2011;31(1):89–96. doi: 10.1523/JNEUROSCI.1034-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Huang YH, Lau LF, Huganir RL. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci. 2004;24(45):10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlew R, Brasier DJ, Feldman DE, Philpot BD. Presynaptic NMDA receptors: newly appreciated roles in cortical synaptic function and plasticity. Neuroscientist. 2008;14(6):609–625. doi: 10.1177/1073858408322675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins SL, Kenny AV, Stephenson FA. Delineation of additional PSD-95 binding domains within NMDA receptor NR2 subunits reveals differences between NR2A/PSD- 95 and NR2B/PSD-95 association. Neuroscience. 2009;158(1):89–95. doi: 10.1016/j.neuroscience.2007.12.051. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004(255):re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103(6):945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Dumas TC. Developmental regulation of cognitive abilities: modified composition of a molecular switch turns on associative learning. Prog Neurobiol. 2005;76(3):189–211. doi: 10.1016/j.pneurobio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Wang Y, Yasuda RP, Kameyama K, Huganir RL, Wolfe BB, et al. Alterations in subunit expression, composition, and phosphorylation of striatal N-methyl- D-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson's disease. Mol Pharmacol. 2000;57(2):342–352. [PubMed] [Google Scholar]
- Elias GM, Elias LA, Apostolides PF, Kriegstein AR, Nicoll RA. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc Natl Acad Sci U S A. 2008;105(52):20953–20958. doi: 10.1073/pnas.0811025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563(Pt 2):345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Feldmeyer D, Takahashi T, Cull-Candy SG. NMDA-receptor channel diversity in the developing cerebellum. Nature. 1994;368(6469):335–339. doi: 10.1038/368335a0. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Kato A, Lovett C, Tonegawa S, Watanabe M. Retention of NMDA receptor NR2 subunits in the lumen of endoplasmic reticulum in targeted NR1 knockout mice. Proc Natl Acad Sci U S A. 2003;100(8):4855–4860. doi: 10.1073/pnas.0830996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardoni F, Picconi B, Ghiglieri V, Polli F, Bagetta V, Bernardi G, et al. A critical interaction between NR2B and MAGUK in L-DOPA induced dyskinesia. J Neurosci. 2006;26(11):2914–2922. doi: 10.1523/JNEUROSCI.5326-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar PA, Bustamante ML, Silva H, Aboitiz F. Molecular mechanisms underlying glutamatergic dysfunction in schizophrenia: therapeutic implications. J Neurochem. 2009;111(4):891–900. doi: 10.1111/j.1471-4159.2009.06325.x. [DOI] [PubMed] [Google Scholar]
- Gerber DJ, Hall D, Miyakawa T, Demars S, Gogos JA, Karayiorgou M, et al. Evidence for association of schizophrenia with genetic variation in the 8p21.3 gene, PPP3CC, encoding the calcineurin gamma subunit. Proc Natl Acad Sci U S A. 2003;100(15):8993–8998. doi: 10.1073/pnas.1432927100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladding CM, Raymond LA. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol Cell Neurosci. 2011;48(4):308–320. doi: 10.1016/j.mcn.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll RA. Distinct Modes of AMPA Receptor Suppression at Developing Synapses by GluN2A and GluN2B: Single-Cell NMDA Receptor Subunit Deletion In Vivo. Neuron. 2011;71(6):1085–1101. doi: 10.1016/j.neuron.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Bard L, Choquet D. Surface trafficking of N-methyl-D-aspartate receptors: physiological and pathological perspectives. Neuroscience. 2009;158(1):4–18. doi: 10.1016/j.neuroscience.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D, Stephenson FA, Verrier D, Manzoni OJ, Chavis P. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J Neurosci. 2007;27(38):10165–10175. doi: 10.1523/JNEUROSCI.1772-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, et al. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103(49):18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12(7):824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11(10):682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AZ, Pettit DL. Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. J Physiol. 2007;584(Pt 2):509–519. doi: 10.1113/jphysiol.2007.137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton CJ, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron. 2005;46(2):261–274. doi: 10.1016/j.neuron.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Henson MA, Roberts AC, Perez-Otano I, Philpot BD. Influence of the NR3A subunit on NMDA receptor functions. Prog Neurobiol. 2010;91(1):23–37. doi: 10.1016/j.pneurobio.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10(9):647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Horak M, Wenthold RJ. Different roles of C-terminal cassettes in the trafficking of fulllength NR1 subunits to the cell surface. J Biol Chem. 2009;284(15):9683–9691. doi: 10.1074/jbc.M807050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh KH, Wenthold RJ. Turnover analysis of glutamate receptors identifies a rapidly degraded pool of the N-methyl-D-aspartate receptor subunit, NR1, in cultured cerebellar granule cells. J Biol Chem. 1999;274(1):151–157. doi: 10.1074/jbc.274.1.151. [DOI] [PubMed] [Google Scholar]
- Jones ML, Leonard JP. PKC site mutations reveal differential modulation by insulin of NMDA receptors containing NR2A or NR2B subunits. J Neurochem. 2005;92(6):1431–1438. doi: 10.1111/j.1471-4159.2004.02985.x. [DOI] [PubMed] [Google Scholar]
- Jung SY, Kim J, Kwon OB, Jung JH, An K, Jeong AY, et al. Input-specific synaptic plasticity in the amygdala is regulated by neuroligin-1 via postsynaptic NMDA receptors. Proc Natl Acad Sci U S A. 2010;107(10):4710–4715. doi: 10.1073/pnas.1001084107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew JN, Richards JG, Mutel V, Kemp JA. Developmental changes in NMDA receptor glycine affinity and ifenprodil sensitivity reveal three distinct populations of NMDA receptors in individual rat cortical neurons. J Neurosci. 1998;18(6):1935–1943. doi: 10.1523/JNEUROSCI.18-06-01935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie V, Roder JC. The involvement of the NMDA receptor D-serine/glycine site in the pathophysiology and treatment of schizophrenia. Neurosci Biobehav Rev. 2010;34(3):351–372. doi: 10.1016/j.neubiorev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J Neurosci. 1994;14(5 Pt 2):3180–3194. doi: 10.1523/JNEUROSCI.14-05-03180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 2004;24(28):6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R. New developments on the role of NMDA receptors in Alzheimer's disease. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.09.001. [Epub Sep, 29 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta JA, Ashby MC, Sanz-Clemente A, Roche KW, Isaac JT. mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron. 2011;70(2):339–351. doi: 10.1016/j.neuron.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Armstrong N. Structure and function of glutamate receptor ion channels. Annu Rev Physiol. 2004;66:161–181. doi: 10.1146/annurev.physiol.66.050802.084104. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Omkumar RV, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB. Identification of a phosphorylation site for calcium/calmodulindependent protein kinase II in the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1996;271(49):31670–31678. doi: 10.1074/jbc.271.49.31670. [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125(4):775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29(1):157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47(6):845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski K, Fu Z, Losi G, Hawkins LM, Luo J, Chang K, et al. Relationship between availability of NMDA receptor subunits and their expression at the synapse. J Neurosci. 2002;22(20):8902–8910. doi: 10.1523/JNEUROSCI.22-20-08902.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauner C, Kohr G. Triheteromeric NR1/NR2A/NR2B receptors constitute the major Nmethyl- D-aspartate receptor population in adult hippocampal synapses. J Biol Chem. 2011;286(9):7558–7566. doi: 10.1074/jbc.M110.182600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveendran R, Devi Suma Priya S, Mayadevi M, Steephan M, Santhoshkumar TR, Cheriyan J, et al. Phosphorylation status of the NR2B subunit of NMDA receptor regulates its interaction with calcium/calmodulin-dependent protein kinase II. J Neurochem. 2009;110(1):92–105. doi: 10.1111/j.1471-4159.2009.06108.x. [DOI] [PubMed] [Google Scholar]
- Ronicke R, Mikhaylova M, Ronicke S, Meinhardt J, Schroder UH, Fandrich M, et al. Early neuronal dysfunction by amyloid beta oligomers depends on activation of NR2Bcontaining NMDA receptors. Neurobiol Aging. 2010;32(12):2219–2228. doi: 10.1016/j.neurobiolaging.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Sanhueza M, Fernandez-Villalobos G, Stein IS, Kasumova G, Zhang P, Bayer KU, et al. Role of the CaMKII/NMDA Receptor Complex in the Maintenance of Synaptic Strength. J Neurosci. 2011;31(25):9170–9178. doi: 10.1523/JNEUROSCI.1250-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20(3):1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Clemente A, Matta JA, Isaac JT, Roche KW. Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron. 2010;67(6):984–996. doi: 10.1016/j.neuron.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimemi A, Fine A, Kullmann DM, Rusakov DA. NR2B-containing receptors mediate cross talk among hippocampal synapses. J Neurosci. 2004;24(20):4767–4777. doi: 10.1523/JNEUROSCI.0364-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci. 2001;21(9):3063–3072. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8(8):1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A, et al. Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell. 1998;92(2):279–289. doi: 10.1016/s0092-8674(00)80921-6. [DOI] [PubMed] [Google Scholar]
- Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Kohr G. C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci. 2000;20(12):4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarna N, Borgland SL, Wang J, Phamluong K, Auberson YP, Bonci A, et al. Ethanol alters trafficking and functional N-methyl-D-aspartate receptor NR2 subunit ratio via H-Ras. J Biol Chem. 2005;280(36):31450–31459. doi: 10.1074/jbc.M504120200. [DOI] [PubMed] [Google Scholar]
- Tang TT, Badger JD, 2nd, Roche PA, Roche KW. Novel approach to probe subunitspecific contributions to N-methyl-D-aspartate (NMDA) receptor trafficking reveals a dominant role for NR2B in receptor recycling. J Biol Chem. 2010;285(27):20975–20981. doi: 10.1074/jbc.M110.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TT, Yang F, Chen BS, Lu Y, Ji Y, Roche KW, et al. Dysbindin regulates hippocampal LTP by controlling NMDA receptor surface expression. Proc Natl Acad Sci U S A. 2009;106(50):21395–21400. doi: 10.1073/pnas.0910499106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95(3):1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34(2):255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science. 1995;268(5212):873–876. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, et al. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 1998;79(2):555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Vikman KS, Rycroft BK, Christie MJ. Switch to Ca2+-permeable AMPA and reduced NR2B NMDA receptor-mediated neurotransmission at dorsal horn nociceptive synapses during inflammatory pain in the rat. J Physiol. 2008;586(2):515–527. doi: 10.1113/jphysiol.2007.145581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu S, Fu Y, Wang JH, Lu Y. Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nat Neurosci. 2003;6(10):1039–1047. doi: 10.1038/nn1119. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Fritschy JM, Mohler H, Benke D. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem. 1997;68(2):469–478. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- Wessell RH, Ahmed SM, Menniti FS, Dunbar GL, Chase TN, Oh JD. NR2B selective NMDA receptor antagonist CP-101,606 prevents levodopa-induced motor response alterations in hemi-parkinsonian rats. Neuropharmacology. 2004;47(2):184–194. doi: 10.1016/j.neuropharm.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Johnson-Venkatesh EM, Zhang H, Parent JM, Sutton MA, Umemori H. Multiple forms of activity-dependent competition refine hippocampal circuits in vivo. Neuron. 2011;70(6):1128–1142. doi: 10.1016/j.neuron.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington's disease. Physiol Rev. 2010;90(3):905–981. doi: 10.1152/physrev.00041.2009. [DOI] [PubMed] [Google Scholar]