Abstract
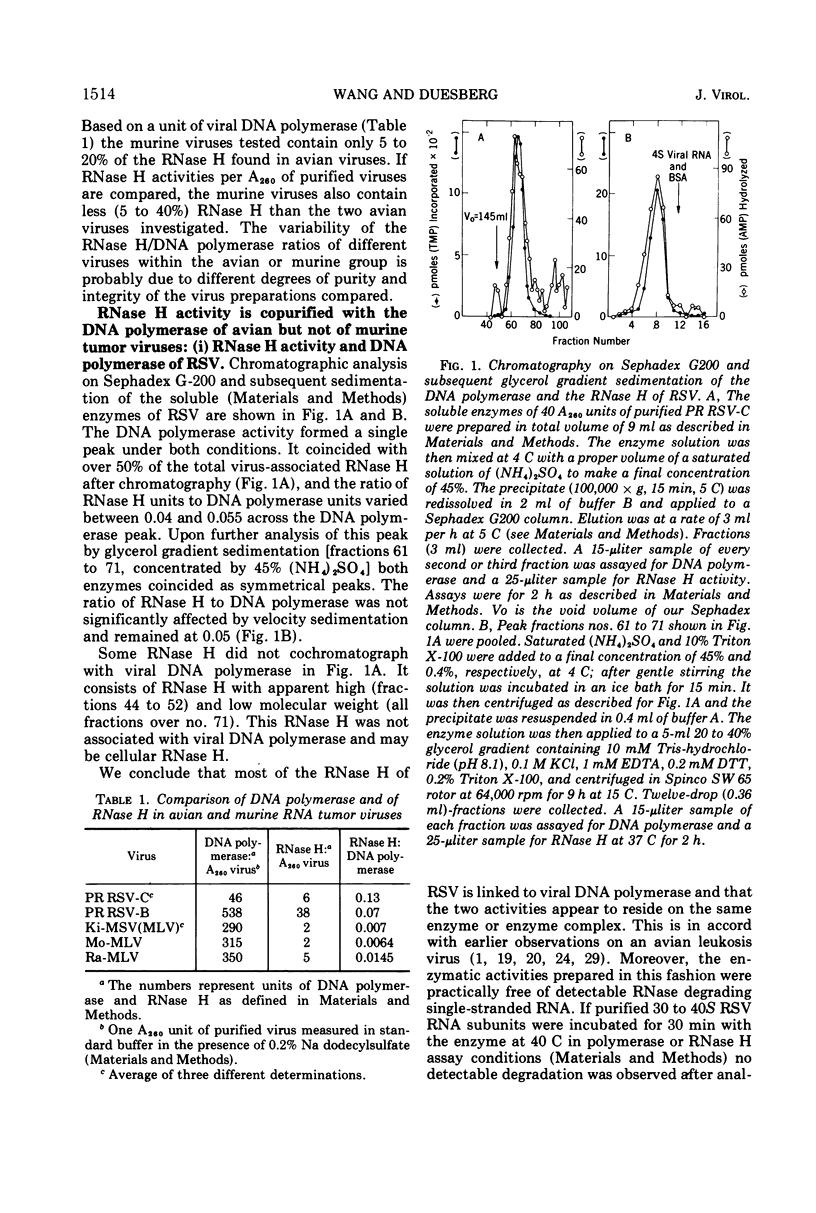
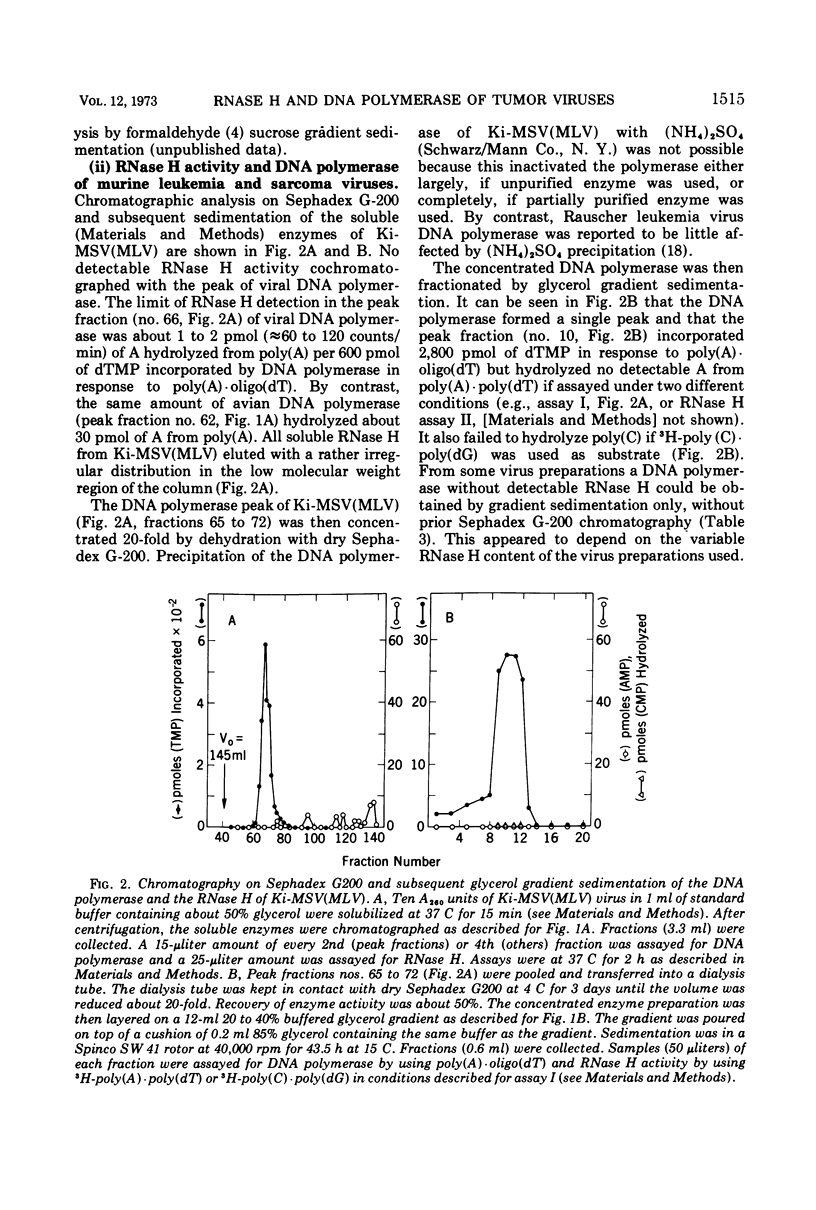
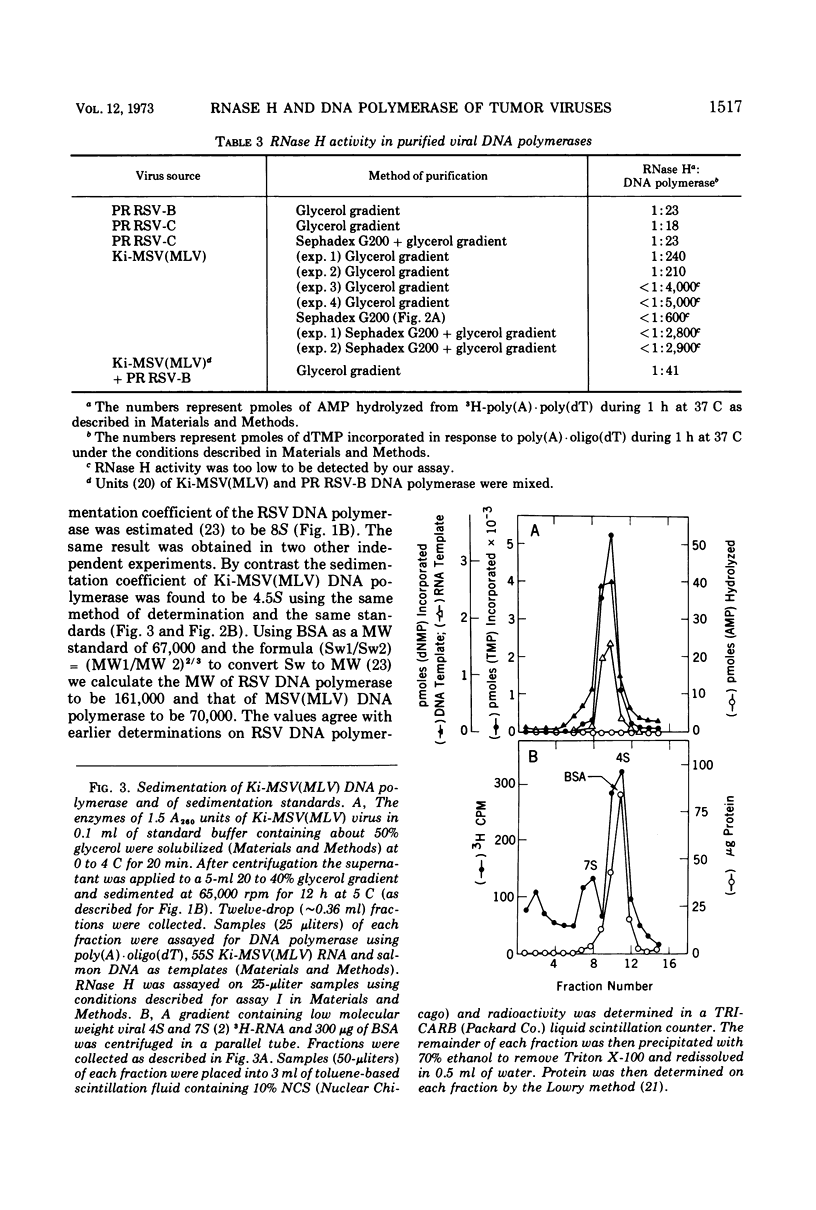
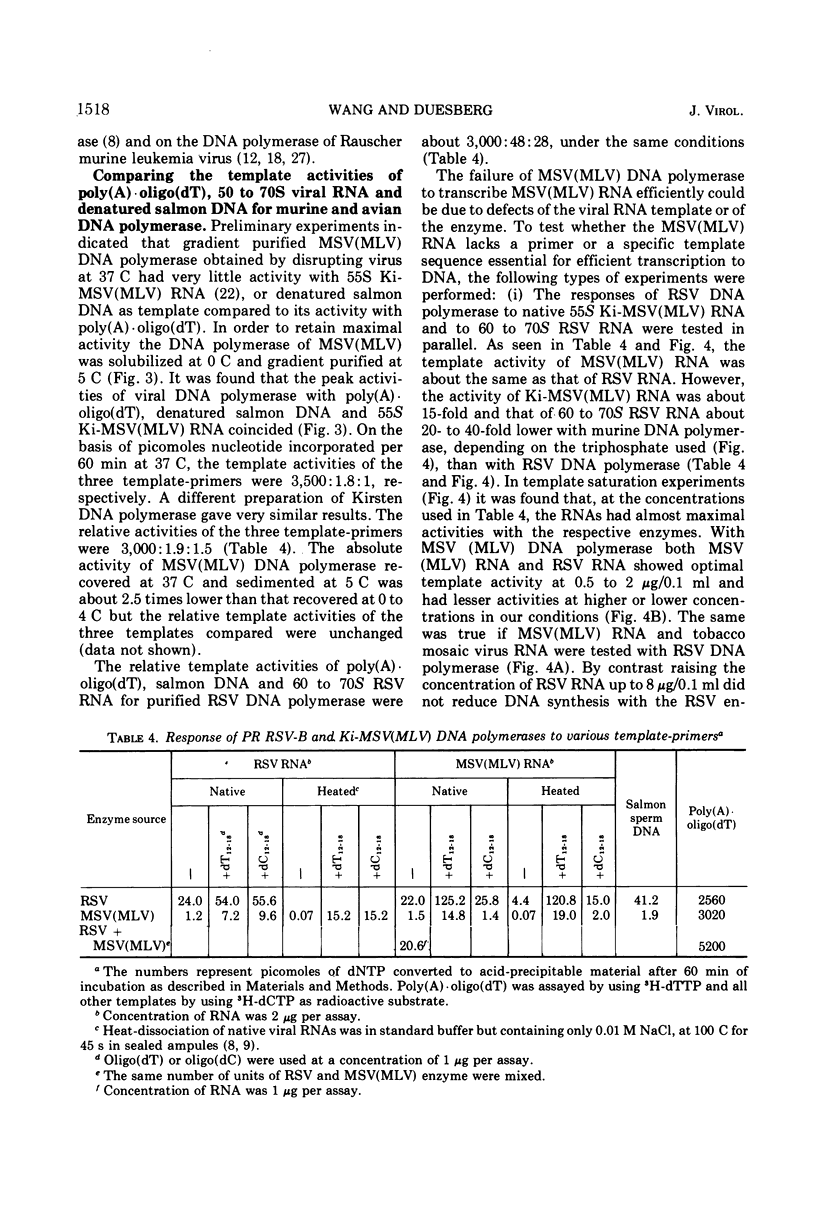
Kirsten murine sarcoma-leukemia virus (Ki-MSV[MLV]) was found to contain less RNase H per unit of viral DNA polymerase than avian Rous sarcoma virus (RSV). Upon purification by chromatography on Sephadex G-200 and subsequent glycerol gradient sedimentation the avian DNA polymerase was obtained in association with a constant amount of RNase H. By contrast, equally purified DNA polymerase of Ki-MSV(MLV) and Moloney [Mo-MSV(MLV)] lacked detectable RNase H if assayed with two homopolymer and phage fd DNA-RNA hybrids as substrates. On the basis of picomoles of nucleotides turned over, the ratio of RNase H to purified avian DNA polymerase was 1:20 and that of RNase H to purified murine DNA polymerase ranged between <1:2,800 and 5,000. Based on the same activity with poly (A)·oligo(dT) the activity of the murine DNA polymerase was 6 to 60 times lower than that of the avian enzyme with denatured salmon DNA template or with avian or murine viral RNA templates assayed under various conditions (native, heat-dissociated, with or without oligo(dT) and oligo(dC) and at different template enzyme ratios). The template activities of Ki-MSV(MLV) RNA and RSV RNA were enhanced uniformly by oligo(dT) but oligo(dC) was much less efficient in enhancing the activity of MSV(MLV) RNA than that of RSV RNA. It was concluded that the purified DNA polymerase of Ki-MSV(MLV) differs from that of Rous sarcoma virus in its lack of detectable RNase H and in its low capacity to transcribe viral RNA and denatured salmon DNA. Some aspects of these results are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Smoler D. F. Association of an endoribonuclease with the avian myeloblastosis virus deoxyribonucleic acid polymerase. J Biol Chem. 1972 Nov 25;247(22):7282–7287. [PubMed] [Google Scholar]
- Bishop J. M., Levinson W. E., Sullivan D., Fanshier L., Quintrell N., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. II. The 7 S RNA. Virology. 1970 Dec;42(4):927–937. doi: 10.1016/0042-6822(70)90341-7. [DOI] [PubMed] [Google Scholar]
- Bondurant M. C., Hackett A. J., Schaffer F. L. Infectivity and RNA patterns as functions of high- and low-dilution passage of murine sarcoma-leukemia virus: evidence for autointerference within an oncornavirus population. J Virol. 1973 May;11(5):642–647. doi: 10.1128/jvi.11.5.642-647.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Duesberg P. Role of subunits of 60 to 70S avian tumor virus ribonucleic acid in its template activity for the viral deoxyribonucleic acid polymerase. J Virol. 1972 Jul;10(1):23–31. doi: 10.1128/jvi.10.1.23-31.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Canaani E. Complementarity between Rous sarcoma virus (RSV) RNA and the in vitro-synthesized DNA of the virus-associated DNA polymerase. Virology. 1970 Nov;42(3):783–788. doi: 10.1016/0042-6822(70)90325-9. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson H. L., Robinson W. S., Huebner R. J., Turner H. C. Proteins of Rous sarcoma virus. Virology. 1968 Sep;36(1):73–86. doi: 10.1016/0042-6822(68)90118-9. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P., Helm K. V., Canaani E. Comparative properties of RNA and DNA templates for the DNA polymerase of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2505–2509. doi: 10.1073/pnas.68.10.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P., Helm K. V., Canaani E. Properties of a soluble DNA polymerase isolated from Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):747–751. doi: 10.1073/pnas.68.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., McDonnell J. P., Levinson W. E., Bishop J. M. Purification and characterization of the deoxyribonucleic acid polymerase associated with Rous sarcoma virus. Biochemistry. 1972 Jun 6;11(12):2334–2342. doi: 10.1021/bi00762a020. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Aaronson S. A., Martin M. A. Heterogeneity of murine leukemia virus in vitro DNA; detection of viral DNA in mammalian cells. Science. 1971 Jun 25;172(3990):1353–1355. doi: 10.1126/science.172.3990.1353. [DOI] [PubMed] [Google Scholar]
- Gerwin B. I., Milstien J. B. An oligonucleotide affinity column for RNA-dependent DNA polymerase from RNA tumor viruses. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2599–2603. doi: 10.1073/pnas.69.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. A single subunit from avian myeloblastosis virus with both RNA-directed DNA polymerase and ribonuclease H activity. Proc Natl Acad Sci U S A. 1973 Jan;70(1):230–234. doi: 10.1073/pnas.70.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. Ribonuclease H: a ubiquitous activity in virions of ribonucleic acid tumor viruses. J Virol. 1972 Dec;10(6):1136–1142. doi: 10.1128/jvi.10.6.1136-1142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausen P., Stein H. Ribonuclease H. An enzyme degrading the RNA moiety of DNA-RNA hybrids. Eur J Biochem. 1970 Jun;14(2):278–283. doi: 10.1111/j.1432-1033.1970.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Hurwitz J., Leis J. P. RNA-dependent DNA polymerase activity of RNA tumor viruses. I. Directing influence of DNA in the reaction. J Virol. 1972 Jan;9(1):116–129. doi: 10.1128/jvi.9.1.116-129.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W., Crouch R. Degradation of DNA RNA hybrids by ribonuclease H and DNA polymerases of cellular and viral origin. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3360–3364. doi: 10.1073/pnas.69.11.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leis J. P., Berkower I., Hurwitz J. Mechanism of action of ribonuclease H isolated from avian myeloblastosis virus and Escherichia coli. Proc Natl Acad Sci U S A. 1973 Feb;70(2):466–470. doi: 10.1073/pnas.70.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mölling K., Bolognesi D. P., Bauer H., Büsen W., Plassmann H. W., Hausen P. Association of viral reverse transcriptase with an enzyme degrading the RNA moiety of RNA-DNA hybrids. Nat New Biol. 1971 Dec 22;234(51):240–243. doi: 10.1038/newbio234240a0. [DOI] [PubMed] [Google Scholar]
- Stein H., Hausen P. Enzyme from calf thymus degrading the RNA moiety of DNA-RNA Hybrids: effect on DNA-dependent RNA polymerase. Science. 1969 Oct 17;166(3903):393–395. doi: 10.1126/science.166.3903.393. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Murine sarcoma and leukemia viruses: genetic differences determined by RNA-DNA hybridization. Virology. 1971 Nov;46(2):480–484. doi: 10.1016/0042-6822(71)90048-1. [DOI] [PubMed] [Google Scholar]
- Tronick S. R., Scolnick E. M., Parks W. P. Reversible inactivation of the deoxyribonucleic acid polymerase of Rauscher leukemia virus. J Virol. 1972 Oct;10(4):885–888. doi: 10.1128/jvi.10.4.885-888.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Levinson W. E., Bishop J. M. Extent of transcription by the RNA-dependent DNA polymerase of Rous sarcoma virus. Nat New Biol. 1971 Sep 1;233(35):19–21. doi: 10.1038/newbio233019a0. [DOI] [PubMed] [Google Scholar]
- Watson K. F., Mölling K., Bauer H. Ribonuclease H activity present in purified DNA polymerase from avian myeloblastosis virus. Biochem Biophys Res Commun. 1973 Mar 5;51(1):232–240. doi: 10.1016/0006-291x(73)90533-0. [DOI] [PubMed] [Google Scholar]



