Abstract
Excessive visceral adipose tissue appears to trigger a cascade of metabolic disturbances that seem to coexist with ectopic fat storage in muscle, liver, heart and the ß-cell. Therefore, the reduction of visceral adipose tissue potentially plays a pivotal role in the treatment of the metabolic syndrome. The purpose of this systematic review and meta-analysis is to describe the overall effect of exercise on visceral adipose tissue and to provide an overview of the effect of different exercise regimes, without caloric restriction, on visceral adipose tissue in obese persons. A systematic literature search was performed according to the PRISMA statement for reporting systematic reviews and meta-analyses. The initial search resulted in 87 articles after removing duplicates. After screening on title, abstract and full-text 15 articles (totalling 852 subjects) fulfilled the a priori inclusion criteria. The quality of each eligible study was assessed in duplicate with “The Critical Review Form for Quantitative Studies”. Using random-effects weights, the standardized mean difference (Hedge's g) of the change in visceral adipose tissue was −0.497 with a 95% confidence interval of −0.655 to −0.340. The Z-value was −6.183 and the p-value (two tailed) was <0.001. A subgroup analysis was performed based on gender, type of training and intensity. Aerobic training of moderate or high intensity has the highest potential to reduce visceral adipose tissue in overweight males and females. These results suggest that an aerobic exercise program, without hypocaloric diet, can show beneficial effects to reduce visceral adipose tissue with more than 30 cm2 (on CT analysis) in women and more than 40 cm2 in men, even after 12 weeks.
Introduction
Obesity (BMI ≥30.0 kg.m− 2) affects more than one third of Americans and a fifth of Europe's population. The prevalence of obesity has reached epidemic proportions with rates of severe childhood obesity that have tripled in the last 25 years.[1], [2] Obesity is associated with an increased risk of comorbidities and increased risk of premature death.[3], [4] Over the last several years, increasing attention has been paid to abdominal adiposity and its association with increased mortality.[5], [6] Visceral adipose tissue (VAT) seems to be the most pathogenic fat depot and is considered to play a central role in the metabolic syndrome. Adipose tissue is not only considered an energy storage organ but is now recognized as an endocrine and paracrine organ that plays an active role in energy homeostasis through the release of a large number of cytokines and bioactive mediators.[7] These novel risk factors and markers can not only influence body weight homeostasis but also insulin resistance, diabetes, lipid metabolism, inflammation, explaining premature atherosclerosis in obesity.[8] This has caused the hypothesis on the underlying mechanisms of the metabolic syndrome to shift towards an adipose tissue disease (adiposopathy) or lipotoxicity.[9] Exercise plays a key role in the prevention and treatment of overweight[10] and the non-pharmacological treatment of dyslipidemia.[11] It is also well established that participation in regular physical activity improves blood glucose control and can prevent or delay type 2 diabetes [12] and has the potential to reduce blood pressure.[13] Evidence for the positive effect of exercise on novel risk factors of the metabolic syndrome such as disturbances in adipokine secretion and low-grade inflammation confirms the importance of exercise in the treatment of the ‘new concept’ metabolic syndrome.[14] Reduction of body weight by lifestyle intervention is often modest, thus therapies which give the best result in decreasing VAT should be favoured over, or combined with, others. In keeping with earlier findings that reported the need for a higher volume on RCT's on the influence of physical activity in abdominal fat[15], in this review literature was screened to determine the effect of different exercise regimes (without hypocaloric diet) on visceral adipose tissue in overweight and obese adults.
Methods
The systematic literature search was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [16].
Study selection
Any randomized (RCT) and non-randomized controlled trials (non-RCT) or clinical trials (CT) meeting the subsequent specifications were included. Trials were included if the mean age of participants (males and females) was older than 18 years (adults, no upper age limit) and if the mean BMI at baseline was above 25.0 kg.m−2. Studies with one or more cohorts participating in aerobic or resistance exercise (physical activity) were eligible for inclusion in the meta-analysis. Physical activity was defined as a program that included voluntary aerobic or resistance exercise at “low to moderate” or “vigorous” intensity for at least two sessions per week. Study inclusion was limited to four discrete measurements of visceral adipose tissue: 1) Computerized Tomography (CT scan), 2) Magnetic Resonance Imaging (MRI), 3) dual energy x-ray absorptiometry (DXA), and 4) Ultrasound (US). These tests were selected because of their documented validity and reliability for assessments as well as reported prevalence in the literature.[17], [18] The studies were expected to conduct a (supervised) physical activity intervention without dietary intervention and to include all information needed for further meta-analysis in order to be considered for inclusion. Diet-only and supplementation-only studies or studies not meeting the inclusion criteria (e.g. physical activity intervention less than 8 weeks) were excluded. If a study examined the effects on visceral adipose tissue in diet and exercise groups, only the data of the exercise intervention arm was included in the final analysis.
Data sources and search strategies
Databases that were systematically searched were Pubmed, SPORTDiscus, Pedro and Cochrane. The following search strategy was conducted (adapted for each database): (Overweight OR Obesity) AND (Exercise OR “Physical activity” OR “Exercise therapy” OR “Resistance training” OR “Aerobic training”) AND (“Visceral adipose tissue” OR “Intra-abdominal fat”).
Studies published in English, German, French and Dutch were included. The date range was from 1990 to August 2012.
Reference lists were checked for any topic-related relevant studies.
Hand searching and screening for abstracts and citations from annual scientific conferences relating to exercise science were not performed.
The corresponding author of a study was contacted if needed to obtain any missing information or data. If authors could not be reached or if the data were no longer available, the trial was not included in the meta-analysis.
Screening and data-extraction form
All citations identified by electronic databases were organized and the duplicates were deleted. Initially, two investigators independently screened the results from the electronic searches in order to select potentially relevant citations based on titles and abstracts. The kappa statistic was used to evaluate the chance-adjusted inter-reviewer agreement (Kappa = 0.94). Inter-reviewer disagreements about study eligibility were resolved through consensus. For articles with relevant citations or with titles/abstracts that were not sufficient for deciding on inclusion/exclusion, the full-text articles were retrieved and evaluated. All studies selected at the first screening step were read and abstracted independently by three reviewers. Differences between the reviewers were resolved by consensus or referred to the third reviewer if necessary.
The following study characteristics were extracted from the articles: publication year, journal, study design, BMI, gender, type of intervention, study size, study duration, volume of physical activity, intensity of physical activity and change in VAT. Missing information was requested from authors by email.
Quality assessment
The quality of each eligible study was assessed in duplicate. Disagreements were resolved by mediation, if necessary with input from a third investigator.
The Critical Review Form for Quantitative Studies (Mc Master University 1998)[16] was used for quality assessment, resulting in a maximum score of 15. Only studies with a score of 8 or higher were included.
Statistical analysis
A meta-analysis with a random-effects model (specified a priori), accounting for possible heterogeneity between the studies, was used to examine the overall effect size of physical activity on visceral adipose tissue.
Effect sizes (change in VAT) of the uncontrolled and controlled studies were calculated as standardized mean differences and expressed as Hedge's g to correct for overestimating the true effect. The 95% confidence intervals [95%CI] were calculated for the individual studies and the overall estimate.
Subgroup analyses were conducted to assess the influence of different co-variates, such as the intensity of physical activity, on the overall estimate of VAT change. Meta regression was used to assess the possible influence of the duration (expressed as weeks) of intervention on the effect sizes of the 15 studies under investigation.
The Cochran's Q statistic and I2 were calculated to assess the degree of heterogeneity across studies. Publication bias was assessed using visual analysis of the funnel plot and by formal testing for funnel plot asymmetry using the ‘trim and fill’ and the ‘fail ’n safe' algorithms. For all analyses, P values less than 0.05 were considered significant. All calculations and plots were conducted using the CMA-2 software (Comprehensive Meta-Analysis 2nd version, Biostat, Englewood, NJ, USA).
For purpose of clinical interpretation the overall estimate of a meta-analysis on a subgroup of five controlled studies which used the same measurement scale (cm2) was re-expressed in the original units following the guidelines as described in the Cochrane handbook for systematic reviews of interventions[19]. Baseline data of the McTiernan study[20] was used to calculate a pooled standard deviation for the female and male experimental and control groups as well as for the combined gender groups.
Results
Overview of included studies for the meta-analysis
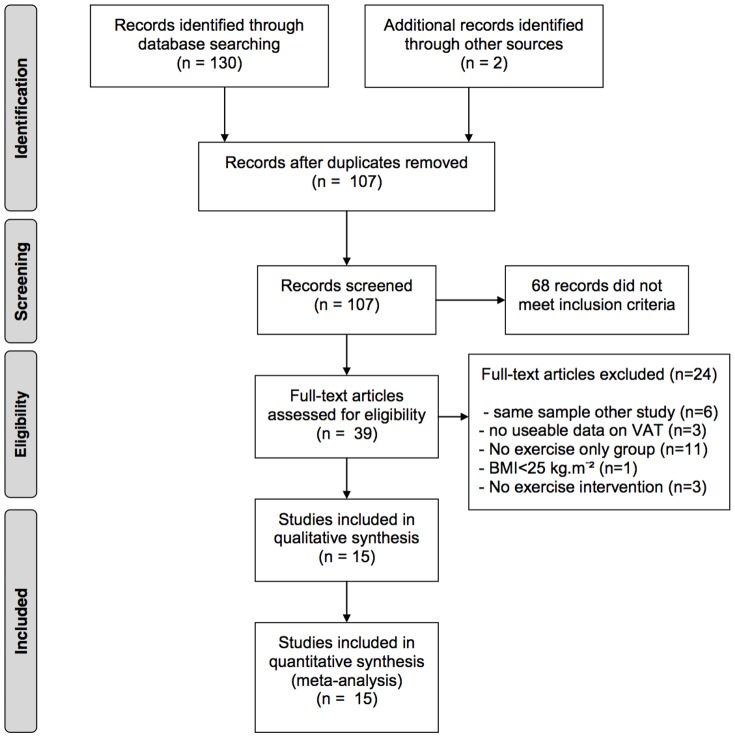
The initial search resulted in 107 articles after removing duplicates. After screening on title, abstract and full-text 15 articles (totalling 852 subjects in the exercise-only groups) fulfilled the a priori set inclusion criteria (Fig. 1). Because all studies that used dual energy x-ray absorptiometry or ultrasound did not fulfill one or more inclusion criteria, all of the 15 included studies used CT scan or MRI to quantify VAT.
Figure 1. Four-phase flow diagram of the systematic reviewing process.
Study quality
All of the 15 studies included in the qualitative analysis (fig 1) scored more than the a priori defined limit of 8/15 (range = 15 to 10), assessed with the “Critical Review Form–Quantitative studies” assessment instrument. In five of the fifteen studies, the reviewers independently agreed on all the items. For the other studies the degree of disagreement ranged between 1 and 5 items (mostly content validity related items). Disagreement was solved by consensus in all cases.
Meta-analysis
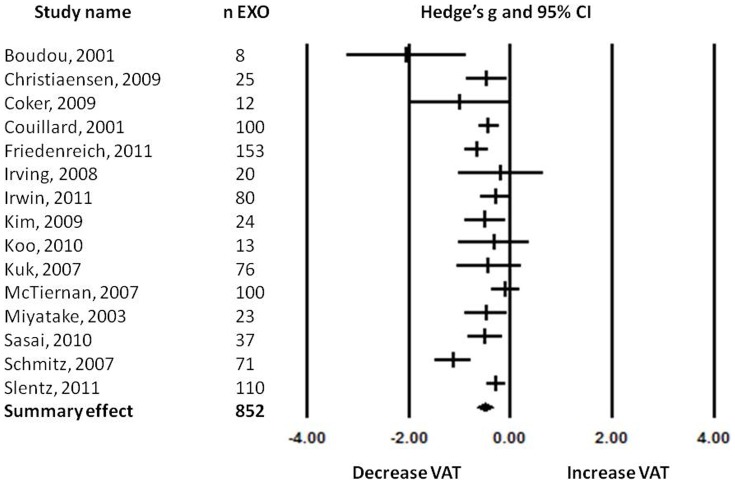
Data were extracted by a reviewer and presented in a spreadsheet to two other reviewers. The data were discussed until consensus was reached and analyzed in work meetings. All fifteen studies analyzed the effect of exercise on visceral adipose tissue (Table 1). The results of these studies were analyzed using the random effects model because of the high heterogeneity between studies (Fig 2). The standardized mean difference (Hedge's g) of the change in visceral adipose tissue after a physical activity intervention was −0.497 with a 95% confidence interval (95%CI) of −0.655 to −0.340. The Z-value was −6.183 and the p-value (two tailed) was <0.001. Heterogeneity analysis showed low to moderate, but significant, heterogeneity between studies (Cochran's Q = 37.317; degrees of freedom of Q (df(Q)) = 14; p = 0.001; I2 = 62.484).
Table 1. Overview of the studies included in the meta-analysis.
| Studies (Year) | N EXO (M/F) | Age (y) | BMI (kg/m2) | Training | Intensity | Frequency | Duration/session | Duration intervention | Assessment VAT | Results VAT |
| Boudou et al.[21] (2001) | 8 (8/0) | 45.4±7.2 | 29.6±4.6 | 2x/w aerobic1x/w interval | aerobic: 75% VO2peak interval: −5×2 min 85% VO2peak−3 min 50% VO2peak | 3x/week | aerobic: 45 min interval: 25 min | 10 weeks | MRI L4–L5 | from 153.25 cm2 ±38.55 to 84.20 cm2 ±21.30 |
| Couillard et al.[51] (2001) | 100 (100/0) | High TG/low HDL: 42±14 Isolated high TG: 30±14 | HIGH TG/LOW HDL: 27.4±4.4 Isolated high TG: 28.8±4.2 | aerobic | 55–75% VO2max | 3/week | 50 min | 5 months | CT L4–L5 | High TG/low HDL: −10.8 cm2±21.1 Isolated high TG: −5.0 cm2±17.2 |
| Irwin ML et al.[36] (2003) | 80 (0/80) | 61.0 | 30.5 | combination | 60–75% HRmax | 5/week | 45 min | 12 months | CT L4–L5 | −8.5 g/cm2 |
| Miyatake et al.[52] (2003) | 23 (23/0) | 45.2±7.5 | 29.0±2.3 | aerobic | 50%–65% HRmax | 1/week and increasing steps with 1000/day | N.A. | 5 months | CT at level umbiculus | from 108.7 cm2±49.1 to 85.9 cm2±40.9 |
| Kuk et al. [53] (2007) | 76 (0/76) | On average 58y | On average 31 | aerobic | 50%VO2max | 3–4/week | 4–8–12 kcal/kg/w | 6 months | CT L4–L5 | −0.02 kg |
| McTiernan et al. [20] (2007) | 100 (51/49) | F: 54.4±7.1 M: 56.2±6.7 | F: 28.9±5.5 M: 29.7±3.7 | aerobic | 60–85% HRmax | 6/week | 60 min | 12 months | CT L4–L5 | −12.2 cm2 (−7.5%) |
| Schmitz et al. [54] (2007) | 71 (0/71) | 36±5 | 29.4±0.4 | strength | 3×8–10 reps/exercise | 2/week | 60 min | 12 months | CT L2–L3 | −2.99% |
| Irving BA et al.[24] (2008) | 20 (0/20) | 51±9 | 34±6 | aerobic | low: rpe 10–12 high: rpe 15–17 | 5/week | 300–400 kcal/session | 16 weeks | CT L4–L5 | High Intensity: from 173 cm2±73 to 148 cm2±59 Moderate Intensity: no significant changes |
| Christiansen et al.[55] (2009) | 25 (0/25) | 37.2±7 | 34.3 | aerobic | 70% HRres | 3/week | 60–75 min | 12 weeks | Multislice MRI, femur to T8–T9 | −18% |
| Coker et al. [22] (2009) | 12 (6/6) | HI: 73±2 MI: 70±1 | HI: 30±1 MI: 28±1 | aerobic | HI: 75% VO2peakMI: 50% VO2peak | 4–5/week | 1000 kcal/week | 12 weeks | CT L4–L5 | High Intensity: −39 cm2±11Moderate Intesity: no change |
| Kim et al. [37] (2009) | 24 (24/0) | 49.4±9.6 | 30.7±3.3 | aerobic | 60–70% Hrmax | 3/week | 60 min | 12 weeks | CT at level umbiculus | from 197.1 cm2 to 165.7 cm2 (±16%) |
| Koo et al. [56] (2010) | 13 (0/13) | 59±4 | 28.0±2.7 | aerobic | brisk walking±500 kcal/d | 7/week | 120 min | 12 weeks | CT L4–L5 | −29,7%±23.3% |
| Sasai et al.[57] (2010) | 37 (37/0) | 47.6±8.6 | Low Volume: 31.0±4.1High Volume: 29.3±2.0 | aerobic | 65–80% HRmax | 3/week | 60–90 min | 12 weeks | CT at level umbilicus | High Volume: −30.0 cm2 ±23.4 Low Volume: −17,8 cm2±37.5 |
| Friedenreich et al.[23] (2011) | 153 (0/153) | 61.2±5.4 | 29.1±4.5 | aerobic | 62% HRres | 3.6/week | 45 min | 12 months | CT at level umbilicus | −16.5 cm2 |
| Slentz et al. [45] (2011) | 110 | ST: 49.7 ±11.4 AT: 49.5 ±9.8 COMB: 46.9 ±10.0 | ST: 30.5 ±3.4AT: 30.4 ±3.2COMB: 30.7 ±3.4 | strengthaerobiccombination | 75% VO2peak (14 kcal/kg/w) | 2–3/week | ST: 3 sets/day, 8–12 reps/set, 8 exercisesAT: eq. To 19.2 km/week | 8 months | CT at level L4 pedicle | ST: + 0.8 cm2 ±19AT: −15.9 cm2 ±34COMB: −10.9 cm2 ±9 |
Sample sizes represent exercise-only groups with results on VAT. ** Statistical significant results (p<0.05) are marked in bold.
Figure 2. Forest plot of the effects found in the individual studies and the overall effect.
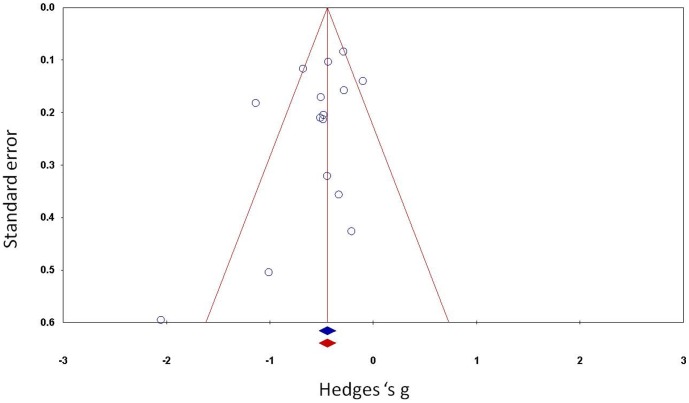
Through a funnel plot of standard error by Hedge's g, a graphic representation of heterogeneity was obtained (Fig 3). Using the ‘fail’n safe' algorithm, it becomes clear that there was no publication bias in the analysis because 405 extra studies would be needed to get the p-value to the alpha level.
Figure 3. Funnel plot of the standard error by Hedge's g.
Three of the analyzed studies (Boudou 2001, Cooker 2009, Koo 2010) had small sample sizes (n<14) and could thereby overestimate the general effect of the therapy. Conducting a sensitivity analysis by removing these studies, still revealed a decrease in visceral adipose tissue of −0.464 Hedge's g after a physical activity intervention program (95%CI: −0.313 to −0.616, p<0.01). By making this advanced analysis, heterogeneity decreased, however only slightly, to Q = 28.741 (with p = 0.002, I2 = 61.727).
Subgroup analysis and Meta-Regression
Controlled versus uncontrolled
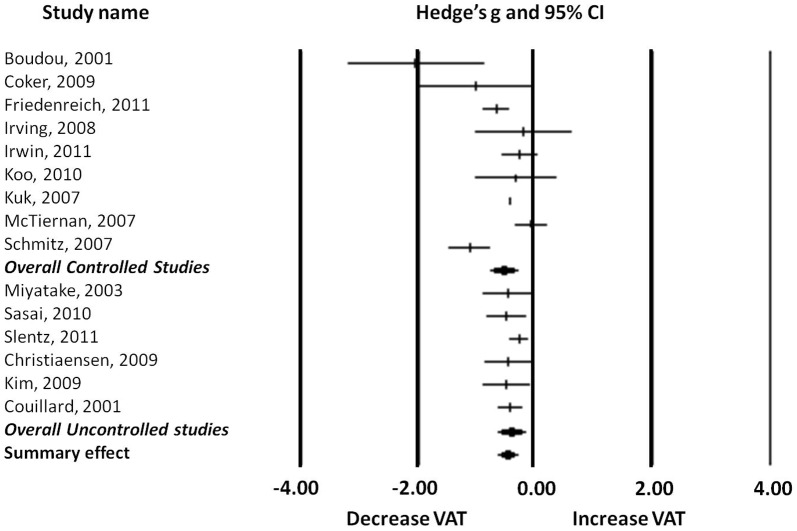
For the first subgroup analysis, the studies were divided in nine controlled and six uncontrolled trials (Fig 4). The analysis of these subgroups revealed that the controlled studies found a greater effect when comparing the results of VAT of the control group with those of the intervention group (Hedge's g = −0.561, 95%CI: −0.332 to −0.791, p<0.01) than uncontrolled studies in which the effect was compared between pre and post exercise intervention VAT values (Hedge's g = −0.437, 95%CI: −0.207 to −0.667, p<0.01).
Figure 4. Forest plot of the subgroup analysis: controlled and uncontrolled studies.
Heterogeneity analysis showed significant moderate to high heterogeneity between controlled studies (Cochran's Q = 32.888; df(Q) = 8; p<0.001; I2 = 75.675) but homogeneity between uncontrolled studies (Cochran's Q = 2.469; df(Q) = 5; p = 0.781; I2 = 0.000).
Gender
In female studies the decrease in VAT was −0.550 Hedge's g (95%CI: −0.269 to −0.831, p<0.01) while in male studies the decrease in VAT was −0.589 Hedge's g (95%CI: −0.276 to −0.896, p<0.001). In two studies where there were mixed samples of males and females, a lower overall decrease of VAT was observed. (Hedge's g = −0.330, 95%CI: −0.045 to −0.706, p = 0.085). Heterogeneity analysis showed significant low to moderate heterogeneity in mixed (Cochran's Q = 10.021; df(Q) = 4; p = 0.040; I2 = 60.085), male (Cochran's Q = 22.007; df(Q) = 6; p = 0.001; I2 = 72.736) and female studies (Cochran’s Q = 25.878; df(Q) = 8; p = 0.001; I2 = 69.086).
By removing the data of the subjects who received strength training or mixed (aerobic and strength) training, heterogeneity between studies decreased, especially in female and mixed gender study populations (Cochran’s Q = 13.279; df(Q) = 6; p = 0.039; I2 = 54.815 vs. Cochran’s Q = 6.113; df(Q) = 2; p = 0.047; I2 = 67.282). The effect of physical activity stayed significant in both male (Hedge’s g = −0.545, 95%CI: −0.286 to −0.805, p<0.001) and female studies (Hedge’s g = −0.334, 95%CI: −0.083 to −0.585, p<0.001). However, in the mixed study groups, the effect of physical activity did not reach significance (Hedge’s g = −0.396, 95%CI: −0.054 to −0.847, p = 0.085).
Training Modality
All studies were categorized in “aerobic training”-studies, “strength training”-studies or “combined” subgroups. This subgroup analysis showed that the decrease in VAT in the aerobic training subgroup was higher (Hedge’s g = −0.550, 95%CI: −0.332 to −0.768, p<0.001) compared to the strength training subgroup (Hedge’s g = −0.529, 95%CI: −0.003 to −1.054, p = 0.049).
However, in the “combined” subgroups, the decrease in VAT was no longer significant (Hedge’s g = −0.301, 95%CI: 0.221 to −0.823, p = 0.258).
Heterogeneity between aerobic studies was significant low to moderate (Cochran’s Q = 39.121; df(Q) = 16; p = 0.001; I2 = 59.101) while significant low to high heterogeneity was observed between strength studies (Cochran’s Q = 23.824; df(Q) = 1; p<0.001; I2 = 95.803). Homogeneity was found between combination studies (Cochran's Q = 0.034; df(Q) = 1; p = 0.853; I2 = 0.000).
Training Intensity
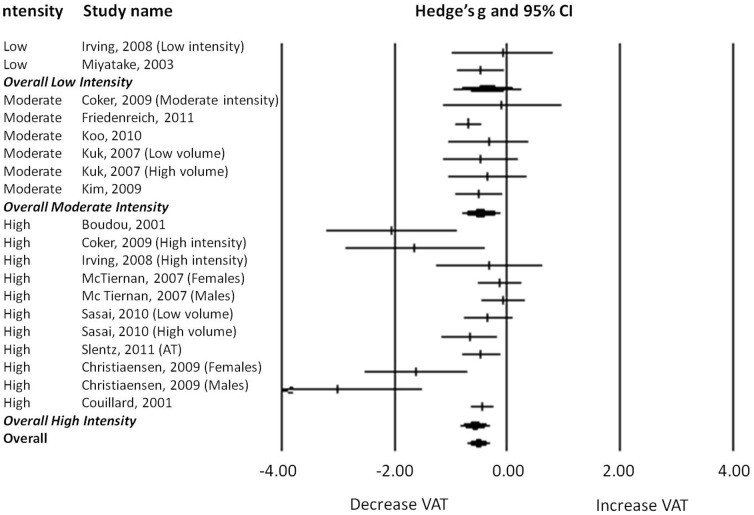
A final subgroup analysis was made through the categorization for intensity of all aerobic studies in “high intensity”, “moderate intensity” and “low-intensity” studies (Fig 5). Following cut-off values were used: 1) “high intensity”: when training most of the time at an intensity≥70% of maximal heart rate (HRmax) or more than 55% of maximal oxygen consumption (VO2max) or 60–80% of the heart rate reserve (HRres); 2) “moderate intensity”: when training most of the time at an intensity between 60–70% HRmax or 45−55% VO2max and 3)“low intensity”: when training most of the time at an intensity<60% HRmax or <45% VO2max.
Figure 5. Forest plot of the subgroup analysis: low intensity, moderate intensity and high intensity studies.
This analysis showed that a certain threshold of physical activity intensity should be reached to obtain a decrease in VAT. Only in studies in which moderate and high intensity-training was used, the effect on VAT was significant (“moderate intensity”-training: Hedge's g = −0.473, 95%CI: −0.140 to −0.806, p = 0.005; “high intensity”-training: Hedge's g = −0.588, 95%CI: −0.336 to −0.840, p<0.001).
Heterogeneity analysis showed significant low to moderate heterogeneity between “high intensity”-studies (Cochran's Q = 35.524; df(Q) = 10; p<0.001; I2 = 0.144) and homogeneity between “low intensity”-studies (Cochran's Q = 0.627; df(Q) = 1; p = 0.428; I2 = <0.001) and “moderate intensity”-studies (Cochran's Q = 2.659; df(Q) = 5; p = 0.751; I2 = <0.001).
The regression model showed no significant effect of duration of the intervention on VAT decrease ( = 0.836)
Re-expression of the estimate for clinical purpose
The five controlled studies (totalling 569 participants) using cm2 as the measuring scale of VAT[20]–[24] showed an overall estimate of Hedge's g = −0.630 representing a pooled effects size of −37.1 cm2 (females), −46.5 cm2 (males) and −42.1 cm2 (combined gender groups) respectively.
Discussion
Summary of findings
The present systematic review and meta-analysis is the first to investigate the effect of exercise without diet on visceral adipose tissue specifically in overweight and obese adults, with subgroup analyses for gender and intensity. All studies included in the meta-analysis used CT scan or MRI to assess VAT. There seems to be a strong association between visceral adipose tissue and increased risk for diseases such as cardiovascular disease[25], [26], type 2 diabetes[27] and non-alcoholic fatty liver disease[28]. A Cochrane review[29], states that a strong association exists between physical activity and improved cardiovascular disease risk factors, independent of weight reduction. Reduction of visceral adipose tissue may play a pivotal role in the pathophysiological mechanisms of this association.[30] Therefore, it is of great clinical interest to know if exercise is suited to reduce VAT because this could be an important clinical target, independent of (large) weight loss, which is often difficult to achieve and maintain.
This meta-analysis showed that a decrease of visceral adipose tissue can be obtained by exercise without diet in people with overweight and obesity. Aerobic exercise of moderate to vigorous intensity seems to have a greater effect on VAT than low intensity aerobic exercise or strength training. This is in line with the findings of Ismael et al.[31] who concluded in their meta-analysis that aerobic exercise is central for exercise programs aimed at reducing VAT. The study of Ismael et al. included predominantly studies with overweight and obese participants, but also to some extent studies with normal weight participants. Differences between the present meta-analysis and the meta-analysis of Ismael et al. are that the present meta-analysis 1) focused exclusively on overweight and obese individuals, 2) only included exercise-only intervention groups, 3)required a minimum of 8 weeks of exercise for inclusion, 4) presented results of subgroup analyses for gender and training intensity, 5) included studies published up to August 2012 and 6) re-expressed Hedge's g in cm2 for clinical interpretation.
Despite the large heterogeneity between studies, demonstrated by the sensitivity analyses, an overall effect of physical activity was found in this meta-analysis. Heterogeneity could possibly be explained by the diversity in study designs, training protocols and characteristics of subjects (age, BMI, gender, randomization,...). As demonstrated by Okura et al. abdominal fat reduction in response to weight loss can be modified by obesity phenotype (intra-abdominal versus abdominal subcutaneous fat storage).[32] The diversity of obesity phenotypes could also play a role in the heterogeneity that was found in this meta-analysis. Also ethnicity or race can play a role in obesity phenotype and more specifically in visceral adipose tissue storage[33], [34] In this meta-analysis most studies had Caucasian or Asian participants. A subgroup analysis revealed no significant difference in reduction of VAT between these 2 groups (results not shown).
Training volume
As described in the 2009 position stand of the American College of Sports Medicine (ACSM), moderate-intensity physical activity of >250 min.wk−1 has been associated with clinically significant weight loss.[35]
In the studies of McTiernan et al.[20] and Irwin et al.[36], participants trained five to six times per week for 45 to 60 minutes, which was twice as much as in the studies of Friedenreich et al.[23] and Kim et al[37]. Surprisingly, the higher training volume didn′t result in a higher reduction of VAT or total fat percentage. This is in contrast with the findings of Friedenreich et al.[23] who concluded that a minimum dose of physical activity must be achieved to yield a reduction in VAT while high volume training programs resulted in a higher reduction of VAT. The totally or partially self reported adherence to the exercise programs using activity diaries in the McTiernan et al.[20] and Irwin et al.[36] studies may have confounded the results by an adherence overestimation.
Training intensity
In the subgroup analysis for training intensity, only the studies with an aerobic exercise-only protocol were included. The results showed that there seems to be a threshold for intensity in order to have an effect on the reduction of VAT.
Although both absolute and relative (i.e.% of VO2max) exercise intensities play important roles in the regulation of substrate metabolism, the relative exercise intensity plays a major role in determining the proportions of carbohydrate and fat oxidized by the working muscles.[38] However, individual characteristics, such as nutritional status, may explain a large part of the variation in maximal rates of fat oxidation during exercise.[39] Also the fact that increasing exercise intensity can increase postexercise energy expenditure and fat oxidation should be taken into account.[40] The cut-offs to classify intensity in relation to a decrease in adipose tissue, as used in this meta-analysis, remain therefore somewhat arbitrary. An association has been reported between the volume of physical activity and weight loss[41], with indications for a possible ‘dose-response relationship’ between exercise intensity and increase in lean body mass. Also an association between cardiorespiratory fitness and diminished abdominal adiposity has been described[42] and between the amount of physical activity and the risk for metabolic syndrome[43]. However, the direct association between exercise intensity and reduction in visceral fat has not been investigated as a primary goal extensively. Gutin et al.[44] reported no clear effect of the intensity of physical training on the reduction of visceral and total-body adiposity. The study of Irving et al.[24] is one of few studies to report on the effect of exercise intensity in obese adults with abdominal visceral fat as primary outcome parameter.
Gender
There were an equal number of male and female studies. Only two mixed gender studies (Coker et al.[22]; Slentz et al.[45]) fulfilled the a priori set inclusion criteria, making it difficult to generalize the findings of this subgroup analysis. The analysis results suggested that in mixed gender studies, the effect of physical activity on VAT is smaller. Participants in those two studies showed only a minor loss of body weight while their reduction in relative body fatness was not mentioned.
This meta-analysis confirmed the need for gender-specific approaches and outcomes of obesity treatment in general, as previously stated by Lovejoy et al.[46] and more specific in the treatment of abdominal obesity. Furthermore, the results of this meta-analysis showed that males yield a higher profit of exercise on VAT than women corroborating the findings of Redman et al.[47]. The latter found more effect of caloric restriction and of the combination of caloric restriction and aerobic exercise in men then in women. In this context, one should take into account the facts that obesity phenotype can have an impact on abdominal fat reduction[32] and that men tend to be more likely to have significant amounts of abdominal fat, and to be more susceptible to abdominal adiposity[48].
Aerobic exercise and strength training
Only two combined aerobic and strength training studies fulfilled the inclusion criteria (Irwin et al.[36]; Slentz et al.[45]). The meta-analysis showed that such a combination yields only a modest reduction in VAT. This is somewhat unexpected because both aerobic training and strength training separately proved to have a significant effect on reduction of VAT. In the study of Slentz et al.[45], the combined training was not found to be superior to the aerobic training but the effects of both training types were similar.
Study strengths and limitations
A structured study protocol was developed and utilized for the search strategy, study selection, data extraction and statistical analysis. When the description of the methods was vague or insufficient data were given, the corresponding authors were contacted. All 15 included studies received scores of 10 or higher on the “Critical Review Form–Quantitative studies” and could thereby be considered to be of sufficient methodological quality.
Despite of all efforts, there are some limitations of this review that need to be acknowledged. In the assessment of the quality, the items with the highest disagreement between both scorers were “detailed description of the sample”, “justifying of the sample”, “avoiding of contamination” and “avoiding of co-intervention”. Only in a few studies, a power measurement of the sample had been done and often recruitment of the sample was not specified. There were also few studies that objectively assessed adherence to the exercise program and nutritional intake.
The strengths of this meta-analysis are that it focuses on the effects of exercise specifically in overweight and obese adults and provides information based on subgroup analyses for gender and training intensity. In a relentless effort to make standardized mean differences more clinical interpretable, Hedge's g were re-expressed as units of VAT, more specifically cm2.
Based on the Hedge's g, it seems that the 5 controlled clinical trials that used cm2 as unit for VAT, slightly overestimate the effect of exercise on reduction of VAT compared to the total of 9 controlled clinical trials (−0.630 versus −0.561). Taking that into account, the results of this meta-analysis show that exercise without diet has the potential to reduce VAT with >30 cm2 in females and >40 cm2 in males. Okauchi et al. demonstrated in a study in 2336 Japanese men that a reduction of VAT within 1 year was associated with a significant decrease in the number of metabolic risk factors, with a decrease of >30 cm2 showing the highest correlation coefficient.[49]
The results of the meta-regression of the duration of intervention on the decrease of VAT, seem to suggest that the effect size does not increase by prolonging the intervention period beyond 12 weeks. However, it remains unclear what the effect of the duration of intervention is on the risk of VAT regain. Regain of VAT during the follow-up period after an initial loss of VAT is clearly a pitfall, as demonstrated by Koga et al.[50], which can be predicted by fluctuation (not absolute values) in daily exercise regimens, during the training-education period.
Conclusion
This systematic review and meta-analysis shows that an exercise program without hypocaloric diet has the potential to reduce visceral adipose tissue.
There seem to be gender differences in decrease of visceral adipose tissue by exercise which could be related to obesity phenotype.
Combining aerobic training with strength training does not result in a higher decrease of visceral adipose tissue. The intensity of a training program should be at least moderate to vigorous. Recommendations for future studies are to take possible confounding factors (such as gender, obesity phenotype, training intensity, type of training) into account and to carefully assess adherence to the training program and nutritional protocol.
Supporting Information
Extended data table including ethnicit and co-morbidities.
(XLSX)
Prisma checklist.
(DOCX)
Funding Statement
The authors have no support or funding to report.
References
- 1. Haslam DW, James WP (2005) Obesity. Lancet 366: 1197–1209. [DOI] [PubMed] [Google Scholar]
- 2. Skelton JA, Cook SR, Auinger P, Klein JD, Barlow SE (2009) Prevalence and Trends of Severe Obesity Among US Children and Adolescents. Acad Pediatr [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, et al. (2006) Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 355: 763–778. [DOI] [PubMed] [Google Scholar]
- 4. Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, et al. (2003) Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 138: 24–32. [DOI] [PubMed] [Google Scholar]
- 5. Janssen I, Katzmarzyk PT, Ross R (2004) Waist circumference and not body mass index explains obesityrelated health risk. Am J Clin Nutr 79: 379–384. [DOI] [PubMed] [Google Scholar]
- 6. Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, et al. (2008) General and abdominal adiposity and risk of death in Europe. N Engl J Med 359: 2105–2120. [DOI] [PubMed] [Google Scholar]
- 7. Ahima RS (2006) Adipose tissue as an endocrine organ. Obesity (Silver Spring) 14 Suppl 5: 242S–249S. [DOI] [PubMed] [Google Scholar]
- 8. Van Gaal LF, Mertens IL, De Block CE (2006) Mechanisms linking obesity with cardiovascular disease. Nature 444: 875–880. [DOI] [PubMed] [Google Scholar]
- 9. Oda E (2008) The metabolic syndrome as a concept of adipose tissue disease. Hypertens Res 31: 1283–1291. [DOI] [PubMed] [Google Scholar]
- 10. Surmi BK, Hasty AH (2008) Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol 3: 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brewer HB Jr, Santamarina-Fojo S (2003) Clinical significance of high-density lipoproteins and the development of atherosclerosis: focus on the role of the adenosine triphosphate-binding cassette protein A1 transporter. Am J Cardiol 92: 10K–16K. [DOI] [PubMed] [Google Scholar]
- 12. Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, et al. (2010) Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 33: e147–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whelton SP, Chin A, Xin X, He J (2002) Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 136: 493–503. [DOI] [PubMed] [Google Scholar]
- 14. You T, Nicklas BJ (2008) Effects of exercise on adipokines and the metabolic syndrome. Curr Diab Rep 8: 7–11. [DOI] [PubMed] [Google Scholar]
- 15. Kay SJ, Fiatarone Singh MA (2006) The influence of physical activity on abdominal fat: a systematic review of the literature. Obes Rev 7: 183–200. [DOI] [PubMed] [Google Scholar]
- 16. Law M, Stewart D, Letts L, Pollock N, Bosch J, et al. (1998) Critical Review Form, Quantitative Studies. McMaster University [Google Scholar]
- 17. Shuster A, Patlas M, Pinthus JH, Mourtzakis M (2012) The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol 85: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL (2012) Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring) 20: 1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(2010) Cochrane Handbook for Systematic Reviews of Interventions; Higgins J, Green S, editors: John Wiley & Sons Ltd. [Google Scholar]
- 20. McTiernan A, Sorensen B, Irwin ML, Morgan A, Yasui Y, et al. (2007) Exercise effect on weight and body fat in men and women. Obesity (Silver Spring) 15: 1496–1512. [DOI] [PubMed] [Google Scholar]
- 21. Boudou P, de Kerviler E, Erlich D, Vexiau P, Gautier JF (2001) Exercise training-induced triglyceride lowering negatively correlates with DHEA levels in men with type 2 diabetes. Int J Obes Relat Metab Disord 25: 1108–1112. [DOI] [PubMed] [Google Scholar]
- 22. Coker RH, Williams RH, Kortebein PM, Sullivan DH, Evans WJ (2009) Influence of exercise intensity on abdominal fat and adiponectin in elderly adults. Metab Syndr Relat Disord 7: 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Friedenreich CM, Woolcott CG, McTiernan A, Terry T, Brant R, et al. (2011) Adiposity changes after a 1-year aerobic exercise intervention among postmenopausal women: a randomized controlled trial. Int J Obes (Lond) 35: 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Irving BA, Davis CK, Brock DW, Weltman JY, Swift D, et al. (2008) Effect of exercise training intensity on abdominal visceral fat and body composition. Med Sci Sports Exerc 40: 1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mathieu P, Lemieux I, Despres JP (2010) Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther 87: 407–416. [DOI] [PubMed] [Google Scholar]
- 26. Ritchie SA, Connell JM (2007) The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis 17: 319–326. [DOI] [PubMed] [Google Scholar]
- 27. Freemantle N, Holmes J, Hockey A, Kumar S (2008) How strong is the association between abdominal obesity and the incidence of type 2 diabetes?. Int J Clin Pract 62: 1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verrijken A, Francque S, Mertens I, Talloen M, Peiffer F, et al. (2010) Visceral adipose tissue and inflammation correlate with elevated liver tests in a cohort of overweight and obese patients. Int J Obes (Lond) 34: 899–907. [DOI] [PubMed] [Google Scholar]
- 29. Shaw K, Gennat H, O'Rourke P, Del Mar C (2006) Exercise for overweight or obesity. Cochrane Database Syst Rev CD003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, et al. (2008) Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 28: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 31. Ismail I, Keating SE, Baker MK, Johnson NA A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev 13: 68–91. [DOI] [PubMed] [Google Scholar]
- 32. Okura T, Nakata Y, Lee DJ, Ohkawara K, Tanaka K (2005) Effects of aerobic exercise and obesity phenotype on abdominal fat reduction in response to weight loss. Int J Obes (Lond) 29: 1259–1266. [DOI] [PubMed] [Google Scholar]
- 33. Carroll JF, Fulda KG, Chiapa AL, Rodriquez M, Phelps DR, et al. (2009) Impact of race/ethnicity on the relationship between visceral fat and inflammatory biomarkers. Obesity (Silver Spring) 17: 1420–1427. [DOI] [PubMed] [Google Scholar]
- 34. Despres JP, Couillard C, Gagnon J, Bergeron J, Leon AS, et al. (2000) Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol 20: 1932–1938. [DOI] [PubMed] [Google Scholar]
- 35. Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, et al. (2009) American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 41: 459–471. [DOI] [PubMed] [Google Scholar]
- 36. Irwin ML, Yasui Y, Ulrich CM, Bowen D, Rudolph RE, et al. (2003) Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. Jama 289: 323–330. [DOI] [PubMed] [Google Scholar]
- 37. Kim MK, Tomita T, Kim MJ, Sasai H, Maeda S, et al. (2009) Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol 106: 5–11. [DOI] [PubMed] [Google Scholar]
- 38. Holloszy JO, Kohrt WM, Hansen PA (1998) The regulation of carbohydrate and fat metabolism during and after exercise. Front Biosci 3: D1011–1027. [DOI] [PubMed] [Google Scholar]
- 39. Gonzalez JT, Stevenson EJ (2012) New perspectives on nutritional interventions to augment lipid utilisation during exercise. Br J Nutr 107: 339–349. [DOI] [PubMed] [Google Scholar]
- 40. Warren A, Howden EJ, Williams AD, Fell JW, Johnson NA (2009) Postexercise fat oxidation: effect of exercise duration, intensity, and modality. Int J Sport Nutr Exerc Metab 19: 607–623. [DOI] [PubMed] [Google Scholar]
- 41. Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, et al. (2004) Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE-a randomized controlled study. Arch Intern Med 164: 31–39. [DOI] [PubMed] [Google Scholar]
- 42. Ross R, Katzmarzyk PT (2003) Cardiorespiratory fitness is associated with diminished total and abdominal obesity independent of body mass index. Int J Obes Relat Metab Disord 27: 204–210. [DOI] [PubMed] [Google Scholar]
- 43. Ekelund U, Brage S, Franks PW, Hennings S, Emms S, et al. (2005) Physical activity energy expenditure predicts progression toward the metabolic syndrome independently of aerobic fitness in middle-aged healthy Caucasians: the Medical Research Council Ely Study. Diabetes Care 28: 1195–1200. [DOI] [PubMed] [Google Scholar]
- 44. Gutin B, Barbeau P, Owens S, Lemmon CR, Bauman M, et al. (2002) Effects of exercise intensity on cardiovascular fitness, total body composition, and visceral adiposity of obese adolescents. Am J Clin Nutr 75: 818–826. [DOI] [PubMed] [Google Scholar]
- 45. Slentz CA, Bateman LA, Willis LH, Shields AT, Tanner CJ, et al. (2011) Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab 301: E1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lovejoy JC, Sainsbury A (2009) Sex differences in obesity and the regulation of energy homeostasis. Obes Rev 10: 154–167. [DOI] [PubMed] [Google Scholar]
- 47. Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, et al. (2007) Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab 92: 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Power ML, Schulkin J (2008) Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. Br J Nutr 99: 931–940. [DOI] [PubMed] [Google Scholar]
- 49. Okauchi Y, Nishizawa H, Funahashi T, Ogawa T, Noguchi M, et al. (2007) Reduction of visceral fat is associated with decrease in the number of metabolic risk factors in Japanese men. Diabetes Care 30: 2392–2394. [DOI] [PubMed] [Google Scholar]
- 50. Koga R, Tanaka M, Tsuda H, Imai K, Abe S, et al. (2008) Daily exercise fluctuations and dietary patterns during training predict visceral fat regain in obese women. Am J Med Sci 336: 450–457. [DOI] [PubMed] [Google Scholar]
- 51. Couillard C, Despres JP, Lamarche B, Bergeron J, Gagnon J, et al. (2001) Effects of endurance exercise training on plasma HDL cholesterol levels depend on levels of triglycerides: evidence from men of the Health, Risk Factors, Exercise Training and Genetics (HERITAGE) Family Study. Arterioscler Thromb Vasc Biol 21: 1226–1232. [DOI] [PubMed] [Google Scholar]
- 52. Miyatake N, Takahashi K, Wada J, Nishikawa H, Morishita A, et al. (2003) Daily exercise lowers blood pressure and reduces visceral adipose tissue areas in overweight Japanese men. Diabetes Res Clin Pract 62: 149–157. [DOI] [PubMed] [Google Scholar]
- 53. Kuk JL (2007) Associations between abdominal adiposity, exercise, morbidity, and mortality. Applied Physiology, Nutrition & Metabolism 32: 1210–1211. [Google Scholar]
- 54. Schmitz KH, Hannan PJ, Stovitz SD, Bryan CJ, Warren M, et al. (2007) Strength training and adiposity in premenopausal women: strong, healthy, and empowered study. Am J Clin Nutr 86: 566–572. [DOI] [PubMed] [Google Scholar]
- 55. Christiansen T, Paulsen SK, Bruun JM, Overgaard K, Ringgaard S, et al. (2009) Comparable reduction of the visceral adipose tissue depot after a diet-induced weight loss with or without aerobic exercise in obese subjects: a 12-week randomized intervention study. Eur J Endocrinol 160: 759–767. [DOI] [PubMed] [Google Scholar]
- 56. Koo BK, Han KA, Ahn HJ, Jung JY, Kim HC, et al. (2010) The effects of total energy expenditure from all levels of physical activity vs. physical activity energy expenditure from moderate-to-vigorous activity on visceral fat and insulin sensitivity in obese Type 2 diabetic women. Diabet Med 27: 1088–1092. [DOI] [PubMed] [Google Scholar]
- 57. Sasai H, Katayama Y, Nakata Y, Eto M, Tsujimoto T, et al. (2010) Physical activity and intra-abdominal fat reduction: effects of age, obesity phenotype and vigorous physical activity. Japanese Journal of Physical Fitness & Sports Medicine 59: 68–68. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Extended data table including ethnicit and co-morbidities.
(XLSX)
Prisma checklist.
(DOCX)