Abstract
The pinewood nematode, Bursaphelenchus xylophilus, is one of the greatest threats to coniferous forests worldwide, causing severe ecological damage and economic loss. The biology of B. xylophilus is similar to that of its closest relative, B. mucronatus, as both species share food resources and insect vectors, and have very similar morphological characteristics, although little pathogenicity to conifers has been associated with B. mucronatus. Using both nuclear and mitochondrial DNA markers, we show that B. xylophilus and B. mucronatus form distinct phylogenetic groups with contrasting phylogeographic patterns. B. xylophilus presents lower levels of intraspecific diversity than B. mucronatus, as expected for a species that evolved relatively recently through geographical or reproductive isolation. Genetic diversity was particularly low in recently colonised areas, such as in southwestern Europe. By contrast, B. mucronatus displays high levels of genetic diversity and two well-differentiated clades in both mitochondrial and nuclear DNA phylogenies. The lack of correlation between genetic and geographic distances in B. mucronatus suggests intense gene flow among distant regions, a phenomenon that may have remained unnoticed due to the reduced pathogenicity of the species. Overall, our findings suggest that B. xylophilus and B. mucronatus have different demographic histories despite their morphological resemblance and ecological overlap. These results suggest that Bursaphelenchus species are a valuable model for understanding the dispersion of invasive species and the risks posed to native biodiversity and ecosystems.
Introduction
The pinewood nematode (PWN), Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae), is the causal agent of the widespread pine wilt disease (PWD), which causes severe ecological and economic losses in coniferous forests [1], [2]. The PWN causes the death of host trees in less than one year after infection under appropriate environmental conditions. On the contrary, little pathogenicity to conifers has been associated with its closest related species, Bursaphelenchus mucronatus, despite both species having similar morphological and biological features [3]–[6]. The phylogeny and evolution of the PWN species complex [7], which includes both B. xylophilus, B. mucronatus and a few other species within the genus Bursaphelenchus, has produced inconsistent results depending on the genetic marker under analysis [8]. Some doubts still remain concerning the taxonomic status of these species, particularly given that B. xylophilus and B. mucronatus can generate hybrids [9]–[12].
These nematodes are transmitted from tree to tree by wood-inhabiting longhorn beetles that belong mainly to the genus Monochamus (Coleoptera: Cerambycidae). The intensification of world trade in recent decades is responsible not only for introduction of PWD but also for the expansion of B. xylophilus via short- and long-distance dispersals through transportation of PWN infected wood, including unprocessed logs, wooden crates, pallets and dunnage [13]–[16]. The impact of human-mediated processes in the evolutionary history of this important plant pathogen is not well understood. B. xylophilus is considered to be native to North America [17], where local conifers are mostly resistant or tolerant to this nematode [18]. However, its introduction in Japan at the beginning of the 20th century and later in mainland China, Taiwan and Korea had a dramatic impact on the newly invaded environment, causing massive mortality of native pine trees, namely Pinus thunbergii and P. densiflora [2]. A similar epidemic has also been occurring in Portugal [19], where B. xylophilus is devastating vast areas of maritime pine (P. pinaster) since 1999. The nematode has already spread to Madeira Island [20] and Spain [21], [22], representing an increasing threat to European forests. In contrast, B. mucronatus is widely distributed throughout the northern hemisphere, and is a prevalent species in the cooler areas of central and northern Europe without causing damage to the local trees. It has been proposed that B. mucronatus originated in Eurasia [17], but almost nothing is known about its intraspecific phylogeny. Studies on the molecular genetics of these nematode species are usually restricted to a geographic location and/or a single molecular marker [23]–[26]. A better understanding of the evolutionary relationships between Bursaphelenchus species is therefore needed. In this study, we provide new insights into the intraspecific phylogeny of B. xylophilus and B. mucronatus using mitochondrial and nuclear DNA data from isolates of different world regions.
Materials and Methods
Nematode samples and DNA extraction
B. xylophilus isolates were obtained across mainland Portugal and Madeira Island and other world regions where it has been reported (North America, Japan, China and Korea). B. mucronatus isolates were obtained in Portugal and Germany in order to increase the worldwide dataset available at GenBank (Figure 1, Table 1 and Table S1 in File S1). Nematodes were extracted from wood samples using the Whitehead & Hemming tray method [27] and identified based on diagnostic morphological characters [28]. Nematodes were then hand-picked, washed several times with sterilised distilled water and transferred to cultures of the fungus Botrytis cinerea grown on malt extract agar medium and incubated at 25°C [29]. Subcultures of each nematode isolate were regularly performed by transferring small plugs with nematodes to new malt extract agar medium colonised with B. cinerea. Portuguese B. xylophilus isolates were established between 2005 and 2010 (Table 1). For this study, hundreds of nematodes were gathered from a subculture of each isolate, without separation according to sex or developmental stage, and washed several times in sterilised distilled water. Nematodes were then concentrated by centrifugation and the resulting supernatant was removed leaving the pellet containing the nematodes (±6,000). DNA was extracted from the pool of nematodes as previously described [30]. No specific permits were required for the described field studies. Collection sites were not privately owned or protected and did not involve endangered or protected species.
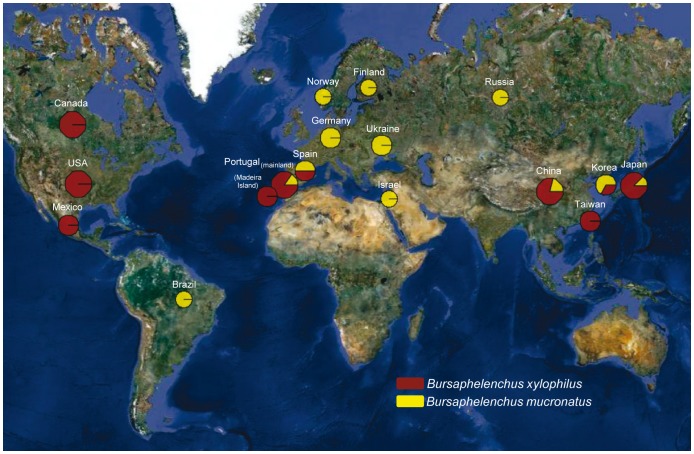
Figure 1. Geographical distribution of Bursaphelenchus xylophilus and B. mucronatus isolates.
Pie chart symbols show the relative proportion of isolates from both species analysed in the present study.
Table 1. Mitochondrial and nuclear DNA sequences from Bursaphelenchus xylophilus (Bx) and B. mucronatus (Bm) isolates used in the present study.
| Isolate code | Isolate origin | Culture | GenBank accession numbers (genetic marker) |
| BxCa188 | Canada (Quebec) | AY508071 (COI), AY508108 (28S rRNA) | |
| BxCa185 | Canada | AY508068 (COI), AY508105 (28S rRNA) | |
| BxCa187 | Canada (New Brunswick) | AY508070 (COI), AY508107 (28S rRNA) | |
| BxCaBC | Canada (British Columbia) | AB108439 (ITS-2) | |
| BxCaFIDS | Canada | AB108441 (ITS-2) | |
| BxCaQ52A | Canada (Quebec) | AB108444 (ITS-2) | |
| BxCaStJ | Canada | AB108445 (ITS-2) | |
| BxCaBXCANADA | Canada | EF446946 (ITS-2), EF446935 (28S rRNA) | |
| BxCaCA | Canada | JF317229 (ITS-2), JF317239 (28S rRNA) | |
| BxCaQ52 | Canada | JF317230 (ITS-2), JF317241 (28S rRNA) | |
| BxCaBC2 | Canada | JF317231 (ITS-2), JF317240 (28S rRNA) | |
| BxCaBxCAN | Canada | EU295503 (28S rRNA) | |
| BxUSA618 | USA | 2008 | JN596443 (COI), JN596460 (ND5), JN596470 (s-rRNA) |
| BxUSA745 | USA | 2008 | JN596444 (COI), JN596461 (ND5) |
| BxUSA30697 | USA | JF317255 (COI) | |
| BxUSA4049 | USA | JF317256 (COI), JF317232 (ITS-2) | |
| BxUSA121AD | USA | JF317257 (COI), JF317234 (ITS-2), JF317244 (28S rRNA) | |
| BxUSAMO | USA | AB108442 (ITS-2) | |
| BxUSABXUSA2 | USA | EF446951 (ITS-2), EF446940 (28S rRNA) | |
| BxUSAUS10 | USA | JF317243 (28S rRNA) | |
| BxUSA39906 | USA | JF317245 (28S rRNA) | |
| BxJT4 | Japan (Iwate) | <2008 | JN596430 (COI), JN596458 (ND5), AB108446/AB277207 (ITS-2) |
| BxJ10 | Japan | <2001 | JN596429 (COI), JN596459 (ND5), JN596466 (s-rRNA) |
| BxJpS10 | Japan (Shimane) | AB067766 (COI), AB277206/U92464 (ITS-2) | |
| BxJp186 | Japan (Mito) | AY508069 (COI), AY508106 (28S rRNA) | |
| BxJpAB050051 | Japan (Akita, Ohmori) | AB050051 (ITS-2) | |
| BxJpAB050052 | Japan (Niigata, Murakami) | AB050052 (ITS-2) | |
| BxJpAB050053 | Japan (Ibaraki, Tsukuba) | AB050053 (ITS-2) | |
| BxJpKyoto1 | Japan (Kyoto) | AB050054 (ITS-2) | |
| BxJpKyoto2 | Japan (Kyoto) | AB050055 (ITS-2) | |
| BxJpAB050056 | Japan (Yamaguchi, Tokuyama) | AB050056 (ITS-2) | |
| BxJpAB050057 | Japan (Ehime, Imabari) | AB050057 (ITS-2) | |
| BxJpAB050058 | Japan (Nagasaki, Shimabara) | AB050058 (ITS-2) | |
| BxJpAB050059 | Japan (Okinawa, Kunigami) | AB050059 (ITS-2) | |
| BxJpOk-2 | Japan (Okinawa) | AB108443 (ITS-2) | |
| BxJpC14-5 | Japan (Chiba, Ichinomiya) | AB277203 (ITS-2) | |
| BxJpOKD1 | Japan (Okayama, Okayama) | AB277205 (ITS-2) | |
| BxJpBCMUBX18 | Japan (Aichi, Nissin, Iwasaki-cho) | AB294736 (ITS-2) | |
| BxJpAY347913 | Japan | AY347913 (ITS-2) | |
| BxJpBXJ1 | Japan | EF446943 (ITS-2), EF446934 (28S rRNA) | |
| BxJpXylT4 | Japan | DQ356002 (28S rRNA) | |
| BxJpBxJAP | Japan | EU295504 (28S rRNA) | |
| BxCSD | China | <2009 | JN596428 (COI), JN596456 (ND5), JN596465 (s-rRNA) |
| BxChBXC | China | AB108440 (ITS-2) | |
| BxChAY347911 | China (Xiangshan, Zhejiang) | AY347911 (ITS-2) | |
| BxChAY347912 | China (Nanjing, Jiangsu) | AY347912 (ITS-2) | |
| BxChBXCNJ3 | China (Nanjing, Jiangsu) | EF446944 (ITS-2), EF446929 (28S rRNA) | |
| BxChBXCAJ | China (Mingguang, Anhui) | EF446945 (ITS-2), EF446942 (28S rRNA) | |
| BxChBXCSC | China (Changdao, Shandong) | EF446947 (ITS-2), EF446932 (28S rRNA) | |
| BxChBXCNJ2 | China (Nanjing, Jiangsu) | EF446948 (ITS-2), EF446941 (28S rRNA) | |
| BxChBXCGD | China (Dongguan, Guangdong) | EF446950 (ITS-2), EF446933 (28S rRNA) | |
| BxChBXCZZ | China (Zhoushan, Zhejiang) | EF446952 (ITS-2), EF446937 (28S rRNA) | |
| BxChXM_1 | China (Fujian) | EU259322 (ITS-2) | |
| BxChDQ364687 | China (Xiangshan) | DQ364687 (28S rRNA) | |
| BxChBXCNJ1 | China (Nanjing, Jiangsu) | EF446930 (28S rRNA) | |
| BxChBXCNJ4 | China (Nanjing, Jiangsu) | EF446931 (28S rRNA) | |
| BxChBxLYG | China (Lianyungang) | EU295491 (28S rRNA) | |
| BxTaNe6/05 | China (Taiwan) | AM179515 (ITS-2) | |
| BxTaTWRC | China (Taiwan) | JF317242 (28S rRNA) | |
| BxKAS | South Korea | <2008 | JN596431 (COI), JN596457 (ND5), JN596467 (s-rRNA) |
| BxPt11AS | Portugal (Alcácer do Sal) | 2005 | JN596432 (COI), JN596447 (ND5) |
| BxPt15SC | Portugal (Santiago do Cacém) | 2007 | JN596433 (COI), JN596448 (ND5) |
| BxPt17AS | Portugal (Alcácer do Sal) | 2007 | JN596434 (COI), JN596449 (ND5), JN596468 (s-rRNA) |
| BxPt19SCD | Portugal (Santa Comba Dão) | 2008 | JN596435 (COI), JN596450 (ND5), JN596469 (s-rRNA) |
| BxPt21T | Portugal (Tábua) | 2008 | JN596436 (COI), JN596451 (ND5) |
| BxPt56M | Portugal (Mealhada) | 2009 | JN596438 (COI), JN596446 (ND5) |
| BxPt60OH | Portugal (Oliveira do Hospital) | 2009 | JN596437 (COI), JN596445 (ND5) |
| BxPtHF | Portugal (Herdade de Ferraria) | AB277204 (ITS-2) | |
| BxPtTroia | Portugal (Troia) | AB277208 (ITS-2) | |
| BxPtPT1w | Portugal (Pegões) | AM157747 (ITS-2), AM396580 (28S rRNA) | |
| BxPtBXPOT | Portugal | EF446949 (ITS-2), EF446936 (28S rRNA) | |
| BxMad1F | Portugal (Madeira Island) | 2010 | JN596439 (COI), JN596452 (ND5) |
| BxMad2M | Portugal (Madeira Island) | 2010 | JN596440 (COI), JN596453 (ND5) |
| BxMad3F | Portugal (Madeira Island) | 2010 | JN596441 (COI), JN596454 (ND5) |
| BxMad4SV | Portugal (Madeira Island) | 2010 | JN596442 (COI), JN596455 (ND5) |
| BxSpEFA1 | Spain | HQ646254 (ITS-2) | |
| BxMe39906-1 | Mexico | JF317253 (COI) | |
| BxMe39906-2 | Mexico | JF317254 (COI) | |
| BxMe39906 | Mexico | JF317233 (ITS-2) | |
| BmJpM | Japan | AB067765 (COI) | |
| BmJp163 | Japan | AY508049 (COI), AY508086 (28S rRNA) | |
| BmJp424B | Japan | JF317260 (COI), JF317235 (ITS-2), JF317246 (28S rRNA) | |
| BmChAY347915 | China (Hong Kong) | AY347915 (ITS-2) | |
| BmChAY347916 | China (Fuyang, Zhejiang) | AY347916 (ITS-2) | |
| BmChBMCSC | China (Zhoushan, Zhejiang) | EF446953 (ITS-2), EF446938 (28S rRNA) | |
| BmChXM | China (Fujian) | EU296624 (ITS-2) | |
| BmKo39571 | South Korea | JF317261 (COI), JF317236 (ITS-2), JF317247 (28S rRNA) | |
| BmKoAY347914 | South Korea | AY347914 (ITS-2) | |
| BmPt1 | Portugal | 2008 | JN596463 (s-rRNA) |
| BmPt2 | Portugal | 2008 | JN596464 (s-rRNA) |
| BmSp860A | Spain | JF317262 (COI) | |
| BmG1 | Germany | <2001 | JN596427 (COI), JN596462 (s-rRNA) |
| BmG166 | Germany (Zusmarshausen) | AY508052 (COI), AY508089 (28S rRNA) | |
| BmG167 | Germany (Grunberg) | AY508053 (COI), AY508090 (28S rRNA) | |
| BmG168 | Germany (Zusmarshausen) | AY508054 (COI), AY508091 (28S rRNA) | |
| BmFi165 | Finland | AY508051 (COI), AY508088 (28S rRNA) | |
| BmNo164 | Norway (Hanestad) | AY508050 (COI), AY508087 (28S rRNA) | |
| BmUk38624 | Ukraine | JF317258 (COI) | |
| BmUk53106 | Ukraine | JF317238 (ITS-2) | |
| BmRuBMRUSSIAN | Russia | EF446939 (28S rRNA) | |
| BmIs5459 | Israel | JF317237 (ITS-2) | |
| BmBr4228 | Brazil | JF317259 (COI) |
The list includes sequences from the mitochondrial cytochrome c oxidase subunit I (COI), NADH dehydrogenase subunit 5 (ND5) and small subunit ribosomal RNA (s-rRNA) genes and the nuclear internal transcribed spacer 2 (ITS-2) and 28S ribosomal RNA gene (28S rRNA). The accession numbers in bold indicate new sequences obtained in this work.
Polymerase chain reaction (PCR) and DNA sequencing
The three mitochondrial DNA (mtDNA) gene regions, cytochrome c oxidase subunit I (COI or COX1), NADH dehydrogenase subunit 5 (ND5) and small subunit ribosomal RNA (s-rRNA), were amplified using the PCR primers described in Table S2 in File S1. PCR was carried out by combining 2 µl of DNA extract, 1 µl of primer mix (2 mM of each primer) and 5 µl of Multiplex PCR Master Mix (Qiagen GmbH, Germany) in a 10 µl final volume. PCR was performed as follows: an initial denaturation step at 95°C for 15 min, followed by 30 cycles of 30 s at 94°C, 90 s at the lower annealing temperature of the primer pair and 1 min at 72°C and a final extension step of 10 min at 72°C. All amplifications were performed using GeneAmp PCR Systems 2700 equipment (Applied Biosystems, Foster City, CA, USA). Sequencing reactions were performed in both directions by combining 2.5 µl of amplified DNA, 0.5 µl of primer (2.5 µM) and 2 µl of Big Dye Sequencing Kit (Applied Biosystems). The sequencing protocol was performed as previously described [31]. Sequencing reaction products were purified using Sephadex G-50 Fine gel filtration beads (GE Healthcare, UK) and sequenced on an ABI 3130XL Automated Sequencer (Applied Biosystems) following the manufacturer’s recommendations. Electrophoretic data were analysed using the DNA Sequencing Analysis V5.2 software (Applied Biosystems) and did not reveal any traces of mixed templates. Therefore, the pool of±6,000 nematodes used for DNA extraction is assumed to be genetically homogeneous for the markers analysed, considering the detection limit of conventional sequencing. Negative controls were used throughout the DNA extraction and amplification processes. Sequence data were submitted to GenBank with accession numbers JN596427-JN596470.
Phylogenetic analyses
Our sequence dataset was analysed together with homologous sequences retrieved from the NCBI Entrez Nucleotide database (http://www.ncbi.nlm.nih.gov) [8], [21], [32]–[35] using the Geneious v5.4 software [36]. For most sequences retrieved from GenBank, no indication of the sampling locality is available besides the country of origin. In those cases, we used the central point of the geographic area of the country (or island) to represent the sampling area of the isolates (Figure 1). In some analyses, we included sequences from unpublished studies reported as being from Central and South American isolates, although their presence in such regions and their correct identification requires further validation. In total, we analysed 40 sequences from COI, 17 from ND5, 9 from s-rRNA, 57 from internal transcribed spacer 2 (ITS-2) and 39 from the 28S ribosomal RNA (28S rRNA) (Table 1). All sequences from each locus were aligned using the default parameters of the MUSCLE 3.6 software [37]. The final lengths of the sequence alignments used in the following analyses were 453 bp for COI, 434 bp for ND5, 274 bp for s-rRNA, 334 bp for ITS-2 and 519 bp for the 28S rRNA.
Median-joining networks of mtDNA sequences were calculated using the NETWORK V4.6.6.0 software [38] (http://www.fluxus-engineering.com). Default parameters were used in all calculations. Superfluous links and median vectors were purged from the network through the use of the post-processing ‘MP option’ [39]. Bayesian analyses were performed with MrBayes v3.1 software [40], [41] running on the public Bioportal at www.bioportal.uio.no [42]. The Metropolis-coupled Markov chain Monte Carlo process was set so that four independent chains ran simultaneously for 3,000,000 generations, each starting from a random tree. We used the GTR+I+G model with gamma-distributed rate variation across sites approximated by four discrete categories and the program’s default prior probabilities on model parameters. The average standard deviation of split frequencies among the four independent runs at completion was 0.0108 for COI, 0.0057 for ITS-2 and 0.0066 for 28S rRNA trees, suggesting convergence on a stationary distribution. A tree was sampled every 1,000 generations for a total of 12,004 samples over four runs, of which 11,604 were sampled for Bayesian posterior probabilities (‘burn-in’ was empirically determined by checking likelihood values). Maximum-likelihood phylogenetic trees were constructed with the program PHYML [43], available on Geneious v5.4 software, using the GTR+I+G substitution model, 100 bootstrap datasets, four substitution rate categories and optimised tree topology and branch length. The transition/transversion ratios, proportion of invariable sites and gamma distribution parameters were estimated by the program. Phylogenetic trees were drawn using FigTree v1.3.1 software (http://tree.bio.ed.ac.uk/software/figtree). The algorithm developed by Nye et al. [44] was used to compare the topology of alternative phylogenetic trees. The tree comparisons were performed with the Compare2Trees software available at http://www.mas.ncl.ac.uk/~ntmwn/compare2trees/index.html. The map with the location and frequency of samples was obtained using PhyloGeoViz v2.4.5 [45] and Google Maps (Google Inc., Mountain View, CA).
Population genetic analyses
Basic sequence statistics and mismatch distributions [46] were estimated using the DnaSP ver. 5.10 software [47]. Relevant population growth parameters for the prediction of expected mismatch distributions of COI sequences were obtained on a first run and then used in a second run for the final analyses, as previously suggested [48]. Estimates of evolutionary divergence among COI sequences were determined using MEGA5 software [49]. Analyses were performed using the Maximum Composite Likelihood model [50]. The rate variation among sites was modelled with a gamma distribution (shape parameter = 4) and the differences in the composition bias among sequences were considered in evolutionary comparisons [51]. All positions containing gaps and missing data were eliminated from the analyses using the MEGA5 software.
Results and Discussion
Bursaphelenchus xylophilus and B. mucronatus are distinct species
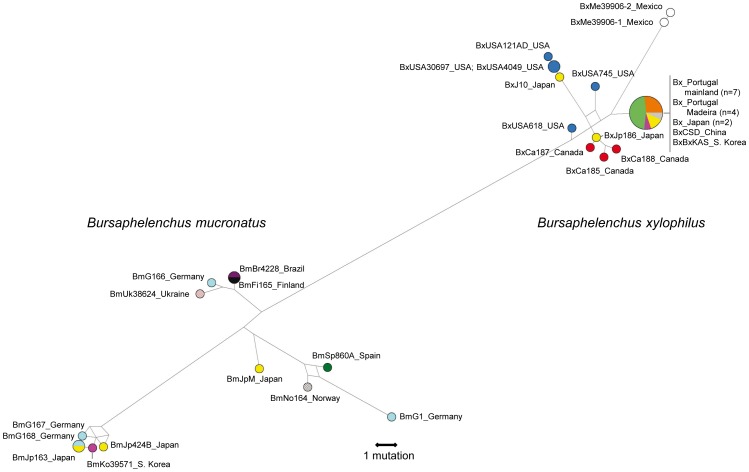
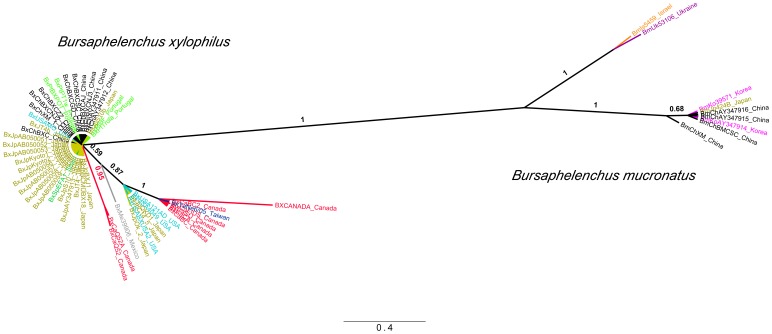
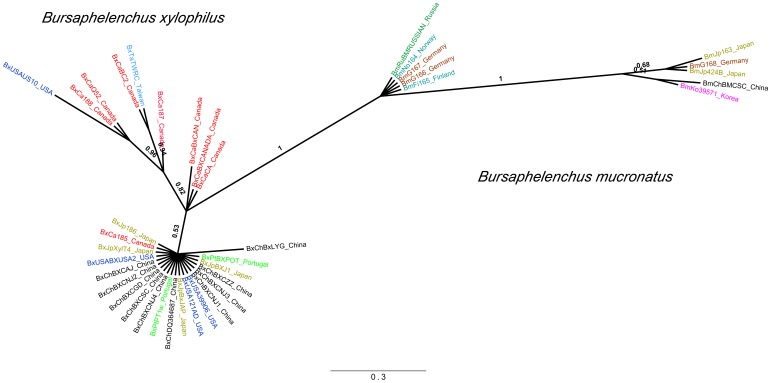
It has been suggested that the ancestor of B. mucronatus and other species of the genus Bursaphelenchus was a free-living nematode inhabiting broad-leaved trees in the eastern part of Eurasia, while B. xylophilus likely originated from a population of B. mucronatus that colonised the North American continent [17]. Our Bayesian and maximum likelihood trees separated all isolates from both species with posterior probabilities and bootstrap values of 1 (Figures 2, 3, 4, Figures S1 to S4 in File S1). The Bayesian and maximum likelihood topologies in each sequence dataset were similar, with an overall topological score of 96.4% for COI and 100% for ITS-2 and 28S rRNA (Figure S5 in File S1). Additionally, mitochondrial haplotypes from isolates of both species were separated by at least 35 mutational steps in the COI median-joining network (Figure 2), which is in agreement with previous results on inter-species mtDNA phylogeny of Bursaphelenchus [8], [17]. This difference was also evident in the mismatch distribution of COI sequences, with a peak of around 35 to 42 pairwise differences resulting from comparisons among isolates of both species (Figure 5). An estimate of the evolutionary divergence between sequences showed that both species diverge by at least 0.091 base substitutions per site (SE = 0.027) in the COI gene (Table S3 in File S1). Therefore, the genetic distance between B. xylophilus and B. mucronatus isolates was always higher than between isolates of the same species. Overall, the examination of both mitochondrial and nuclear genetic markers enabled us to unequivocally recognise that B. xylophilus and B. mucronatus are genetically differentiated species despite their morphological resemblance and ecological overlap.
Figure 2. Median-joining network of mitochondrial cytochrome c oxidase subunit I (COI) haplotypes of Bursaphelenchus xylophilus and B. mucronatus.
The area of the circles is proportional to the frequency of isolates in the sample, and the branch length is proportional to the number of mutations.
Figure 3. Bayesian phylogenetic tree based on internal transcribed spacer 2 (ITS-2) sequences of Bursaphelenchus xylophilus (Bx) and B. mucronatus (Bm).
Support values are given in Bayesian posterior probabilities. The scale bar represents nucleotide substitutions per site.
Figure 4. Bayesian phylogenetic tree based on 28S ribosomal RNA (28S rRNA) gene sequences from Bursaphelenchus xylophilus (Bx) and B. mucronatus (Bm).
Support values are given in Bayesian posterior probabilities. The scale bar represents nucleotide substitutions per site.
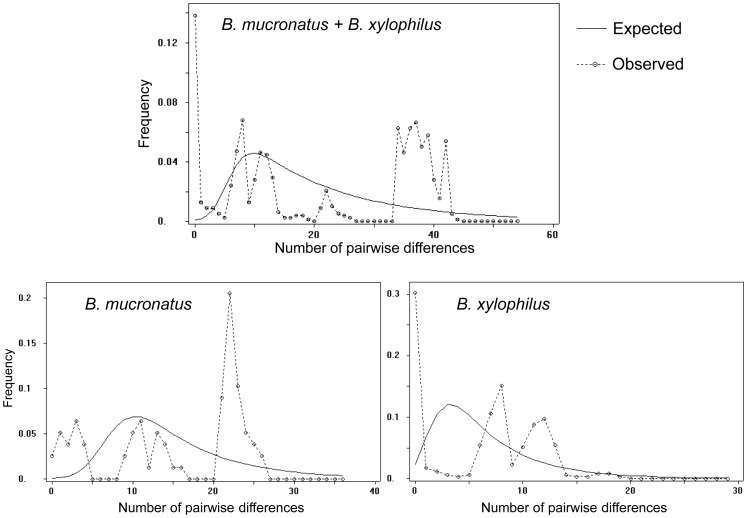
Figure 5. Mismatch distributions of cytochrome c oxidase subunit I (COI) haplotypes for Bursaphelenchus xylophilus and B. mucronatus isolates (combined and separated).
The number of differences between pairs of sequences is given on the horizontal axis with relative frequencies represented on the vertical scale.
Bursaphelenchus xylophilus and B. mucronatus have contrasting patterns of intraspecific diversity
The nucleotide diversity within the COI gene was 0.014 (SD = 0.002) for B. xylophilus and 0.034 (SD = 0.002) for B. mucronatus (Table 2). The average number of nucleotide differences in the COI gene was also lower in B. xylophilus (6.44) than in B. mucronatus (15.30), as clearly shown by the mismatch distribution (Figure 5). The evolutionary divergence among COI sequences indicated that B. xylophilus isolates were not separated by more than 0.045 base substitutions per site, while several B. mucronatus isolates were separated by a higher number of base substitutions, with some reaching 0.065 per site (Table S3 in File S1). Similarly, the nucleotide diversity in nuclear sequences (ITS-2 and 28S rRNA) was lower in B. xylophilus than in B. mucronatus (Table 2). The average number of nucleotide differences was 2.26 for ITS-2 and 1.52 for 28S rRNA in B. xylophilus and 8.57 for ITS-2 and 6.20 for 28S rRNA in B. mucronatus (Table 2). The same result was obtained when the isolates from Central and South America for which classification is doubtful where excluded from the analyses (Table S4 in File S1). There was also a more conserved dispersion of B. xylophilus lineages across phylogenetic trees, with branches separated by smaller numbers of substitutions per site than in B. mucronatus (Figures 2, 3, 4, Figures S1 to S4 in File S1).
Table 2. Summary statistics based on partial sequences of the mitochondrial cytochrome c oxidase subunit I (COI), the nuclear internal transcribed spacer 2 (ITS-2) and the 28S ribosomal RNA (28S rRNA) for Bursaphelenchus xylophilus (Bx) and B. mucronatus (Bm) isolates (combined and separated).
| Genomic region | Bursaphelenchus species | n | Invariable sites | Variable sites | Singleton variable sites | Total number of mutations | Number of haplotypes | Haplotype diversity(standard deviation) | Nucleotide diversity (standard deviation) | Average number of nucleotide differences |
| COI | Bx+Bm | 40 | 384 | 69 | 10 | 79 | 23 | 0.862 (0.054) | 0.047 (0.005) | 21.36 |
| Bx | 27 | 423 | 30 | 9 | 30 | 12 | 0.698 (0.099) | 0.014 (0.002) | 6.44 | |
| Bm | 13 | 417 | 36 | 8 | 41 | 11 | 0.974 (0.039) | 0.034 (0.002) | 15.30 | |
| ITS-2 | Bx + Bm | 57 | 237 | 62 | 13 | 71 | 12 | 0.664 (0.065) | 0.042 (0.009) | 12.54 |
| Bx | 48 | 288 | 21 | 13 | 22 | 8 | 0.538 (0.080) | 0.007 (0.002) | 2.26 | |
| Bm | 9 | 294 | 23 | 3 | 23 | 4 | 0.583 (0.183) | 0.027 (0.011) | 8.57 | |
| 28S rRNA | Bx + Bm | 39 | 489 | 28 | 7 | 28 | 13 | 0.750 (0.070) | 0.013 (0.002) | 6.79 |
| Bx | 29 | 507 | 10 | 6 | 10 | 8 | 0.571 (0.107) | 0.003 (0.001) | 1.52 | |
| Bm | 10 | 507 | 12 | 1 | 12 | 5 | 0.756 (0.130) | 0.012 (0.001) | 6.20 |
Sites with alignment gaps were not considered in computations.
The low levels of intraspecific diversity detected in B. xylophilus by comparison to B. mucronatus may have at least two explanations. First, the high indices of genetic diversity detected in B. mucronatus may result from the existence of two well-defined subclades in mitochondrial and nuclear phylogenies. The presence of two distantly related lineages within B. mucronatus is confirmed by the peak of around 20 to 25 pairwise differences in the mismatch distribution (Figure 5). Second, the levels of genetic diversity in B. xylophilus are reduced, as expected for a species that evolved relatively recently through geographical or reproductive isolation. For instance, low genetic diversity among B. xylophilus isolates from mainland Portugal and Madeira Island was observed using several genetic markers [25], [35], [52], [53] and is in agreement with a drastic reduction in population size at the moment of the introduction of an invasive species.
The worldwide phylogeography of B. xylophilus
The most frequent B. xylophilus COI haplotype was found to be shared by two Japanese, one Chinese, one Korean and all Portuguese (both mainland and Madeira Island) isolates (Figure 2, Figures S1 and S2 in File S1). The mitochondrial haplotype found in Portuguese isolates was absent from North America (the other putative source population), although the sample size in this region is still too small to draw definitive conclusions (Figure 2, Figures S1 and S2 in File S1). Nevertheless, the presence of identical mitochondrial and nuclear DNA sequences in all Portuguese B. xylophilus isolates is in agreement with the hypothesis that a single founder lineage from Asia arrived only once in southwestern Europe. It is unlikely that the founder population was a mixture of several lineages that were all subsequently lost by stochastic effects because the power of genetic drift is reduced on expanding populations. It could be argued that the long-term maintenance of nematodes in culture enhances the genetic differences among isolates due to repeated population bottlenecks. However, all Portuguese isolates collected in different years presented identical DNA sequences for all the genetic markers analysed (Table 1). We also analysed B. xylophilus isolates across the entire range of dispersion in Portugal to guarantee a good representation of all extant lineages. It is therefore clear that repeated population bottlenecks are not affecting the cultured isolates since genetic drift can only occur in the presence of genetic variation.
The recently identified B. xylophilus isolate in northwestern Spain [21], [22] also clustered with the Portuguese isolates, suggesting a common origin followed by local dispersion (Figure 3). Similarly, our phylogeographic investigation indicates that B. xylophilus isolates from Madeira Island are likely to be related to isolates from mainland Portugal (Figure 2), although an independent introduction from Asia (where the same haplotype exists) cannot be completely ruled out. Still, it is likely that the dispersal of this nematode to the Atlantic island has a continental European origin owing to the more intense trade of wood products.
The association between Portuguese and Asian lineages was also detected by the analysis of nuclear DNA markers (Figures 3 and 4, Figures S3 to S4 in File S1). In contrast with the results obtained with mtDNA analysis, a few North American isolates clustered on the Asian/Portuguese branch in phylogenetic trees built using nuclear DNA sequences (Figures 3 and 4, Figures S3 to S4 in File S1). These shared lineages could be those initially introduced in Asia from the North American continent. Nevertheless, nuclear DNA analysis does not completely exclude the possible direct introduction of North American isolates in Europe. Future work with larger samples will be necessary to completely exclude this hypothesis.
The results using different methods of phylogenetic analyses are in agreement with the hypothesis that B. xylophilus as a species originated in North America, as the lineages from Canada and the USA were found on different branches on most phylogenetic trees (Figures 3 and 4, Figures S1 and S2 in File S1). This is clear when analysing both mitochondrial and nuclear DNA, particularly in the COI and ITS-2 trees (Figures 2 and 3, Figures S1 and S2 in File S1), and could explain the two-peaked mismatch distribution of B. xylophilus COI sequences (Figure 5). Moreover, North American B. xylophilus lineages were separated by a high number of base substitutions. For instance, two isolates from the USA diverge by 0.023 (SE = 0.008) base substitutions per site in the COI region (Table S3 in File S1). This high diversity among North American lineages is expected in the native area of a population, while areas where B. xylophilus was recently introduced showed lower levels of genetic diversity due to founder effects [54].
The worldwide phylogeography of B. mucronatus
B. mucronatus haplotypes were found to be widespread and did not show any geographical association (Figure 2, Figures S1 and S2 in File S1). Isolates from distant geographic regions were not necessarily related phylogenetically. In fact, the same COI haplotype was present in isolates sampled at locations as distant as Brazil, Finland, Japan and Germany (Figure 2). Conversely, B. mucronatus isolates within the same region (Germany) belonged to very distant phylogenetic branches of the COI median-joining network (Figure 2). Two of these German haplotypes diverged by 0.065 base substitutions per site (SE = 0.019) in the COI region (Table S3 in File S1). The COI median-joining network (Figure 2) suggests that two or even three haplogroups exist in B. mucronatus (the cluster of haplotypes at the tip of the network and the two interior branches), although additional samples are necessary to clearly exclude the existence of intermediate haplotypes. The Bayesian and maximum likelihood trees built with COI sequences show two clearly separated B. mucronatus branches supported by very high posterior probabilities and bootstrap values (Figures S1 and S2 in File S1). When evolutionarily divergent lineages were co-analysed, they yielded a large number of pairwise nucleotide substitutions, while pairs of haplotypes sharing a common origin matched quite closely. This pattern is clearly visible on the graph artificially mixing B. xylophilus and B. mucronatus isolates with a peak of around 37 nucleotide differences (Figure 5). This phenomenon is also visible on a smaller scale in the mismatch distribution of B. mucronatus, suggesting the existence of two well-differentiated haplogroups.
The observed weak genetic structure accompanied by high levels of diversity reflects the presence of two highly divergent lineages in B. mucronatus and/or that intense gene flow among distant regions may be common in this species and has remained unnoticed due to its reduced pathogenicity. The absence of star-like clusters of haplotypes in median-joining networks (Figure 2, Figures S6 and S7 in File S1) and the multimodal mismatch distribution (Figure 5) excludes the possibility of an abrupt population growth (for instance, from a bottlenecked population) with a recent worldwide dispersion, which would explain the presence of shared haplotypes in different geographic regions. In addition, the shared haplotypes occupy the tips of networks (Figure 2), which indicates that they are relatively recent and do not represent old lineages that still persist in extant populations. Other historical demographic events, such as a wide range selective sweep wherein a given haplotype favoured by selection spreads across the species range, are unlikely because they would lead to low levels of genetic diversity in addition to weak phylogeographical patterns [55].
The different genetic patterns observed in B. xylophilus and B. mucronatus isolates may result from multiple factors that affect their dispersion across short and long distances. A different phylogeography would be expected if B. xylophilus evolved recently from a B. mucronatus population in North America through geographical or reproductive isolation. The higher genetic diversity of B. mucronatus could be the result of an earlier origin in Eurasia [17]. Anthropogenic activities are also important for the spatial and genetic structure of Bursaphelenchus species. The relevance of human-assisted dispersion has already been shown for Asian B. xylophilus isolates using microsatellite data [24] and mathematical modelling [14]–[16]. The spread is facilitated by highways, railways, river ports and lakes, and nematodes are transported within insect vectors or independently within wood itself [2], [13], [14].
The pathogenic nature of B. xylophilus may also impose a different selective pressure on their populations (i.e., reducing their genetic diversity), which is absent in B. mucronatus. Additionally, these nematodes rely on longhorn beetles of the genus Monochamus for its natural dispersal. It is possible that specific host-vector interactions occur in both Bursaphelenchus species conferring them different dispersion capacities. Further studies are necessary to uncover the combination of human activities and ecological factors that shape the different genetic landscapes of B. xylophilus and B. mucronatus.
Supporting Information
Supporting Figures S1 - S7 and Tables S1 - S4.
(PDF)
Acknowledgments
We are grateful to Paulo Vieira and Pedro Barbosa for helping with sample collections.
Funding Statement
This work was partially supported by the Portuguese national project ‘O nemáode-da-madeira-do-pinheiro (NMP), Bursaphelenchus xylophilus’ (Fundo Florestal Permanente) and by research grants to FP (SFRH/BPD/44637/2008) and BA (SFRH/BPD/73108/2010) from the Portuguese Foundation for Science and Technology (FCT). MM was partially supported by the EC 7th Framework project REPHRAME (KBBE.2010.1.4-09, ‘Analysis of the potential of the pine wood nematode (Bursaphelenchus xylophilus) to spread, survive and cause pine wilt in European coniferous forests in support of EU plant health policy’). The Institute of Molecular Pathology and Immunology of the University of Porto (IPATIMUP) is an Associate Laboratory of the Portuguese Ministry of Science, Technology and Higher Education and is partially supported by FCT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dwinell LD (1997) The pinewood nematode: Regulation and mitigation. Annu Rev Phytopathol 35: 153–166. [DOI] [PubMed] [Google Scholar]
- 2.Mota M, Vieira P (2008) Pine wilt disease: a worldwide threat to forest ecosystems. The Netherlands:Springer Verlag . [Google Scholar]
- 3. Cheng XY, Xie PZ, Cheng FX, Xu RM, Xie BY (2009) Competitive displacement of the native species Bursaphelenchus mucronatus by an alien species Bursaphelenchus xylophilus (Nematoda: Aphelenchida: Aphelenchoididae): a case of successful invasion. Biol Invasions 11: 205–213. [Google Scholar]
- 4. Kanzaki N, Futai K (2006) Is Bursaphelenchus mucronatus a weak pathogen to the Japanese red pine? Nematology 8: 485–489. [Google Scholar]
- 5. Maehara N, Aikawa T, Kanzaki N (2011) Inoculation of several Bursaphelenchus xylophilus group nematodes into adult trees of Pinus thunbergii and their survival in the trees. Forest Pathol 41: 477–481. [Google Scholar]
- 6. Mamiya Y, Enda N (1979) Bursaphelenchus mucronatus n.sp (Nematoda, Aphelenchoidadae) from pine wood and its biology and pathogenicity to pine trees. Nematologica 25: 353–361. [Google Scholar]
- 7. Webster JM, Anderson R, Baillie D, Beckenbach K, Curran J, et al. (1990) DNA probes for differentiating isolates of the pinewood nematode species complex. Revue de Nématologie 13: 255–263. [Google Scholar]
- 8. Ye W, Giblin-Davis R, Braasch H, Morris K, Thomas W (2007) Phylogenetic relationships among Bursaphelenchus species (Nematoda: Parasitaphelenchidae) inferred from nuclear ribosomal and mitochondrial DNA sequence data. Mol Phylogenet Evol 43: 1185–1197. [DOI] [PubMed] [Google Scholar]
- 9. Bolla RI, Boschert M (1993) Pinewood Nematode Species Complex - interbreeding potential and chromosome number. J Nematol 25: 227–238. [PMC free article] [PubMed] [Google Scholar]
- 10. Riga E, Beckenbach K, Webster JM (1992) Taxonomic relationships of Bursaphelenchus xylophilus and B. mucronatus based on interspecific and intraspecific cross-hybridization and DNA analysis. Fundam Appl Nematol 15: 391–395. [Google Scholar]
- 11. Rutherford TA, Riga E, Webster JM (1992) Temperature-mediated behavioral relationships in Bursaphelenchus xylophilus, B. mucronatus, and their hybrids. J Nematol 24: 40–44. [PMC free article] [PubMed] [Google Scholar]
- 12. Taga Y, Goto S, Matsunaga K, Togashi K (2011) Temporal changes in characteristics of populations originating from interbreeding between Bursaphelenchus xylophilus and B. mucronatus . Nematology 13: 701–712. [Google Scholar]
- 13. Jones JT, Moens M, Mota M, Li HM, Kikuchi T (2008) Bursaphelenchus xylophilus: opportunities in comparative genomics and molecular host-parasite interactions. Mol Plant Pathol 9: 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robinet C, Roques A, Pan HY, Fang GF, Ye JR, et al. (2009) Role of human-mediated dispersal in the spread of the pinewood nematode in China. Plos One 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robinet C, Van Opstal N, Baker R, Roques A (2011) Applying a spread model to identify the entry points from which the pine wood nematode, the vector of pine wilt disease, would spread most rapidly across Europe. Biol Invasions 13: 2981–2995. [Google Scholar]
- 16. Togashi K, Shigesada N (2006) Spread of the pinewood nematode vectored by the Japanese pine sawyer: modeling and analytical approaches. Popul Ecol 48: 271–283. [Google Scholar]
- 17. Kanzaki N, Kazuyoushi F (2002) A PCR primer set for determination of phylogenetic relationships of Bursaphelenchus species within the xylophilus group. Nematology 4: 35–41. [Google Scholar]
- 18. Rutherford TA, Mamiya Y, Webster JM (1990) Nematode-induced pine wilt disease - factors influencing its occurrence and distribution. Forest Sci 36: 145–155. [Google Scholar]
- 19. Mota MM, Braasch H, Bravo MA, Penas AC, Burgermeister W, et al. (1999) First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1: 727–734. [Google Scholar]
- 20. Fonseca L, Cardoso JMS, Lopes A, Pestana M, Abreu F, et al. (2012) The pinewood nematode, Bursaphelenchus xylophilus, in Madeira Island. Helminthologia 49: 96–103. [Google Scholar]
- 21. Abelleira A, Picoaga A, Mansilla JP, Aguin O (2011) Detection of Bursaphelenchus xylophilus, causal agent of pine wilt disease on Pinus pinaster in Northwestern Spain. Plant Dis 95: 776. [DOI] [PubMed] [Google Scholar]
- 22. Robertson L, Arcos SC, Escuer M, Merino RS, Esparrago G, et al. (2011) Incidence of the pinewood nematode Bursaphelenchus xylophlius Steiner & Buhrer, 1934 (Nickle, 1970) in Spain. Nematology 13: 755–757. [Google Scholar]
- 23. Cheng XY, Cheng FX, Xu RM, Xie BY (2007) Genetic variation in the invasive process of Bursaphelenchus xylophilus (Aphelenchida: Aphelenchoididae) and its possible spread routes in China. Heredity 100: 356–365. [DOI] [PubMed] [Google Scholar]
- 24. Jung J, Han H, Ryu S, Kim W (2010) Microsatellite variation in the pinewood nematode, Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle in South Korea. Genes Genomics 32: 151–158. [Google Scholar]
- 25. Vieira P, Burgermeister W, Mota M, Metge K, Silva G (2007) Lack of Genetic Variation of Bursaphelenchus xylophilus in Portugal Revealed by RAPD-PCR Analyses. J Nematol 39: 118–126. [PMC free article] [PubMed] [Google Scholar]
- 26. Beckenbach K, Blaxter M, Webster JM (1999) Phylogeny of Bursaphelenchus species derived from analysis of ribosomal internal transcribed spacer DNA sequences. Nematology 1: 539–548. [Google Scholar]
- 27. Whitehead A, Hemmings J (1965) A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann Appl Biol 55: 25–38. [Google Scholar]
- 28. EPPO (2009) Diagnostic protocols for regulated pests: Bursaphelenchus xylophilus . Bulletin OEPP/EPPO 31: 61–69. [Google Scholar]
- 29. Fonseca L, Santos M, Santos M, Curtis R, Abreu F, et al. (2008) Morpho-biometrical characterisation of Portuguese Bursaphelenchus xylophilus isolates with mucronate, digitate or round tailed females. Phytopathol Mediterr 47: 223–233. [Google Scholar]
- 30. Braasch H, Schonfeld U, Polomski J, Burgermeister W (2004) Bursaphelenchus vallesianus sp. n. – A new species of the Bursaphelenchus sexdentati group (Nematoda: Parasitaphelenchidae). Nematol Mediterr 32: 71–79. [Google Scholar]
- 31. Pereira F, Queiros S, Gusmao L, Nijman IJ, Cuppen E, et al. (2009) Tracing the history of goat pastoralism: new clues from mitochondrial and Y chromosome DNA in North Africa. Mol Biol Evol 26: 2765–2773. [DOI] [PubMed] [Google Scholar]
- 32. Iwahori H, Tsuda K, Kanzaki N, Izui K, Futai K (1998) PCR-RFLP and sequencing analysis of ribosomal DNA of Bursaphelenchus nematodes related to pine wilt disease. Fundam Appl Nematol 21: 655–666. [Google Scholar]
- 33. Kang JSK, Choi KS, Shin SC, Moon IS, Lee SG, et al. (2004) Development of an efficient PCR-based diagnosis protocol for the identification of the pinewood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae). Nematology 6: 279–285. [Google Scholar]
- 34. Li H, Trinh PQ, Waeyenberge L, Moens M (2009) Characterisation of Bursaphelenchus spp. isolated from packaging wood imported at Nanjing, China. Nematology 11: 375–408. [Google Scholar]
- 35. Mota MM, Takemoto S, Takeuchi Y, Hara N, Futai K (2006) Comparative studies between Portuguese and Japanese isolates of the pinewood nematode, Bursaphelenchus xylophilus . J Nematol 38: 429–433. [PMC free article] [PubMed] [Google Scholar]
- 36.Drummond A, Ashton B, Cheung M, Heled J, Kearse M, et al. (2009) Geneious v.5.4. Available: http://www.geneious com/.
- 37. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bandelt HJ, Forster P, Rohl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16: 37–48. [DOI] [PubMed] [Google Scholar]
- 39. Polzin T, Daneshmand SV (2003) On Steiner trees and minimum spanning trees in hypergraphs. Oper Res Lett 31: 12–20. [Google Scholar]
- 40. Huelsenbeck JP, Ronquist F (2001) MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- 41. Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 42. Kumar S, Skjaeveland A, Orr RJ, Enger P, Ruden T, et al. (2009) AIR: A batch-oriented web program package for construction of supermatrices ready for phylogenomic analyses. BMC Bioinformatics 10: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 44. Nye TMW, Lio P, Gilks WR (2006) A novel algorithm and web-based tool for comparing two alternative phylogenetic trees. Bioinformatics 22: 117–119. [DOI] [PubMed] [Google Scholar]
- 45. Tsai YH (2011) PhyloGeoViz: a web-based program that visualizes genetic data on maps. Mol Ecol Resour 11: 557–561. [DOI] [PubMed] [Google Scholar]
- 46. Rogers AR, Harpending H (1992) Population growth makes waves in the distribution of pairwise genetic differences. Mol Biol Evol 9: 552–569. [DOI] [PubMed] [Google Scholar]
- 47. Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. [DOI] [PubMed] [Google Scholar]
- 48.Rozas J (2009) DNA sequence polymorphism analysis using DnaSP. In: Bioinformatics for DNA Sequence Analysis. pp. 337–350. [DOI] [PubMed] [Google Scholar]
- 49. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci U S A 101: 11030–11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tamura K, Kumar S (2002) Evolutionary distance estimation under heterogeneous substitution pattern among lineages. Mol Biol Evol 19: 1727–1736. [DOI] [PubMed] [Google Scholar]
- 52.Cardoso J, Fonseca L, Abrantes I (2011) Genetic analysis by ITS RFLP and sequencing of pinewood nematode, Bursaphelenchus xylophilus, isolates from Portugal. Abstracts of the 4th International Conference on Mediterranean Pines, Avignon, France180.. [Google Scholar]
- 53. Valadas V, Laranjo M, Barbosa P, Espada M, Mota M, et al. (2012) The pine wood nematode, Bursaphelenchus xylophilus, in Portugal: possible introductions and spread routes of a serious biological invasion revealed by molecular methods. Nematology 14: 899–911. [Google Scholar]
- 54. Metge K, Burgermeister W (2006) Intraspecific variation in isolates of Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) revealed by ISSR and RAPD fingerprints. J Plant Dis Prot 113: 275–282. [Google Scholar]
- 55. Maruyama T, Birky C (1991) Effects of periodic selection on gene diversity in organelle genomes and other systems without recombination. Genetics 127: 449–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figures S1 - S7 and Tables S1 - S4.
(PDF)