Abstract
Circulating tumor cells (CTCs) are highly correlated with the invasive behavior of cancer; as such, the ability to isolate and quantify CTCs is of great biomedical importance. This research presents a multi-stage multi-orifice flow fractionation (MS-MOFF) device formed by combining three single-stage multi-orifice segments designed for separating breast cancer cells from blood. The structure and dimensions of the MS-MOFF were determined by hydrodynamic principles to have consistent Reynolds numbers (Re) at each multi-orifice segment. From this device, we achieved improved separation efficiency by collecting and re-separating non-selected target cells in comparison with the single-stage multi-orifice flow fractionation (SS-MOFF). The recovery of breast cancer cells increased from 88.8% to greater than 98.9% through the multi-stage multi-orifice segments. This device can be utilized to isolate rare cells from human blood, such as CTCs, in a label-free manner solely through the use of hydrodynamic forces.
INTRODUCTION
Tumor cells that originate from a primary tumor mass can circulate in the peripheral blood of cancer patients via intravasation during malignant progression. There is growing evidence that these circulating tumor cells (CTCs) are highly correlated with the invasive behaviors of cancer cells responsible for poor prognosis and the majority of cancer-related deaths.1, 2, 3 The isolation or detection of CTCs can provide valuable clinical information for cancer prognosis, diagnosis, assessment of tumor sensitivity to anticancer drugs, and personalization of anticancer therapy.4
The detection and separation of CTCs are challenging due to their extremely low concentrations in blood, as few as one cell per 109 hematologic cells or 0 to one cell per mL.5, 6, 7, 8, 9 Several microfluidic devices have recently been reported for the detection and isolation of CTCs. For example, microchips containing microposts coated with EpCAM antibody have been used to identify CTCs via immunocapture.10, 11, 12 Microchips enable the detection of CTCs but do not allow for the retrieval of live cells for downstream analysis. Moreover, some tumor cells express low or no EpCAM, resulting in limited binding efficiency.13 A mechanical sieve can isolate CTCs based on size differences.14 Although this method is simple and easy to use, high cell concentrations can lead to blockage, and harvesting captured cells is also inconvenient. Magnetophoretic removal of red blood cells using their intrinsic paramagnetic properties is a good candidate method for the isolation of CTCs9 if white blood cell contamination is tolerable. Dielectrophoretic separation techniques utilizing size and dielectric properties also show promise and several studies such as stem cell discrimination,15 blood cells separation,16 and cancer cell17 were reported lately.
Hydrodynamic sorting, a separation technique based on inertial microfluidics,18 is gaining recognition19, 20 since this process enables continuous and high throughput separation of different particles without the application of external forces. Shear-induced migration of blood cells were fundamentally studied21, 22 and actively applied to blood cell separations.23 This technique is advantageous for CTC separation because of its simple experimental set up and increased operational flow rate (100–300 μL/min) compared to other microfluidic separators. In continuous methods, the sample mixture is continuously fed into the separation channel and subjected to force to spatially separate different cells, and the separated fractions can be continuously and simultaneously collected at different channel outlets. These methods have the potential for high throughput and are easily combined with other upstream and downstream applications to enable the more complex lab-on-a-chip systems to address medical and biological issues.
Previously, we introduced a novel hydrodynamic method using a multi-orifice microchannel for size-based particle separation called multi-orifice flow fractionation (MOFF).24 In MOFF, microparticles are moved laterally due to hydrodynamic inertial forces created by a multi-orifice structure. The extent of lateral movement varies according to particle size, and polymer microspheres can be concentrated at different lateral positions in the microchannel.25 This process enables continuous and high throughput separation of different particles without the application of external forces. Recently, we developed a multi-stage multi-orifice flow fractionation (MS-MOFF) system combining three multi-orifice segments to increase recovery. With this device, we improved recovery and minimized the loss of purity by collecting and re-separating non-selected particles in the first separation. Using polymer beads (7 μm and 15 μm), we found that recovery was successfully increased from 73.2% to 88.7%, while the purity slightly decreased from 91.4% to 89.1% (for 15 μm).26
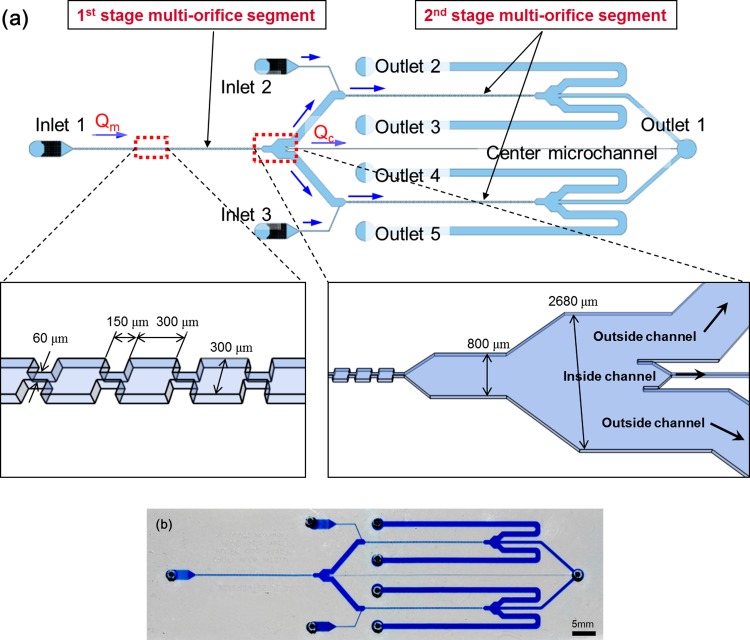
In this paper, we attempted to separate breast cancer cells (MCF-7, a model cell line for CTCs) from blood using the MS-MOFF and investigated the improvement in separation efficiency in comparison with a single-stage multi-orifice flow fractionation (SS-MOFF) (Fig. 1).
Figure 1.
Schematic view of the multi-stage multi-orifice flow fractionation (MS-MOFF) system. At the first MOFF stage, the RBCs and WBCs split into two positions, and the MCF-7 cells focused at the middle of the channel (inside). At the second MOFF stage, RBCs and WBCs directed to the side channel (outside), similar to the first stage. On the other hand, a few MCF-7 cells that travelled through the side channel at the first stage (non-selected target) were directed to the middle of the channel (inside).
MATERIALS AND METHODS
Design and fabrication of the device
The multi-orifice segment is an alternating series of contraction and expansion channels, through which the momentum-change-induced inertial force separates particles.
The channel dimension and fractionation of particles according to the size are correlated with the particle Reynolds number (Rep), a dimensionless number that provides a measure of the ratio of inertial forces to viscous forces, characterizing the motion of fluid in the microchannel. We described the fluid dynamic phenomenon of particles flowing via a multi-orifice channel using Rep, which is expressed as
where is the hydraulic diameter of the channel, is the maximum flow velocity in the channel, ρ is the density of the fluid, and μ is the dynamic viscosity of the fluid.
In previous research, we studied the separable flow rate zones of differently sized particles according to varying dimensions of the orifice channel. Based on this study, we chose the channel dimensions. The widths of the contraction and expansion channels are 60 μm and 300 μm, respectively. Each expansion region is 300 μm in length and is linked to a 150 μm contraction channel. The series of orifice patterns is repeated 80 times and has a 60 μm thickness.
Fig. 2a shows the schematic configurations of the multi-stage multi-orifice flow fractionation (MS-MOFF), and Fig. 2b shows an image of the fabricated device. The MS-MOFF is formed by combining three multi-orifice segments and consists of three inlets, three filters, three multi-orifice segments, and five outlets. The dimensions of the multi-orifice segment in the first stage are same as those of the SS-MOFF. In the second stage, the central channel is 80 μm wide (at Qc/Qm = 45%, for Qc/Qm condition please see the results and discussion) and 60 mm long. Each branched outside channel is 10 mm in length and connects with the second stage MOFF. A sample of blood (smaller cells) spiked with breast cancer cells (larger cells) is injected from inlet 1 using a syringe pump. Then, the target breast cancer cells (MCF-7) are focused onto the centerline of the multi-orifice segments in the first stage and directly transported to the outlet via the center microchannel in the second stage. The mixture, including remaining target cells (remnant MCF-7 cells and most blood cells), flows through the side microchannels. Buffers are added from inlets 2 and 3 to compensate the flow rate through multi-orifice segments in the second stage. The separated target particles are collected to outlet 1, and the other particles are collected to outlets 2 through 5.
Figure 2.
Photographic image of the MS-MOFF fabricated device.
The MS-MOFF device was fabricated using soft-lithography techniques. Six-inch silicon wafers were used as the substrate, and SU-8 (SU-8 2050, MicroChem, MA, USA) was used for the channel master mold. Finally, the MS-MOFF device was replicated with PDMS (Sylgard 184, Dow Corning Corp., MI, USA). A 10:1 volumetric mixture of PDMS and a curing agent was poured into the master mold. After degassing the PDMS mixture, the wafer was placed on a hot plate at 75 °C for 60 min. Then, the cured polymer mixture was removed from the master mold. The MS-MOFF patterned polymer was punched at the inlets and outlets and bonded to clean glass after plasma treatment (plasma generator, Cute-B Plasma, FEMTO Science, Korea).
Sample preparation
Red blood cells (RBCs), white blood cells (WBCs), and MCF-7 breast carcinoma cells were used for the separation mixture. Blood (4 ml) was drawn from healthy volunteers by phlebotomists using EDTA vacutainer tubes, and the RBCs and WBCs were processed using a density gradient separation. RBCs were briefly separated from whole blood using centrifugation and diluted with phosphate buffered saline (PBS) and 2% bovine serum albumin (BSA) to reduce cell aggregation. WBCs were also separated from whole blood using centrifugation. Whole blood cells were first floated on Ficoll-Paque (GE Healthcare Bio-Sciences Corp., Uppsala, Sweden) and then centrifuged for 30 min at 1380 rpm, followed by careful isolation of the buffy coat. The isolated buffy coat was washed and diluted in PBS to remove residual Ficoll-Paque. The final step was an additional centrifugation for 10 min at 980 rpm to reduce platelet contamination. All blood samples were used within 6 h of collection.
The MCF-7 breast carcinoma cell line was chosen as the model for circulating tumor cells (CTCs). Cells were incubated under ordinary cell culture conditions, and the MCF-7 cells were cultured to 80% confluence in Dulbecco's modified Eagle's medium supplemented with high glucose and 1.5 g/l sodium bicarbonate (NaHCO3), 15 mM HEPES buffer, and 10% fetal bovine serum (FBS). A 0.25% trypsin solution was prepared in 150 mM PBS and was used to harvest the MCF-7 cells. The cells were trypsinized, washed, and resuspended in PBS to the same concentration as the other blood cell samples.
Blood samples diluted 1:100 in PBS were spiked with 1 × 104 MCF-7 cells/ml, and the mixture was introduced into the device. The average RBC and MCF-7 cell sizes were 8 μm and 20 μm, respectively. Although there were various types of leukocytes, the average size of neutrophils and lymphocytes, which accounted for most leukocytes (>75%), was about 8 μm.
Experimental setup
A syringe pump (KDS200, KD Scientific Inc., MA, USA) was used to generate a continuous, stable microflow, and syringes were connected to the PDMS microchannel inlets using silicone and aluminum tubes. Table TABLE I. shows the flow rates of the sample inlet (inlet 1), the buffer inlets (inlets 2 and 3), the cancer cell outlet (outlet 1), and the blood cell outlets (outlets 2 through 5). Prior to performing each experiment, the system was degassed by filling with 70% ethanol and PBS solution because the low polarity solvent wetted the hydrophobic PDMS surface.27
TABLE I.
Flow rate (μl/min) of each inlet and outlet at various Qc/Qm conditions.
| Qc/Qm | |||
|---|---|---|---|
| 0.35 | 0.45 | 0.60 | |
| Inlet 1 | 126 | 126 | 126 |
| Inlets 2 and 3 | 170 | 184 | 202 |
| Outlet 1 | 132 | 170 | 228 |
| Outlets 2 through 5 | 164 | 139 | 100 |
An inverted optical microscope (I-70, Olympus Co., Japan) equipped with a halogen lamp was used to observe moving cells in the microchannel that faced the objective lens. Images were captured using a high speed CCD camera (HotShot 1280; NAC Image Technology, CA, USA).
Performance evaluation
After cell separation, performance was evaluated by cell counting after collection at each outlet using an immunocytochemical method. To count cells in the mixed sample, three fluorescence markers were used. The CD45 antibody was used to identify WBCs, DAPI visualized cell nuclei, MCF-7 cells were defined as EpCAM+/DAPI+/CD45- and WBCs were identified as EpCAM-/DAPI+/CD45+. In addition, a cytospin centrifuge (Cytospin 3, Shandon Inc., PA, USA) was employed to smear the collected sample to reduce cell loss during the staining process and enhance fluorescent image quality.28 All of the separated samples were kept on ice, and 50 μl of each sample was cyto-centrifuged for 5 min at 2000 rpm.
RESULTS AND DISCUSSION
Flow rate
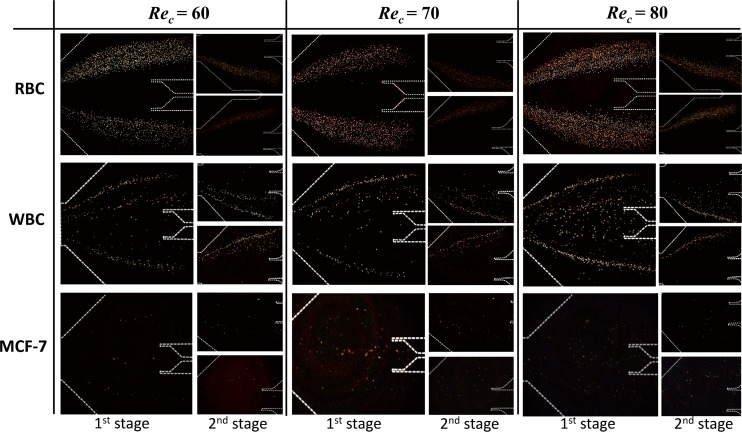
To determine the flow rate (Rec), RBCs, WBCs, and MCF-7 cells were tested at various flow rates (Rec = 60, 70, 80) such that the cells could be concentrated laterally according to size using the MOFF microchannel. The fluid rate flowing through a microchannel can be estimated using the channel Reynolds number (Rec). We used a Rec range from 60 to 80 based on the characteristic length of the contraction channel and a flow rate ranging from 108 to 144 μl/min. Fig. 3 shows the behaviors of three different cells in the separation region after flowing through the MOFF at various Reynolds numbers. To obtain a high contrast image relative to the background, we captured two images at a certain time interval from the same site in the channel to determine the exact concentration of cells. From these two images, all pixels with the same value were removed. As a result, high contrast images depicting the few cells that remained in the channel were obtained.
Figure 3.
Trajectory of cells through the multi-orifice microchannel according to channel Reynolds number (Rec). A picture of the first stage separation region; two small pictures of the upper and lower second stage separation regions. When Rec was 70 at the first stage MOFF, the best separation of MCF-7 and blood cells was achieved at this flow rate condition.
The RBCs bifurcated at the end of the MOFF channel when the Rec ranged from 60 to 70 and were clearly split into two groups. Although RBCs clustered near both sidewalls in the first stage, they gathered to only one side of the wall in the second stage. When the cells were biased to one side due to buffer flow from inlets 2 and 3, they never exceed the centerline of the multi-orifice segment. Thus, the biased cells remained biased to the opposite side of the sheath flow and exited through the outside channel. The overall cell behavioral trend of WBCs was similar to that of the RBCs. Since WBCs have larger diameters than RBCs, some of them moved to the inside channel at the first stage. At the second stage, the WBCs exited one of the outside channels, similar to the RBCs. The MCF-7 cells maintained a certain distance relative to the channel sidewall as a result of a heavy wall effect-induced lift force since the mean diameter of the MCF-7 cells was ∼20 μm, larger than the other cell sizes. When the Rec reached 70, the streamlines were finely focused in the middle of the channel. Since the majority of the MCF-7 cells travelled through the inside channel, they were barely observed in the second stage. However, a few MCF-7 cells that did travel through the side channel at the first stage (non-selected target) were directed to the inside channel at the second stage and exited via outlet 1. As the Rec increased to 80, the focused streamline trajectory of the MCF-7 cells gradually dispersed from the centerline. Thus, we considered the best separation condition for MCF-7 cells from blood cells to be Rec = 70.
Collecting region and separation efficiency
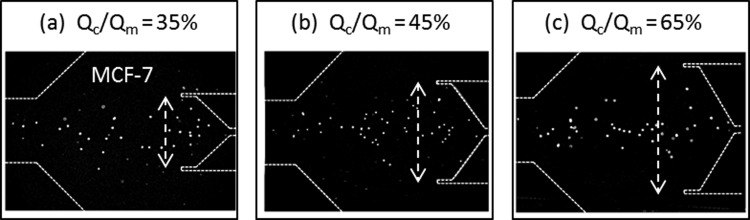
To optimize the collection channel dimensions, we tested various dimensions according to the Qc/Qm condition, where Qc is the flow rate of central collecting channel in the first stage of the MOFF, and Qm is the total flow rate in first stage of the MOFF. The Qc/Qm value represents the portion of the central collecting channel for each segment (Fig. 2a). To maintain Rec in the second stage, the buffer solution was injected into inlets 2 and 3 with a flow rate of 170, 184 and 202 μl/min depending on the Qc/Qm condition (35%, 45%, 65%, respectively). Fig. 4 shows the trajectory of MCF-7 cells through the multi-orifice microchannel according to Qc/Qm condition. As the Qc/Qm increased, which indicates a wider central collection region, a collection of larger cells (MCF-7) that flowed through the middle of the channel was advantageous. Table TABLE II. describes the separation efficiencies of MS-MOFF when Rec was 70. As the central collecting channel became larger, the recovery of MCF-7 cells increased while the recovery of blood cells decreased. Using this device, we separated 88.9 ± 5.2, 98.9 ± 0.4, and 97.5 ± 0.5% of MCF-7 cells when the Qc/Qm was 35, 45, and 60%, respectively. On the other hand, blood cell separation efficiencies were 93.3 ± 2.9, 70.3 ± 4.7, and 35.6 ± 9.3% when the Qc/Qm value was 35, 45, and 60%, respectively. Since we re-separated the non-selected MCF-7 cells using MS-MOFF, the recovery of MCF-7 cells increased from 88.8% (SS-MOFF29) to greater than 93.3% (when Qc /Qm = 35%). The Qc/Qm value had a proportional relationship with MCF-7 cells inside the channel stream and a reciprocal proportional relationship with blood cells outside the channel stream. This indicates that there is a trade-off between MCF-7 cell and blood cell separation efficiency. Therefore, we adopted the Qc/Qm = 45% condition as it showed the highest MCF-7 cell separation efficiency and acceptable blood cell separation efficiency.
Figure 4.
Trajectory of MCF-7 cells through the multi-orifice microchannel according to Qc/Qm condition. Qc/Qm values represent the used portion of the central collecting channel. Qc, flow rate of the central collecting channel in the first MOFF stage; Qm, total flow rate in the first MOFF stage.
TABLE II.
Separation efficiencies of blood and MCF-7 cells in the MS-MOFF depending on the Qc/Qm condition. The inside fraction exits to outlet 1 and the outside fraction to outlets 2 through 5.
| Qc/Qm | |||||
|---|---|---|---|---|---|
| 0.35 | 0.45 | 0.60 | |||
| Recovery | Blood cells | Outlet 1 | 6.7 ± 2.9 | 29.7 ± 4.7 | 64.4 ± 9.3 |
| Outlets 2 through 5 | 93.3 ± 2.9a | 70.3 ± 4.7 | 35.6 ± 9.3 | ||
| MCF-7 | Outlet 1 | 88.9 ± 5.2 | 98.9 ± 0.4 | 97.5 ± 0.5 | |
| Outlets 2 through 5 | 11.1 ± 5.2 | 1.1 ± 0.4 | 2.5 ± 0.5 | ||
Boldface numbers indicate recoveries.
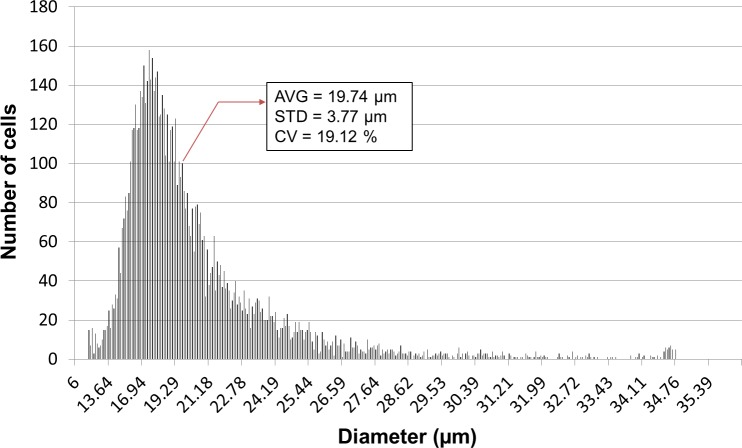
Unlike beads, which are evenly size controlled, cells have a relatively wide size distribution. The MCF-7 cells used in this study had an average diameter of 19.74 ± 3.77 μm and a 19.21% coefficient of variation (Fig. 5). Since hydrodynamic cell separation methods mainly exploit size differences, these methods necessarily have limited separation efficiency. In addition, rare cell separation purity may be low due to the extremely imbalanced initial fraction. For example, the presence of only 0.01 to 1% RBCs can significantly decrease the purity of MCF-7 cells, and we obtained a 0.3% MCF-7 purity as shown in Table TABLE III.. The initial fraction of MCF-7 cells in the injected sample was only 0.1%, and therefore the purity of blood cells at the outlets 2 through 5 was as high as 100%.
Figure 5.
Size distribution of MCF-7 cells used in this study. The histogram was derived using an automated cell counter (Scepter, Millipore Co.). The coefficient of variation was relatively large (19.12%) since cell size depends on various conditions such as cell cycle and the microenvironment.
TABLE III.
Separation efficiency in comparison of SS-MOFF, MOFF+DEP and MS-MOFF.
| SS-MOFF29 |
MOFF+DEP29
|
MS-MOFF
|
||||
|---|---|---|---|---|---|---|
| Recovery | MCF-7 | 88.8 | 75.8 | 0 98.9 a | ||
| Blood | 84.1 | 99.2 | 0 70.3 a | |||
| Purity | MCF-7 | 1.5 | 16.2 | 0.3 | ||
| Blood | 100 | 99.7 | 100 | |||
The value is at Qc/Qm condition of 0.45.
COMPARISON BETWEEN SS-MOFF, MOFF-DEP, AND MS-MOFF
According to our previous report, we described a new method for particle separation according to size using a multi-orifice microchannel.25 This method was demonstrated through confirmation of separation performance using microspheres of three different sizes. This technique is advantageous for continuous processing in that it can be operated by one syringe pump without the use of sheath flow. In addition, the microfluidic channel can be fabricated simply and rapidly by PDMS soft-lithography without supplementing any mechanical or electrical parts. To enhance separation efficiency, we combined this technique with dielectrophoretic cell separation.29 Hydrodynamic separation in the first separation region takes advantage of the massive and high-throughput filtration of blood cells. DEP separation in the second separation region plays a role in precise post-processing to enhance separation efficiency. Slanted electrodes at the bottom of channel selectively shifted the MCF-7 by DEP force originated from applied AC electric field. The serial combination of these two different sorting techniques enabled high-speed continuous flow-through separation without labeling. We observed up to a 163-fold increase in MCF-7 cells at a 126 μl/min flow rate, and RBCs and WBCs were efficiently removed with separation efficiencies of 99.24% and 94.23%, respectively.29
The recovery of MCF-7 cells was higher than the results of SS-MOFF and MOFF-DEP that were performed previously due to the multi-stage MOFF structure (Table TABLE III.). Collecting and re-separating the non-selected target cells through the multi-stage multi-orifice segments improved separation efficiency. However, the purity of MS-MOFF was much lower than the results of our previous MOFF-DEP experiments since MOFF exploits only particle size while DEP can exploit dielectric properties of the particle. As described, MOFF-DEP and MS-MOFF have different advantages and disadvantages, and we hope to derive a more efficient technique that is capable of both high purity and recovery in future work.
CONCLUSIONS
Here, we demonstrated an MS-MOFF technique that continuously separates MCF-7 cancer cells, which serve as a model for CTCs, from blood with improved efficiency. Cells were sorted based on size differences and hydrodynamic behavior in the MOFF channel. We observed up to a 93.3% blood cell removal rate at the Qc/Qm = 0.35 and a 98.9% MCF-7 separation rate at the Qc/Qm = 0.45. These improved separation efficiencies were achieved by collecting and re-separating the non-selected target cells through the multi-stage multi-orifice segments. For a simple operation, if the width of 1st stage channel increases while it remains in 2nd stage and the flow rate of each channel meets the separable flow condition, the buffer injection becomes unnecessary.
The recovery of MCF-7 cells was higher than the results of SS-MOFF and MOFF-DEP29 due to the multi-stage MOFF structure. However, the purity of the MS-MOFF technique was much lower than our previous MOFF-DEP results since MOFF exploits only particle size while DEP can exploit dielectric properties of a particle.
This technique will be useful for clinical applications, such as for the separation of CTCs or rare cells from human blood samples because of its simple experimental set up, high operational flow rate, and capability for the collection of viable cells due to its label-free characteristics.
The higher separable flow rates and blood cell concentration are required owing to the abundance of blood cells and for the minimum operation time. The inertial migration of cancer cell towards equilibrium positions occurred only when the hematocrit level was below 10%.30 It is generally admitted that a hydrodynamic separation in a microfluidic channel requires a dilution to secure the sufficient inter particle distance. However, the dilution of sample leads to increase the sample volume for separation. Although it is difficult to apply a clinical sample without pretreatment in our system because of specific experimental conditions such as flow rate and hematocrit effect, those can be achieved by increasing the cross sectional area of orifice and RBC lysis. The RBC lysis can significantly decreases the hematocrit without further dilution, hence adaptable for hydrodynamic separation.
ACKNOWLEDGMENTS
This work was supported by the Korea Science & Engineering Foundation (KOSEF) grant funded by the Korean government (MEST)(2008-05943, 2011-0016731) and the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1120290).
References
- Pantel K., Brakenhoff R., and Brandt B., Nat. Rev. Cancer 8(5), 329–340 (2008). 10.1038/nrc2375 [DOI] [PubMed] [Google Scholar]
- Georgakoudi I., Solban N., Novak J., Rice W. L., Wei X., Hasan T., and Lin C. P., Cancer Res. 64(15), 5044–5047 (2004). 10.1158/0008-5472.CAN-04-1058 [DOI] [PubMed] [Google Scholar]
- Allard W. J., Matera J., Miller M. C., Repollet M., Connelly M. C., Rao C., Tibbe A. G. J., Uhr J. W., and Terstappen L. W. M. M., Clin. Cancer Res. 10(20), 6897–6904 (2004). 10.1158/1078-0432.CCR-04-0378 [DOI] [PubMed] [Google Scholar]
- Kim S. I. and Jung H. I., J. Breast Cancer 13(2), 125–131 (2010). 10.4048/jbc.2010.13.2.125 [DOI] [Google Scholar]
- Kahn H., Presta A., Yang L., Blondal J., Trudeau M., Lickley L., Holloway C., McCready D., Maclean D., and Marks A., Breast Cancer Res. Treat. 86(3), 237–247 (2004). 10.1023/B:BREA.0000036897.92513.72 [DOI] [PubMed] [Google Scholar]
- Zieglschmid V., Hollmann C., and Böcher O., Crit. Rev. Cl. Lab. Sci. 42(2),155–196 (2005). 10.1080/10408360590913696 [DOI] [PubMed] [Google Scholar]
- Krivacic R. T., Ladanyi A., Curry D. N., Hsieh H. B., Kuhn P., Bergsrud D. E., Kepros J. F., Barbera T., Ho M. Y., Chen L. B., Lerner R. A., and Bruce R. H., Proc. Natl. Acad. Sci. USA 101(29), 10501–10504 (2004). 10.1073/pnas.0404036101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K., Han A., and Frazier A., Biosens. Bioelectron. 21(10), 1907–1914 (2006). 10.1016/j.bios.2006.01.024 [DOI] [PubMed] [Google Scholar]
- Han K., Han S., and Frazier A., Lab Chip 9(20), 2958–2964 (2009). 10.1039/b909753h [DOI] [PubMed] [Google Scholar]
- Nagrath S., Sequist L., Maheswaran S., Bell D., Irimia D., Ulkus L., Smith M., Kwak E., Digumarthy S., and Muzikansky A., Nature 450(7173), 1235–1239 (2007). 10.1038/nature06385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Liu K., Liu J., Yu Z. T. F., Xu X., Zhao L., Lee T., Lee E. K., Reiss J., Lee Y. K., Chung L. W. K., Huang J., Rettig M., Seligson D., Duraiswamy K. N., Shen C. K. F., and Tseng H. R., Angew. Chem. Int. Ed. 50(13), 3084–3088 (2011). 10.1002/anie.201005853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A., Okagbare P., Feng J., Hupert M., Patterson D., Gottert J., McCarley R., Nikitopoulos D., and Murphy M., S. Soper, J. Am. Chem. Soc. 130(27), 8633–8641 (2008). 10.1021/ja8015022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G., Herrler M., Burgess D., Manna E., Krag D., and Burke J., Breast Cancer Res. 10(4), R69 (2008). 10.1186/bcr2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed H., Murray M., Turner J. N., and Caggana M., J. Chromatogr. A 1216(47), 8289–8295 (2009). 10.1016/j.chroma.2009.05.036 [DOI] [PubMed] [Google Scholar]
- Velugotla S., Pells S., Mjoseng H. K., Duffy C. R. E., Smith S., De Sousa P., and Pethig R., Biomicrofluidics 6(4), 044113 (2012). 10.1063/1.4771316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini N., Mernier G., Tornay R., and Renaud P., Biomicrofluidics 5(3), 034122 (2011). 10.1063/1.3640045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V., Jafferji I., Garza M., Melnikova V. O., Hasegawa D. K., Pethig R., and Davis D. W., Biomicrofluidics 6(2), 024133 (2012). 10.1063/1.4731647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo D., Lab Chip 9(21), 3038–3046 (2009). 10.1039/b912547g [DOI] [PubMed] [Google Scholar]
- Di Carlo D., Edd J., Irimia D., Tompkins R., and Toner M., Anal. Chem. 80(6), 2204–2211 (2008). 10.1021/ac702283m [DOI] [PubMed] [Google Scholar]
- Di Carlo D., Irimia D., Tompkins R. G., and Toner M., P. Natl. Acad. Sci. USA 104(48), 18892–18897 (2007). 10.1073/pnas.0704958104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R. and Chang H. C., J. Colloid Interface Sci. 287(2), 647–656 (2005). 10.1016/j.jcis.2005.02.023 [DOI] [PubMed] [Google Scholar]
- Zhou R., Gordon J., Palmer A. F., and Chang H.-C., Biotechnol. Bioeng. 93(2), 201–211 (2006). 10.1002/bit.20672 [DOI] [PubMed] [Google Scholar]
- Hou H. W., Gan H. Y., Bhagat A. A. S., Li L. D., Lim C. T., and Han J., Biomicrofluidics 6(2), 024115 (2012). 10.1063/1.4710992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. S., Song S. H., and Jung H. I., Lab Chip 9(7), 939–948 (2009). 10.1039/b813952k [DOI] [PubMed] [Google Scholar]
- Park J. S. and Jung H. I., Anal. Chem. 81(20), 8280–8288 (2009). 10.1021/ac9005765 [DOI] [PubMed] [Google Scholar]
- Sim T. S., Kwon K., Park J. C., Lee J. G., and Jung H. I., Lab Chip 11(1), 93–99 (2011). 10.1039/c0lc00109k [DOI] [PubMed] [Google Scholar]
- Torres C., Alternative Lithography: Unleashing the Potentials of Nanotechnology (Kluwer Academic, Dordrecht, 2003). [Google Scholar]
- Sanders C., Nelson C., Hove M., and Woods G. L., Diagn. Microbiol. Infect. Dis. 32(2), 111–113 (1998). 10.1016/S0732-8893(98)00075-3 [DOI] [PubMed] [Google Scholar]
- Moon H. S., Kwon K., Kim S. I., Han H., Sohn J., Lee S., and Jung H. I., Lab Chip 11(6), 1118–1125 (2011). 10.1039/c0lc00345j [DOI] [PubMed] [Google Scholar]
- Tanaka T., Ishikawa T., Numayama-Tsuruta K., Imai Y., Ueno H., Yoshimoto T., Matsuki N., and Yamaguchi T., Biomed. Microdevices 14(1), 25–33 (2012). 10.1007/s10544-011-9582-y [DOI] [PubMed] [Google Scholar]