Abstract
Integration of biomaterials into tissues is often disturbed by unopposed activation of macrophages. Immediately after implantation, monocytes are attracted from peripheral blood to the implantation site where they differentiate into macrophages. Inflammatory signals from the sterile tissue injury around the implanted biomaterial mediate the differentiation of monocytes into inflammatory M1 macrophages (M1) via autocrine and paracrine mechanisms. Suppression of sustained M1 differentiation is thought to be crucial to improve implant healing. Here, we explore whether artificial extracellular matrix (aECM) composed of collagen I and hyaluronan (HA) or sulfated HA-derivatives modulate this monocyte differentiation. We mimicked conditions of sterile tissue injury in vitro using a specific cytokine cocktail containing MCP-1, IL-6 and IFNγ, which induced in monocytes a phenotype similar to M1 macrophages (high expression of CD71, HLA-DR but no CD163 and release of high amounts of pro-inflammatory cytokines IL-1β, IL-6, IL-8, IL-12 and TNFα). In the presence of aECMs containing high sulfated HA this monocyte to M1 differentiation was disturbed. Specifically, pro-inflammatory functions were impaired as shown by reduced secretion of IL-1β, IL-8, IL-12 and TNFα. Instead, release of the immunregulatory cytokine IL-10 and expression of CD163, both markers specific for anti-inflammatory M2 macrophages (M2), were induced. We conclude that aECMs composed of collagen I and high sulfated HA possess immunomodulating capacities and skew monocyte to macrophage differentiation induced by pro-inflammatory signals of sterile injury toward M2 polarization suggesting them as an effective coating for biomaterials to improve their integration.
Keywords: artificial extracellular matrix, biomaterial coatings, sulfated hyaluronan, immunomodulation, monocyte into macrophage differentiation, sterile tissue injury
Introduction
Long-lasting and functional integration of biomaterials requires the induction of a normal wound healing response after its implantation. However, the presence of the implant often disturbs this response resulting in acute continuing inflammatory processes and/or chronic foreign body reactions which potentially lead to implant failure.1,2 To improve the healing outcome of implants, novel functionalized materials capable of modulating cellular activities during early and late healing processes gain importance. Here, mimicking the function of native extracellular matrix (ECM) turned out to be a promising strategy.3 Thus, recent approaches focus on the design of artificial ECMs (aECMs) composed of ECM-derived proteins and synthetically sulfated glycosaminoglycans (GAGs).4,5 Sulfated GAGs bind and process signaling factors like cytokines, chemokines and growth factors and regulate their local concentration and enhance, reduce or even abrogate their bioactivity.6 Chemically modified GAGs to which additional sulfate groups were added were shown to differentially bind growth factors and chemokines including human bone morphogenetic protein-4 (BMP-4), transforming growth factor-β1 (TGF-β1) and interleukin 8 (IL-8) and to alter the effectivity of these factors in the induction of specific cell functions.7-9 Bioengineered aECMs composed of GAGs with a specific sulfation pattern which may regulate cellular responses during the healing process of biomaterial implants are therefore supposed to be potent biomaterial coatings for medical applications.2,8 In a recent study, aECM containing artificially sulfated GAGs was demonstrated to modulate the function of dermal fibroblasts, important players of the proliferation phase of the healing response. The aECM promoted the induction of a “proliferative phenotype” in the fibroblasts but no extensive matrix deposition by these cells which was suggested to be beneficial for accelerated wound healing without scarring.10 However, such balanced activation of fibroblasts requires the resolution of inflammatory processes which precedes in the healing response.
Failed or disturbed integration of biomaterials is often associated with prolonged inflammation mediated by macrophages.1 Macrophages are able to develop two different phenotypes, a classically activated, inflammatory phenotype (referred to as M1) and an alternatively activated, anti-inflammatory phenotype (referred to as M2).11 In a normal wound healing response both phenotypes are activated.12 M1 macrophages initiate and amplify the inflammatory response while subsequently M2 macrophages downregulate inflammatory processes and regulate new tissue formation through controlled activation of fibroblasts.13 In disturbed healing of biomaterial implants this tightly regulated activation of M1 and M2 macrophages is often abrogated. Consequently, inflammatory resolution is impeded which results in a foreign body reaction characterized by persistent M1 activation, macrophage fusion and uncontrolled fibroblast proliferation.1,2 Modulating the inflammatory process and here specifically macrophage activity during the biomaterial healing response represents an attractive target to improve implant acceptance and performance.
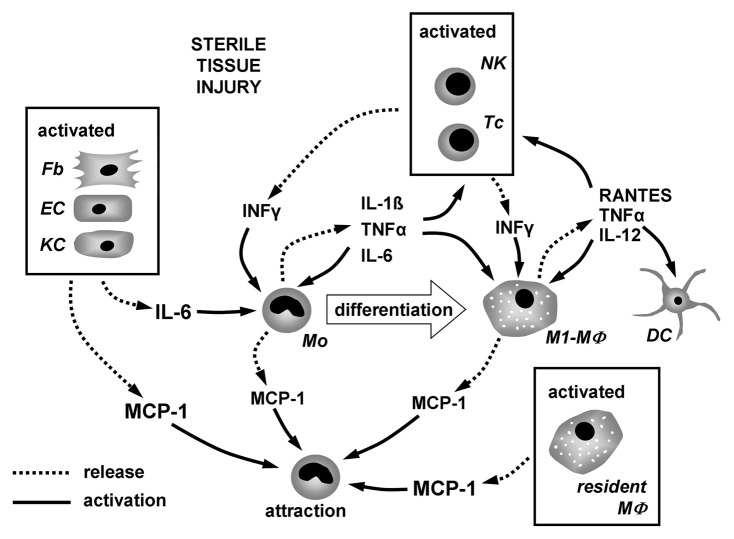
In this study we tested the immunomodulating capacities of aECMs composed of collagen I (coll) and either hyaluronan (HA) or derivatives of HA modified by attaching sulfate groups at low (lsHA) or high (hsHA) levels, respectively. In detail, we addressed the differentiation of monocytes into macrophages under conditions of a sterile inflammation as it occurs after surgical biomaterial implantation. Besides few resident tissue macrophages, the majority of macrophages involved in the biomaterial healing process arise from monocytes that are attracted from peripheral blood to the implantation site where inflammatory mediators regulate their differentiation into macrophages. As implantation of biomaterials is performed under sterile surgical conditions the environment at the implantation site is expected to contain only few pathogen associated molecular patterns (PAMPs) but to be enriched on damage associated molecular patterns (DAMPs). These signals activate tissue cells and resident immune cells to release inflammatory cytokines, chemokines and proteins which determine the monocyte to macrophage differentiation outcome.14,15 The complex interplay of these mediators resulting in monocyte differentiation and macrophage activation thought to occur under conditions of sterile tissue injury is illustrated in Figure 1. It is suggested that these chemokines and cytokines are sequentially and differentially released to promote phase-specific infiltration and activation of leukocyte subsets during the healing response.16 A study of wound healing of adult skin demonstrated that monocyte infiltration peaks at day two of the healing response and is paralleled by strong MCP-1 expression in the wound area.17 Besides MCP-1, which is crucial for the attraction of monocytes, inflammatory cytokines like interferon γ (IFNγ), tumor necrosis factor α (TNFα), IL-1β and IL-6 regulate monocyte differentiation and macrophage activation in the early phase of inflammation during wound repair.18-20 Activated lymphocytes and natual killer cells are a major source of IFNγ, which has pleiotropic effects on monocytes and macrophages. An essential involvement of IFNγ in the wound healing process has been demonstrated by Ishida et al.20 They detected IFNγ in sterile wounds 3 d post-injury and showed that the absence of IFNγ may accelerate the healing process through increased TGF-β production. Implications for wound healing and regulation of monocyte activation have also been shown for MCP-1 and IL-6 secreted by activated tissue cells including keratinocytes, fibroblasts and endothelial cells.21,22 TNFα and IL-1β are released by a variety of activated cells post-injury but predominantly by monocytes and macrophages themselves.23 Both cytokines are regarded as amplifiers of the inflammatory response and to impair wound healing through persistent paracrine/autocrine signaling to monocytes and macrophages.18,24,25
Figure 1. Interplay of inflammatory mediators involved in the process of monocyte to macrophage differentiation under condition of sterile tissue injury. Chemokines and cytokines released from activated tissue cells and lymphocytes attract monocytes and induce their differentiation into macrophages. During the differentiation process monocytes/macrophages release cytokines regulating their differentiation and activation in an autocrine/paracrine fashion.
To mimic monocyte to macrophage differentiation under conditions resembling those of a sterile tissue injury at biomaterial implantation sites in vivo we used a cytokine cocktail composed of MCP-1, IL-6 and IFNγ. These mediators are not preferentially released by monocytes and macrophages and are therefore suggested to be crucial for initial monocyte activation and differentiation into macrophages (Fig. 1). Here, we show that in vitro stimulation of human monocytes with this cytokine cocktail induces their differentiation into macrophages displaying an inflammatory phenotype as it occurs after biomaterial implantation in vivo. Furthermore we demonstrate that this monocyte to macrophage differentiation is modulated in the presence of aECMs containing high sulfated forms of HA.
Results
Monocyte stimulation with a cytokine cocktail composed of MCP-1, IL-6 and IFNγ induces their differentiation toward an inflammatory macrophage phenotype
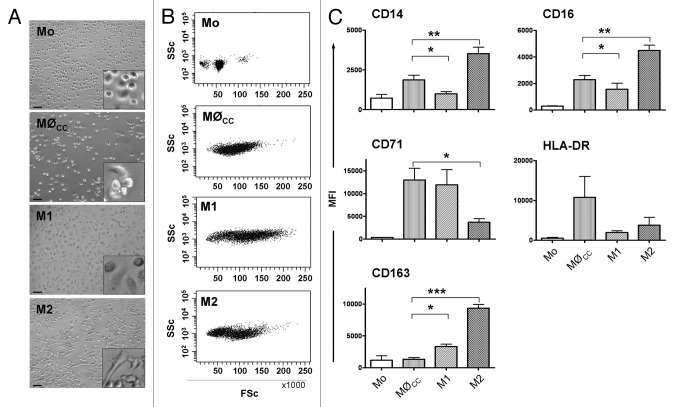
To mimic monocyte differentiation under conditions of early inflammation of a sterile tissue injury after biomaterial implantation we stimulated fresh isolated CD14 positive peripheral blood monocytes with a cytokine cocktail (cc) composed of MCP-1, IL-6 and IFNγ. After six days of culture we characterized the differentiated macrophage population (referred to as MØCC) and compared them to M1 and M2 macrophages, differentiated from monocytes by stimulation with GM-CSF and M-CSF, respectively.26
Figure 2 displays morphological properties of MØCC, M1 and M2 macrophages and fresh isolated monocytes. In contrast to M1 and M2, MØCC appear as round cells that tend to form cluster and only weakly attach to the underlying substrate. The morphology of MØCC resembles that of monocytes, however the cells increased in size during the six days of culture. As seen in the FSc/SSc dotplots in Figure 2, the population of MØCC has remarkably higher FSc values (reflecting cell size) than monocytes. Compared with M1 and M2 macrophages, FSc properties of MØCC are similar to those of M2.
Figure 2. Characteristics of monocytes and MØCC, M1 and M2 macrophages. Monocytes (Mo) were differentiated on bsa in the presence of the cytokine cocktail (cc) consisting of MCP-1, IL-6 and IFNγ, or GM-CSF or M-CSF to generate MØCC, M1 or M2 macrophages, respectively. Representative micrographs (A) and dotplots (B) show fresh isolated monocytes and differentiated macrophages of day 6. (A) Representative micrographs depict Mo as small round cells. MØCC appear larger in size as Mo but still round-shaped and tend to form clusters which are fragile attached to the substrate. M1 appear as large, round cells. M2 display an elongated, spindle-shaped morphology. Scale bars represent 50 µm. Included details are 4× magnified. (B) MØCC, M1 and M2 display higher FSc properties as Mo demonstrating their increase in size, as it occurs during the process of monocyte to macrophage differentiation. (C) Expression of macrophage phenotype specific surface markers was analyzed on fresh isolated monocytes and on MØCC, M1 and M2 on day 6 of differentiation, respectively. Surface marker profile of MØCC is similar to that of M1 macrophages in respect of their expression of CD14, CD16, CD71 and CD163. Data are presented as mean ± SD of three independent experiments. *p < 0.05, **p < 0.01 and ***p < 0,001
For phenotypical characterization of MØCC we analyzed a specific subset of surface markers including CD14, CD16, CD71, CD163 and HLA-DR. The expression of these surface markers differs in M1 and M2 macrophages (Fig. 2C). M2 macrophages express higher levels of CD14, CD16 and CD163 while M1 macrophages express higher levels of CD71. Expression of HLA-DR is similar on both macrophage subsets. Assessing MØCC we found a profile predominantly resembling that of M1 macrophages (Fig. 2C). Specifically, MØCC expressed CD14, CD16, CD71 and CD163 to similar levels as M1 macrophages. Levels of HLA-DR on MØCC are markedly elevated compared with both, M1 and M2. We therefore conclude the cytokine cocktail composed of MCP-1, IL-6 and IFNγ promotes the differentiation of monocytes toward an inflammatory macrophage phenotype. Next, we tested whether this process is modulated by aECMs composed of coll and either HA or low sulfated HA (lsHA) or high sulfated HA (hsHA), respectively.
aECMs have no effect on adhesion characteristics and survival of monocytes and MØCC
Freshly isolated blood monocytes were seeded in the absence of serum on bsa, coll or the aECMs (coll/HA, coll/lsHA and coll/hsHA) for one hour before addition of 10% fetal calf serum and the cytokine cocktail. Within this hour, monocytes were attaching to their underlying substrate in an evenly spread manner and no differences in the adhesion between bsa, coll and the aECMs were observed (data not shown). Supplementation with the cytokine cocktail did not affect monocyte adhesion within the first 24 h of culture; however during the six day differentiation process these cells lost their tight adherence (Fig. 3A). On all substrates cells formed cell clusters which weakly attached to the tissue culture dish. Cell cluster formation was most pronounced on coll/HA. On coll/hsHA MØCC predominantly appeared as single, low adhesive cells. To exclude that low MØCC attachment was associated with cell death we performed a live-death assay by staining the cells with AnnxV/PI. MØCC cultured on control substrates bsa and coll revealed 83 ± 3% and 94 ± 3% AnnxV-/PI- cells, representing viable cells, respectively. Viability of MØCC on coll/HA, coll/lsHA and coll/hsHA was similar to the collagen control ranging from 91–96% (Fig. 3B).
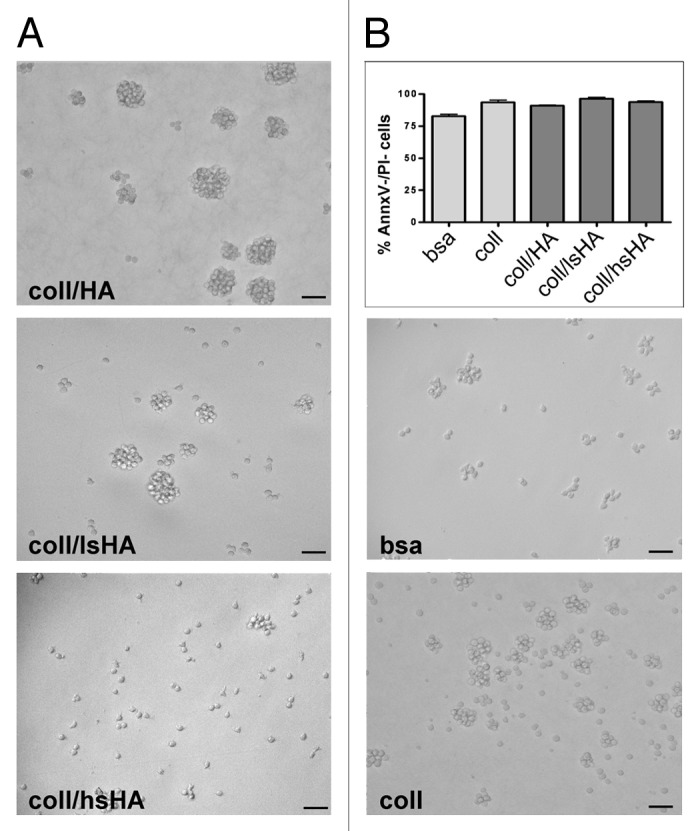
Figure 3. Morphology and viability of MØCC differentiated on aECM. Monocytes were differentiated to MØCC on bsa, coll or different aECMs (coll/HA, coll/lsHA, coll/hsHA) by stimulation with the cytokine cocktail (MCP-1, IL-6, IFNγ). (A) Representative micrographs out of three independent experiments from MØCC on day 6 are depicted. On all substrates MØCC are small, round shaped and only fragile attached. On coll, coll/HA and coll/lsHA MØCC form large clusters while on coll/hsHA MØCC appear predominantly as single cells. (B) Vitality of MØCC was determined on day 6 by labeling the cells with Annexin V (AnnxV) and propidium iodide (PI). Percentage of AnnxV-/PI- cells reflecting the population of viable cells is presented as mean ± SD of three independent experiments. No differences in the viabilty of the MØCC generated on the different substrates were observed.
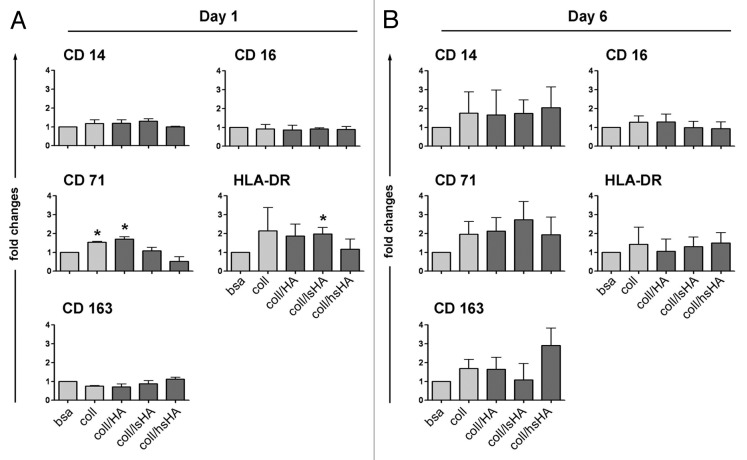
Monocyte to MØCC differentiation on aECMs results in an altered surface marker profile
As shown above, stimulation of monocytes with the cytokine cocktail induced their differentiation to macrophages displaying M1 like characteristics. To evaluate whether the different aECMs exert any modulatory effects on this process we first assessed the expression of CD14, CD16, CD71, CD163 and HLA-DR which are differentially expressed on M1, M2 and MØCC. To detect possible alterations at early and late points in time of the differentiation process we compared MØCC cultured for one day and six days, respectively (Fig. 4). On day 1 the expression of CD71 and HLA-DR is different between MØCC from the two control substrates bsa and coll (Fig. 4A). Both markers are higher expressed on MØCC cultured on coll. Expression levels of CD71 on MØCC cultured on coll/HA are similar to the coll control whereas CD71 levels on coll/lsHA are similar to that of the bsa control. HLA-DR expression on MØCC cultured on coll/HA and coll/lsHA is similar to the coll control. Of note is the reduced expression of CD71 and HLA-DR on MØCC cultured on coll/hsHA which is on the same level as on MØCC from the bsa control (HLA-DR) or even less (CD71). No differences in the expression of CD14, CD16 and CD163 are observed between MØCC from all substrates including controls on day one.
Figure 4. Surface marker profile of MØCC differentiated on aECM. Monocytes were differentiated to MØCC on bsa, coll or different aECMs. Expression of surface markers were analyzed on day one (A) and day 6 (B) of differentiation. Changes in the levels of surface marker expression on MØCC cultured on the different substrates are displayed relative to bsa. Of note is the remarkable induction of CD163 on MØCC differentiated for six days on coll/hsHA. All data are presented as mean ± SD of three independent experiments. *p < 0.05 (compared with bsa).
On day 6 no striking differences between all MØCC in their expression of HLA-DR and CD16 are found. Compared with bsa, expression levels of CD14 and CD71 are upregulated by trend on MØCC differentiated on coll and the aECMs. Of note is the altered expression of CD163 on the different MØCC. This surface protein is slightly elevated on MØCC on bsa and coll/HA and remarkably induced on MØCC grown on coll/hsHA. Expression of CD163 is characteristic for M2 like macrophages indicating an altered differentiation of MØCC on coll/hsHA toward an alternatively activated macrophage phenotype.
Cytokine response in the early and late differentiation process is altered in MØCC cultured on aECMs
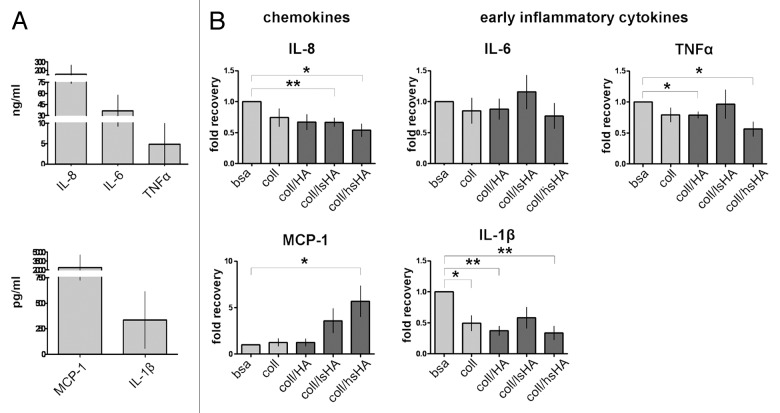
An important function of monocytes and macrophages is the release of a variety of chemokines, cytokines and growth factors, which is decisive for the differentiation and activity of monocytes and macrophages via autocrine and paracrine regulatory mechanisms. Furthermore, this mediator release differs between M1 and M2 macrophages.27 Determining the cytokine profile is therefore an important tool to characterize monocyte differentiation toward specific macrophage phenotypes. Thus, we analyzed the cytokine response of MØCC exposed to the different aECMs on day 1 and day 6 of differentiation.
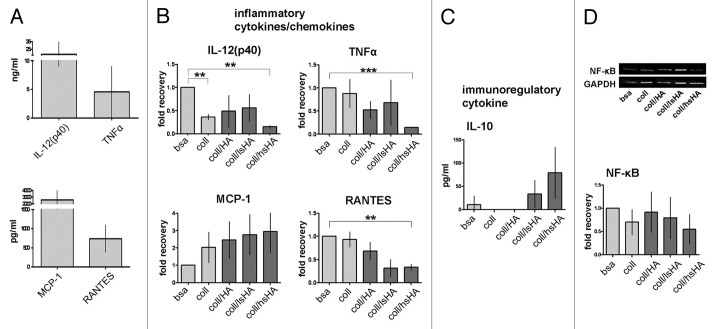
On day 1 we assessed the release of IL-8, MCP-1, IL-1β, IL-6 and TNFα which represent important mediators of early inflammatory processes.18 MØCC differentiated on bsa release these cytokines at high levels (Fig. 5A) and in MØCC differentiated on aECMs this cytokine response is modified (displayed as fold recovery relative to bsa, Fig. 5B): Of note is the increased release of MCP-1 by MØCC differentiated on coll/lsHA and coll/hsHA compared with MØCC from all other substrates. In contrast, release of IL-1β, IL-8 and TNFα by MØCC is reduced on coll and all aECMs, which is significant for IL-1β and TNFα on coll/HA, for IL-8 on coll/lsHA, and for all three cytokines on coll/hsHA. Secretion of IL-6 by MØCC is unaffected on all substrates compared with the bsa control.
Figure 5. Early cytokine response of MØCC differentiated on aECM. Monocytes were induced to differentiate into MØCC on bsa, coll or different aECMs. On day one of differentiation, early cytokine response was determined after LPS stimulation for 5 h (TNFα) or 24 h (all other cytokines). (A) Inflammatory cytokines/chemokines IL-8, MCP-1, IL-1β, IL-6 and TNFα are secreted at high levels. (B) Changes in the levels of released cytokines are presented as fold recovery relative to bsa. MØCC cultured on coll/hsHA show reduced secretion of the early inflammatory cytokines IL-1β and TNFα and the chemoattractant IL-8 while release of chemoattractive MCP-1 is increased in these MØCC and in MØCC cultured coll/lsHA. Secretion of IL-6 in MØCC on all aECMs is similar to bsa and coll. All data are presented as mean ± SD of three to five independent experiments. *p < 0.05 and **p < 0.01.
On day 6 we focused on cytokines which are known to be differently released in different macrophage phenotypes including the inflammatory cytokines TNFα, IL-12, RANTES, MCP-1 and the immunoregulatory cytokine IL-10 (Fig. 6).27 Monocyte differentiation on bsa induced by the cytokine cocktail results in MØCC displaying an inflammatory cytokine profile characterized by high amounts of IL-12, TNFα, MCP-1 and RANTES (Fig. 6A). With exception of MCP-1 release of these inflammatory cytokines is reduced in all MØCC differentiated on aECMs compared with the bsa control (displayed as fold recovery to bsa; Fig. 6B). However, only for MØCC differentiated on coll/hsHA the reduction of the cytokine levels was significant. In contrast, secretion of MCP-1 was increased for MØCC on coll and all aECMs (coll/HA < coll/lsHA < coll/hsHA; Fig. 6B). MØCC differentiated on bsa produce very little amounts of immunregulatory IL-10 while MØCC on coll and coll/HA do not. In MØCC differentiated on coll/lsHA and coll/hsHA the amount of released IL-10 is increased (coll/lsHA < coll/hsHA) but still at low levels (Fig. 6C).
Figure 6. Late cytokine response of MØCC differentiated on aECM. Monocytes were differentiated into MØCC on bsa, coll or different aECMs. On day 6 of differentiation, cytokine response and NF-κB activation were evaluated after LPS stimulation for 20 min (NF-κB), 5 h (TNFa) or 24 h (all other cytokines). (A) MØCC differentiated on bsa release high levels of inflammatory cytokines/chemokines. (B) Changes in the levels of released cytokines are presented as fold recovery relative to bsa. Release of inflammatory cytokines IL-12(p40), RANTES and TNFα is reduced in MØCC on all aECM whereas levels of the chemokine MCP-1 are elevated. Altered cytokine/chemokine release is most pronounced and significant in MØCC on coll/hsHA. (C) MØCC on bsa produce little amounts of immunoregulatory IL-10 which are elevated in MØCC on coll/lsHA and coll/hsHA. (D) Activation of NF-κB is remarkably reduced in MØCC differentiated on coll/hsHA. Levels of NF-κB were determined by densitometric evaluation and calculation relative to GAPDH which was used as loading control. All data are presented as mean ± SD of three (NF-κB: five) independent experiments. *p < 0.05, **p < 0.01 and ***p < 0.001.
In summary, we observe for MØCC differentiated on coll/hsHA consistently reduced secretion of the early inflammatory mediators IL-8, IL-1β and TNFα (except MCP-1) while IL-6 release is unaffected on all aECMs. On day 6, we find that in fully matured MØCC on coll/hsHA the release of the pro-inflammatory cytokines IL-12, TNFα and RANTES is reduced while levels of the immunoregulatory cytokine IL-10 are increased.
Since gene expression of inflammatory cytokines is regulated by the transcription factor NF-κB,28 we analyzed the NF-κB expression in MØCC and found nearly 50% reduced protein expression levels of NF-κB in MØCC on coll/hsHA compared with bsa control (Fig. 6D).
Discussion
Bioengineered aECMs have been shown to modulate cellular responses, i.e., of fibroblasts and mesenchymal stroma cells, and were highlighted as functional coating to improve biomaterial integration and healing.2,810,29 In this study we tested for immunmodulatory effects of different aECMs composed of a collagen matrix and native HA or HA artificially sulfated at low or high levels on the differentiation of monocytes into macrophages induced by a cytokine cocktail mimicking conditions of a sterile inflammation. The cytokine cocktail was composed of MCP-1, IL-6, and IFNγ which were shown by different studies to attract monocytes in sterile wounds and to prime and activate them.16,17,19-22
Here, we demonstrate that treatment of human monocytes with the cytokine cocktail containing MCP-1, IL-6 and IFNγ stimulates their activation and differentiation in vitro. During the differentiation process into macrophages, monocytes acquire new properties and functions; i.e., they gain adhesive properties, enlarge in size and express a different set of surface markers.30 Likewise, after stimulation with the cytokine cocktail for six days, monocytes were increased in size and displayed macrophage specific surface markers such as CD16, CD71 and HLA-DR indicating their differentiation into macrophages.30,31 However, they did not properly adhere and spread on the underlying substrate. Adhesion is regarded as a critical factor for monocyte survival and differentiation in vitro and loss of adherence is often associated with cell death.30,32 Apoptosis rate of monocytes treated with the cytokine cocktail was not increased compared with those stimulated with GM-CSF and M-CSF, respectively (data not shown). Recent reports demonstrated that monocytes differentiate into macrophages even if they are non-adherent when they receive exogenous survival and differentiation factors which are provided by the cytokine cocktail.33 In turn, monocytes do survive without stimulation when they are able to adhere to tissue culture plastic. Haskill et al. showed that adhesion to plastic allows monocytes to produce autocrine survival factors.34 To prevent such artificial monocyte stimulation due to plastic adherence we coated the tissue culture plates with bsa before seeding the monocytes. We assume that the bsa surface provides no appropriate adhesion sites for monocytes resulting in their aggregation to cell clusters as also described for the differentiation of monocytes on low adhesive substrates like coatings of sP(EO-stat-PO) or HA.35,36 However, monocytes stimulated with GM-CSF or M-CSF attached and spread well on the bsa surface which questions a low adhesive nature of bsa. As adhesion of monocytes treated with cytokine cocktail was also weak on coll and all aECMs we think that monocyte activation with the cytokine cocktail favors cell-to-cell interactions and formation of multi-cell clusters during the differentiation process. After six days in culture MØCC on bsa had developed a phenotype similar to inflammatory M1 macrophages with respect to their low expression of CD14, CD16 and CD163 and their high expression of CD71 and, upon activation, release high amounts of IL-1β, IL-6, IL-12, TNFα but no IL-10.26,37
Next, we tested whether the different aECMs are capable to modulate this monocyte to macrophage differentiation. Therefore, monocytes were seeded on the aECMs, stimulated with the cytokine cocktail and surface marker profile and cytokine response upon MØCC activation were assessed at early and late points in time during differentiation. The aECMs are composed of specific forms of HA that are incorporated in a matrix of collagen I which serves as structural scaffold. In a previous study collagen I (coll) was shown to promote monocyte to macrophage differentiation.38 In accordance with this study we found upregulated expression of the CD71 and HLA-DR on monocytes cultured on coll one day after stimulation with the cytokine cocktail suggesting an acceleration of the differentiation process of monocytes into inflammatory MØCC, which was further substantiated by the surface marker profile on day 6 of culture. However, MØCC cultured on coll showed an unexpected cytokine response after their activation characterized by a reduced release of inflammatory IL-1β, IL-8 and TNFα on day 1 and IL-12p40 and TNFα on day 6. These findings indicate that coll alone modulates monocyte to macrophage differentiation induced by the cytokine cocktail but with different effects on macrophage surface phenotype and cytokine release. Such divergent modulation was also observed for monocytes differentiated on coll/HA. As phenotypical and functional characteristics of MØCC kept on this aECM are nearly identical to those kept on coll we speculate that the modulatory impact of coll/HA was mediated predominantly by the coll component. Compared with coll and all other aECMs, MØCC differentiated on the collagen matrix containing low sulfated HA show a slightly increased release of the inflammatory cytokines IL-1β, IL-6, MCP-1, IL-12p40 and TNFα upon their activation. This suggests that low sulfated HA exerts influence on MØCC functions and particularly the cytokine response. However, since levels of inflammatory cytokine on this aECM were mostly higher than on coll we assume that coll/lsHA may support the inflammatory cytokine response. In contrast, on coll/hsHA both early and late inflammatory cytokine responses (except MCP-1, IL-6) of MØCC are significantly reduced compared with the control. The expression of CD163 as well as the induction of IL-10 production, both characteristic markers for regulatory M2,26,37 suggests a profound impact of coll/hsHA on the monocyte to macrophage differentiation. Reduced activation of NF-κB, which regulates gene transcription of inflammatory cytokines including IL-1β, IL-12 and TNFα points to altered intracellular signaling cascades.28 These may be induced via direct action of coll/hsHA on specific monocyte receptors or through modified signaling of the cytokine cocktail to the monocytes. High sulfated HA may bind MCP-1, IL-6 and INFy and thus alter the bioactivity of these cytokines on monocytes as shown for TGF-β on mesenchymal stroma cells and for IL-8 on granulocytes.9,29 However data on a putative modulation of MCP-1, IL-6 or INFy activity by coll/hsHA are elusive. Further studies need to clarify the mechanism by which the aECM modulates monocyte/macrophage functions.
Aim of this study was to test aECMs composed of collagen I and HA or differently sulfated HA derivatives for their immunmodulatory capabilities on the process of monocyte to macrophage differentiation. Artificial ECMs are intended to be used as coating for biomaterials to improve their functional integration into healthy tissue.2,8,10 Persistent activation of inflammatory macrophages resulting in chronic inflammation is viewed as a major reason for aberrant biomaterial healing.1,2 As the majority of these macrophages differentiates from monocytes that migrate to the implantation site the modulation, of this differentiation process represents an attractive target to improve biomaterial healing. We identified the aECM composed of collagen I and high sulfated HA to effectively modify monocyte differentiation induced by the cytokine cocktail that promotes polarization into inflammatory M1 macrophages. Moreover macrophages differentiated on coll/hsHA, display typical characteristics of regulatory M2 macrophages, i.e., reduced release of inflammatory cytokines (IL-1β, IL-8, IL-12p40, TNFα and RANTES) but upregulation of IL-10 and MCP-1 as well as expression of CD163.26,37 Although these results purely base on investigations in vitro, we believe that our data are of relevance for in vivo effects due to mimicking the environment of sterile injury post biomaterial implantation. Inducing monocyte to macrophage differentiation in the presence of physiological amounts of MCP-1, IL-6 and IFNγ is thought to resemble an in vivo situation more than well-established artificial in vitro systems, i.e., tissue culture plastic or non-physiological amounts of single factors. We therefore speculate that coll/hsHA applied as coating for biomaterials may also modulate differentiation of monocytes at the implantation site. It has been shown that the initial population of macrophages found in wound sites post-injury is decisive for the extent of scar formation and that their depletion results in minimal scarring.39 Investigations on the remodelling process following biomaterial implantation revealed favorable tissue remodelling and less scar formation to be associated with higher M1/M2 ratios in the early healing process.40 We suggest that an early switch in monocyte differentiation from M1 toward M2 macrophages as it could be promoted by coll/hsHA may facilitate the healing of biomaterials and the restoration of functional tissue. However, sulfated HA has been described to possess anticoagulant activities which may affect the healing response adversely.41 Therefore, we propose to examine effects of coll/hsHA on wound healing in in vivo models.
Conclusion
Goal of this in vitro study was to characterize aECM in respect of their immunomodulating capacities and to identify matrices that may benefit healing of biomaterials. Artificial ECMs composed of collagen I and high sulfated HA were found to modulate the differentiation of monocytes into inflammatory macrophages induced by signals of a sterile inflammation. Moreover, macrophages differentiated on this aECM display phenotypic and functional characteristics of regulatory M2 which are crucial for inflammatory resolution and tissue remodeling in the biomaterial healing response. We therefore suggest the aECM composed of collagen and high-sulfated HA as promising immunomodulating coating for biomaterials and recommend its further testing in in vivo models.
Materials and Methods
Preparation of aECM
Native HMW-HA from Streptococcus was obtained from Aqua Biochem Dessau. Low and high sulfated HA were synthesized from native HMW-HA of the same batch as described elsewhere.7,8 In the course of the sulfation process the native HA is continuously degraded resulting in sulfated HA products of lower molecular weight. Following purification by dialyses low and high sulfated HA derivatives with small PD values ranging in size from 50 to 30 kDa are obtained. Characterization of the sulfated HA products was performed as described in Hintze et al.7,8 Characteristics of the GAGs are shown in Table 1.
Table 1. Composition of control substrates and aECM and characteristics of GAGs.
| Name | Coll/GAG [%] | Used GAG | Characteristics of GAGs |
||||
|---|---|---|---|---|---|---|---|
| S (%) | DSS | MW (Da) | PD | ||||
|
Control |
bsa |
0/0 |
Non |
/ |
/ |
/ |
/ |
| coll |
100/0 |
Non |
/ |
/ |
/ |
/ |
|
| aECM | coll/HA |
88/12 |
Native HA |
0 |
0 |
1,174,865 |
4.80 |
| coll/lsHA |
96/4 |
Low-sulfated HA |
6.61 ± 0.9 |
1.1 ± 0.1 |
28,728 ± 3,292 |
3.23 ± 1.8 |
|
| coll/hsHA | 88/12 | High-sulfated HA | 13.44 ± 0.1 | 3.25 ± 0.2 | 50,435 ± 1,004 | 1.71 ± 0.01 | |
Preparation and characterization of aECM was performed as described elsewhere.10,29 Briefly, in vitro fibrillogenesis of rat collagen I (BD Bioscience, Cat. No. 354249) was induced in 24-well-tissue-culture-plates in the presence of native HA or the sulfated HA derivatives, respectively. Therefore, acid-solubilised collagen at a concentration of 1 mg/ml was mixed with an equal volume of 1 mg/ml HA or the respective same molar concentrations of disaccharide units of sulfated GAGs solubilised in ice-cold 60 mM phosphate buffer pH 7.4. After fibrillogenesis for 16–18 h at 37°C the aECMs were dried on the well plates, washed two times with deionised water and dried again. Characterization of the generated coatings by the methods of Lowry revealed a stable content of collagen I for up to 8 d. The aECMs contained a stable content of collagen of > 90% of the original amount. In contrast, compared with the original amount there were only 5–9% of sulfated GAG and 54% of HA associated to the aECMs after washing. We observed a significant desorption after 1 h incubation in PBS at 37°C with only 2–12% of the original amount of disaccharide units detected in the aECM coatings. However, at later points in time the GAG content only marginally decreased further. Therefore, immediately before utilizing the aECMs for cell culture assays, the coatings were incubated with PBS for 1 h at 37°C. The resulting aECMs were composed of 88–96% collagen and 4–12% GAG. Nomenclature and composition of the different aECMs after 1 h of desorption are summarized in Table 1.
As control substrates coatings of bovine serum albumin (bsa) and collagen I (coll) were used. Both were prepared in 24-well-tissue-culture-plates. For the bsa coating, 1% bsa (ROTH, Cat. No. CP77.1) dissolved in sterile water was used. After 30 min incubation at room temperature the bsa solution was removed and the coating was dried for another 30 min. Collagen coatings were prepared by in vitro fibrillogenesis of rat collagen I in phosphate buffer pH 7.4 as described above.
Monocyte isolation and culture conditions
EDTA blood was taken from healthy human volunteers in compliance with institutional ethical use protocols. Human peripheral blood mononuclear cells were isolated from the blood by densitiy-gradient centrifugation (Ficoll-Paque PLUS, GE Healthcare, Cat. No. 17-1440-03). Monocytes were enriched using anti-CD14 microbeads (Miltenyi Biotec, Cat. No. 130-050-201), suspended in culture medium (RPMI-1640 containing stable glutamine (Biochrom AG, Cat. No. FG1215) supplemented with 1% penicillin/streptamycin and seeded on the different aECMs or bsa at 400,000 cells in 500 μl per well. Under serum-free conditions monocytes were allowed to adhere to the substrates for 1h at 37°C and 5% CO2 in a humidified atmosphere before addition of 500 μl culture medium supplemented with 20% FCS. Monocytes were stimulated either with a cytokine cocktail composed of 10 ng/ml recombinant human IFN-γ (Promokine, Cat. No. C-60724), 1ng/ml recombinant human IL-6 (PeproTech, Cat. No. 200-06) and 10 pg/ml recombinant human MCP-1 (eBioscience, Cat. No. 14-8398-62), or with 50 U/ml GM-CSF (Leukine, Cat. No. NDC50419-002-33) or with 50 ng/ml M-CSF (eBioscience, Cat. No. 14-8789-80) and cultured at 37°C and 5% CO2 in a humidified atmosphere. To characterize phenotype and function of the monocytes, surface marker profile and cytokine response upon additional stimulation with LPS was assessed on day one and day six of culture.
Flow cytometry
Surface marker profile of monocytes was determined in a flow cytometric assay. Therefore, monocytes were removed from their underlying substrate by incubation with 5 mM EDTA for 30 min at room temperature and careful rinsing with PBS. Cells were washed with PBS and incubated with fluorescence labeled antibodies, diluted in PBS according to the manufacturer’s protocols for 30 min at 4°C. The following antibodies were used: mouse anti-human CD14-PE, mouse anti-human CD16-APC-Cy7, mouse anti-human HLA-DR-FITC (all BD PharMingen, Cat. No. 555398, 557873 and 555866), mouse anti-human CD71-FITC (Biolegend, Cat. No. 347304), mouse anti-human CD163-PE (eBioscience, Cat. No. 12-1639-73), and corresponding isotype controls -FITC, -PE or -APC (all from BD PharMingen, Cat. No. 555748, 555749 and 555751). To determine cell viability and to exclude dead monocytes from the analysis of surface marker expression, 7AAD (7-Amino-Actinomycin D; BD PharMingen, Cat. No. 559925) was added to the samples which were then incubated for another 10 min at 4°C. Samples of 1 × 105 cells were analyzed using a FACS Canto II flow cytometer (BD Bioscience).
Determination of viability
Cell viability, apoptosis and necrosis on day six was determined by detaching the cells with 5 mM EDTA and labeling them with FITC conjugated Annexin V (AnnxV, kindly provided by Prof M. Herrmann, Erlangen) and propidium iodide for 30 min at 4°C. Flow cytometric analysis reveals three populations of cells representing viable (AnnxV-/PI-), apoptotic (AnnxV+/PI-) and necrotic (AnnxV+/PI+) cells, respectively.
ELISA
To determine the cytokine response of monocytes on day 1 and day 6, cells were stimulated with LPS at 100 ng/ml for 5 h (detection of TNFα release) and 24 h, respectively. Supernatants were collected and cell-free aliquots were frozen at -20°C until examination. The following cytokines were quantified by ELISA according to manufacturer’s instructions: IL-6, IL-8, IL-10, MCP-1, RANTES (all purchased from PeproTech, Cat. No. 900-K16, 900-K18, 900-K21, 900-K31, 900-K33), IL-1β and TNFα, (eBioscience, Cat. No. 88-7010-88, 88-7346-88), and IL-12(p40) (BD Biosciences, Cat. No. 555171).
Western blot
Cell extracts of macrophages from day six stimulated with LPS at 100 ng/ml for 20 min were obtained by cooled lysis with RIPA-buffer (50 mM HEPES, 150 mM NaCl, 5 mM EDTA, 1 mM EGTA, 1% Triton ×100, 0.1% SDS, 1% Deoxycholate, 1 mM dithiothreitol). Protein lysates were separated by 8% sodium dodecyl sulfate polyacrylamide gel electrophoreses (SDS-PAGE) and blotted on Amersham Hybond-ECL membranes (GE Healthcare, Cat. No. RPN2020D). The membranes were then incubated with the primary antibody for NF-κB (rabbit NF-κB p65, Santa Cruz, Cat. No. SC56735) diluted in 5% milk buffer. GAPDH served as reference protein and was detected by mouse-anti-human GAPDH (Millipore, Cat. No. MAB374). As secondary antibody anti-mouse IRDye 800CW and anti-rabbit IRDye 680LT (both Li-Cor Bioscience, Cat. No. 926-32210 and 926-68021) were used. Membranes were analyzed on a LI-COR Odyssey Scanner (LI-COR Biosciences) and band densities were quantified using the Odyssey 3.0 analytical software (LI-COR Biosciences)
Statistical analysis
All presented data were derived from at least three different experiments with different donors. Results were prepared with the graphic program GraphPad Prism4 and are presented as mean ± standard deviation. For statistical evaluation data were analyzed using matched paired t-test: *p < 0.05, **p < 0.01 and ***p < 0.001.
Acknowledgments
This work was funded by the German Research Council (DFG SFB-TR67, projects A2, A3, B3 and a medical student scholarship to E.R.). We thank Aline Katzschner and Heike Zimmermann for excellent technical assistance in preparing the aECMs.
Glossary
Abbreviations:
- aECM
artificial ECM
- bsa
bovine serum albumin
- cc
cytokine cocktail
- coll
collagen
- ECM
extracellular matrix
- GAGs
glycosaminoglycans
- FSc
forward scatter
- GM-CSF
granulocyte macrophage colony stimulating factor
- HA
hyaluronan
- IL
interleukin
- HLA
human leucocyte antigen
- IFNγ
interferon gamma
- KC
keratinocytes
- LPS
lipopolysaccharide
- M-CSF
macrophage colony stimulating factor
- MCP
monocyte chemotactic protein
- MFI
mean fluorescence index
- Mo
monocytes
- MØCC
macrophages induced by the cytokine cocktail
- M1
inflammatory macrophages
- M2
anti-inflammatory macrophages
- NF-κB
nuclear factor “kappa-light-chain-enhancer” of activated B-cells
- NK
natural killer cells
- RANTES
regulated upon activation, Normal T-cell expressed and secreted
- ROS
reactive oxygen species
- SSc
side scatter
- TGF-β
transforming growth factor beta
- Tc
T cells
- TNFα
tumor necrosis factor alpha
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/22855
References
- 1.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692–709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 3.Turner NJ, Yates AJ, Jr., Weber DJ, Qureshi IR, Stolz DB, Gilbert TW, et al. Xenogeneic extracellular matrix as an inductive scaffold for regeneration of a functioning musculotendinous junction. Tissue Eng Part A. 2010;16:3309–17. doi: 10.1089/ten.tea.2010.0169. [DOI] [PubMed] [Google Scholar]
- 4.Rammelt S, Heck C, Bernhardt R, Bierbaum S, Scharnweber D, Goebbels J, et al. In vivo effects of coating loaded and unloaded Ti implants with collagen, chondroitin sulfate, and hydroxyapatite in the sheep tibia. J Orthop Res. 2007;25:1052–61. doi: 10.1002/jor.20403. [DOI] [PubMed] [Google Scholar]
- 5.Schneiders W, Reinstorf A, Biewener A, Serra A, Grass R, Kinscher M, et al. In vivo effects of modification of hydroxyapatite/collagen composites with and without chondroitin sulphate on bone remodeling in the sheep tibia. J Orthop Res. 2009;27:15–21. doi: 10.1002/jor.20719. [DOI] [PubMed] [Google Scholar]
- 6.Streuli C. Extracellular matrix remodelling and cellular differentiation. Curr Opin Cell Biol. 1999;11:634–40. doi: 10.1016/S0955-0674(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 7.Hintze V, Moeller S, Schnabelrauch M, Bierbaum S, Viola M, Worch H, et al. Modifications of hyaluronan influence the interaction with human bone morphogenetic protein-4 (hBMP-4) Biomacromolecules. 2009;10:3290–7. doi: 10.1021/bm9008827. [DOI] [PubMed] [Google Scholar]
- 8.Hintze V, Miron A, Moeller S, Schnabelrauch M, Wiesmann HP, Worch H, et al. Sulfated hyaluronan and chondroitin sulfate derivatives interact differently with human transforming growth factor-β1 (TGF-β1) Acta Biomater. 2012;8:2144–52. doi: 10.1016/j.actbio.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Schlorke D, Thomas L, Samsonov SA, Huster D, Arnhold J, Pichert A. The influence of glycosaminoglycans on IL-8-mediated functions of neutrophils. Carbohydr Res. 2012;356:196–203. doi: 10.1016/j.carres.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 10.van der Smissen A, Hintze V, Scharnweber D, Moeller S, Schnabelrauch M, Majok A, et al. Growth promoting substrates for human dermal fibroblasts provided by artificial extracellular matrices composed of collagen I and sulfated glycosaminoglycans. Biomaterials. 2011;32:8938–46. doi: 10.1016/j.biomaterials.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamson R. Role of macrophages in normal wound healing: an overview. J Wound Care. 2009;18:349–51. doi: 10.12968/jowc.2009.18.8.43636. [DOI] [PubMed] [Google Scholar]
- 13.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–57. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–37. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 16.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–21. [PubMed] [Google Scholar]
- 17.Engelhardt E, Toksoy A, Goebeler M, Debus S, Bröcker EB, Gillitzer R. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol. 1998;153:1849–60. doi: 10.1016/S0002-9440(10)65699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–25. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 19.Grellner W, Georg T, Wilske J. Quantitative analysis of proinflammatory cytokines (IL-1beta, IL-6, TNF-alpha) in human skin wounds. Forensic Sci Int. 2000;113:251–64. doi: 10.1016/S0379-0738(00)00218-8. [DOI] [PubMed] [Google Scholar]
- 20.Ishida Y, Kondo T, Takayasu T, Iwakura Y, Mukaida N. The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. J Immunol. 2004;172:1848–55. doi: 10.4049/jimmunol.172.3.1848. [DOI] [PubMed] [Google Scholar]
- 21.Kupper TS, Min K, Sehgal P, Mizutani H, Birchall N, Ray A, et al. Production of IL-6 by keratinocytes. Implications for epidermal inflammation and immunity. Ann N Y Acad Sci. 1989;557:454–64, discussion 464-5. doi: 10.1111/j.1749-6632.1989.tb24038.x. [DOI] [PubMed] [Google Scholar]
- 22.Lu Z, Li Y, Jin J, Zhang X, Lopes-Virella MF, Huang Y. Toll-like receptor 4 activation in microvascular endothelial cells triggers a robust inflammatory response and cross talk with mononuclear cells via interleukin-6. Arterioscler Thromb Vasc Biol. 2012;32:1696–706. doi: 10.1161/ATVBAHA.112.251181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hübner G, Brauchle M, Smola H, Madlener M, Fässler R, Werner S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine. 1996;8:548–56. doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- 24.Ishida Y, Kondo T, Kimura A, Matsushima K, Mukaida N. Absence of IL-1 receptor antagonist impaired wound healing along with aberrant NF-kappaB activation and a reciprocal suppression of TGF-beta signal pathway. J Immunol. 2006;176:5598–606. doi: 10.4049/jimmunol.176.9.5598. [DOI] [PubMed] [Google Scholar]
- 25.Mori R, Kondo T, Ohshima T, Ishida Y, Mukaida N. Accelerated wound healing in tumor necrosis factor receptor p55-deficient mice with reduced leukocyte infiltration. FASEB J. 2002;16:963–74. doi: 10.1096/fj.01-0776com. [DOI] [PubMed] [Google Scholar]
- 26.Verreck FA, de Boer T, Langenberg DM, van der Zanden L, Ottenhoff TH. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J Leukoc Biol. 2006;79:285–93. doi: 10.1189/jlb.0105015. [DOI] [PubMed] [Google Scholar]
- 27.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8:837–48. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 29.Hempel U, Hintze V, Möller S, Schnabelrauch M, Scharnweber D, Dieter P. Artificial extracellular matrices composed of collagen I and sulfated hyaluronan with adsorbed transforming growth factor β1 promote collagen synthesis of human mesenchymal stromal cells. Acta Biomater. 2012;8:659–66. doi: 10.1016/j.actbio.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Becker S, Warren MK, Haskill S. Colony-stimulating factor-induced monocyte survival and differentiation into macrophages in serum-free cultures. J Immunol. 1987;139:3703–9. [PubMed] [Google Scholar]
- 31.Andreesen R, Brugger W, Scheibenbogen C, Kreutz M, Leser HG, Rehm A, et al. Surface phenotype analysis of human monocyte to macrophage maturation. J Leukoc Biol. 1990;47:490–7. doi: 10.1002/jlb.47.6.490. [DOI] [PubMed] [Google Scholar]
- 32.Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24:425–39. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- 33.Pham TH, Langmann S, Schwarzfischer L, El Chartouni C, Lichtinger M, Klug M, et al. CCAAT enhancer-binding protein beta regulates constitutive gene expression during late stages of monocyte to macrophage differentiation. J Biol Chem. 2007;282:21924–33. doi: 10.1074/jbc.M611618200. [DOI] [PubMed] [Google Scholar]
- 34.Haskill S, Johnson C, Eierman D, Becker S, Warren K. Adherence induces selective mRNA expression of monocyte mediators and proto-oncogenes. J Immunol. 1988;140:1690–4. [PubMed] [Google Scholar]
- 35.Bartneck M, Heffels KH, Pan Y, Bovi M, Zwadlo-Klarwasser G, Groll J. Inducing healing-like human primary macrophage phenotypes by 3D hydrogel coated nanofibres. Biomaterials. 2012;33:4136–46. doi: 10.1016/j.biomaterials.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 36.Tsai IY, Kuo CC, Tomczyk N, Stachelek SJ, Composto RJ, Eckmann DM. Human macrophage adhesion on polysaccharide patterned surfaces. Soft Matter. 2011;7:3599–606. doi: 10.1039/c0sm01353f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol. 2007;178:5245–52. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- 38.Jacob SS, Shastry P, Sudhakaran PR. Monocyte-macrophage differentiation in vitro: modulation by extracellular matrix protein substratum. Mol Cell Biochem. 2002;233:9–17. doi: 10.1023/A:1015593232347. [DOI] [PubMed] [Google Scholar]
- 39.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Müller W, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–77. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 40.Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012;8:978–87. doi: 10.1016/j.actbio.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magnani A, Albanese A, Lamponi S, Barbucci R. Blood-interaction performance of differently sulphated hyaluronic acids. Thromb Res. 1996;81:383–95. doi: 10.1016/0049-3848(96)00009-6. [DOI] [PubMed] [Google Scholar]