Abstract
Biomedical field is constantly requesting for new biomaterials, with innovative properties. Natural polymers appear as materials of election for this goal due to their biocompatibility and biodegradability. In particular, materials found in marine environment are of great interest since the chemical and biological diversity found in this environment is almost uncountable and continuously growing with the research in deeper waters. Moreover, there is also a slower risk of these materials to pose illnesses to humans.
In particular, sulfated polysaccharides can be found in marine environment, in different algae species. These polysaccharides don’t have equivalent in the terrestrial plants and resembles the chemical and biological properties of mammalian glycosaminoglycans. In this perspective, are receiving growing interest for application on health-related fields. On this review, we will focus on the biomedical applications of marine algae sulfated polymers, in particular on the development of innovative systems for tissue engineering and drug delivery approaches.
Keywords: marine biomaterials, sulfated polysaccharides, algae polysaccharides, biopolymers, glycosaminoglycans (GAG), biomedical, tissue engineering
Introduction
As a response to trauma or tissue disease, the human body tries to remodel the injured tissue, but for many situations, these efforts result in dysfunctionality and then tissue failure.1
To address this serious health problem, scientists have dedicated great attention aiming at developing alternative therapeutic solutions, to overcome the drawbacks of the current clinical practices (prosthesis, autografts, allografts, xenografts, with insufficient properties, site morbidity, donor scarcity and risk of immune rejection as main drawbacks). In this regard, regenerative medicine arose as a hot medical topic, with Langer and Vacanti defining tissue engineering, in their seminal paper, as a multidisciplinary approach that uses principles of engineering and life sciences to create a new tissue.2 Following the general strategy, a porous structure is developed in which cells are seeded/cultured, under certain conditions, with defined biochemical and mechanical cues, until obtaining a functional tissue substitute to be subsequently implanted in the patient injury site.
Such porous structures—called scaffolds—are requested to exhibit certain properties, such as biocompatibility both in as-implanted form and after degradation, which should occur in controlled manner, present appropriate mechanical properties to meet the needs of the tissue to be regenerated, adequate surface properties to enhance interaction with cells and optimal three-dimensional structure (porosity, pore size and interconnectivity). Thus, different processing technologies have been proposed to prepare such structures,3,4 for instance foaming,5,6 freeze-drying,7-9 fiber extrusion and bonding,10,11 three-dimensional printing,12,13 porogen leaching,14-16 in situ pore forming,17 particle aggregation,18-20 electrospinning,21,22 supercritical fluids technology23,24 and use of ionic liquids.25
Together with several techniques, a wide range of materials have been also proposed, including natural and synthetic polymers or a conjugation of both. More recently, considering their predominant existence in the extracellular matrix, but also because of their low immunogenicity and enhanced interaction with growth factors, attention has been devoted to glycosaminoglycans, namely hyaluronic acid and chondroitin sulfate.26,27 Despite the incomplete understanding of the interactions between cells and extracellular matrix, namely at molecular level, it is known that glycosaminoglycans modulate the adhesion of progenitor cells and their subsequent differentiation and gene expression. In particular, degree of sulfation, molecular weight and structural composition influence cell behavior, with changes being observed in different physiological but also pathological processes.27
In fact, degree of sulfation seems to play such a relevant role that recent efforts have been focusing on the preparation and further use of highly sulfated glycosaminoglycans derivatives, in particular by introducing sulfate features in hyaluronic acid or increasing sulfate degree of chondroitin sulfate. Interesting findings have been observed, as the enhanced binding of growth factors to sulfate hyaluronic acid when comparing with chondroitin sulfate with the same sulfation degree, which has been attributed to differences in the sulfation pattern.28 This is in agreement with the findings of Gama and coworkers, where the precise position of sulfate groups resulted in specific ligand-receptor interactions, according to a kind of sulfation code.29 This specificity results in different cellular behavior: sulfate hyaluronic acid was observed to promote proliferation of dermal fibroblasts,26 but on the case of rat calvarial osteoblasts such proliferation was hindered.30 It is noteworthy that the mentioned improved proliferation of fibroblasts was followed by a reduced extracellular matrix expression, which might be quite interesting for skin regeneration, namely by promoting wound closure while hindering scar formation.26 Besides effects on cell adhesion and proliferation, sulfate groups were shown to also influence cell differentiation: matrices of collagen II and sulfate hyaluronic acid stimulate osteogenic differentiation of human mesenchymal stem cells.31 In order to shed more light on the mechanism of influence of sulfate groups on cell behavior, the specific effect of sulfate groups, disconnected from the glycosaminoglycan environment, was also assessed. Sulfate-rich surfaces were prepared with self-assembled monolayers of ω-sulfatealkanethiols, which were shown to influence cell morphology and mobility with enhanced formation of filopodia in both bone marrow derived mesenchymal stem cells and adipose derived stem cells.32
Having in mind this huge potential of sulfate groups, research communities have also been looking at other ways to achieve glycosaminoglycans and sulfated polysaccharides besides chemical modification. In fact, such polysaccharides can be found in marine organisms, in particular macroalgae.33 Besides being an under-exploited resource, marine environments also possess an enormous chemical and biological variety, thus being an extraordinary source of biomaterials.34 On the particular case of sulfated polysaccharides bearing glycosaminoglycan-like biological properties, different structures can be found in marine macroalgae, and its marine origin promises potentially safer polymers when compared with mammalian alternatives,33 since their associated risk of posing diseases to humans is not an issue. In the present review, focus will be made on the most representative sulfate polysaccharides that can be obtained from each of the three main classes of macroalgae, mainly carrageenans, ulvan and fucoidan from red, green and brown algae, respectively, and the attempts to use them further, particularly in biomedical applications, including tissue engineering approaches.
Sulfated Polysaccharides from Red Algae
Carrageenans are sulfated polysaccharides that occur as matrix material in several species of red seaweeds (Rhodophyceae), with main sources being Chondrus crispus, Gigartina, Eucheuma cottonii and spinosum, and that can be extracted with water or aqueous alkali methods.35-37 Chemically, these hydrophilic colloids are highly sulfated galactans, with sulfate content varying between 15% and 40%,38 with a primary structure based on an alternating sequence of β (1–4) and α (1–3) linked D-galactose residues,39 resulting in polymers with molecular weight ranging from 105 to 106 Da.40,41 The number and position of sulfate groups in the repeating galactose units allows the classification of carrageenans in three main commercially relevant families: kappa (κ), iota (ι) and lambda (λ). Table 1 summarizes the main characteristic features of each of these carrageenan families, as well as a representative scheme of the repeating unit structure.
Table 1. Structural formula and typical properties of κ-, ι-, and λ- carrageenans196,197.

It should be stressed that carrageenans can be quite heterogeneous, either due to differing molecular structures within the chains, to differing chains within the seaweed (hybrids) or to algae species, ecophysiology and seasonality or even extraction conditions.42,43 Thus a wide variety of materials can be obtained from them, including hydrogels with a vast range of properties.44
In fact, the gel formation is one of the most relevant properties in carrageenans, even though not possible with λ-carrageenan (being although used as thickening agent to control viscosity). The gelling mechanism is not known in detail, but its high hydration capacity, the structural type, temperature, polymer concentration and the presence of cations are key factors.45-47 κ-Carrageenan hydrogels are thermoreversible48 and can be formed by ionic gelation through interaction with cations, particularly with potassium (K+), which inhibits the electrostatic repulsion between the neighboring negatively charged helices, allowing their aggregation (fig. 1).49,50 By their turn, ι-carrageenan hydrogels exhibit the interesting feature of spontaneously reforming once the mechanical disruption action has stopped,51 called thixotropy, very useful in certain applications, such as cosmetic emulsions.
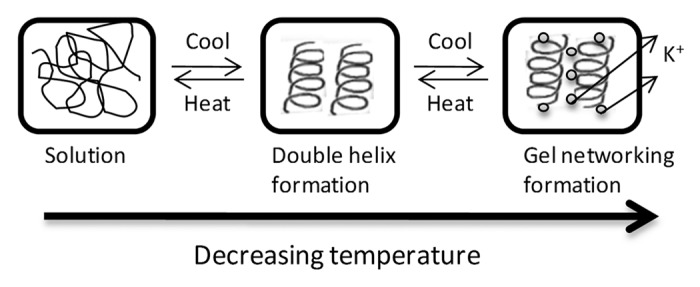
Figure 1. Gelation model of κ-carrageenan (adapted from refs194,195): by decreasing temperature of carrageenan solution, a coil-to-helix conformational transition is enhanced; with further decrease in temperature, in the presence of cations such as potassium, an organization and aggregation of helices is promoted, forming a gel network.
The versatility of carrageenans is also patent on its several processing and formulation routes. Besides hydrogels, carrageenans can also be processed into fibers by wet spinning, into membranes by casting and further crosslinking or into porous structures by freeze drying.52 In addition, the combination of two different families of carrageenans has also been tested, for instance on the production of microscale fibers.53 Hydrogel systems based on carrageenan and other materials from natural algae origin, namely alginate, have been developed into different formats (beads/fibers);52,54,55 fibers resulting from wet-spinning carrageenan into chitosan or vice versa are also possible, including together with carbon nanotubes to improve significantly their mechanical properties, in which active ingredients can be trapped;56 chitosan/carrageenan/tripolyphosphate nanoparticles exhibiting small size and high positive charge have been produced by polyelectrolyte complexation/ionic gelation;57 Fe3O4 nanoparticles with κ-carrageenan58,59 and spheres of carrageenans crosslinked by paramagnetic ions (Ho3+)60 have also been developed.
The chemical reactivity of carrageenans, mainly due to the sulfate groups, as well as the diversity of carrageenan structures just described, can justify the application of carrageenans in numerous applications.61,62 For example, their reactivity with proteins, due to interactions between the sulfate groups of the carrageenan and the charged groups of the protein, is present in the interaction with casein in milk, extremely important in the dairy industry;63,64 their ability to bind heavy metals through covalent, electrostatic or redox reaction is useful for removing them from contaminated waters, with clear environmental impact;65,66 their unique properties of interaction with polyols can be useful to control the texture of any formulation of polyols.67 Nevertheless, their major industrial applications is as thickening, emulsifier, gelling and stabilizing agents, such as for example, in salad dressings, processed meat,68,69 abovementioned dairy industry,70-73 and personal care products,74,75 but also in pharmaceuticals formulations,76,77 being considered a good substitute for gelatin.
In addition to the mentioned applications, the use of carrageenans in the biomedical field is also being explored, mainly taking advantage of their biological activities mostly related to the sulfate content.78,79 In this sense, the antioxidant activity of κ-carrageenan has been investigated,80 as well as the protective activity against viral, fungal and bacterial infections.81 Based on these known biological activities, carrageenans have been tested as treatments for respiratory weakness, from the common cold until influenza viruses, including the pandemic H1N1 influenza strain,82 but also against other viruses, such as dengue virus, hepatitis A virus and African swine fever virus,83,84 or even as topical microbicide targeting HIV and herpes viruses.85,86 In other works, the in vivo antitumor and immunomodulation activities of carrageenan has been studied, with low molecular weight molecules showing the highest activities.87 Furthermore, carrageenan has recently been used in a clinical trial to significantly reduce serum cholesterol and triglyceride levels,88 presenting anticoagulant properties,89,90 and also a regulatory role, namely on growth factors.91,92 Nevertheless, carrageenan biocompatibility has been questioned in the literature, with examples of inflammatory response being presented.93,94 However, it should be noted that food-grade carrageenan and degraded carrageenan (low molecular weight) have completely different toxicological properties,95,96 and not all the studies of acute toxicity have sufficient details on dose, type of carrageenan, seaweed source or extraction procedures used.97 Thus, carrageenan biocompatibility is an open debate.
From all these facts, the use of carrageenans in diverse medical applications has been considered, and the tissue engineering field is no exception, with a few works reporting its use in the recent years and more are expected to come.98 In this perspective, carrageenan has been considered for growth factor/drug delivery systems,99,100 and for immobilization of enzymes,101 but also in encapsulation of several cell types for their in vivo delivery,102,103 envisioning cartilage regeneration. In fact, the structural resemblance of these naturally occurring sulfated polymers to the cartilage extracellular matrix components, glycosaminoglycans (GAGs), may confer biochemical reactivity that urge to determine. Addressing this, hydrogel based on κ-carrageenan was studied for the encapsulation of human-adipose-derived stem cells, human nasal chondrocytes, or a chondrocytic cell line, proving to be a good support for cell culture, viability, cartilage matrix extracellular formation and chondrogenic differentiation.100,103
Sulfated Polysaccharides from Green Algae
As in other macroalgae classes, sulfated polysaccharides can be also found in green algae, but with more complex and diverse chemistries.104,105 In fact, different genus or species may synthesize distinct sulfated polysaccharides with distinct sugar composition.104,106 In this regard, Percival propose a division of these green algae in diverse groups according to the synthesized sulfated polysaccharide, namely (sulfated) glucuronoxylorhamnans, glucuronoxylorhamnogalactans and xyloarabinogalactans.104 However, an accurate division is much more complex: literature is rich in research works reporting sulfated polysaccharides extracted from different green algae (as exemplified in Table 2). For instance, Codium fragile is known to possess a sulfated heteropolysaccharide mainly composed of arabinose and galactan moieties.104,107,108 On the other hand, a pyruvylated galactan sulfate was obtained from Codium yezoense109 and Codium fragile,110,111 whereas a sulfated mannan was extracted from Codium vermilara112 but sulfated arabinan and sulfated arabinogalactan were the ones identified in Codium dwarkense Boergs extracts.113 Within other genus, such variability can also be found.110,114-117 A genus of green algae receiving particular attention for the extraction of sulfated polysaccharide is Ulva, from which ulvan can be obtained, a polysaccharide mostly composed of rhamnose, uronic acid and xylose.118-125
Table 2. Examples of sulfated polysaccharides extracted and identified in green algae.
| Alga | Polysaccharide(s) | References |
|---|---|---|
|
Bryopsis plumosa |
Rhamnan sulfate |
198 |
|
Chaetomorpha aerea |
Sulfated galactan |
147 |
|
Codium dwarkense |
Sulfated arabinan Sulfated arabinogalactan |
113 |
|
Codium fragile |
Sulfated arabinogalactans Pyruvylated galactan sulfate |
104,107,108 |
|
Codium vermilara |
Sulfated mannan |
112 |
|
Codium yezoense |
Pyruvylated galactan sulfate |
109,111,140 |
|
Monostroma latissimum |
Rhamnan sulfate |
115-117 |
|
Monostroma nitidum |
Rhamnan sulfate |
110,114 |
|
Ulva lactuca |
Sulfated rhaman |
118,119 |
|
Ulva rigida |
Sulfated rhaman |
123,124 |
|
Ulva rotundata |
Sulfated rhaman |
120,121 |
| Enteromorpha compressa | Sulfated rhaman | 122,125 |
This variety of chemistries that can be found in sulfated polysaccharides from green algae is substantiated by the numerous oligosaccharide moieties identified as constituting the basic structural units of these complex polysaccharides. In an interesting review paper,106 Stengel and coworkers highlight this variability and its relevance and impact in prospect applicative science based on algal origin molecules.106 In fact, such variability, attributed to taxonomic, ecological or environmental issues,106,126-128 should always be regarded. Despite this chemical variability, certain biological effects are common. In fact, sulfated polysaccharides are commonly investigated for their biological properties, and the ones obtained from green algae are no exception. A summary of reported activities demonstrated in these polysaccharides is presented in Table 3.
Table 3. Biological effects associated with sulfated polysaccharides from green algae.
| Activity | Alga | References |
|---|---|---|
| Antioxidant |
Caulerpa cupressoides C. prolifera C. sertularioides Chaetomorpha moniligera Codium fragile C. isthmocladum Enteromorpha intestinalis Ulva pertusa U. lactuca |
129-135 |
| Antitumoral and antiproliferative activities |
Caulerpa cupressoides C. prolifera C. sertularioides Codium isthmocladum Enteromorpha intestinalis Ulva lactuca |
129,131,136 |
| Immunostimulating |
Codium tomentosum C. fragile Enteromorpha sp Ulva rigida |
137-141 |
| Anticoagulant |
Bryopsis plumosa Boodlea composite Caulerpa cupressoides C. prolifera C. sertularioides C. okamurai C. brachypus C. racemosa C. taxifolia C. scalpelliformis C. veravalensis C. peltata Chaetomorpha media C. torta Cladophora fascicularis Codium isthmocladum C. divaricatum C. adhaerence C. latum C. fragile Enteromorpha clathrata E. compressa Monostroma latissimum M. nitidum Ulva lactuca U. fasciata U. reticulata Valoniopsis pachynema |
115-117,129,131,150-152 |
| Antihyperlipidemic |
Ulva pertusa U. lactuca |
130,143-145 |
| Antiviral |
Codium fragile Gayralia oxysperma Monostroma nitidum Ulva sp U. lactuca |
110,111,131,146-148 |
For instance, these polysaccharides exhibit antioxidant effects, as was recently reported in several research works, describing sulfated polysaccharides with superoxide and hydroxyl radicals scavenging activity, reducing power and able to chelate metals.129-135 Antitumoral activity and antiproliferative effects have also been described and associated with these polysaccharides.129,131,136 Another important features of these polysaccharides are their immunostimulating ability, similar to other algal polysaccharides,137-141 as well as their heparin-like character.105 Besides, these polysaccharides are largely studied for their antihyperlipidemic activities,130,142-145 or antiviral effects.111,131,146-148
Although common to the several sulfated polysaccharides extracted from green algae, the expression of those biological activities is dependent on different sugar composition, molecular weight and sulfate content,149 and thus, as abovementioned, on genus, species and ecological and environmental factors. Several studies stress this variability regarding heparin-like behavior according to the genus and species of the studied algae,115-117,129,131,150-152 but similar variability can be found on anticoagulant150-152 and antioxidant activities,133-135 as well as on antiproliferative effect, which was shown to be strongly related with the polysaccharide sulfate content.129
Within this scenario, an attractive use and exploitation of green algae would take advantage of these biological properties and translate them into applications with pharmacological and medical relevance. However, among the three main divisions of macroalgae, green algae remain a rather underexploited biomass, particularly in areas where other algal origin polysaccharides have already proven their value. A striking example of commercial success is carrageenan (as discussed in the previous section).
Alongside its biological activity and potential pharmaceutical use, green algae sulfated polysaccharides may also be used for biomedical applications, in areas as demanding as regenerative medicine. In this particular arena, both their biological activities and their resemblance with glycosaminoglycans might position these polysaccharides in an advantageous point. In this regard, some important research work has already been performed related with polysaccharide modification, processing and biomaterial development, particularly using ulvan as a starting material. Described ulvan structures include nanofibers,153 membranes,154 particles,155 hydrogels156 and 3D porous structures.157 The applicability of these structures may range from drug delivery to wound dressing or bone tissue engineering.153-157
Sulfated Polysaccharides from Brown Algae
Brown macroalgae are rich in polysaccharides such as alginic acids (alginate) or laminarins (laminarans), but also sulfated fucans, namely fucoidan, which are potential therapeutic agents.158 Fucoidan is found in the cells walls of these algae, representing 5% to 20% of the algae dry weight.159 Its history starts in 1913, when it was named fucoidin by Kylin.160 Later on, McNeely named it fucoidan following polysaccharide nomenclature,161,162 but according to a simple PubMed™ search, only around the 1970s this polymer was mentioned for the first time in the medical literature.
The extraction of fucoidan from brown algae, as of other sulfated polysaccharides from the respective macroalgae, is hot-water based, with precipitation with salts or organic solvents.34 Nevertheless, the production of valuable polysaccharides follows a more complex procedure, adding additional steps to render more pure materials, dependent on their further use. In the case of fucoidan, its extraction procedure has been methodically studied to develop economically and industrially viable systems (fig. 2). The process can be divided in three steps: milling seaweeds, extraction/purification (involves multiple, extended aqueous extractions and acidic solutions and may include calcium to promote the alginate precipitation and obtain fucoidan) and drying/careful storage. This type of extraction can obtain yields of fucoidan (%) ranging from 0.26% to 20% of algal biomass dry weight.163 The physic-chemical characteristics of the extracted fucoidan are dependent on the severity of the treatments in the extraction such as temperature, reaction time, concentration of the chemicals, as well as on inherent factors of algae, such as species and size of algae, local climate and environmental factors.164-166
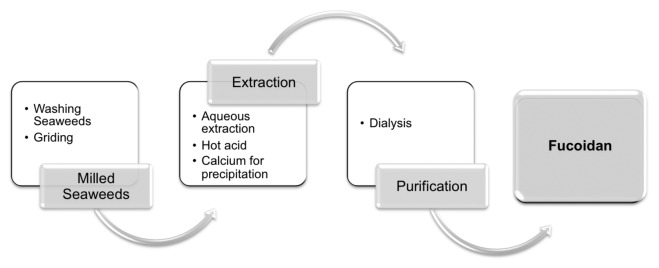
Figure 2. Extraction of fucoidan from brown seaweeds
Different chemical structures have been proposed for this polysaccharide, since its discovery by Kylin. Fucoidan is a heteropolysaccharide and its composition differs depending on the sources and seasonality. The chemical composition of most fucoidan is complex. Fucoidan structure can be divided into two groups depending on their sources: one group includes Laminaria species that have their central chains composed by (1→3)-linked α-L-fucopyranose residues; a second group includes fucoidan isolated from Ascophyllum and Fucus species that have their central chains composed of repeating (1→3) and (1→4) linked α-L-fucopyranose residues.158,167 Besides fucose and sulfate, the presence of monosaccharide residues such as mannose, galactose, glucose, xylose and uronic acids were also identified.165 Nevertheless, the chemical structure of fucoidan can be schematically illustrated as showed in figure 3.
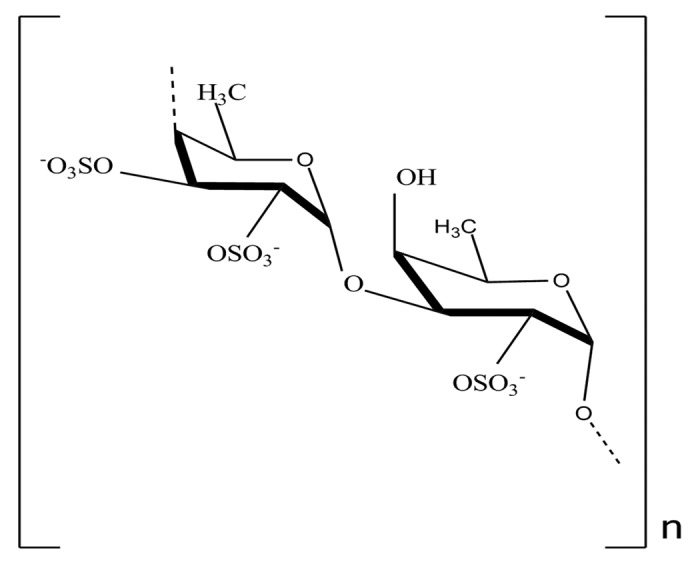
Figure 3. Chemical structure of fucoidan (Adapted from ref180)
The molecular weight of fucoidan varies from 13 kDa to 950 kDa164 and it depends also on many factors such as source, season and extraction method from which they are obtained. In addition, knowledge on the solubility and rheological properties of fucoidan is important for different applications.168 In this perspective, recent studies reported that rheological characteristics and viscosity of fucoidan are different according to the algae species, but independent of the molecular weight and proportion of sulfates and uronic acids.169,170 One of those studies, developed by Rioux and coworkers, report that fucoidan may not be capable to form a gel, but a viscous solution.170
As for the previously reviewed sulfated polysaccharides, several different bioactivities have been attributed to fucoidan and its oligosaccharides. These bioactivities include anti-tumoral effects,171 anti-coagulant,172-175 anti-viral176-179 and anti-inflamatory activities.171 These properties are related to molecular size, type of sugar, sulfation degree and molecular geometry.
Based on the reported activities, fucoidan has been founding application mainly in cosmetic industry (skin exfoliation, acne treatment, hair hydration and tooth paste), food industry (dietetic fibers, cholesterol reducer, functional fibers, sports beverage and processed meat products) and biopharmaceutical industry (immunologic, antiviral and anticoagulant).180-182 Moreover, fucoidan is presently emerging as a popular potential and natural ingredient to be used in cosmeceuticals industry. Some epidemiological studies suggested that fucoidan has some skin protecting, antioxidant and anti-aging activities.183
The interest in fucoidan has also been extended to biomedical-related fields, such as drug delivery, nanomedicine and tissue engineering applications. For instance, Sezer and coworkers reported the development of a new microspheres delivery system based on cross-linking of fucoidan with chitosan (Fucosphere), with extent of drug release being dependent on the concentrations of the polymers and protein.184 Alternatively, other authors reported the development of chitosan/fucoidan pH-sensitive nanoparticles for oral administration of drugs.185 The complexation of fucoidan with chitosan has been explored on the development of nanoparticles with increased potential for the delivery of anticoagulant agents taking advantage of its antithrombotic agents.185 In addition to delivery of bioactive agents, it was also reported the ability of fucoidan to stimulate the production of hepatocyte growth factor (HGF),186 thus reinforcing the potential of this algae-derived biomaterial for health-related applications.
Regarding tissue engineering, in particular considering cell support systems (since drug delivery and growth factor regulatory roles are also relevant), most of the studies investigate fucoidan in combination with different polymers as e.g., chitosan,187 alginate188 or polycaprolactone (PCL),189,190 processed into hydrogels, scaffolds, films and nanofibers. This may relate to the great solubility of fucoidan in water and the difficulty in forming gels. In this regard, the mixture with natural or synthetic polymers, modified structure or chemical cross-linking is critical for its further application. For instance, Sezer and coworkers propose a fucoidan-chitosan hydrogel to be applied as burn injuries healing accelerator on rabbits.191 The authors report the use of chitosan due to its hydrogel forming properties and advantageous use in applications as wound dressing material, adding to the anti-coagulant activity of fucoidan, among other properties. By their turn, Murakami and coworkers developed a hydrogel sheet by blending alginate, chitosan and fucoidan, aiming to stimulate rapid wound healing in rats.188 Besides polysaccharides, fucoidan has been also blended with polyesters, namely PCL, following a general melt-plotted process.189 This rapid-prototyping methodology provided a system with an appropriate pore structure for bone tissue regeneration, where low molecular weight fucoidan was used to induce not only cell proliferation but additionally influence on osteoconductive properties including alkaline phosphatase activity, collagen Type I expression and mineral deposition. Alternatively, micro/nanofibrous scaffolds of PCL and fucoidan have been produced by using an electrospinning process, aimed for application in bone regenerative medicine.190
Final Remarks
Since ancient times, human efforts to treat and recover tissues have been constinuously made.192 Among these, regenerative medicine has brought new hopes for the treatment of inumerous diseases and conditions. Although the tissue engineering and stem cell industry is now close to breaking even,193 technological challenges remain high.
Many tissue-engineered products (TEPs) under development rely on the use of medical devices and/or materials to assure its efficacy. So, the quest for new and improved materials remains a pivotal pillar for the development and marketing authorization of many products. Modulating and controlling cell response through novel biomaterials chemistry and surface is an attractive approach for development of new technology platforms that can, in principle, sustain intellectual property development strategies. In this regard, biomaterials that can bring exquisite properties and improve biological performance of TEPs will continue to justify research and investment.
Like never before, marine species constitute an inspirational template for development of new biomedical technology. The evolution of animal and vegetal species in marine ecossytems has originated an extensive library of biomolecules with enormous human application potential.
Marine polysaccharides remain an untapped reservoir for development of novel biomaterials.
Sulfate groups can effectively modulate cell behavior in tissue regeneration contexts, which may constitute an opportunity for exploiting the clinical potential of marine origin sulfated polysaccharides. These polymers do not have a true mammalian analog and exhibit a high application potential across many different regenerative medicine applications.
The realization of clinical potential of these polysaccharides will be a long and challenging road, as the regulatory context of medical devices and advanced therapy medicinal products, in particular, are very demanding. The lack of industrial scale extraction and purification of many of these molecules remains an obstacle for their application development. In fact, a fundamental requirement for any clinical application will be related with development and validation of manufacturing methods. In some cases, the extraction route may not be a possible manufacturing strategy, as the scarcity of the raw materials, attainable purity levels or final cost may be incompatible with industrialization. Synthesis of surrogate or close analog molecules may be, in some cases, the only cost effective approach.
In spite of the manufacturing strategy adopted, the natural provenience and novelty of these materials imposes a strict control of their purity, stability and safety, which imply extensive and, above all, expensive studies. More than proving additional and or incremental benefits in discrete application contexts, the challenge ahead for any sulfated polysaccharide will be to gain its status as a new biomaterial. For that, sulfated polysaccharides will have to demonstrate outstanding application performance, scalable manufacturing, remarkable cost-benefit potential, while addressing a tangible market opportunity.
Acknowledgments
The authors wish to acknowledge the financial support of ERDF through POCTEP Project 0330_IBEROMARE_1_P and Atlantic Area Project 2011-1/164 MARMED, as well as from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement KBBE-2010-266033 (SPECIAL project). Portuguese Foundation for Science and Technology is also gratefully acknowledged for fellowships of E.G.P., S.S.S. and T.H.S.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/22947
References
- 1.Wagner WR. Soft material design and development to facilitate desirable cardiovascular tissue remodelling. E-MRS 2012 Fall Meeting. Warsaw, Poland, 2012. [Google Scholar]
- 2.Langer R, Vacanti JP. Tissue. Eng Sci. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Dalton PD, Woodfield T, Hutmacher DW. Snapshot: Polymer scaffolds for tissue engineering. Biomaterials. 2009;30:701–2. doi: 10.1016/j.biomaterials.2009.01.049. [DOI] [PubMed] [Google Scholar]
- 4.Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues--state of the art and future perspectives. J Biomater Sci Polym Ed. 2001;12:107–24. doi: 10.1163/156856201744489. [DOI] [PubMed] [Google Scholar]
- 5.Gomes ME, Ribeiro AS, Malafaya PB, Reis RL, Cunha AM. A new approach based on injection moulding to produce biodegradable starch-based polymeric scaffolds: morphology, mechanical and degradation behaviour. Biomaterials. 2001;22:883–9. doi: 10.1016/S0142-9612(00)00211-8. [DOI] [PubMed] [Google Scholar]
- 6.Mooney DJ, Baldwin DF, Suh NP, Vacanti JP, Langer R. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17:1417–22. doi: 10.1016/0142-9612(96)87284-X. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira JM, Silva SS, Mano JF, Reis RL. Innovative technique for the preparation of porous bilayer hydroxyapatite/chitosan scaffolds for osteochondral applications. Key Eng Mater. 2006;309-311:927–30. doi: 10.4028/www.scientific.net/KEM.309-311.927. [DOI] [Google Scholar]
- 8.Yan LP, Oliveira JM, Oliveira AL, Caridade SG, Mano JF, Reis RL. Macro/microporous silk fibroin scaffolds with potential for articular cartilage and meniscus tissue engineering applications. Acta Biomater. 2012;8:289–301. doi: 10.1016/j.actbio.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 9.Reys LL, Silva SS, Oliveira JM, Frias AM, Mano JF, Silva TH, et al. Valorization of Chitosan from Squid Pens and Further Use on the Development of Scaffolds for Biomedical Applications. Int J Artif Organs. 2011;34:704. [Google Scholar]
- 10.Gomes ME, Holtorf HL, Reis RL, Mikos AG. Influence of the porosity of starch-based fiber mesh scaffolds on the proliferation and osteogenic differentiation of bone marrow stromal cells cultured in a flow perfusion bioreactor. Tissue Eng. 2006;12:801–9. doi: 10.1089/ten.2006.12.801. [DOI] [PubMed] [Google Scholar]
- 11.Mikos AG, Bao Y, Cima LG, Ingber DE, Vacanti JP, Langer R. Preparation of poly(glycolic acid) bonded fiber structures for cell attachment and transplantation. J Biomed Mater Res. 1993;27:183–9. doi: 10.1002/jbm.820270207. [DOI] [PubMed] [Google Scholar]
- 12.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–43. doi: 10.1016/S0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 13.Salgado AJ, Sousa RA, Oliveira JT, Silva N, Neves NM, Reis RL, et al. Novel tissue engineering 3D scaffolds for spinal cord injury based on starch/polycaprolactone blends: Development and preliminary assessment of their biological performance. Tissue Eng. 2007;13:1736–7. [Google Scholar]
- 14.Correlo VM, Boesel LF, Pinho E, Costa-Pinto AR, Alves da Silva ML, Bhattacharya M, et al. Melt-based compression-molded scaffolds from chitosan-polyester blends and composites: Morphology and mechanical properties. J Biomed Mater Res A. 2009;91:489–504. doi: 10.1002/jbm.a.32221. [DOI] [PubMed] [Google Scholar]
- 15.Mikos AG, Sarakinos G, Leite SM, Vacanti JP, Langer R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials. 1993;14:323–30. doi: 10.1016/0142-9612(93)90049-8. [DOI] [PubMed] [Google Scholar]
- 16.Mikos AG, Thorsen AJ, Czerwonka LA, Bao Y, Langer R, Winslow DN, et al. Preparation and Characterization of Poly(L-Lactic Acid) Foams. Polymer (Guildf) 1994;35:1068–77. doi: 10.1016/0032-3861(94)90953-9. [DOI] [Google Scholar]
- 17.Martins AM, Santos MI, Azevedo HS, Malafaya PB, Reis RL. Natural origin scaffolds with in situ pore forming capability for bone tissue engineering applications. Acta Biomater. 2008;4:1637–45. doi: 10.1016/j.actbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Borden M, Attawia M, Khan Y, Laurencin CT. Tissue engineered microsphere-based matrices for bone repair: design and evaluation. Biomaterials. 2002;23:551–9. doi: 10.1016/S0142-9612(01)00137-5. [DOI] [PubMed] [Google Scholar]
- 19.B Malafaya PP, Pedro AJ, Peterbauer A, Gabriel C, Redl H, Reis RL. Chitosan particles agglomerated scaffolds for cartilage and osteochondral tissue engineering approaches with adipose tissue derived stem cells. J Mater Sci Mater Med. 2005;16:1077–85. doi: 10.1007/s10856-005-4709-4. [DOI] [PubMed] [Google Scholar]
- 20.Miranda ES, Silva TH, Reis RL, Mano JF. Nanostructured natural-based polyelectrolyte multilayers to agglomerate chitosan particles into scaffolds for tissue engineering. Tissue Eng Part A. 2011;17:2663–74. doi: 10.1089/ten.tea.2010.0635. [DOI] [PubMed] [Google Scholar]
- 21.Martins A, Chung S, Pedro AJ, Sousa RA, Marques AP, Reis RL, et al. Hierarchical starch-based fibrous scaffold for bone tissue engineering applications. J Tissue Eng Regen Med. 2009;3:37–42. doi: 10.1002/term.132. [DOI] [PubMed] [Google Scholar]
- 22.Puppi D, Piras AM, Chiellini F, Chiellini E, Martins A, Leonor IB, et al. Optimized electro- and wet-spinning techniques for the production of polymeric fibrous scaffolds loaded with bisphosphonate and hydroxyapatite. J Tissue Eng Regen Med. 2011;5:253–63. doi: 10.1002/term.310. [DOI] [PubMed] [Google Scholar]
- 23.Duarte ARC, Mano JF, Reis RL. Supercritical fluids in biomedical and tissue engineering applications: a review. Int Mater Rev. 2009;54:214–22. doi: 10.1179/174328009X411181. [DOI] [Google Scholar]
- 24.Duarte ARC, Mano JF, Reis RL. Perspectives on: Supercritical Fluid Technology for 3D Tissue Engineering Scaffold Applications. J Bioact Compat Polym. 2009;24:385–400. doi: 10.1177/0883911509105796. [DOI] [Google Scholar]
- 25.Silva SS, Santos TC, Cerqueira MT, Marques AP, Reys LL, Silva TH, et al. The use of ionic liquids in the processing of chitosan/silk hydrogels for biomedical applications. Green Chem. 2012;14:1463–70. doi: 10.1039/c2gc16535j. [DOI] [Google Scholar]
- 26.van der Smissen A, Hintze V, Scharnweber D, Moeller S, Schnabelrauch M, Majok A, et al. Growth promoting substrates for human dermal fibroblasts provided by artificial extracellular matrices composed of collagen I and sulfated glycosaminoglycans. Biomaterials. 2011;32:8938–46. doi: 10.1016/j.biomaterials.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Salbach J, Rachner TD, Rauner M, Hempel U, Anderegg U, Franz S, et al. Regenerative potential of glycosaminoglycans for skin and bone. J Mol Med (Berl) 2012;90:625–35. doi: 10.1007/s00109-011-0843-2. [DOI] [PubMed] [Google Scholar]
- 28.Hintze V, Miron A, Moeller S, Schnabelrauch M, Wiesmann HP, Worch H, et al. Sulfated hyaluronan and chondroitin sulfate derivatives interact differently with human transforming growth factor-β1 (TGF-β1) Acta Biomater. 2012;8:2144–52. doi: 10.1016/j.actbio.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M, Vaidehi N, et al. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol. 2006;2:467–73. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- 30.Kunze R, Rösler M, Möller S, Schnabelrauch M, Riemer T, Hempel U, et al. Sulfated hyaluronan derivatives reduce the proliferation rate of primary rat calvarial osteoblasts. Glycoconj J. 2010;27:151–8. doi: 10.1007/s10719-009-9270-9. [DOI] [PubMed] [Google Scholar]
- 31.Hintze V, Miron A, Möller S, Schnabelrauch M, Heinemann S, Worch H, et al. Artificial extracellular matrices of collagen and sulphated hyaluronan enhance the differentiation of human mesenchymal stem cells in the presence of dexamethasone. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1528. In press. [DOI] [PubMed] [Google Scholar]
- 32.da Costa DS, Pires RA, Frias AM, Reis RL, Pashkuleva I. Sulfonic groups induce formation of filopodia in mesenchymal stem cells. J Mater Chem. 2012;22:7172–8. doi: 10.1039/c2jm15762d. [DOI] [Google Scholar]
- 33.Senni K, Pereira J, Gueniche F, Delbarre-Ladrat C, Sinquin C, Ratiskol J, et al. Marine polysaccharides: a source of bioactive molecules for cell therapy and tissue engineering. Mar Drugs. 2011;9:1664–81. doi: 10.3390/md9091664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva TH, Alves A, Ferreira BM, Oliveira JM, Reys LL, Ferreira RF, et al. Materials of marine origin: a review on polymers and ceramics of biomedical interest. Int Mater Rev. 2012;57:276–306. doi: 10.1179/1743280412Y.0000000002. [DOI] [Google Scholar]
- 35.Pereira L, Amado AM, Critchley AT, van de Velde F, Ribeiro-Claro PJA. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman) Food Hydrocoll. 2009;23:1903–9. doi: 10.1016/j.foodhyd.2008.11.014. [DOI] [Google Scholar]
- 36.Hoefler AC. Hydrocolloids St. Paul: Eagan Press, 2004:7-25. [Google Scholar]
- 37.Rivera-Carro H, Craigie JS, Shacklock PF. Influence of tissue source and growth rates on dry weight and carrageenan composition of Chondrus crispus (Gigartinales, Rhodophyta) Hydrobiologia. 1990;204-205:533–8. doi: 10.1007/BF00040282. [DOI] [Google Scholar]
- 38.Falshaw R, Bixler HJ, Johndro K. Structure and performance of commercial kappa-2 carrageenan extracts: I. Structure analysis. Food Hydrocoll. 2001;15:441–52. doi: 10.1016/S0268-005X(01)00066-2. [DOI] [Google Scholar]
- 39.Nickerson MT, Darvesh R, Paulson AT. Formation of calcium-mediated junction zones at the onset of the sol-gel transition of commercial κ-carrageenan solutions. J Food Sci. 2010;75:E153–6. doi: 10.1111/j.1750-3841.2010.01519.x. [DOI] [PubMed] [Google Scholar]
- 40.Glicksman M. Utilization of seaweed hydrocolloids in the food industry. Hydrobiologia. 1987;151-152:31–47. doi: 10.1007/BF00046103. [DOI] [Google Scholar]
- 41.Harding SE, Day K, Dhami R, Lowe PM. Further observations on the size, shape and hydration of kappa-carrageenan in dilute solution. Carbohydr Polym. 1997;32:81–7. doi: 10.1016/S0144-8617(96)00167-1. [DOI] [Google Scholar]
- 42.van de Velde F. Structure and function of hybrid carrageenans. Food Hydrocoll. 2008;22:727–34. doi: 10.1016/j.foodhyd.2007.05.013. [DOI] [Google Scholar]
- 43.Delattre C, Fenoradosoa TA, Michaud P. Galactans: an overview of their most important sourcing and applications as natural polysaccharides. Brazilian Archives of Biology and Technology. 2011;54:1075–92. [Google Scholar]
- 44.Whistler RL. BeMiller JN. Carbohydrate Chemistry for Food Scientists. St. Paul MN: AACC/Eagan Press, 1997. [Google Scholar]
- 45.Tuvikene R, Truus K, Kollist A, Volobujeva O, Mellikov E, Pehk T. Gel-forming structures and stages of red algal galactans of different sulfation levels. J Appl Phycol. 2008;20:527–35. doi: 10.1007/s10811-007-9229-9. [DOI] [Google Scholar]
- 46.Yuguchi Y, Thu Thuy TT, Urakawa H, Kajiwara K. Structural characteristics of carrageenan gels: temperature and concentration dependence. Food Hydrocoll. 2002;16:515–22. doi: 10.1016/S0268-005X(01)00131-X. [DOI] [Google Scholar]
- 47.Harding SE, Day K, Dhami R, Lowe PM. Further observations on the size, shape and hydration of kappa-carrageenan in dilute solution. Carbohydr Polym. 1997;32:81–7. doi: 10.1016/S0144-8617(96)00167-1. [DOI] [Google Scholar]
- 48.Pekcan Ö, Kara S, Arda E. Cation effects on phase transition of kappa-iota-carrageenan hybrids: a photon transmission study. Compos Interfaces. 2007;14:1–19. doi: 10.1163/156855407779230344. [DOI] [Google Scholar]
- 49.Wang Q, Rademacher B, Sedlmeyer F, Kulozik U. Gelation behaviour of aqueous solutions of different types of carrageenan investigated by low-intensity-ultrasound measurements and comparison to rheological measurements. Innov Food Sci Emerg Technol. 2005;6:465–72. doi: 10.1016/j.ifset.2005.05.002. [DOI] [Google Scholar]
- 50.Kara S, Arda E, Pekcan Ö. Monovalent and Divalent Cation Effects on Phase Transitions of ι-carrageenan. J Bioact Compat Polym. 2007;22:42–61. doi: 10.1177/0883911506073361. [DOI] [Google Scholar]
- 51.Morris ER, Rees DA, Robinson G. Cation-specific aggregation of carrageenan helices: Domain model of polymer gel structure. J Mol Biol. 1980;138:349–62. doi: 10.1016/0022-2836(80)90291-0. [DOI] [PubMed] [Google Scholar]
- 52.Popa EG, Gomes ME, Reis RL. Cell delivery systems using alginate--carrageenan hydrogel beads and fibers for regenerative medicine applications. Biomacromolecules. 2011;12:3952–61. doi: 10.1021/bm200965x. [DOI] [PubMed] [Google Scholar]
- 53.Kong L, Ziegler GR. Fabrication of κ-Carrageenan fibers by Wet Spinning: Spinning Parameters. Materials. 2011;4:1805–17. doi: 10.3390/ma4101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mohamadnia Z, Zohuriaan-Mehr MJ, Kabiri K, Jamshidi A, Mobedi H. Ionically cross-linked carrageenan-alginate hydrogel beads. J Biomater Sci Polym Ed. 2008;19:47–59. doi: 10.1163/156856208783227640. [DOI] [PubMed] [Google Scholar]
- 55.Mohamadnia Z, Zohuriaan-Mehr MJ, Kabiri K, Jamshidi A, Mobedi H. pH-Sensitive IPN Hydrogel Beads of Carrageenan-Alginate for Controlled Drug Delivery. J Bioact Compat Polym. 2007;22:342–56. doi: 10.1177/0883911507078519. [DOI] [Google Scholar]
- 56.Granero AJ, Razal JM, Wallace GG, in het Panhuis M. Conducting gel-fibres based on carrageenan, chitosan and carbon nanotubes. J Mater Chem. 2010;20:7953–6. doi: 10.1039/c0jm00985g. [DOI] [Google Scholar]
- 57.Rodrigues S. Costa AMRd, Grenha A. Chitosan/carrageenan nanoparticles: Effect of cross-linking with tripolyphosphate and charge ratios. Carbohydr Polym. 2012;89:282–9. doi: 10.1016/j.carbpol.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Daniel-da-Silva AL, Trindade T, Goodfellow BJ, Costa BFO, Correia RN, Gil AM. In situ synthesis of magnetite nanoparticles in carrageenan gels. Biomacromolecules. 2007;8:2350–7. doi: 10.1021/bm070096q. [DOI] [PubMed] [Google Scholar]
- 59.Daniel-da-Silva AL, Lóio R, Lopes-da-Silva JA, Trindade T, Goodfellow BJ, Gil AM. Effects of magnetite nanoparticles on the thermorheological properties of carrageenan hydrogels. J Colloid Interface Sci. 2008;324:205–11. doi: 10.1016/j.jcis.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 60.Winkleman A, Bracher PJ, Gitlin I, Whitesides GM. Fabrication and Manipulation of Ionotropic Hydrogels Crosslinked by Paramagnetic Ions. Chem Mater. 2007;19:1362–8. doi: 10.1021/cm062626f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van de Velde F, Antipova AS, Rollema HS, Burova TV, Grinberg NV, Pereira L, et al. The structure of kappa/iota-hybrid carrageenans II. Coil-helix transition as a function of chain composition. Carbohydr Res. 2005;340:1113–29. doi: 10.1016/j.carres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 62.van de Velde F, Knutsen SH, Usov AI, Rollema HS, Cerezo AS. 1H and 13C high resolution NMR spectroscopy of carrageenans: application in research and industry. Trends Food Sci Technol. 2002;13:73–92. doi: 10.1016/S0924-2244(02)00066-3. [DOI] [Google Scholar]
- 63.Waaland JR, Lembi CA. Algae and human affairs. New York: Cambridge University Press, 1988. [Google Scholar]
- 64.Ribeiro KO, Rodrigues MI, Sabadini E, Cunha RL. Mechanical properties of acid sodium caseinate-ι-carrageenan gels: effect of co-solute addition. Food Hydrocoll. 2004;18:71–9. doi: 10.1016/S0268-005X(03)00043-2. [DOI] [Google Scholar]
- 65.Guven KC, Akyuz K, Yurdun T. Selectivity of heavy metal binding by algal polysaccharides. Toxicol Environ Chem. 1995;47:65–70. doi: 10.1080/02772249509358127. [DOI] [Google Scholar]
- 66.Burdin KS, Bird KT. Heavy metal accumulation by carrageenan and agar producing algae. Bot Mar. 1994;37:467–70. doi: 10.1515/botm.1994.37.5.467. [DOI] [Google Scholar]
- 67.Gekko K, Mugishima H, Koga S. Effects of sugars and polyols on the sol-gel transition of k-carrageenan: calorimetric study. Int J Biol Macromol. 1987;9:146–52. doi: 10.1016/0141-8130(87)90042-0. [DOI] [Google Scholar]
- 68.Bixler HJ. The Carrageenan Connection IV. Br Food J. 1994;96:12–7. doi: 10.1108/00070709410060763. [DOI] [Google Scholar]
- 69.van de Velde F, Lourenco ND, Pinheiro HM, Bakker M. Carrageenan: A food-grade and biocompatible support for immobilisation techniques. Adv Synth Catal. 2002;344:815–35. doi: 10.1002/1615-4169(200209)344:8<815::AID-ADSC815>3.0.CO;2-H. [DOI] [Google Scholar]
- 70.Michon C, Chapuis C, Langendorff V, Boulenguer P, Cuvelier G. Structure evolution of carrageenan/milk gels: effect of shearing, carrageenan concentration and nu fraction on rheological behavior. Food Hydrocoll. 2005;19:541–7. doi: 10.1016/j.foodhyd.2004.10.018. [DOI] [Google Scholar]
- 71.Langendorff V, Cuvelier G, Michon C, Launay B, Parker A. De kruif CG. Effects of carrageenan type on the behaviour of carrageenan/milk mixtures. Food Hydrocoll. 2000;14:273–80. doi: 10.1016/S0268-005X(99)00064-8. [DOI] [Google Scholar]
- 72.Snoeren THM. Kappa-Carrageenan: a study on its physico-chemical properties, sol-gel transition and interaction with milk proteins. Wageningen: Veenman, 1976. [Google Scholar]
- 73.Vega C, Dalgleish DG, Goff HD. Effect of κ-carrageenan addition to dairy emulsions containing sodium caseinate and locust bean gum. Food Hydrocoll. 2005;19:187–95. doi: 10.1016/j.foodhyd.2004.05.003. [DOI] [Google Scholar]
- 74.Tye RJ. Industrial and non-food uses for carrageenan. Carbohydr Polym. 1989;10:259–80. doi: 10.1016/0144-8617(89)90066-0. [DOI] [Google Scholar]
- 75.Gu YS, Decker EA, McClements DJ. Influence of pH and carrageenan type on properties of β-lactoglobulin stabilized oil-in-water emulsions. Food Hydrocoll. 2005;19:83–91. doi: 10.1016/j.foodhyd.2004.04.016. [DOI] [Google Scholar]
- 76.Grenha A, Gomes ME, Rodrigues M, Santo VE, Mano JF, Neves NM, et al. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J Biomed Mater Res A. 2010;92:1265–72. doi: 10.1002/jbm.a.32466. [DOI] [PubMed] [Google Scholar]
- 77.Ahmad Bani-Jaber LA-A, Alkhatib H, Al-Khalidi B. Prolonged Intragastric Drug Delivery Mediated by Eudragit E-Carrageenan Polyelectrolyte Matrix Tablets. AAPS PharmSciTech. 2011;12:354–61. doi: 10.1208/s12249-011-9595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pittman KA, Goldberg L, Coulston F. Carrageenan: the effect of molecular weight and polymer type on its uptake, excretion and degradation in animals. Food Cosmet Toxicol. 1976;14:85–93. doi: 10.1016/S0015-6264(76)80249-0. [DOI] [PubMed] [Google Scholar]
- 79.Opoku G, Qiu X, Doctor V. Effect of oversulfation on the chemical and biological properties of kappa carrageenan. Carbohydr Polym. 2006;65:134–8. doi: 10.1016/j.carbpol.2005.12.033. [DOI] [Google Scholar]
- 80.Yuan H, Song J, Zhang W, Li X, Li N, Gao X. Antioxidant activity and cytoprotective effect of κ-carrageenan oligosaccharides and their different derivatives. Bioorg Med Chem Lett. 2006;16:1329–34. doi: 10.1016/j.bmcl.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 81.Vera J, Castro J, Gonzalez A, Moenne A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar Drugs. 2011;9:2514–25. doi: 10.3390/md9122514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leibbrandt A, Meier C, König-Schuster M, Weinmüllner R, Kalthoff D, Pflugfelder B, et al. Iota-carrageenan is a potent inhibitor of influenza A virus infection. PLoS One. 2010;5:e14320. doi: 10.1371/journal.pone.0014320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de S.F-Tischer PC Talarico LB, Noseda MD, Pita B. Guimarães SM, Damonte EB, Duarte MER. Chemical structure and antiviral activity of carrageenans from Meristiella gelidium against herpes simplex and dengue virus. Carbohydr Polym. 2006;63:459–65. doi: 10.1016/j.carbpol.2005.09.020. [DOI] [Google Scholar]
- 84.García-Villalón D, Gil-Fernández C. Antiviral activity of sulfated polysaccharides against African swine fever virus. Antiviral Res. 1991;15:139–48. doi: 10.1016/0166-3542(91)90031-L. [DOI] [PubMed] [Google Scholar]
- 85.Buck CB, Thompson CD, Roberts JN, Müller M, Lowy DR, Schiller JT. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006;2:e69. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Turville SG, Aravantinou M, Miller T, Kenney J, Teitelbaum A, Hu L, et al. Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity. PLoS One. 2008;3:e3162. doi: 10.1371/journal.pone.0003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou G, Sun Y, Xin H, Zhang Y, Li Z, Xu Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol Res. 2004;50:47–53. doi: 10.1016/j.phrs.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 88.Panlasigui LN, Baello OQ, Dimatangal JM, Dumelod BD. Blood cholesterol and lipid-lowering effects of carrageenan on human volunteers. Asia Pac J Clin Nutr. 2003;12:209–14. [PubMed] [Google Scholar]
- 89.Farias WR, Valente AP, Pereira MS, Mourão PA. Structure and anticoagulant activity of sulfated galactans. Isolation of a unique sulfated galactan from the red algae Botryocladia occidentalis and comparison of its anticoagulant action with that of sulfated galactans from invertebrates. J Biol Chem. 2000;275:29299–307. doi: 10.1074/jbc.M002422200. [DOI] [PubMed] [Google Scholar]
- 90.Shanmugam M, Mody KH. Heparinoid-active sulphated polysaccharides from marine algae as potential blood anticoagulant agents. Curr Sci. 2000;79:1672–83. [Google Scholar]
- 91.Hoffman R. Carrageenans inhibit growth-factor binding. Biochem J. 1993;289:331–4. doi: 10.1042/bj2890331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen HM, Gao Y, Yan XJ. [Carrageenan oligosaccharides inhibit growth-factor binding and heparanase activity] Yao Xue Xue Bao. 2011;46:280–4. [PubMed] [Google Scholar]
- 93.Morris CJ. Carrageenan-induced paw edema in the rat and mouse. Methods Mol Biol. 2003;225:115–21. doi: 10.1385/1-59259-374-7:115. [DOI] [PubMed] [Google Scholar]
- 94.Borthakur A, Bhattacharyya S, Dudeja PK, Tobacman JK. Carrageenan induces interleukin-8 production through distinct Bcl10 pathway in normal human colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G829–38. doi: 10.1152/ajpgi.00380.2006. [DOI] [PubMed] [Google Scholar]
- 95.Cicala C, Morello S, Alfieri A, Vellecco V, Marzocco S, Autore G. Haemostatic imbalance following carrageenan-induced rat paw oedema. Eur J Pharmacol. 2007;577:156–61. doi: 10.1016/j.ejphar.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 96.Sakaguchi Y, Shirahase H, Kunishiro K, Ichikawa A, Kanda M, Uehara Y. Effect of combination of nitric oxide synthase and cyclooxygenase inhibitors on carrageenan-induced pleurisy in rats. Life Sci. 2006;79:442–7. doi: 10.1016/j.lfs.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 97.Weiner ML. Toxicological properties of carrageenan. Agents Actions. 1991;32:46–51. doi: 10.1007/BF01983307. [DOI] [PubMed] [Google Scholar]
- 98.Williams PA, Phillips GO. Education NEWIoH. Gums and Stabilisers for the Food Industry 13. Cambridge: RSC, 2006. [Google Scholar]
- 99.Santo VE, Frias AM, Carida M, Cancedda R, Gomes ME, Mano JF, et al. Carrageenan-based hydrogels for the controlled delivery of PDGF-BB in bone tissue engineering applications. Biomacromolecules. 2009;10:1392–401. doi: 10.1021/bm8014973. [DOI] [PubMed] [Google Scholar]
- 100.Rocha PM, Santo VE, Gomes ME, Reis RL, Mano JF. Encapsulation of adipose-derived stem cells and transforming growth factor-β1 in carrageenan-based hydrogels for cartilage tissue engineering. J Bioact Compat Polym. 2011;26:493–507. doi: 10.1177/0883911511420700. [DOI] [Google Scholar]
- 101.Desai PD, Dave AM, Devi S. Entrapment of lipase into K-carrageenan beads and its use in hydrolysis of olive oil in biphasic system. J Mol Catal, B Enzym. 2004;31:143–50. doi: 10.1016/j.molcatb.2004.08.004. [DOI] [Google Scholar]
- 102.Popa EG, Gomes ME, Reis RL. In vitro and in vivo biocompatibility evaluation of k-carrageenan hydrogels aimed at applications in regenerative medicine. Histology and Histopathology Cellular and Molecular Biology. 2011;26(supplement 1):62. [Google Scholar]
- 103.Popa E, Reis R, Gomes M. Chondrogenic phenotype of different cells encapsulated in κ-carrageenan hydrogels for cartilage regeneration strategies. Biotechnol Appl Biochem. 2012;59:132–41. doi: 10.1002/bab.1007. [DOI] [PubMed] [Google Scholar]
- 104.Percival E. The polysaccharides of green, red and brown seaweeds: their basic structure, biosynthesis and function. Br Phycol J. 1979;14:103–17. doi: 10.1080/00071617900650121. [DOI] [Google Scholar]
- 105.Pomin VH, Mourão PAS. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology. 2008;18:1016–27. doi: 10.1093/glycob/cwn085. [DOI] [PubMed] [Google Scholar]
- 106.Stengel DB, Connan S, Popper ZA. Algal chemodiversity and bioactivity: sources of natural variability and implications for commercial application. Biotechnol Adv. 2011;29:483–501. doi: 10.1016/j.biotechadv.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 107.Ciancia M, Quintana I, Vizcargüénaga MI, Kasulin L, de Dios A, Estevez JM, et al. Polysaccharides from the green seaweeds Codium fragile and C. vermilara with controversial effects on hemostasis. Int J Biol Macromol. 2007;41:641–9. doi: 10.1016/j.ijbiomac.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 108.Love J, Percival E. Polysaccharides of Green Seaweed Codium Fragile. 2. Water-Soluble Sulphated Polysaccharides. J Chem Soc. 1964:3338–45. doi: 10.1039/jr9640003338. [DOI] [Google Scholar]
- 109.Bilan MI, Vinogradova EV, Shashkov AS, Usov AI. Structure of a highly pyruvylated galactan sulfate from the Pacific green alga Codium yezoense (Bryopsidales, Chlorophyta) Carbohydr Res. 2007;342:586–96. doi: 10.1016/j.carres.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 110.Lee J-B, Koizumi S, Hayashi K, Hayashi T. Structure of rhamnan sulfate from the green alga Monostroma nitidum and its anti-herpetic effect. Carbohydr Polym. 2010;81:572–7. doi: 10.1016/j.carbpol.2010.03.014. [DOI] [Google Scholar]
- 111.Ohta Y, Lee J-B, Hayashi K, Hayashi T. Isolation of sulfated galactan from Codium fragile and its antiviral effect. Biol Pharm Bull. 2009;32:892–8. doi: 10.1248/bpb.32.892. [DOI] [PubMed] [Google Scholar]
- 112.Fernández PV, Estevez JM, Cerezo AS, Ciancia M. Sulfated β-d-mannan from green seaweed Codium vermilara. Carbohydr Polym. 2012;87:916–9. doi: 10.1016/j.carbpol.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 113.Siddhanta AK, Shanmugam M, Mody KH, Goswami AM, Ramavat BK. Sulphated polysaccharides of Codium dwarkense Boergs. from the west coast of India: chemical composition and blood anticoagulant activity. Int J Biol Macromol. 1999;26:151–4. doi: 10.1016/S0141-8130(99)00079-3. [DOI] [PubMed] [Google Scholar]
- 114.Harada N, Maeda M. Chemical structure of antithrombin-active Rhamnan sulfate from Monostrom nitidum. Biosci Biotechnol Biochem. 1998;62:1647–52. doi: 10.1271/bbb.62.1647. [DOI] [PubMed] [Google Scholar]
- 115.Li H, Mao W, Zhang X, Qi X, Chen Y, Chen Y, et al. Structural characterization of an anticoagulant-active sulfated polysaccharide isolated from green alga Monostroma latissimum. Carbohydr Polym. 2011;85:394–400. doi: 10.1016/j.carbpol.2011.02.042. [DOI] [Google Scholar]
- 116.Mao W, Li H, Li Y, Zhang H, Qi X, Sun H, et al. Chemical characteristic and anticoagulant activity of the sulfated polysaccharide isolated from Monostroma latissimum (Chlorophyta) Int J Biol Macromol. 2009;44:70–4. doi: 10.1016/j.ijbiomac.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 117.Zhang HJ, Mao WJ, Fang F, Li HY, Sun HH, Chen Y, et al. Chemical characteristics and anticoagulant activities of a sulfated polysaccharide and its fragments from Monostroma latissimum. Carbohydr Polym. 2008;71:428–34. doi: 10.1016/j.carbpol.2007.06.012. [DOI] [Google Scholar]
- 118.Lahaye M, Jegou D. Chemical and physical-chemical characteristics of dietary fibres from Ulva lactuca (L.) Thuret and Enteromorpha compressa (L.) Grev. J Appl Phycol. 1993;V5:195–200. doi: 10.1007/BF00004017. [DOI] [Google Scholar]
- 119.Lahaye M, Jegou D, Buleon A. Chemical characteristics of insoluble glucans from the cell wall of the marine green alga Ulva lactuca (L.) Thuret. Carbohydr Res. 1994;262:115–25. doi: 10.1016/0008-6215(94)84008-3. [DOI] [Google Scholar]
- 120.Robic A, Bertrand D, Sassi JF, Lerat Y, Lahaye M. Determination of the chemical composition of ulvan, a cell wall polysaccharide from Ulva spp. (Ulvales, Chlorophyta) by FT-IR and chemometrics. J Appl Phycol. 2009;21:451–6. doi: 10.1007/s10811-008-9390-9. [DOI] [Google Scholar]
- 121.Robic A, Rondeau-Mouro C, Sassi JF, Lerat Y, Lahaye M. Structure and interactions of ulvan in the cell wall of the marine green algae Ulva rotundata (Ulvales, Chlorophyceae) Carbohydr Polym. 2009;77:206–16. doi: 10.1016/j.carbpol.2008.12.023. [DOI] [Google Scholar]
- 122.Ray B. Polysaccharides from Enteromorpha compressa: Isolation, purification and structural features. Carbohydr Polym. 2006;66:408–16. doi: 10.1016/j.carbpol.2006.03.027. [DOI] [Google Scholar]
- 123.Ray B, Lahaye M. Cell-wall polysaccharides from the marine green alga Ulva “rigida” (Ulvales, Chlorophyta). Chemical structure of ulvan. Carbohydr Res. 1995;274:313–8. doi: 10.1016/0008-6215(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 124.Ray B, Lahaye M. Cell-wall polysaccharides from the marine green alga Ulva rigida (Ulvales, Chlorophyta). Extraction and chemical composition. Carbohydr Res. 1995;274:251–61. doi: 10.1016/0008-6215(95)00138-J. [DOI] [PubMed] [Google Scholar]
- 125.Chattopadhyay K, Mandal P, Lerouge P, Driouich A, Ghosal P, Ray B. Sulphated polysaccharides from Indian samples of Enteromorpha compressa (Ulvales, Chlorophyta): Isolation and structural features. Food Chem. 2007;104:928–35. doi: 10.1016/j.foodchem.2006.12.048. [DOI] [Google Scholar]
- 126.Lewis LA, McCourt RM. Green algae and the origin of land plants. Am J Bot. 2004;91:1535–56. doi: 10.3732/ajb.91.10.1535. [DOI] [PubMed] [Google Scholar]
- 127.Domozych DS, Stewart KD, Mattox KR. The comparative aspects of cell wall chemistry in the green algae (Chlorophyta) J Mol Evol. 1980;15:1–12. doi: 10.1007/BF01732578. [DOI] [PubMed] [Google Scholar]
- 128.Popper ZA, Tuohy MG. Beyond the green: understanding the evolutionary puzzle of plant and algal cell walls. Plant Physiol. 2010;153:373–83. doi: 10.1104/pp.110.158055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Costa LS, fidelis GP, Cordeiro SL, Oliveira RM, Sabry DA, Câmara RBG, et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed Pharmacother. 2010;64:21–8. doi: 10.1016/j.biopha.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 130.Devaki T, Sathivel A, BalajiRaghavendran HR. Stabilization of mitochondrial and microsomal function by polysaccharide of Ulva lactuca on D-Galactosamine induced hepatitis in rats. Chem Biol Interact. 2009;177:83–8. doi: 10.1016/j.cbi.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 131.El-Baky HHA, Baz FKE, Baroty GSE. Potential Biological Properties of Sulphated Polysaccharides Extracted from the Macroalgae Ulva lactuca L. Academic Journal of Cancer Research. 2009;2:1–11. [Google Scholar]
- 132.Kuda T, Ikemori T. Minerals, polysaccharides and antioxidant properties of aqueous solutions obtained from macroalgal beach-casts in the Noto Peninsula, Ishikawa, Japan. Food Chem. 2009;112:575–81. doi: 10.1016/j.foodchem.2008.06.008. [DOI] [Google Scholar]
- 133.Qi H, Liu X, Ma J, Zhang Q, Li Z. In vitro antioxidant activity of acetylated derivatives of polysaccharide extracted from Ulva pertusa (Cholorophta) Journal of Medicinal Plants Research. 2010;4:2445–51. [Google Scholar]
- 134.Qi H, Zhang Q, Zhao T, Chen R, Zhang H, Niu X, et al. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int J Biol Macromol. 2005;37:195–9. doi: 10.1016/j.ijbiomac.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 135.Qi H, Zhao T, Zhang Q, Li Z, Zhao Z, Xing R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta) J Appl Phycol. 2005;17:527–34. doi: 10.1007/s10811-005-9003-9. [DOI] [Google Scholar]
- 136.Jiao L, Jiang P, Zhang L, Wu M. Antitumor and immunomodulating activity of polysaccharides from Enteromorpha intestinalis. Biotechnology and Bioprocess Engineering. 2010;15:421–8. doi: 10.1007/s12257-008-0269-z. [DOI] [Google Scholar]
- 137.Castro R, Piazzon MC, Zarra I, Leiro J, Noya M, Lamas J. Stimulation of turbot phagocytes by Ulva rigida C. Agardh polysaccharides. Aquaculture. 2006;254:9–20. doi: 10.1016/j.aquaculture.2005.10.012. [DOI] [Google Scholar]
- 138.Castro R, Zarra I, Lamas J. Water-soluble seaweed extracts modulate the respiratory burst activity of turbot phagocytes. Aquaculture. 2004;229:67–78. doi: 10.1016/S0044-8486(03)00401-0. [DOI] [Google Scholar]
- 139.Kim J-K, Cho ML, Karnjanapratum S, Shin I-S, You SG. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int J Biol Macromol. 2011;49:1051–8. doi: 10.1016/j.ijbiomac.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 140.Lee J-B, Ohta Y, Hayashi K, Hayashi T. Immunostimulating effects of a sulfated galactan from Codium fragile. Carbohydr Res. 2010;345:1452–4. doi: 10.1016/j.carres.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 141.Leiro JM, Castro R, Arranz JA, Lamas J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int Immunopharmacol. 2007;7:879–88. doi: 10.1016/j.intimp.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 142.Bocanegra A, Bastida S, Benedí J, Ródenas S, Sánchez-Muniz FJ. Characteristics and nutritional and cardiovascular-health properties of seaweeds. J Med Food. 2009;12:236–58. doi: 10.1089/jmf.2008.0151. [DOI] [PubMed] [Google Scholar]
- 143.Pengzhan Y, Ning L, Xiguang L, Gefei Z, Quanbin Z, Pengcheng L. Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta) Pharmacol Res. 2003;48:543–9. doi: 10.1016/S1043-6618(03)00215-9. [DOI] [PubMed] [Google Scholar]
- 144.Pengzhan Y, Quanbin Z, Ning L, Zuhong X, Yanmei W, Zhi'en L. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J Appl Phycol. 2003;15:21–7. doi: 10.1023/A:1022997622334. [DOI] [Google Scholar]
- 145.Sathivel A, Raghavendran HRB, Srinivasan P, Devaki T. Anti-peroxidative and anti-hyperlipidemic nature of Ulva lactuca crude polysaccharide on D-galactosamine induced hepatitis in rats. Food Chem Toxicol. 2008;46:3262–7. doi: 10.1016/j.fct.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 146.Cassolato JEF, Noseda MD, Pujol CA, Pellizzari FM, Damonte EB, Duarte MER. Chemical structure and antiviral activity of the sulfated heterorhamnan isolated from the green seaweed Gayralia oxysperma. Carbohydr Res. 2008;343:3085–95. doi: 10.1016/j.carres.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 147.Pierre G, Sopena V, Juin C, Mastouri A, Graber M, Maugard T. Antibacterial activity of a sulfated galactan extracted from the marine alga Chaetomorpha aerea against Staphylococcus aureus. Biotechnology and Bioprocess Engineering. 2011;16:937–45. doi: 10.1007/s12257-011-0224-2. [DOI] [Google Scholar]
- 148.Kaeffer B, Bénard C, Lahaye M, Blottière HM, Cherbut C. Biological properties of ulvan, a new source of green seaweed sulfated polysaccharides, on cultured normal and cancerous colonic epithelial cells. Planta Med. 1999;65:527–31. doi: 10.1055/s-1999-14009. [DOI] [PubMed] [Google Scholar]
- 149.Yang L, Zhang L-M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr Polym. 2009;76:349–61. doi: 10.1016/j.carbpol.2008.12.015. [DOI] [Google Scholar]
- 150.Mao W-J, Fang F, Li H-Y, Qi X-H, Sun H-H, Chen Y, et al. Heparinoid-active two sulfated polysaccharides isolated from marine green algae Monostroma nitidum. Carbohydr Polym. 2008;74:834–9. doi: 10.1016/j.carbpol.2008.04.041. [DOI] [Google Scholar]
- 151.Shanmugam M, Ramavat BK, Mody KH, Oza RM, Tewari A. Distribution of heparinoid-active sulphated polysaccharides in some Indian marine green algae. Indian J Mar Sci. 2001;30:222–7. [Google Scholar]
- 152.Hayakawa Y, Hayashi T, Lee J-B, Srisomporn P, Maeda M, Ozawa T, et al. Inhibition of thrombin by sulfated polysaccharides isolated from green algae. Biochim Biophys Acta. 2000;1543:86–94. doi: 10.1016/S0167-4838(00)00193-X. [DOI] [PubMed] [Google Scholar]
- 153.Toskas G, Hund R-D, Laourine E, Cherif C, Smyrniotopoulos V, Roussis V. Nanofibers based on polysaccharides from the green seaweed Ulva Rigida. Carbohydr Polym. 2011;84:1093–102. doi: 10.1016/j.carbpol.2010.12.075. [DOI] [Google Scholar]
- 154.Alves A, Pinho ED, Neves NM, Sousa RA, Reis RL. Processing ulvan into 2D structures: cross-linked ulvan membranes as new biomaterials for drug delivery applications. Int J Pharm. 2012;426:76–81. doi: 10.1016/j.ijpharm.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 155.Alves A, Duarte ARC, Mano JF, Sousa RA, Reis RL. PDLLA enriched with ulvan particles as a novel 3D porous scaffold targeted for bone engineering. J Supercrit Fluids. 2012;65:32–8. doi: 10.1016/j.supflu.2012.02.023. [DOI] [Google Scholar]
- 156.Morelli A, Chiellini F. Ulvan as a New Type of Biomaterial from Renewable Resources: Functionalization and Hydrogel Preparation. Macromol Chem Phys. 2010;211:821–32. doi: 10.1002/macp.200900562. [DOI] [Google Scholar]
- 157.Alves A, Sousa RA, Reis RL. Processing of degradable ulvan 3D porous structures for biomedical applications. J Biomed Mater Res A. 2012 doi: 10.1002/jbm.a.34403. In press. [DOI] [PubMed] [Google Scholar]
- 158.Hennequart F, O'Connell E, Spence J, Tuohy GM. Brown Macro-alage. Aqua Feeds:Formulation & Beyond. 2004;1:14–8. [Google Scholar]
- 159.Vera J, Castro J, Gonzalez A, Moenne A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar Drugs. 2011;9:2514–25. doi: 10.3390/md9122514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kylin H. The biochemistry of seaweed. Hoppe Seylers Z Physiol Chem. 1913;83:171–97. doi: 10.1515/bchm2.1913.83.3.171. [DOI] [Google Scholar]
- 161.McNeely WH. Fucoidan. In: Whistler RL, BeMiller JM, eds. Industrial Gums. New York: Academic Press, 1959:117-21. [Google Scholar]
- 162.Berteau O, Mulloy B. Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology. 2003;13:29R–40R. doi: 10.1093/glycob/cwg058. [DOI] [PubMed] [Google Scholar]
- 163.Foley SA, Mulloy B, Tuohy MG. An unfractionated fucoidan from Ascophyllum nodosum: extraction, characterization, and apoptotic effects in vitro. J Nat Prod. 2011;74:1851–61. doi: 10.1021/np200124m. [DOI] [PubMed] [Google Scholar]
- 164.Holtkamp A. Isolation, Characterisation, Modification and Application of Fucoidan from Fucus vesiculosus. Braunschweig: Technischen Universit[UNKNOWN ENTITY &adie;]t Carolo Wilhelmina, 2009. [Google Scholar]
- 165.Li B, Lu F, Wei X, Zhao R. Fucoidan: structure and bioactivity. Molecules. 2008;13:1671–95. doi: 10.3390/molecules13081671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.fitton JH, Irhimeh M, Falk N. Macroalgal Fucoidan Extracts: A New Opportunity for Marine Cosmetics. Cosmetics & Toiletries. 2007;125:55–64. [Google Scholar]
- 167.Jiao G, Yu G, Zhang J, Ewart HS. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs. 2011;9:196–223. doi: 10.3390/md9020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rioux LE, Turgeon SL, Beaulieu M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr Polym. 2007;69:530–7. doi: 10.1016/j.carbpol.2007.01.009. [DOI] [Google Scholar]
- 169.Tako M. Studies on the gelation mechanism of polysaccharides, and development and application of fucoidan from commercially cultured Cladosiphon okamuranu. J Appl Glycosci. 2009;56:17–27. doi: 10.5458/jag.56.17. [DOI] [Google Scholar]
- 170.Rioux LE, Turgeon SL, Beaulieu M. Rheological characterisation of polysaccharides extracted from brown seaweeds. J Sci Food Agric. 2007;87:1630–8. doi: 10.1002/jsfa.2829. [DOI] [Google Scholar]
- 171.Siddhanta AK, Murthy ASK. Bioactive polysaccharides from marine brown algae (Phaeophyceae) J Indian Chem Soc. 2001;78:431–7. [Google Scholar]
- 172.Dobashi K, Nishino T, Fujihara M, Nagumo T. Isolation and preliminary characterization of fucose-containing sulfated polysaccharides with blood-anticoagulant activity from the brown seaweed Hizikia fusiforme. Carbohydr Res. 1989;194:315–20. doi: 10.1016/0008-6215(89)85032-3. [DOI] [PubMed] [Google Scholar]
- 173.Farias WR, Valente AP, Pereira MS, Mourão PA. Structure and anticoagulant activity of sulfated galactans. Isolation of a unique sulfated galactan from the red algae Botryocladia occidentalis and comparison of its anticoagulant action with that of sulfated galactans from invertebrates. J Biol Chem. 2000;275:29299–307. doi: 10.1074/jbc.M002422200. [DOI] [PubMed] [Google Scholar]
- 174.Grauffel V, Kloareg B, Mabeau S, Durand P, Jozefonvicz J. New natural polysaccharides with potent antithrombic activity: fucans from brown algae. Biomaterials. 1989;10:363–8. doi: 10.1016/0142-9612(89)90127-0. [DOI] [PubMed] [Google Scholar]
- 175.Silva TM, Alves LG, de Queiroz KC, Santos MG, Marques CT, Chavante SF, et al. Partial characterization and anticoagulant activity of a heterofucan from the brown seaweed Padina gymnospora. Braz J Med Biol Res. 2005;38:523–33. doi: 10.1590/S0100-879X2005000400005. [DOI] [PubMed] [Google Scholar]
- 176.Baba M, Snoeck R, Pauwels R, de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1988;32:1742–5. doi: 10.1128/AAC.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lapshina L, Reunov A, Nagorskaya V, Zvyagintseva T, Shevchenko N. Inhibitory effect of fucoidan from brown alga Fucus evanescens on the spread of infection induced by tobacco mosaic virus in tobacco leaves of two cultivars. Russ J Plant Physiol. 2006;53:246–51. doi: 10.1134/S1021443706020154. [DOI] [Google Scholar]
- 178.Lee J-B, Hayashi K, Maeda M, Hayashi T. Antiherpetic activities of sulfated polysaccharides from green algae. Planta Med. 2004;70:813–7. doi: 10.1055/s-2004-827228. [DOI] [PubMed] [Google Scholar]
- 179.Witvrouw M, De Clercq E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen Pharmacol. 1997;29:497–511. doi: 10.1016/S0306-3623(96)00563-0. [DOI] [PubMed] [Google Scholar]
- 180.Wijesinghe WAJP, Jeon Y-J. Biological activities and potential industrial applications of fucose rich sulfated, polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr Polym. 2012;88:13–20. doi: 10.1016/j.carbpol.2011.12.029. [DOI] [Google Scholar]
- 181.Nagai TY. Preparation and functional properties of beverages made from sea algae. Food Chem. 2003;81:327–32. doi: 10.1016/S0308-8146(02)00426-0. [DOI] [Google Scholar]
- 182.López-López I, Bastida S, Ruiz-Capillas C, Bravo L, Larrea MT, Sánchez-Muniz F, et al. Composition and antioxidant capacity of low-salt meat emulsion model systems containing edible seaweeds. Meat Sci. 2009;83:492–8. doi: 10.1016/j.meatsci.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 183.Kim S-K, Ravichandran YD, Khan SB, Kim YT. Prospective of the Cosmeceuticals Derived from Marine Organisms. Biotechnology and Bioprocess Engineering. 2008;13:511–23. doi: 10.1007/s12257-008-0113-5. [DOI] [Google Scholar]
- 184.Sezer AD, Akbuğa J. Fucosphere--new microsphere carriers for peptide and protein delivery: preparation and in vitro characterization. J Microencapsul. 2006;23:513–22. doi: 10.1080/02652040600687563. [DOI] [PubMed] [Google Scholar]
- 185.Lee EJ, Khan SA, Lim K-H. Chitosan-nanoparticle preparation by polyelectrolyte complexation. World Journal of Engineering. 2009;6(Supplement):541. [Google Scholar]
- 186.Nakamura S, Nambu M, Ishizuka T, Hattori H, Kanatani Y, Takase B, et al. Effect of controlled release of fibroblast growth factor-2 from chitosan/fucoidan micro complex-hydrogel on in vitro and in vivo vascularization. J Biomed Mater Res A. 2008;85:619–27. doi: 10.1002/jbm.a.31563. [DOI] [PubMed] [Google Scholar]
- 187.Sezer AD, Hatipoglu F, Cevher E, Oğurtan Z, Baş AL, Akbuğa J. Chitosan film Containing Fucoidan as a Wound Dressing for Dermal Burn Healing: Preparation and In Vitro/In Vivo Evaluation. AAPS PharmSciTech. 2007;8:E94–101. doi: 10.1208/pt0802039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Murakami K, Aoki H, Nakamura S, Nakamura S-i, Takikawa M, Hanzawa M, et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials. 2010;31:83–90. doi: 10.1016/j.biomaterials.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 189.Jin G, Kim GH. Rapid-prototyped PCL/fucoidan composite scaffolds for bone tissue regeneration: design, fabrication, and physical/biological properties. J Mater Chem. 2011;21:17710–8. doi: 10.1039/c1jm12915e. [DOI] [Google Scholar]
- 190.Lee JS, Jin GH, Yeo MG, Jang CH, Lee H, Kim GH. Fabrication of electrospun biocomposites comprising polycaprolactone/fucoidan for tissue regeneration. Carbohydr Polym. 2012;90:181–8. doi: 10.1016/j.carbpol.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 191.Sezer AD, Cevher E, Hatipoğlu F, Oğurtan Z, Baş AL, Akbuğa J. Preparation of fucoidan-chitosan hydrogel and its application as burn healing accelerator on rabbits. Biol Pharm Bull. 2008;31:2326–33. doi: 10.1248/bpb.31.2326. [DOI] [PubMed] [Google Scholar]
- 192.Nahmias Y, Yarmush M. Tissue engineering application in general surgery. In: Meyer U, Meyer T, Handschel J, Wiesmann HP, eds. Fundamentals of Tissue Engineering and Regenerative Medicine. Berlin: Springer, 2009:855-68. [Google Scholar]
- 193.Jaklenec A, Stamp A, Deweerd E, Sherwin A, Langer R. Progress in the tissue engineering and stem cell industry “are we there yet?”. Tissue Eng Part B Rev. 2012;18:155–66. doi: 10.1089/ten.teb.2011.0553. [DOI] [PubMed] [Google Scholar]
- 194.Kara S, Tamerler C, Bermek H, Pekcan Ö. Cation effects on sol-gel and gel-sol phase transitions of kappa-carrageenan-water system. Int J Biol Macromol. 2003;31:177–85. doi: 10.1016/S0141-8130(02)00080-6. [DOI] [PubMed] [Google Scholar]
- 195.Mangione MR, Giacomazza D, Bulone D, Martorana V, San Biagio PL. Thermoreversible gelation of kappa-carrageenan: relation between conformational transition and aggregation. Biophys Chem. 2003;104:95–105. doi: 10.1016/S0301-4622(02)00341-1. [DOI] [PubMed] [Google Scholar]
- 196.Prajapati S, Patel L, Patel A. Carrageenan: A Naturally Occurring Routinely Used Excipient. Pharmaceutical Reviews. 2007;5 [Google Scholar]
- 197.Rowe RC, Sheskey PJ, Weller PJ. Handbook of pharmaceutical excipients. London: Pharmaceutical Press, 2003. [Google Scholar]
- 198.Ciancia M, Alberghina J, Arata PX, Benavides H, Leliaert F, Verbruggen H, et al. Characterization of cell wall polysaccharides of the coencocytic green seaweed Bryopsis plumosa (Bryopsidaceae, Chlorophyta) from the argentine coast. J Phycol. 2012;48:326–35. doi: 10.1111/j.1529-8817.2012.01131.x. [DOI] [PubMed] [Google Scholar]


