Abstract
One of the backbones in nanomedicine is to deliver drugs specifically to unhealthy cells. Drug nanocarriers can cross physiological barriers and access different tissues, which after proper surface biofunctionalization can enhance cell specificity for cancer therapy. Recent developments have highlighted the potential of mesoporous silica (PSiO2) and silicon (PSi) nanoparticles for targeted drug delivery. In this review, we outline and discuss the most recent advances on the applications and developments of cancer therapies by means of PSiO2 and PSi nanomaterials. Bio-engineering and fine tuning of anti-cancer drug vehicles, high flexibility and potential for sophisticated release mechanisms make these nanostructures promising candidates for “smart” cancer therapies. As a result of their physicochemical properties they can be controllably loaded with large amounts of drugs and coupled to homing molecules to facilitate active targeting. The main emphasis of this review will be on the in vitro and in vivo studies.
Keywords: mesoporous silica, mesoporous silicon, nanoparticles, cell targeting, functionalization, cancer therapy, in vivo, in vitro, drug delivery
Introduction
Cancer is a very complex disease and is the leading cause of death in economically developed countries and the second leading cause of death in developing countries.1 According to the World Health Organization, cancer accounted for 7.6 million deaths (around 13% of all deaths) in 2008 (www.who.int/mediacentre/factsheets/fs297/en) and is estimated to have caused almost 2 million deaths in the US and Europe in 2011,1 making cancer one of the leading causes of death worldwide. Cancer deaths in the European Union countries are estimated to be near 1.3 million in 2012,2 and deaths from cancer worldwide are projected to continue rising, with an estimated 13.1 million deaths in 2030 (http://globocan.iarc.fr).
Cancer is known to be developed via a multistep carcinogenesis process entailing numerous cellular physiological systems, such as cell signaling and apoptosis.3 Cancer has a physiological barrier like vascular endothelial pores, heterogeneous blood supply, heterogeneous architecture, etc. For a treatment to be successful, it is very important to get over these barriers. As far as cancer therapeutics is concerned, the most common cancer treatments are restricted to chemotherapy, radiation and surgery, which are severely fraught with challenges concerned with deleterious side effects of anticancer agents caused by their non-specific tissue distribution, inefficient drug concentrations reaching the tumor site, intolerable cytotoxicity, limited ability to monitor therapeutic responses and development of multiple drug resistance (MDR) acquired upon repeated chemotherapeutic cycles.4-6 Rapid elimination by the immune system, enzymatic degradation and poor targeting efficiency are some of the main obstacles to be overcome before nanomedicines are fully used clinically. In order to be effective in cancer treatment, anticancer drugs should first (after administration) be able to reach the desired tumor tissues through the penetration of barriers in the body with minimal loss of volume or activity in the blood circulation, and then, after reaching the tumor tissue, drugs should have the ability to selectively kill tumor cells without affecting healthy cells.2,7
Targeted cancer therapy is designed to disrupt the function of specific molecules needed for carcinogenesis and tumor growth, and thus, either killing or preventing the growth of cancer cells.8,9 Targeted cancer therapy may be more effective and less harmful to healthy cells than conventional chemotherapy. For example, cellular targeting of antibodies or specific ligands is based on the capability of the targeting agents to selectively bind to the cell surface to trigger receptor-mediated endocytosis.3,5 Thus, the drug delivery system along with the therapeutic agent would be delivered to the interior of a given cell type. This is also especially relevant as most of the commonly used anticancer drugs have serious side-effects due to unspecific action on healthy cells.
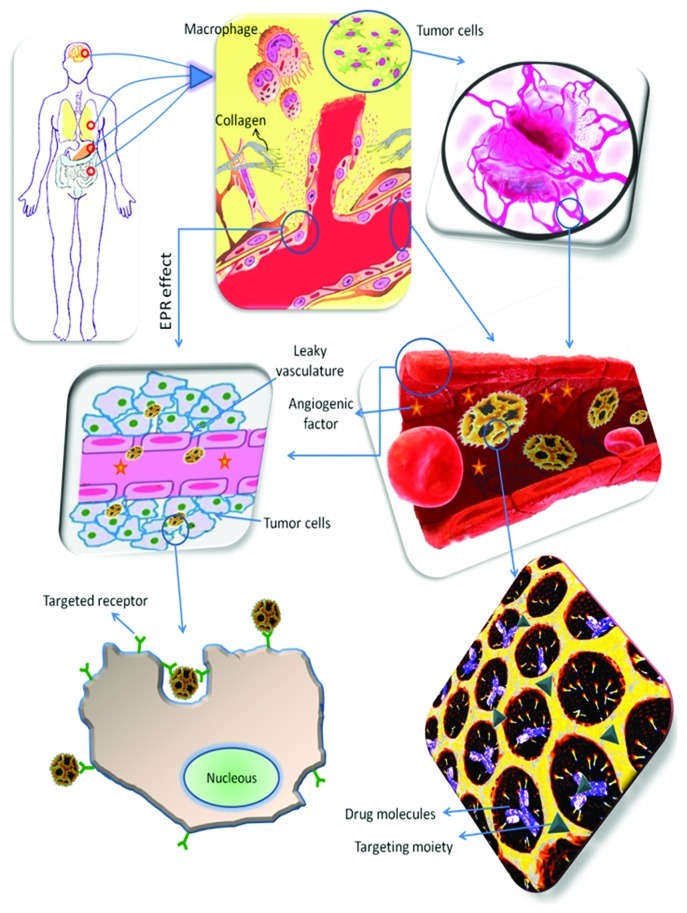
The key parameters for successful treatment using nanodelivery systems are essential selectivity, biological activity, efficiency of uptake and drug concentration.6 In principle, nanoparticulate delivery systems can be used to target anticancer drugs to tumor tissues by either passive or active targeting (Fig. 1). Passive targeting refers to the accumulation of a drug or drug carrier system at a desired site owing to physicochemical or pharmacological factors due to the inherent size of the nanoparticles, the enhanced permeability and retention (EPR) effect and the tumor microenvironment, enhancing drug bioavailability and efficacy due to the functional differences between normal and tumor cells. On the other hand, active targeting involves the attachment of a moiety, such as a monoclonal antibody or a ligand, to deliver a drug to pathological sites or to cross biological barriers based on molecular recognition processes. The cell-surface antigen or receptor should be homogeneously and exclusively expressed on tumor cells, and should not be shed into the blood circulation.5 Targeted nano cancer therapies are used to block the growth and spread of cancer by, for example, interfering directly with specific molecules involved in tumor growth and progression or indirectly, by stimulating the immune system to recognize and destroy cancer cells, either by using small-molecule drugs or monoclonal antibodies.7,10-13
Figure 1. Schematic overview of the events taking place in cancer tissues and some of the strategies for providing a rational nanoparticulate drug delivery system with both passive and active targeting. Nanoparticulate delivery systems can be used to target and accumulate anticancer drugs to tumor tissues (e.g., brain, lung, stomach and intestine) by passive targeting; making use of the leaky vasculature and the EPR effect to reach the cancer cells. Alternatively, nanoparticulate delivery systems can also be used to target and accumulate anticancer drugs to tumor tissues by active targeting; making use of the targeting moieties attached to the surface of the nanoparticulate systems to deliver drugs to pathological sites or to cross biological barriers based on molecular recognition processes to target the receptors of the cancer cells.
Over the past few years, a variety of functional nanostructures, such as mesoporous materials (e.g., silica- and silicon-based) come to the fore to circumvent the problems associated with the currently practiced therapeutic modalities for cancer-specific targeting, imaging and therapy. These materials have attracted great attention in the scientific community due to their unique physicochemical properties and potential biomedical applications.14-19 The complexity of health diseases has triggered the close collaboration of different research areas, such as engineering, nano(bio)technology and nanomedicine. Mesoporous materials have also been proposed as drug delivery carriers of a wide variety of therapeutic agents and lately with particular emphasis in the nanomedicine field.15,20 These mesoporous systems are designed to carry and release their payloads to a specific location in the body and at a controllable release rate, without compromising the patient’s health. This is only feasible if the mesoporous material itself is biocompatible and biodegradable.21-23 The pore diameters of these mesoporous materials can be tuned to 2−50 nm allowing high payloads of therapeutic molecules and protecting them from premature release and degradation before reaching a specific site where the payload is then controlled release with an effective concentration of pharmacological relevance.15,16,18,24
The most remarkable properties of the mesoporous silicon (PSi) and silica (PSiO2) materials as nanodelivery systems are their high surface-to-volume ratio, large surface area (up to 700-1,000 m2/g), large pore volume (> 0.9 cm3/g),14,18 possessing a stable and rigid framework with excellent chemical, thermal and mechanical stability. In this respect, the mesoporous materials act as reservoirs for storing the therapeutic molecules and can be easily tailored via different pore size and surface chemistries, for selective storage.15,18 In addition, both the exterior of the particle and the interior pore surfaces can also be easily functionalized with different biomolecules for targeting therapy and site-specific delivery.15,24-26 Thus, the cellular uptake can be maximized by tuning the shape, size, pore or surface functionalization of the mesoporous based materials.
Although the majority of the studies found in the literature have been focused on the structure, morphology, surface properties and size of both PSi and PSiO2 for controlled drug delivery applications and in cancer treatments, several studies have also demonstrated the biosafety and biocompatibility of these materials both in vitro and in vivo.27 In this review, we will present and discuss the most recent works on PSi and PSiO2 based nanomaterials for cancer therapy. Detailed information on the preparation and characterization of PSi17-19,28 and PSiO214-16,29 materials can be found extensively in the literature and will not be revised herein. Instead, we will focus our work on the most recent applications of PSi and PSiO2, in particular what regards to the biofunctionalization of the surface of the mesoporous nanomaterials for controlled drug delivery and targeting therapy. Several examples addressing the mesoporous materials as drug delivery vehicles, challenges in cell targeting and cancer therapy, including therapeutic applications, intracellular uptake and trafficking as well as biodistribution, degradation and clearance will be presented.
Si-Vehicles for Controlled Drug Delivery
The application of porous nanomaterials in the field of drug delivery has attracted much interest over the latest decades. Immense advances in the morphology control and surface modification of inorganic-based delivery vehicles, such as PSiO2 and PSi nanoparticles, as well as the increased knowledge regarding physiological factors affecting a favorable drug delivery system, have opened new possibilities for more efficient treatment via this burgeoning area of research.18,24,30-33
In practice, the PSiO2 and PSi materials differ in their fabrication techniques: PSiO2 materials are synthesized through a so-called “bottom-up” approach, whereas PSi materials are produced by a so-called “top-down” approach.17,18,28,34,35 The mesoporous materials have the advantage of delivering large dosages of poorly water-soluble drugs without premature release complications. This is because of their large surface area (≥ 300 m2/g) and large pore volume (> 0.9 cm3/g). PSi and PSiO2 can act as reservoirs for storing the hydrophobic drug molecules and can be easily tailored—via the size and surface chemistries of the pores—for selective storage of different molecules of interest. PSi materials are produced by a top-down approach by electrochemical anodization.17,18,28 PSi particles have irregular pore structure, but the surface of the as-anodized, hydrogen-terminated PSi is not stable, and thus, there is a need for subsequent surface treatment. The most common surface treatments of PSi are oxidation (thermally oxidized-PSi) and stabilization by thermal carbonization or hydrocarbonization which render the PSi materials hydrophilic or hydrophobic surface properties.17,34-41 PSiO2 synthesis processes utilize different template systems to direct the silica molecules into a mesoscopically ordered yet amorphous structure contain very unidirectional and uniform pore channel structures. The surface chemistries of these PSiO2 materials consist of siloxane groups (–Si–O–Si–), with the oxygen on the surface, and of three forms of silanol groups (–Si–OH).42,43 In terms of drug delivery, PSi/PSiO2-based materials provide a possibility to tailor the carrier structure and the surface composition according to the different needs. The pore size can be modified to fit the size of the drug molecule that will be loaded into the porous material, as well as to achieve the aimed release profile. The release profile can be controlled also via different surface treatments of the materials, leading to desired interactions between the porous carrier and the loaded substance. The surface treatment can also affect the loading of the molecules into the pores via hydrophobic-hydrophilic interactions. The pore diameters of PSi can vary from few nanometers to micrometers, however in drug delivery applications the mesopores (2–50 nm) are the most studied and used. The PSiO2 exhibit materials highly ordered two-dimensional tube-like pore structures with pore diameters typically between 1.5–30 nm.
Since cancer is a unique disease which has been causing the most challenges in terms of proper drug therapy, scientists are working hard to overcome the imperfections by rapidly developing nanovehicles and cell targeting moieties to alleviate long lasting medical deficiencies that hinder therapeutic effect of anticancer medicines.5,8,44-47 In this section, some important therapeutic aspects of controlled drug delivery systems based on PSi and PSiO2 nanoparticles are discussed in detail with the aim to highlight the undeniable role of these favorable particles in the future progress of cancer therapy. Controlled drug delivery is intended to improve the efficacy and reduce the potential side effects of drug molecules.32 PSi and PSiO2 nanoparticles, due to their low toxicity, high porosity, and convenient surface chemistry, have been used as carriers for many drug molecules that suffer from low bioavailability (as is the case for 40–60% of the anticancer drug candidates) because of their poor solubility, poor permeability through the biological membranes, high first pass metabolism effect and rapid clearance. Overcoming these defects as well as releasing therapeutic molecules in a suitable concentration at the desired target site in a predetermined time are the main features that a desirable drug delivery system should meet.
The release profile designed for controlled drug delivery systems depends on the desired biodistribution and achieved minimum effective concentration. Usually, drugs are released from intact or degraded nanocarriers by erosion, desorption or diffusion. For cancer therapy, the aim is to release the drug into the interstitial fluid, tumor surface or directly into the intracellular space. When drugs encapsulated in nanoparticles are meant to be delivered directly to the cell cytosol, endocytosis needs to occur from plasma membrame to the lysosome, where the particles degrade and release their payloads. When studying drug delivery of cytostatic drugs, it is more relevant to study their biological effect in cancer cell death instead of studying the concentration of the drug as a function of time.24
PSi-based materials for controlled drug delivery
The success in drug delivery using PSi depends on its hydrophilicity/hydrophobicity, pore size, surface chemistry, surface charge, physicochemical attributes of the loaded molecule and loading method.17-19,28 These properties can be tuned to achieve diverse controlled and temporal drug release profiles.
The drug loading into the PSi structure can be achieved by different methods, yielding different drug release profiles.32 The first strategy for drug loading into the PSi structure can be achieved by covalent attachment, in which the payload can be released only when the covalent bonds break or the supporting PSi matrix is degraded, achieving a prolonged drug release. Another drug loading method is by physical adsorption of the drug into the inner pore walls of suitably modified PSi particles. For example, Gu et al. loaded simultaneously an anticancer drug, doxorubicin and super-paramagnetic iron oxide by simple adsorption into intrinsically luminescent PSi nanoparticles (LPSiNPs) to achieve localized delivery of the drug.48 The molecules were strongly adsorbed to the particles’ surface and were not removed after being rinsed with water. Instead, when rinsed with phosphate buffer saline solution, the drug release was observed for several days. It is noteworthy that to control and precisely tune the drug release profiles is also possible by using a so-called “gate-keeping” approach, which consists in the incorporation of a responsive polymer or other pH-sensitive compound attached to the surface of the PSi (or PSiO2) structures.44,49-52
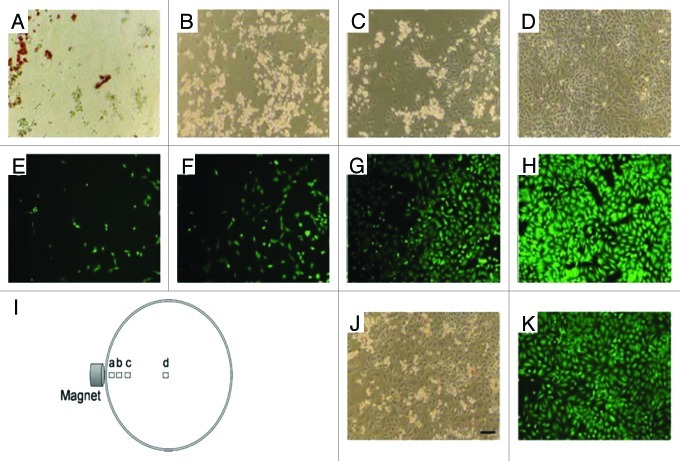
In addition, the magnetic and fluorescent properties of modified PSi-based particles are capable to be manipulated with an external magnetic field and tracked by fluorescence imaging.48 Taking advantage of these features, the feasibility of targeted drug delivery is tested guided by a magnetic field. For example, doxorubicin-loaded magnetic LiPSi were added to HeLa cells in a Petri dish and then guided with a rare earth permanent magnet to the edge of the Petri dish. After 24 h of incubation, the results showed that the particles were still accumulated at the edge of the Petri dish and that cell death was localized in the vicinity of the particles (Fig. 2).
Figure 2. Phase contrast (A–D) and fluorescence (stained with Calcein AM) microscope images of HeLa cells incubated for 24 h with doxorubicin-loaded magnetic LiPSi, showing magnetically guided (H) and without guidance (J and K) delivery of doxorubicin. The position of each image relative to the external magnet is depicted in I). Scale bar for all images is 100 µm. Reprinted with permission from reference 48.
PSiO2 for controlled drug delivery
PSiO2 based materials have been widely used for controling the intracellular delivery of anticancer drugs,16,24-26,33,45,53,54 taking advantage of their unique properties. This offers various possibilities for, e.g., gate-keeping functions, in order to minimize premature release and to control the drug delivery at the target site with very minor harmful effects over non-cancerous cells. This gate-keeper system is based on the reversible opening and closing of the pores of the particles by surface modification, allowing drug release as a response to different kinds of stimuli. These stimuli can be divided into two main classes: (1) systems that are trigger by external stimuli and (2) systems that are trigger by means of differences in chemical conditions of external and internal cell environments.
Among systems triggered by internal or external stimuli are pH,55 oxidation-reduction,56 enzymatic degradation,57 temperature,58 electricity,59 magnetic fields60 and photoirradiation50 responses. The pH-responsive systems have a different behavior toward pH depending on the administration route. For example, when administered orally, gate-keepers must show the ability of remaining intact against the harsh acidic conditions of the stomach without premature drug release. In the case of intravenous administration of nanosystems for intracellular drug delivery, the PSiO2 nanoparticles should retain the drugs inside the pores when circulating in the bloodstream, but allow the drug release from the pores in the acidic environment of tumors and intracellular compartments.25 In this respect, a recent study has demonstrated that the coating of PSiO2 nanoparticles containing a pH-responsive polymer shell formed by chitosan/polymethacrylic acid was able to protect and stabilize the PSiO2 nanoparticles under pH-values ranging from 5 to 8, as well as in the physiological saline.44 The release of the anticancer drug doxorubicin was much faster at pH 5.5 than at pH 7.4. Similarly, Zhu et al. have recently developed an enzyme-triggered drug delivery system based on a cytosine-phosphodiester-guanine oligodeoxinucleotide (CpG ODN) capped hollow PSiO2 nanoparticles.61 The drug release was achieved by degradation of the CpG ODN after the addition of deoxyribonuclease, and the rate of degradation could be controlled by changing the enzyme concentration.
Using photoirradiation as an external stimulus, Yang et al. have also recently developed a novel system that presents triggered delivery by near-infrared light (NIR) for controlled drug release toward cancer cells.50 The complex structure was formed by a PSiO2 nanoparticle framework containing gold nanorods (GNR), which can absorb NIR photoenergy, and its surface was modified with aptamer DNA, which served as a capping and targeting agent. By using a 26-mer guanine-rich oligonuclueotide DNA aptamer, which is already in phase II f clinical trials for relapsed or refractory acute myeloid leukemia and for renal cell carcinoma, the authors showed that the modified PSiO2 nanoparticles formed a stable G-quadruplex structure and bound with high affinity to nucleolin, an overexpressed molecule in tumor cancer cells. In addition, by using another 12-mer oligonucleotide (DNA-1) complementary to the 3′ ending extension covalently attached to the surface of the PSiO2 nanoparticles, both identical DNA regions assembled, resulting in a linker anchored on the PSiO2 nanoparticle surface; the G-quadruplex served as a pore gate-keeper trapping the guest molecules within the pore channels. The GNR transformed the photoenergy from a laser beam into phototermal heat, rendering a general increase in the particles’ temperature that led to a DNA dehybridation and G-quadruplex release, thus unblocking the PSiO2 nanopores and readily delivering the drug payload (Fig. 3). This multifunctional platform also showed in vitro the feasibility of its use as a nanocarrier for targeted and non-invasive remote-controlled drug delivery system in cancer cells.50
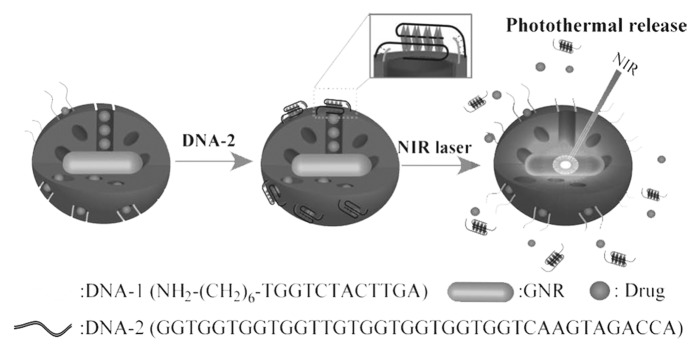
Figure 3. Schematic of a NIR light-triggered release of guest molecules from the pores of an aptamer-covered PSiO2-based nanovehicle. Reprinted with permission from reference 50.
To overcome the problems related to the tumor resistance, Andrew et al. focused on the development of biocompatible and biodegradable nanostructured PSiO2 films of bevacizumab prepared by electrochemical etching and thermal oxidation in air at 800°C.62 It was shown that bevacizumab adsorbed to the surface of PSiO2 was released ca. 98% in its active form over a period of one month. Although the aim of this study was to cure age-related macular degeneration, the primary cause of blindness in the developed world, via bevacizumab-loaded PSiO2 nanovehicles, the results showed the feasibility of this system to be expanded for possible anti-angiogenesis cancer immunotherapy.
Pharmacokinetic and Pharmacodynamic Considerations of Porous Si-Based Materials
Owing to some restricting factors for efficient therapeutic effect of conventional chemotherapy agents, including limited aqueous solubility, lack of selectivity of anticancer drugs and DR, the focus on profitable effects of nanotechnology based devices is higher than at any time in the past. Although medical nanodevices are in a unique position to leverage their abilities to provide desirable cancer treatment technologies by exploiting appropriate materials in nanodelivery systems, it has still to address many of the challenges that the researchers face for cancer therapy.63-65 For example, despite positive effects of multiple drug loading in PSiO2 nanoparticles, this combination therapy may synergistically enhance side effects by manifold mechanisms. The unwanted side effects are due to the attainable cytotoxic chemotherapeutics that are not completely selective for tumor cells, and therefore, there is a high probability of damaging the normal cells, in particular the replicating ones like gastrointestinal epithelia, bone marrow and hair follicles.63-65 In addition to this challenge, there are also some other hindrances for nanoparticulate therapy, which have to be meticulously be taken into account and they include the presence of the reticuloendothelial system (RES) and epithelial/endothelial membranes, cellular drug extrusion mechanisms, tumor vascular architecture, interstitial pressure gradients, transport across the extracellular matrix, stromal impediments, specificity and density of tumor surface receptors and tumor heterogenecity.
In the next sections, we discuss some of the therapeutic properties of Si-based materials and some of the strategies presented in the literature to by-pass the biological barriers.63,64,66
Biocompatibility
Exposure to PSi and PSiO2 based materials is an increasing reality due to their increased interest in exploring the usage of these materials as drug delivery carriers.23,27,36,38-41 However, there is still minimal information on the adverse effects induced by these materials, particularly in vivo. The intravenous administration of nanoparticles faces multiple biological elements and boundaries as they travel to the targeted tissue/organs/cells. Blood-born cells, including erythrocytes, white blood cells (e.g., monocytes and neutrophils), tissue macrophages and endothelial cells aligning the vessel walls are cells that come into close and immediate contact with intravenously administered nanoparticulates.
Chemophysical properties of nanomaterials such as size, shape, surface area and structure have been studied as modifiers of particles’ biocompatibility.23 Even though particle size is considered to be one of the most influential parameters in nanoparticle biocompatibility, its exact relationship with the in vivo toxic effects is still uncertain.30 Generally, smaller nanoparticles have greater hemolytic potential than larger ones. This effect has been studied on red blood cells,67 where after 3 h of exposure, particles of 25 and 93 nm in size induced higher toxicity than particles of 155 and 225 nm, at a concentration of 3.125–1.600 µg/ml. The same effect was observed in a cytotoxicity study performed over human breast-cancer and African green monkey kidney cell lines with particles ranging from 190 to 1220 nm.68
It is known that the biocompatibility of PSi depends on its porosity and pore size. While PSi with porosity above 70% dissolves in all the simulated body fluids, except stomach, PSi porosities below 70% is bioactive and slowly biodegradable, very low porosity PSi and macroporous silicon are bioinert materials. The biocompatibility of PSi-based materials has been assessed by measuring the cytotoxicity, reactive oxygen species (ROS) and inflammatory responses in several cancer cell lines.23,36,38,40,41 For example, RAW 264.7 macrophage cells incubated with thermally hydrocarbonized-PSi and thermally oxidized-PSi nanoparticles showed that the effects were size- and concentration-dependent.36,38,40,41 However, the in vivo administration of unmodified PSi nanoparticles was extensively detected in the liver and spleen without major toxicity effects,38,40,69 and the toxicity of other mesoporous-based particulates was dependent on the administration route, with intraperitoneal and intravenous routes being deadly to mice, while the subcutaneous route showed no obvious toxicity on the animals.21
The PSiO2 nanoparticle concentration in the body also plays an important role in its biocompatibility. Studies of short-term biodistribution of PSiO2 nanoparticles (50−100 nm, positively charged-surface functionalized particles) in rats demonstrated that the toxic effects where detected when doses over 200 mg/kg were administered intraveneously.70 PSiO2 nanoparticles tended to accumulate mainly in the liver (35.3%) for up to 3 mo. These results suggest that PSiO2 nanoparticles are resistant to decomposition and are biocompatible in vivo at low concentrations.24-26
Surface properties have also greatly influenced the biocompatibility of particles. Therefore, it is important to take the particle’s surface charge in consideration. Particles with cationic charge have a higher endocytosis efficiency due to the higher affinity to negatively charged cell surfaces.24 Cationic particles induce a higher immune response and cytotoxicity, presenting a facilitated transvascular transport to tumor tissues, whereas neutral particles show longer circulation times and interstitial transport in tumors and particles with a higher negative charge that can easily escape from endosomal entrapment.70,71
PSiO2 nanoparticles present a small percentage of silanol groups on their surface which are able to interact with biological molecules altering their structures.71 Moreover, when administered intraveously, these groups are responsible for a hemolytic effect. To overcome this issue, and thus, improve their biocompatibility, some changes in the surface of PSiO2 nanoparticles have been performed, such as lipid coating51,72,73 or PEGylation.67,74 Polyethylene glycol (PEG) forms a hydrophilic layer around the particles, which improves the biocompatibility by hiding silanol groups of the surface,67 as well as by diminishing hemolysis, cytotoxicity and endocytosis of PSiO2 nanoparticles.74 Nevertheless, PEGylated nanoparticles may cause hypersensitivity reactions because of the production of specific anti-PEG IgM.75 It has also been reported that the surface functionalization of PSiO2 nanoparticles with surfactants tended to enhance anticancer drug loading capacities, causing cytotoxicity against MCF-7 cancer cells and changes in the proliferative activity of the cells.76 These effects were dependent of the concentration, incubation time and type of surfactant used.
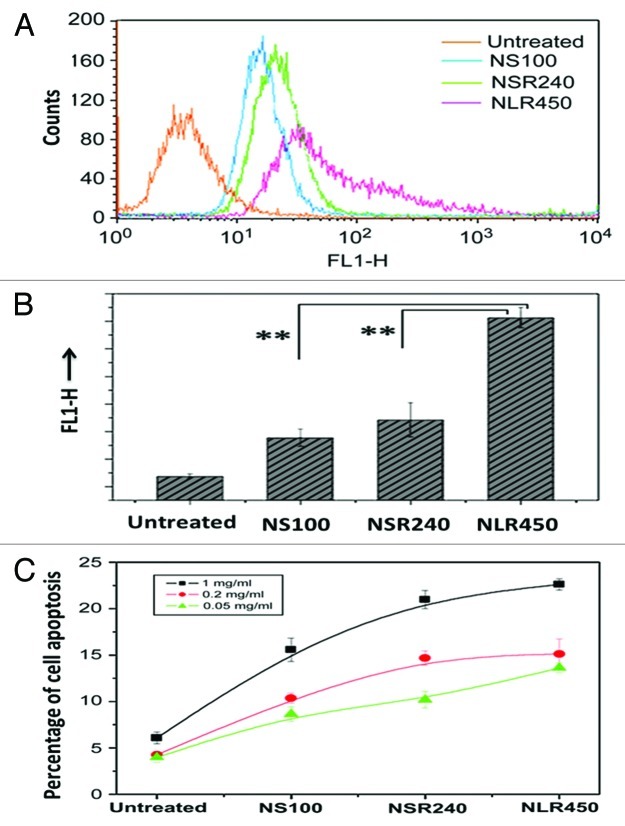
Current studies have also shown that the shape of the nanoparticles influence the cell−nanoparticle interactions and the in vivo particle bio-translocation,77-79 although there is still need for more accurate research in this direction. A recent study on the cytotoxicity of PSiO2 nanoparticles with diameters from 80 to 150 nm and different aspect ratios performed in murine macrophages, human lung carcinoma cells and human erythrocytes, found that the aspect ratio had neither significant effect on the particles’ acute cytotoxicity and cellular uptake, nor inhibited the cell proliferation or damage its plasma membrane integrity.78 However, higher aspect ratio particles showed lower hemolytic toxicity. On the other hand, Huang et al. demonstrated that diverse aspect ratios of PSiO2 nanoparticles, including sphere-shaped (100 nm), short rod-shaped (240 nm) and long rod-shaped (450 nm) PSiO2 nanoparticles, affected the extent and rate of internalization of the particles into A375 human melanoma cells (Fig. 4).77 The higher aspect ratios of rod-shaped particles affected the cell functions, such as cellular uptake and apoptosis, in higher extent than sphere-shaped particles.
Figure 4. (A) Quantification of cellular uptake and apoptosis of different shaped PSiO2 by flow cytometry analysis of different prepared PSiO2 nanoparticles: sphere-shaped, NS (100 nm), short rod-shaped, NSR (240 nm) and long rod-shaped, NLR (450 nm). (B) Quantitative measurement (fluorescence) of particle numbers in cells (data are presented as mean ± s.d; statistical significance for the comparison of the number of internalized particles between different shaped particles, **p < 0.1). (C) Influence of different concentrations of shaped nanoparticles on early apoptosis of A375 cells. Reprinted with permission from reference 77.
Cellular uptake and trafficking
In order to understand the response of biological cells to nanoparticles, it is crucial to learn about the mechanisms of cellular uptake and intracellular trafficking.10 The cell membrane is a complex system consisting of lipids, proteins, cholesterol and receptors, which presents a net negative surface charge.16 Hence, the surface potential of the nanocarrier as well as the receptors attached to the cell membrane are the features that define cell uptake and trafficking in animal cells. Moreover, it has been demonstrated that the cell uptake of nanoparticles, besides depending on the dosage and time, also depends on the cell type,80 particle size,81 shape,77 surface charge45 and surface chemistry.82 Concerning the size of the nanoparticles, there are some studies that link the size of different vehicles with cell uptake and from which it can be deduced that depending on the cell line, the size limits for endocytosis of particles may vary.45,83-86 Particles smaller than 200 nm are internalized by cells through endocytic mechanisms, while bigger particles when internalized they are uptaked by either endocytosis or phagocytosis.
Regarding the PSiO2 nanoparticles, several studies have been reported describing the relationship between particle size, surface modifications and targeting moieties, and cellular uptake.23 PSiO2 nanoparticle uptake was found to take place via a clarthrin-mediated endocytosis, but the surface modifications of the particles led to different endocytosis mechanisms, e.g., amine- and guanidinium-functionalized PSiO2 nanoparticles suffered a clarthrin and caveolae-independent endocytosis, while folic acid (FA)-functionalized PSiO2 nanoparticles experienced a FA receptor-mediated endocytosis, which increased particles’ uptake by cancer cells.24,53,87 In addition, it has been demonstrated that FITC-PSiO2 nanoparticle internalization is also cell type-, concentration- and time-dependent.80,87 Lu et al. further demonstrated the energy dependency of the uptake process by showing the higher particle uptake efficiency of cells at 37°C compared with 4°C, and the effect of some metabolic inhibitors in surpressing the PSiO2 nanoparticle uptake in human pancreatic cells.46
PSiO2 nanoparticle endocytosis and intracellular trafficking pathways have been followed by confocal fluorescence microscopy.45,80,88 The endocytosis led to the formation of a vesicle which captured the particles and internalized them into the cytosol rendering an endosome. Then, the endosome content was either recycled back to the extracellular environment or transported to secondary endosomes that fused with lysosomes.88 The PSiO2 nanoparticles could escape from the endolysosomes entering the cytosol, where the drug payload of PSiO2 nanoparticle could then be released. Generally, the negatively charged materials are able to escape more effeciently from the endosomes than the positively charged PSiO2 nanoparticles which usually remain trapped within the endosomes.80
To overcome the nanoparticle retention within the endosomes, surface modification of the nanoparticles have been developed to escape endosomal uptake, for example, the modification of the surface of the nanoparticles with amino groups can interfere with the proton sponge effect by creating a proton osmotic influx inside the endosome that is able to break it down, allowing the escape of the particles.89
Biodistribution, degradation and clearance of nanoparticles
In order to evaluate the suitability of nanoparticles as carriers for drug delivery applications is necessary to obtain detailed knowledge about their biodistribution and in vivo behavior. Various factors have to be considered as possible disrupters of the nanoparticles’ biodistribution such as the administration route, the particle size, its composition and its surface charge.31,32 It is possible to tune these features to improve the nanoparticles’ biodistribution with the goal of targeting their effect to the tumor tissue. In this case, it is crucial to take into account the special physiological conditions that surround the tumor cells. Capillary blood vessels that irrigate normal body tissues are approximately 5 µm wide and their walls present pores with a diameter of mainly around 9 nm, although a small percentage of them reach 50 nm in diameter.90 Therefore, in order for the particles to circulate through the bloodstream at this capillary level, their design must be accordingly small. However, tumor and inflammatory tissues and RES organs (liver, spleen and bone marrow), present pores with a diameter of 100 nm due to the absence of basal lamina and are present in the walls of normal tissue vessels. Therefore, particles up to a similar size (100 nm) can easily penetrate the tumor and inflammatory tissues, whereas bigger particles cannot trespass the wall of a normal-tissue vessel. Moreover, becuase tumor tissues do not present a lymphatic system for eliminating lipophilic and polymeric materials, particles that penetrate inside the tumor cannot be eliminated easily.91,92 Both facts cause an EPR effect for nanoparticles between 50 and 100 nm in size.
In the case of the RES organs, the EPR effect is limitated to the nanoparticle biodistribution, because it diminishes their blood circulation time. Both the surface modification of the nanoparticles and the reduction in particle size have been evaluated to overcome such limitation.41,69 The surface composition has been shown to affect the biodistribution of doxorrubicin-loaded LPSiNPs with a particle size smaller than 200 nm when administered intravenously.69 The biodistribution and histological studies performed by monitoring the NIR fluorescence of the particles showed that while regular particles accumulated mainly in the spleen rather than in the liver, dextran-coated LPSiNPs tended to accumulate in the tumor site when administered to tumor-bearing nude mice.
It has been extensively reported that PSiNPs are readily biodegraded into silicic acid,17,18,28 which is a natural compound of the human body and that can be cleared from the blood through the urine. PSi degradation rate is directly correlated with the particle’s size diameter and pore.93 However, in the case of the particles with diameters around 100 nm presenting pores sizes between 5 and 20 nm, the stability of the particles does not strongly depend on the pore size.69 Moreover, the PSiNPs ranging from 80 to 120 nm, besides presenting an EPR and enhanced tumor uptake, are large enough to avoid renal clearance. Nevertheless, the biodegradation properties of the PSiNPs provide a safe clearance from the body, and their biodegradability rate, which is often too fast, limits their half-life, thus reducing their in vivo delivery efficiency.
Although the half-life of some modified PSi particles have been reported,69,94 there is still a lack of systematic studies on the stabilizing processes of the surface of the particles to avoid rapid clearance from the body. In this respect, Hon et al. developed two thermal oxidation processes: (1) peroxidation using rapid thermal processing (RTP) and (2) postoxidation using hot aqueous baths.93 To measure the degradation rate of the different types of PSiNPs, they digested and analyzed the concentration of dissolved Si in a phosphate buffer saline solution at a constant temperature of 37°C. Thermal oxidation performed in particles with an average size of 100 nm and average pore size of 5 nm showed an increase in the half-life of the PSiNPs while avoiding alteration of their chemical properties. Both oxidation processes led to the formation of an inert silica layer that protected the Si core from degradation. The longer was the process the thicker the oxidation layer, and thus, the longer the half-life of the particle. When combined, the maximum improvement in half-life of the particle appeared after 90 to 180s of RTP and 18 h of postoxidation, increasing from 10 min up to 3 h.
In another study, the effect of the silica-coating on the half-life of the particles was also evaluated.93 Instead of oxidizing the Si particles, a layer of silica was chemically grown over a hydroxyl PSi surface-terminated. Athough the silica-coated PSi particles increased the half-life of the particles from 10 min to 8 h, an increase in the coating decreased the solubility of the particles due to the thick silica shell around the particles and, hence, creating a higher Si density.
In addition, there have been studies on both passive accumulation and active targeting of functionalized PSiO2 nanoparticles to tumors. The ideal situation would be to achieve a synergic effect of both passive and active targeting of PSiO2 nanoparticles to the tumor in order to improve the biodistribution of the drug to the tumor tissue. Passive tumor accumulation was demonstrated using fluorescent and magnetic PEGylated PSiO2 nanoparticles (97 nm)95 and surface-bound magnetite nanocrystals PSiO2 nanoparticles (70 nm)96 which were administered intravenously to mice bearing subcutaneously xenotransplanted MCF-7 tumors. After 2 to 24 h of the injection both particles accumulated in the tumor due to an EPR effect in the tumor. PSiO2 nanoparticles particles functionalized with magnetite nanocrystals also accumulated in the liver, spleen and lungs, which was attributed to phagocytosis by macrophages.
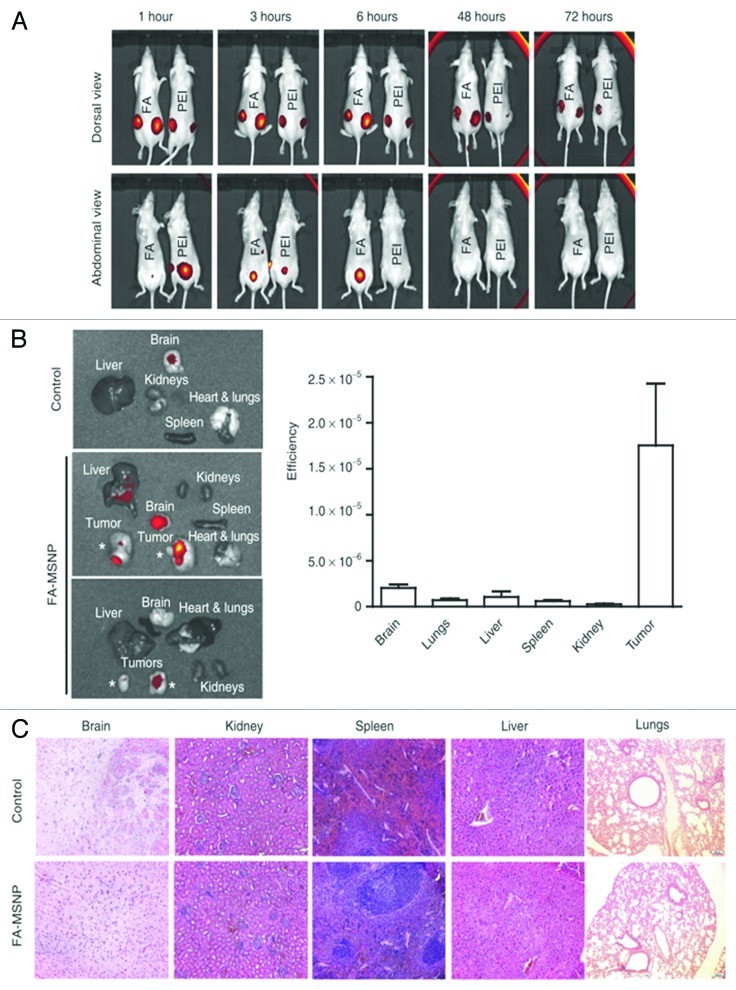
Other studies have demonstrated that folate-functionalized PSiO2 nanoparticles is a feasible mechanism to stimulate the tumor accumulation of the drug.47 This effect has been demonstrated in mice bearing different xenotransplanted tumors, such as MDF-7 subcutaneous tumor PANC-1 and MicPaca-2, or MDA-MB-231 breast cancer tumor, after intravenous administration of the folate-functionalized PSiO2 nanoparticles.33,97 For example, folate-tagged PSiO2 nanoparticles injected perotumorally to mice bearing MDA-MB-231 subcutaneous tumors remained within the tumor much longer period than non-folate modified PSiO2 nanoparticles, which were washed out from the tumor in 72 h (Fig. 5).33 This effect was attributed to a faster cell uptake of folate-modified PSiO2 nanoparticles that minimized the tumor wash out.
Figure 5. (A) In vivo imaging of mice injected peritumoral with poly(ethylene imine) (PEI)-PSiO2 nanoparticles ( = MSNPs) or FA-MSNPs. Images of the abdominal and dorsal area demonstrate accumulation of fluorescence in the bladder and in the tumors, respectively. Images of the abdominal area show elimination of fluorescence within 48 h after injections. (B) Ex vivo analyses (left) and quantification of fluorescence intensity (right) in organs from mice injected intravenous with FA-MSNPs. (C) Histological analysis of brain, kidney, spleen, liver, and lungs of untreated mice and FA-MSNPs-treated mice showed no morphological changes. PSiO2 nanoparticles accumulated in the tumors were biocompatible, biodegradable and eliminated through renal excretion. Reprinted with permission from reference 33.
The expected low silica concentration in body fluids, under sink conditions, renders a fast dissolution of the PSiO2 nanoparticles. However, the rate of dissolution of these particles also depends on the size, surface functionalization and degree of silica condensation. The silica dissolution may be either adsorbed by the body or excreted in urine and feces through the bile duct in the form of silica acid or oligomeric silica species.47,98 Even though the renal cut-off is 5 nm, it has been found that depending on the particle size, partially degraded PSiO2 nanoparticles with dimensions similar to the original administered particles appeared in urine.33,99,100 However, the excretion process is still not very well-known.
The influence of the surface charge on the in vivo clearance of the PSiO2 nanoparticles has also been demonstrated. Negatively charged particles PSiO2 nanoparticle-TA-ICG (tagged with the NIR dye, indocyanine green, ICG), with a zeta (ζ)-potential of −17.6 mV and a particle size from 50 to 100 nm were tracked in vivo.101 The biodistribution and clearance studies showed that the particles mainly accumulated in the liver and, in a lesser extent, in the kidneys, lung, spleen and heart. The same experiment was performed using positively charged PSiO2 nanoparticle-NH2-ICG with a ζ-potential of +34.4 mV and a size from 50 to 100 nm.102 The results showed that 10 min after intravenous administration almost all particles were accumulated in the liver. After 60 min the particles had moved through the bilateral duct to the duodenum and after 4 h they appeared mainly in the jejunum and duodenum instead of in the liver. After 3 d, the excretions of the animal were analyzed, and the content of silica in feces was higher than 60%, but silica was not found in the urine. Similarly, after 4 d of intravenous administration of phosphonate-PSiO2 nanoparticles, the silica content found in feces and urine was similar to the injected silica.
In summary, while the clearance of positively charged PSiO2 nanoparticles stayed in the body less than 1 h after injection, lower surface charged particles remained days in the animal body. Finally, PEGylated surface-modified particles have been shown to prevent phagocytosis, avoiding removal of the particles from the circulation. Biodistribution and urine excretion assessment of PEG-PSiO2 nanoparticles (80−360 nm) found that the particles mainly accumulate in liver and spleen.103 Fewer particles were accumulated in the lungs and even less in the kidneys and heart. Nevertheless, PEG-PSiO2 nanoparticles accumulated in lesser extent than PSiO2 nanoparticles in these organs. Other studies have also demonstrated the increase in the half-life of PSiO2 nanoparticles from 15 min to 3 h after the PEG surface functionalizatons.104,105
Cell Targeting
Tumor targeting moieties and specific indications
Despite great advances, cancer therapy still suffers from a major challenge associated to the low therapeutic concentration of the drugs reaching the subcellular compartments of a target tissue, resulting from the lack of target selectivity.106,107 To develop an effective therapeutic system with a higher probability of extravasation, it is desiring to fabricate targeted particles with a size defined in the range of nanometer108 in order to avoid unwanted side-effects by the anticancer drug on healthy cells. Among the outstanding advantages of porous nanomaterials, the ability of surface functionalization with targeting moieties is the most exciting favorable result reported in the literature, which works as caps for sustained release of various cargos to cancerous cells and highlights the paramount importance of porous materials as a relevant platform for a wide range of pharmaceutical compounds.
Interestingly, it is becoming significantly difficult to reject the fact that all nanomedicines currently used in different cancer therapies show some aspects of targeting either passively or actively. In recent years, the surface modification of porous nanoparticles with various targeting ligands,24,25 e.g., peptides, DNA aptamers, sugars, monoclonal antibodies, F, and small molecules, have been reported in the literature with many promising and successful results. For the success in active targeting of porous nanovehicles, it is necessary to make a reasonable balance between ligand content and surface exposure in order to hold some promise toward reduced immunogenecity and clearance, high affinity binding to the receptors expressed on the surface of cancerous cells, enhanced interactions with the target cells, minimized interactions with healthy tissues, and consequently, improving cellular uptake and reducing drug resistance of the diseased cells.33,109 For tumor targeting, small molecular ligands can be attached before drug loading in either aqueous or organic solvent; however, organic solvent is preferred owing to less effect on the silica matrix. By contrast, peptide-based ligands conjugate to vehicle after drug loading in an aqueous solvent with the aim to guarantee the activity of targeting moiety.25 In this case, it is crucial to retain the binding activity while the condition used during the conjugation process must prevent denaturation of the protein.24,25
Currently, considerably work has been devoted to generate cell targeted drug delivery systems by utilizing specific ligands relying on the capability of selective conjugation to the surface area of the cells and to trigger receptor-mediated endocytosis.45,77,82,86,110 However, problems associated to the appropriate multi-functionalization still remain due to the limited attachment sites on the particle’s surface as well as the possibility for stability reduction during the functionalization steps.7,25,30,45,82,95 In this respect, the interest has shifted toward particles with a great number of terminal functional groups. For example, surface modified PSiO2 nanoparticles by hyperbranching polymerization of PEI followed by fluorescent and FA conjugation, were introduced by Rosenholm et al. with the aim of producing non-cytotoxic targeting into cancer cells.53 In this study, high positively charged functional end groups of primary amines provided by PEI could be use for gene delivery due to the destabilization of the lysosomal membranes, and thus, enhancing endosomal escape. In addition, folate receptors were selected as the targeting ligand due to their high abundance in many different kinds of cancer cells in comparison to normal ones. The results showed that HeLa cervical carcinoma cells internalized an extensive number of PSiO2 nanoparticles of 400 nm (the particle size was chosen due to the unspecific cellular uptake and high cytotoxicity of previously studied silica particles of smaller size),45,111 and that the fraction of the internalized nanoparticles by cancerous cells was considerably high.112 Furthermore, FITC/PEI- and FITC/PEI/FA-functionalized PSiO2 nanoparticles were upataked by ca. 70% of the cells, mainly resulting from electrostatic attraction between the positively charged particles and the negatively charged HeLa cell membranes; ca. 20% of the FITC/PEI-modified PSiO2 nanoparticles incubated with the cells remaining fluorescent after trypan blue quenching, while a 2-fold increase to 40% was observed for the FITC/PEI/FA-modified PSiO2 nanoparticles incubated with the cells (Fig. 6).
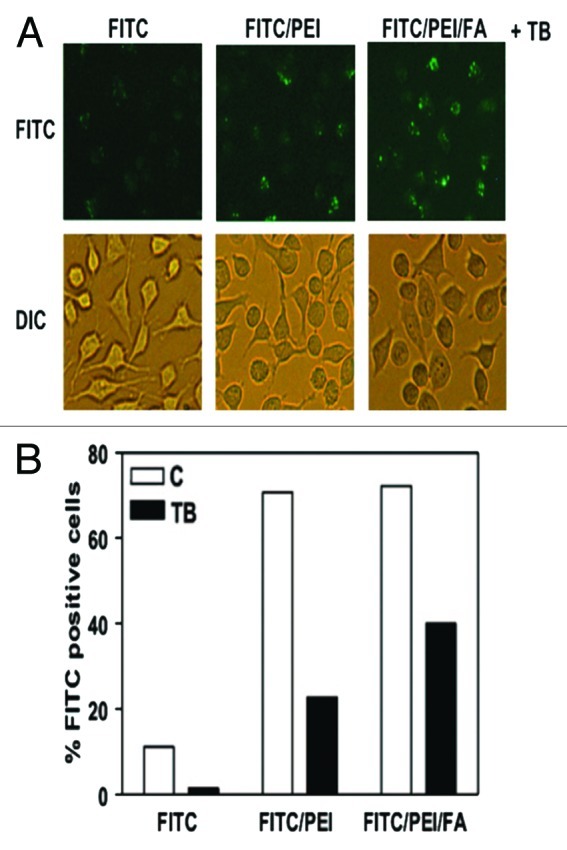
Figure 6. Conjugation of FA with PSiO2 nanoparticle showed improved endocytosis in HeLa cells. HeLa cells treated with PSiO2 nanoparticles at 10 µg/ml for 2 h and the extracellular binding of the nanoparticles was quenched with trypan blue (FITC, pristine FITC-labeled particle; FITC/PEI, PEI-functionalized FITC-labeled particle; FITC/PEI/FA, FA-conjugated PEI-functionalized FITC-labeled particle). Fluorescence microscopy was used to image FITC-labeled particles inside the cells and differential interference contrast microscopy for cellular morphology evaluations (A). Flow cytometry was used to detect the number of HeLa cells with endocytosed PSiO2 nanoparticles in control or trypan blue quenched cells (B). Reprinted with permission from reference 53.
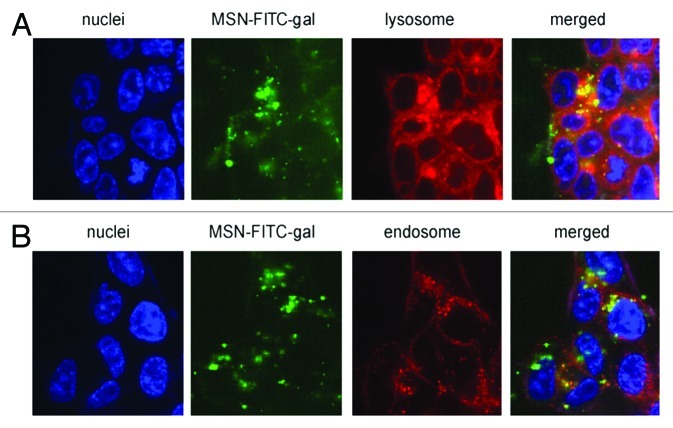
Another interesting area of research in cancer therapy is the combination of drug delivery, targeting and photodynamic therapy in the same nanosystem. In this respect, Gary-Bobo et al. reported the first evidence of a synergic anticancer effect of PSiO2 nanoparticles covalently encapsulated with both a photosensitizer (porphyrin) and a drug (camptothecin) in order to prepare lectin-targeted PSiO2 nanoparticles.113 When the PSiO2 nanoparticles were functionalized with galactose, the confocal microscopy experiments displayed an enhanced PSiO2 nanoparticle uptake by endosomal and lysosomal compartments of colorectal cancer cells (HCT-116) (Fig. 7). Compare with single therapy, this study showed a significant enhancement of cancer cell death effect by combining drug delivery and photodynamic therapy. Therefore, this proof of principle indicates that the simultaneous use of two different therapeutic mechanisms within the same nanocarrier may lead to very efficient cancer cell death.
Figure 7. Confocal microscopy images after 6 h incubation of living HCT-116 colorectal cancer cells with FITC-galactose-modified PSiO2 nanoparticles ( = MSN-FITC-gal) at 37°C. Merged pictures of both section A and B indicated the co-localization (yellow) of FITC-nanoparticles (green) with lysosomal or endosomal markers, respectively. Reprinted with permission from reference 113.
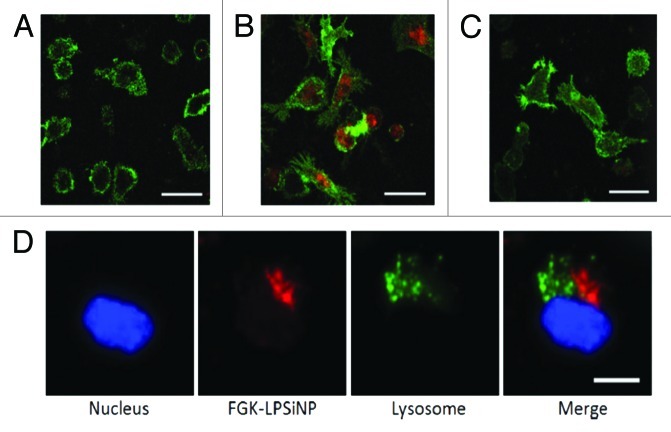
Despite the promising results of targeted porous nanomaterials, these nanosystems still encounter several challenges that nullify the best outcome of the developed nanosystems. Therefore, there is an important and unmet need for using porous nanoparticles as nanovaccines to treat cancer. Immunotherapy is an alternative strategy to retrieve harmful unwelcome results through intentionally activation of the body’s own immune system to fight against cancer.114,115 In this respect, despite unexplored experimental studies owing to the restricted understanding of the interactions between the nanomaterials and the immune system, Gu et al. used an engineered LPSiNPs to activate antigen presenting cells in order to alter the potency of immunomodulators.116 FGK45 immunomodulators (an agonist antibody of CD40) can bind to antigen presenting cell receptors of CD40 to improve the activation of B-cells; thus, a 30–40-fold increase in the cellular response to the nanoparticle-based stimulators compared with free FGK45 was observed, when FGK-LPSiNPs where readily taken up by antigen presenting cells (Fig. 8). Figure 8A shows limited presence of bare LPSiNPs in the mouse bone marrow-derived dendritic cells, while the FGK loaded counterparts exhibited much higher uptake of nanoparticles under the same conditions (Fig. 8B). To evluate how FGK45 binding improved the internalization and induced endocytosis of FGK-LPSiNPs, cells where pre-treated with free FGK45 for 30 min before incubation with FGK-LPSiNPs for 1.5 h at 37°C, resulting in substantial block for the nanoparticles internalization (Fig. 8C). Overall, these results suggested the feasibility of utilizing nanostructured PSiNPs for a specific tumor targeting ligand to remarkably enhance the tumor targeting efficiency of such nanosystems.
Figure 8. Fluorescence microscope images of mouse bone marrow derived dendritic cells treated with (A) LPSiNPs or (B) FGK-LPSiNPs for 1.5 h at 37°C. (C) 30 min pretreatment of the bone marrow-derived dendritic cells (green) with free FGK45 inhibits uptake of FGK-LPSiNPs incubated with the cells for 1.5 h at 37°C. FGK-LPSiNPs were detected by their intrinsic visible/near-infrared photoluminescence (red, λex = 405 nm and λem = 700 nm). The scale bars are 40 µm. (D) Distribution of FGK-LPSiNPs bone marrow-derived dendritic cells after 1.5 h incubation at 37°C. The lysosomes are stained in green (LysoTracker; Invitrogen), the nucleus in blue and FGK-LPSiNPs in red. The scale bar is 10 µm. Reprinted and modified with permission from reference 116.
In comparison with unfunctionalized PSiO2, it is now demonstrated that functionalized PSiO2 can load larger amounts of protein and also provide an interactive and confined environment such that the loaded protein activity is the highest.117-121 In functionalized PSiO2 nanocomposites, it is also possible to control the release profile of the encapsulated proteins based on their functional groups and pore sizes.53 Lei et al. demonstrated that due to their comprehensive non-covalent interactions, antibodies have the ability to spontaneously load in functionalized PSiO2 composites with super high density (0.4–0.8 mg of antibody/mg of functionalized PSiO2) and then gradual release, helping to develop innovative cancer nano-immunotherapy strategies for treating many diseases.121
One interesting effort for evaluating the effect of EPR on PSiO2 nanoparticle-mediated cancer therapy was reported by Meng et al., who showed sufficient doxorubicin delivery to cancer cells by PEG/PEI-coated PSiO2 nanoparticles of 50 nm of particle size.122 In addition, it was demonstrated the important role of the EPR for cancer therapy with ca. of 38% apoptosis induced by the nanoparticles compared with 13% of the free drug.
Because of the unique properties, in vivo studies are expected in the future to demonstrate the ability of PSiO2 nanoparticles and PSi for targeted cancer immunotherapy. Although a full discussion about this topic is beyond the scope of this review, it is important to emphasize that there is a crucial need for reconciling the application of nano-immunomodulatory with concerns regarding biocompatibility and toxicity of the nanoparticles. Generally, the inflammation associated to the nanoparticles is considered as unwanted side effect, but it can be considered very advantageous in nano-immunotherapy protocols.123
Advantages, Disadvantages and Concerns Associated to Porous Si-based Nanomaterials
The application of porous materials for cancer therapy has been emerging as a new interesting field of interdisciplinary research among chemistry, medicine, material science, biology, pharmacology and toxicology, and are expecting to bring a major progress to alleviate unsolved issues related to cancer therapy.63 PSi and PSiO2 based materials are among the most interesting compounds which can provide more opportunities for on-demand cancer therapy and pave the road toward simple treatment of challenging diseases.10,26
The availability of silica and silicon in a broad range, their versatility, non-toxicity, biocompatibility, biodegradability, high surface area and pore volume, homogenous distribution of guest molecules into porous space, the ability for surface charge control, free dispersion throughout the body and capability to be tailored to fit a desire purpose make them suitable options as emerging nanovectors.14,17,19,24-26 In addition, the higher level of multi-functional integration to improve the efficacy of cancer drugs via a pre-defined step-by-step therapeutic strategy including the escape from the immune system of the host, finding their target in damaged tissues, entering to the intracellular space of the cells, drug release in a proper manner, and in a final step, ease of excretion from urine in the form of silicic acid or oligomeric silica species.24-26,93,124 Another attractive and profitable attribute of porous nanomaterials well documented in the literature is the satisfactory drug loading capacity, accompanied by facile control of the material characteristic scale such as pore and particle size in the nanometer range.26,124 Furthermore, the rigid frame of PSiO2 nanoparticles and their acceptable stability allow for long resistance to mechanical stress and harsh pH conditions.24,25
The increment toward lower MDR is another advantage achieved by the porous nanoparticles, increasing the success of cancer therapy. For example, Chen et al.,125 used PSiO2 nanoparticles to study the simultaneous MDR effect of doxorubicin and Bcl-2-targeted siRNA on A2780/AD human ovarian cancer cells and on the Bcl-2 mRNA silence, and consequently, the suppression of non-pump resistance. They also observed a significant improvement in the anticancer action of doxorubicin with the minimal premature release in the extracellular region, decreasing the drug side effects. Overall, the abovementioned advantages have been resulting in enhanced application of porous materials in cancer therapy since the beginning of the millennium.15,124,126
Despite all the advantages and developments, misunderstandings and complex parameters such as lack of pharmacokinetic-pharmacodynamic studies concerning biodistribution, clearance, therapeutic efficacy and safety are important paramteres that need further attention in the quest of providing competent porous nanoparticles which can move from the bench to beside.26 As an evidence for the misunderstandings related to porous materials, it has been observed that, under physiological conditions, porous nanoparticles can dissolve from the inside out leaving the initial particle size virtually intact under static conditions,127 resulting in the detection of particles with dimensions similar to their injected correspondents in urine. These findings are rather surprising considering that the renal cut-off is around 5 nm, which makes unclear the exact mechanism of excretion of such kind of particles.
There are also some concerns about the absolute safety of PSiO2 nanoparticles. For example, the major drawback in terms of the hemocompatibility of PSiO2 nanoparticles is attributed to the surface density of silanol groups interacting with the surface of the phospholipids of the red blood cell membranes resulting in hemolysis.128 This harmful effect can be minimized by surface PEGylation of the PSiO2 nanoparticles, but not completely.128 Generally, it is accepted that the biocompatibility and safety of PSiO2 nanoparticles will depend on size, morphology, surface chemistry, composition, dosage and the administration route used.33 Therefore, all these parameters must be taken into account to lead to a minimum of adverse effects possible.
Other disadvantage is related to metabolic changes induced by PSiO2 nanoparticles, leading to melanoma promotion.129 This phenomenon results from reduced endogenous ROS and upregulation of antiapoptotic molecules (nanoparticles may regulate and cause tumor growth via a ROS dependent manner). In addition, different results in different in vitro and in vivo studies,25,26 depending on the type and physicochemical attributes of the applied silica- or silicon-based porous nanoparticles, is another issue of this type of nanocarriers. Thus, efforts need to move toward finding a general characteristic and outcome for each type of porous nanocarriers. Besides, as described in recently published works, the cell type specificity is a challenge that must be improved for these types of materials.25,26,54
Finally, it is noteworthy to mention that in addition to these drawbacks, there is a crucial concern about whether in vitro success of porous nanomaterials can also be reproduced in vivo. Generally, in the first decade of this century, researches have been focused more on the basic characteristics of porous materials and their ability to deliver different kinds of anticancer drugs in cultured cells but, unfortunately, there has been little experimental data about in vivo fate of silica- or silicon-based nanovehicles, limiting our knowledge about the clinical capability of porous nanomaterials to deliver and release the chemotherapeutic molecules to cancerous cells in the body through active targeting or EPR effect.
Summary and Future Outlook
In this review we highlighted and provided some examples of the recent advances in the biofunctionalization of PSi and PSiO2 nanomaterials used for potential cancer therapy. These nanocarriers have attracted great attention in the scientific community due to their unique properties and potential application in drug delivery applications and cancer treatment and diagnostics.130,131 It is now well-acknowledged that the high specific area, high pore volume, tunable pore structures, and physicochemical stability render these materials excellent multifunctionalities. Furthermore, these materials can be strictly designed for triggering a proper response and in the future deliver the payloads according to the clinical needs of the patient and pathology. Interfacing these nanostructures with biological entities is a significant advance to resolve many key challenges being faced by the mankind, which include the development of novel drug delivery vehicles for early diagnosis, prognosis and treatment of complicated human diseases, such as cancer diseases. PSi and PSiO2 nanoprobes not only enable the detection of lesions at cellular and molecular levels, but can specifically be targeted to a tumor, sense pathophysiological defects in tumor cells, deliver therapeutic genes or drugs based on tumor characteristics, respond to external triggers to release the agent and identify residual tumor cells without any deleterious consequences on healthy cells.
Although great research advances have been achieved in the last decade regarding these mesoporous materials with important findings suggestion the potential of these materials, the biomedical application of these materials is only feasible with a deep understanding on the in vivo biocompatibility/toxicity and in vivo biodistribution. Future studies should focus on more clinically-oriented programs to confirm or dismiss the pre-clinical results on cancer applications. Nevertheless, the current findings are already rather encouraging in order to develop PSi and PSiO2 based materials for targeting of drugs to cancer tumors that can be further tailored toward clinical translation.
Acknowledgments
H.A.S. acknowledges financial support from the Academy of Finland (decision numbers 252215 and 256394) and Centre for International Mobility, CIMO (decision no. TM-12-8201).
Glossary
Abbreviations:
- CpG ODN
cytosine-phosphodiester-guanine oligodeoxinucleotide
- EPR
enhanced permeability and retention
- FA
folic acid
- FGF
fibroblast growth factor
- GNR
gold nanorods
- HGF
hepatocyte growth factor
- HIF-1a
hypoxia inducible factor
- LPSiNPs
luminescent porous silicon nanoparticles
- MDR
multiple drug resistance
- MMP
matrix metalloproteinase
- MRI
magnetic resonance imaging
- MSNP
mesoporous silica nanoparticle
- NIR
near-infrared light
- PEG
polyethylene glycol
- PEI
poly(ethylene imine)
- PlGF
placenta growth factor
- PSi
mesoporous silicon
- PSiO2
mesoporous silica
- RES
reticuloendothelial system
- ROS
reactive oxygen species
- RTP
rapid thermal processing
- VEGF
vascular endothelial growth factor
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/22347
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2012. Ann Oncol. 2012;23:1044–52. doi: 10.1093/annonc/mds024. [DOI] [PubMed] [Google Scholar]
- 3.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2:750–63. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 4.Patil YB, Swaminathan SK, Sadhukha T, Ma L, Panyam J. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials. 2010;31:358–65. doi: 10.1016/j.biomaterials.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misra R, Acharya S, Sahoo SK. Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug Discov Today. 2010;15:842–50. doi: 10.1016/j.drudis.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Yang L, Chen ZG, Shin DM. Application of nanotechnology in cancer therapy and imaging. CA Cancer J Clin. 2008;58:97–110. doi: 10.3322/CA.2007.0003. [DOI] [PubMed] [Google Scholar]
- 7.Manasmita D, Debasish M, Maiti T, Basak A, Pramanik P. Bio-functionalization of magnetite nanoparticles using an aminophosphonic acid coupling agent: new, ultradispersed, iron-oxide folate nanoconjugates for cancer-specific targeting. Nanotech. 2008;19:415101. doi: 10.1088/0957-4484/19/41/415101. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 9.Hamidi M, Shahbazi MA, Rostamizadeh K. Copolymers: efficient carriers for intelligent nanoparticulate drug targeting and gene therapy. Macromol Biosci. 2012;12:144–64. doi: 10.1002/mabi.201100193. [DOI] [PubMed] [Google Scholar]
- 10.Chou LY, Ming K, Chan WC. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40:233–45. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 11.von Maltzahn G, Park JH, Lin KY, Singh N, Schwöppe C, Mesters R, et al. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat Mater. 2011;10:545–52. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JH, von Maltzahn G, Xu MJ, Fogal V, Kotamraju VR, Ruoslahti E, et al. Cooperative nanomaterial system to sensitize, target, and treat tumors. Proc Natl Acad Sci U S A. 2010;107:981–6. doi: 10.1073/pnas.0909565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–64. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 14.Vallet-Regí M. Nanostructured mesoporous silica matrices in nanomedicine. J Intern Med. 2010;267:22–43. doi: 10.1111/j.1365-2796.2009.02190.x. [DOI] [PubMed] [Google Scholar]
- 15.Vallet-Regí M, Balas F, Arcos D. Mesoporous materials for drug delivery. Angew Chem Int Ed Engl. 2007;46:7548–58. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]
- 16.Vivero-Escoto JL, Slowing II, Trewyn BG, Lin VS. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small. 2010;6:1952–67. doi: 10.1002/smll.200901789. [DOI] [PubMed] [Google Scholar]
- 17.Santos HA, Bimbo LM, Lehto VP, Airaksinen AJ, Salonen J, Hirvonen J. Multifunctional porous silicon for therapeutic drug delivery and imaging. Curr Drug Discov Technol. 2011;8:228–49. doi: 10.2174/157016311796799053. [DOI] [PubMed] [Google Scholar]
- 18.Salonen J, Kaukonen AM, Hirvonen J, Lehto VP. Mesoporous silicon in drug delivery applications. J Pharm Sci. 2008;97:632–53. doi: 10.1002/jps.20999. [DOI] [PubMed] [Google Scholar]
- 19.Godin B, Tasciotti E, Liu X, Serda RE, Ferrari M. Multistage nanovectors: from concept to novel imaging contrast agents and therapeutics. Acc Chem Res. 2011;44:979–89. doi: 10.1021/ar200077p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev. 2012;41:2590–605. doi: 10.1039/c1cs15246g. [DOI] [PubMed] [Google Scholar]
- 21.Hudson SP, Padera RF, Langer R, Kohane DS. The biocompatibility of mesoporous silicates. Biomaterials. 2008;29:4045–55. doi: 10.1016/j.biomaterials.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunzmann A, Andersson B, Thurnherr T, Krug H, Scheynius A, Fadeel B. Toxicology of engineered nanomaterials: focus on biocompatibility, biodistribution and biodegradation. Biochim Biophys Acta. 2011;1810:361–73. doi: 10.1016/j.bbagen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Bimbo LM, Peltonen L, Hirvonen J, Santos HA. Toxicological Profile of Therapeutic Nanodelivery Systems. Curr Drug Metab. 2012;13:1068–86. doi: 10.2174/138920012802850047. [DOI] [PubMed] [Google Scholar]
- 24.Rosenholm JM, Sahlgren C, Lindén M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles--opportunities & challenges. Nanoscale. 2010;2:1870–83. doi: 10.1039/c0nr00156b. [DOI] [PubMed] [Google Scholar]
- 25.Rosenholm JM, Sahlgren C, Lindén M. Multifunctional mesoporous silica nanoparticles for combined therapeutic, diagnostic and targeted action in cancer treatment. Curr Drug Targets. 2011;12:1166–86. doi: 10.2174/138945011795906624. [DOI] [PubMed] [Google Scholar]
- 26.Rosenholm JM, Mamaeva V, Sahlgren C, Lindén M. Nanoparticles in targeted cancer therapy: mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine (Lond) 2012;7:111–20. doi: 10.2217/nnm.11.166. [DOI] [PubMed] [Google Scholar]
- 27.Jaganathan H, Godin B. Biocompatibility assessment of Si-based nano- and micro-particles. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2012.05.008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salonen J, Lehto V-P. Fabrication and chemical surface modification of mesoporous silicon for biomedical applications. Chem Eng J. 2008;137:162–72. doi: 10.1016/j.cej.2007.09.001. [DOI] [Google Scholar]
- 29.Zhang J, Li X, Rosenholm JM, Gu HC. Synthesis and characterization of pore size-tunable magnetic mesoporous silica nanoparticles. J Colloid Interface Sci. 2011;361:16–24. doi: 10.1016/j.jcis.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 30.Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24:1504–34. doi: 10.1002/adma.201104763. [DOI] [PubMed] [Google Scholar]
- 31.Betty CA. Porous silicon: a resourceful material for nanotechnology. Recent Pat Nanotechnol. 2008;2:128–36. doi: 10.2174/187221008784534514. [DOI] [PubMed] [Google Scholar]
- 32.Anglin EJ, Cheng L, Freeman WR, Sailor MJ. Porous silicon in drug delivery devices and materials. Adv Drug Deliv Rev. 2008;60:1266–77. doi: 10.1016/j.addr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mamaeva V, Rosenholm JM, Bate-Eya LT, Bergman L, Peuhu E, Duchanoy A, et al. Mesoporous silica nanoparticles as drug delivery systems for targeted inhibition of Notch signaling in cancer. Mol Ther. 2011;19:1538–46. doi: 10.1038/mt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinnari P, Mäkilä E, Heikkilä T, Salonen J, Hirvonen J, Santos HA. Comparison of mesoporous silicon and non-ordered mesoporous silica materials as drug carriers for itraconazole. Int J Pharm. 2011;414:148–56. doi: 10.1016/j.ijpharm.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Santos HA, Salonen J, Bimbo LM, Lehto VP, Peltonen L, Hirvonen J. Mesoporous materials as controlled drug delivery formulations. J Drug Deliv Sci Tech. 2011;21:139–55. [Google Scholar]
- 36.Bimbo LM, Mäkilä E, Laaksonen T, Lehto VP, Salonen J, Hirvonen J, et al. Drug permeation across intestinal epithelial cells using porous silicon nanoparticles. Biomaterials. 2011;32:2625–33. doi: 10.1016/j.biomaterials.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Bimbo LM, Mäkilä E, Raula J, Laaksonen T, Laaksonen P, Strommer K, et al. Functional hydrophobin-coating of thermally hydrocarbonized porous silicon microparticles. Biomaterials. 2011;32:9089–99. doi: 10.1016/j.biomaterials.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Bimbo LM, Sarparanta M, Santos HA, Airaksinen AJ, Mäkilä E, Laaksonen T, et al. Biocompatibility of thermally hydrocarbonized porous silicon nanoparticles and their biodistribution in rats. ACS Nano. 2010;4:3023–32. doi: 10.1021/nn901657w. [DOI] [PubMed] [Google Scholar]
- 39.Sarparanta M, Mäkilä E, Heikkilä T, Salonen J, Kukk E, Lehto VP, et al. ¹⁸F-labeled modified porous silicon particles for investigation of drug delivery carrier distribution in vivo with positron emission tomography. Mol Pharm. 2011;8:1799–806. doi: 10.1021/mp2001654. [DOI] [PubMed] [Google Scholar]
- 40.Sarparanta MP, Bimbo LM, Mäkilä EM, Salonen JJ, Laaksonen PH, Helariutta AM, et al. The mucoadhesive and gastroretentive properties of hydrophobin-coated porous silicon nanoparticle oral drug delivery systems. Biomaterials. 2012;33:3353–62. doi: 10.1016/j.biomaterials.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 41.Bimbo LM, Sarparanta M, Mäkilä E, Laaksonen T, Laaksonen P, Salonen J, et al. Cellular interactions of surface modified nanoporous silicon particles. Nanoscale. 2012;4:3184–92. doi: 10.1039/c2nr30397c. [DOI] [PubMed] [Google Scholar]
- 42.Limnell T, Santos HA, Mäkilä E, Heikkilä T, Salonen J, Murzin DY, et al. Drug delivery formulations of ordered and nonordered mesoporous silica: comparison of three drug loading methods. J Pharm Sci. 2011;100:3294–306. doi: 10.1002/jps.22577. [DOI] [PubMed] [Google Scholar]
- 43.Limnell T, Heikkilä T, Santos HA, Sistonen S, Hellstén S, Laaksonen T, et al. Physicochemical stability of high indomethacin payload ordered mesoporous silica MCM-41 and SBA-15 microparticles. Int J Pharm. 2011;416:242–51. doi: 10.1016/j.ijpharm.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 44.Tang H, Guo J, Sun Y, Chang B, Ren Q, Yang W. Facile synthesis of pH sensitive polymer-coated mesoporous silica nanoparticles and their application in drug delivery. Int J Pharm. 2011;421:388–96. doi: 10.1016/j.ijpharm.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Slowing I, Trewyn BG, Lin VS. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J Am Chem Soc. 2006;128:14792–3. doi: 10.1021/ja0645943. [DOI] [PubMed] [Google Scholar]
- 46.Lu J, Liong M, Sherman S, Xia T, Kovochich M, Nel AE, et al. Mesoporous silica nanoparticles for cancer therapy: energy-dependent cellular uptake and delivery of paclitaxel to cancer cells. Nanobiotechnology. 2007;3:89–95. doi: 10.1007/s12030-008-9003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu J, Liong M, Li Z, Zink JI, Tamanoi F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small. 2010;6:1794–805. doi: 10.1002/smll.201000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gu L, Park JH, Duong KH, Ruoslahti E, Sailor MJ. Magnetic luminescent porous silicon microparticles for localized delivery of molecular drug payloads. Small. 2010;6:2546–52. doi: 10.1002/smll.201000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J, Sailor MJ. Chitosan hydrogel-capped porous SiO2 as a pH responsive nano-valve for triggered release of insulin. Adv Funct Mater. 2009;19:733–41. doi: 10.1002/adfm.200800921. [DOI] [Google Scholar]
- 50.Yang X, Liu X, Liu Z, Pu F, Ren J, Qu X. Near-infrared light-triggered, targeted drug delivery to cancer cells by aptamer gated nanovehicles. Adv Mater. 2012;24:2890–5. doi: 10.1002/adma.201104797. [DOI] [PubMed] [Google Scholar]
- 51.Wang LS, Wu LC, Lu SY, Chang LL, Teng IT, Yang CM, et al. Biofunctionalized phospholipid-capped mesoporous silica nanoshuttles for targeted drug delivery: improved water suspensibility and decreased nonspecific protein binding. ACS Nano. 2010;4:4371–9. doi: 10.1021/nn901376h. [DOI] [PubMed] [Google Scholar]
- 52.Wu EC, Park JH, Park J, Segal E, Cunin F, Sailor MJ. Oxidation-triggered release of fluorescent molecules or drugs from mesoporous Si microparticles. ACS Nano. 2008;2:2401–9. doi: 10.1021/nn800592q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenholm JM, Meinander A, Peuhu E, Niemi R, Eriksson JE, Sahlgren C, et al. Targeting of porous hybrid silica nanoparticles to cancer cells. ACS Nano. 2009;3:197–206. doi: 10.1021/nn800781r. [DOI] [PubMed] [Google Scholar]
- 54.Slowing II, Vivero-Escoto JL, Wu CW, Lin VS. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev. 2008;60:1278–88. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Park C, Oh K, Lee SC, Kim C. Controlled release of guest molecules from mesoporous silica particles based on a pH-responsive polypseudorotaxane motif. Angew Chem Int Ed Engl. 2007;46:1455–7. doi: 10.1002/anie.200603404. [DOI] [PubMed] [Google Scholar]
- 56.Luo Z, Cai K, Hu Y, Zhao L, Liu P, Duan L, et al. Mesoporous silica nanoparticles end-capped with collagen: redox-responsive nanoreservoirs for targeted drug delivery. Angew Chem Int Ed Engl. 2011;50:640–3. doi: 10.1002/anie.201005061. [DOI] [PubMed] [Google Scholar]
- 57.Schlossbauer A, Kecht J, Bein T. Biotin-avidin as a protease-responsive cap system for controlled guest release from colloidal mesoporous silica. Angew Chem Int Ed Engl. 2009;48:3092–5. doi: 10.1002/anie.200805818. [DOI] [PubMed] [Google Scholar]
- 58.Murakami K, Yu X, Watanabe S, Kato T, Inoue Y, Sugawara K. Synthesis of thermosensitive polymer/mesoporous silica composite and its temperature dependence of anion exchange property. J Colloid Interface Sci. 2011;354:771–6. doi: 10.1016/j.jcis.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 59.Zhu Y, Liu H, Li F, Ruan Q, Wang H, Fujiwara M, et al. Dipolar molecules as impellers achieving electric-field-stimulated release. J Am Chem Soc. 2010;132:1450–1. doi: 10.1021/ja907560y. [DOI] [PubMed] [Google Scholar]
- 60.Hu SH, Liu TY, Huang HY, Liu DM, Chen SY. Magnetic-sensitive silica nanospheres for controlled drug release. Langmuir. 2008;24:239–44. doi: 10.1021/la701570z. [DOI] [PubMed] [Google Scholar]
- 61.Zhu Y, Meng W, Hanagata N. Cytosine-phosphodiester-guanine oligodeoxynucleotide (CpG ODN)-capped hollow mesoporous silica particles for enzyme-triggered drug delivery. Dalton Trans. 2011;40:10203–8. doi: 10.1039/c1dt11114k. [DOI] [PubMed] [Google Scholar]
- 62.Andrew JS, Anglin EJ, Wu EC, Chen MY, Cheng L, Freeman WR, et al. Sustained Release of a Monoclonal Antibody from Electrochemically Prepared Mesoporous Silicon Oxide. Adv Funct Mater. 2010;20:4168–74. doi: 10.1002/adfm.201000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanaka T, Decuzzi P, Cristofanilli M, Sakamoto JH, Tasciotti E, Robertson FM, et al. Nanotechnology for breast cancer therapy. Biomed Microdevices. 2009;11:49–63. doi: 10.1007/s10544-008-9209-0. [DOI] [PubMed] [Google Scholar]
- 64.Scanlon KJ. Cancer gene therapy: challenges and opportunities. Anticancer Res. 2004;24(2A):501–4. [PubMed] [Google Scholar]
- 65.Farrell D, Ptak K, Panaro NJ, Grodzinski P. Nanotechnology-based cancer therapeutics--promise and challenge--lessons learned through the NCI Alliance for Nanotechnology in Cancer. Pharm Res. 2011;28:273–8. doi: 10.1007/s11095-010-0214-7. [DOI] [PubMed] [Google Scholar]
- 66.Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol. 2010;624:25–37. doi: 10.1007/978-1-60761-609-2_3. [DOI] [PubMed] [Google Scholar]
- 67.Lin YS, Haynes CL. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J Am Chem Soc. 2010;132:4834–42. doi: 10.1021/ja910846q. [DOI] [PubMed] [Google Scholar]
- 68.He Q, Zhang Z, Gao Y, Shi J, Li Y. Intracellular localization and cytotoxicity of spherical mesoporous silica nano- and microparticles. Small. 2009;5:2722–9. doi: 10.1002/smll.200900923. [DOI] [PubMed] [Google Scholar]
- 69.Park JH, Gu L, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mater. 2009;8:331–6. doi: 10.1038/nmat2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu SH, Lin YS, Hung Y, Chou YH, Hsu YH, Chang C, et al. Multifunctional mesoporous silica nanoparticles for intracellular labeling and animal magnetic resonance imaging studies. Chembiochem. 2008;9:53–7. doi: 10.1002/cbic.200700509. [DOI] [PubMed] [Google Scholar]
- 71.Slowing II, Wu CW, Vivero-Escoto JL, Lin VS. Mesoporous silica nanoparticles for reducing hemolytic activity towards mammalian red blood cells. Small. 2009;5:57–62. doi: 10.1002/smll.200800926. [DOI] [PubMed] [Google Scholar]
- 72.Liu J, Stace-Naughton A, Jiang X, Brinker CJ. Porous nanoparticle supported lipid bilayers (protocells) as delivery vehicles. J Am Chem Soc. 2009;131:1354–5. doi: 10.1021/ja808018y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ashley CE, Carnes EC, Phillips GK, Padilla D, Durfee PN, Brown PA, et al. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat Mater. 2011;10:389–97. doi: 10.1038/nmat2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He Q, Zhang J, Shi J, Zhu Z, Zhang L, Bu W, et al. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials. 2010;31:1085–92. doi: 10.1016/j.biomaterials.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 75.Moghimi SM, Hamad I. Liposome-mediated triggering of complement cascade. J Liposome Res. 2008;18:195–209. doi: 10.1080/08982100802309552. [DOI] [PubMed] [Google Scholar]
- 76.He Q, Shi J, Chen F, Zhu M, Zhang L. An anticancer drug delivery system based on surfactant-templated mesoporous silica nanoparticles. Biomaterials. 2010;31:3335–46. doi: 10.1016/j.biomaterials.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 77.Huang X, Teng X, Chen D, Tang F, He J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials. 2010;31:438–48. doi: 10.1016/j.biomaterials.2009.09.060. [DOI] [PubMed] [Google Scholar]
- 78.Yu T, Malugin A, Ghandehari H. Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano. 2011;5:5717–28. doi: 10.1021/nn2013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tao Z, Toms B, Goodisman J, Asefa T. Mesoporous silica microparticles enhance the cytotoxicity of anticancer platinum drugs. ACS Nano. 2010;4:789–94. doi: 10.1021/nn9015345. [DOI] [PubMed] [Google Scholar]
- 80.Huang DM, Hung Y, Ko BS, Hsu SC, Chen WH, Chien CL, et al. Highly efficient cellular labeling of mesoporous nanoparticles in human mesenchymal stem cells: implication for stem cell tracking. FASEB J. 2005;19:2014–6. doi: 10.1096/fj.05-4288fje. [DOI] [PubMed] [Google Scholar]
- 81.Lu F, Wu SH, Hung Y, Mou CY. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small. 2009;5:1408–13. doi: 10.1002/smll.200900005. [DOI] [PubMed] [Google Scholar]
- 82.Tao Z, Toms BB, Goodisman J, Asefa T. Mesoporosity and functional group dependent endocytosis and cytotoxicity of silica nanomaterials. Chem Res Toxicol. 2009;22:1869–80. doi: 10.1021/tx900276u. [DOI] [PubMed] [Google Scholar]
- 83.Popat A, Liu J, Hu Q, Kennedy M, Peters B, Lu GQ, et al. Adsorption and release of biocides with mesoporous silica nanoparticles. Nanoscale. 2012;4:970–5. doi: 10.1039/c2nr11691j. [DOI] [PubMed] [Google Scholar]
- 84.Zhang H, Li Z, Xu P, Wu R, Wang L, Xiang Y, et al. Synthesis of novel mesoporous silica nanoparticles for loading and release of ibuprofen. J Control Release. 2011;152(Suppl 1):e38–9. doi: 10.1016/j.jconrel.2011.08.108. [DOI] [PubMed] [Google Scholar]
- 85.Kiekens F, Eelen S, Verheyden L, Daems T, Martens J, Van Den Mooter G. Use of ordered mesoporous silica to enhance the oral bioavailability of ezetimibe in dogs. J Pharm Sci. 2012;101:1136–44. doi: 10.1002/jps.23016. [DOI] [PubMed] [Google Scholar]
- 86.Gan Q, Dai D, Yuan Y, Qian J, Sha S, Shi J, et al. Effect of size on the cellular endocytosis and controlled release of mesoporous silica nanoparticles for intracellular delivery. Biomed Microdevices. 2012;14:259–70. doi: 10.1007/s10544-011-9604-9. [DOI] [PubMed] [Google Scholar]
- 87.Chung TH, Wu SH, Yao M, Lu CW, Lin YS, Hung Y, et al. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials. 2007;28:2959–66. doi: 10.1016/j.biomaterials.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 88.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 89.He X, Nie H, Wang K, Tan W, Wu X, Zhang P. In vivo study of biodistribution and urinary excretion of surface-modified silica nanoparticles. Anal Chem. 2008;80:9597–603. doi: 10.1021/ac801882g. [DOI] [PubMed] [Google Scholar]
- 90.Taylor AE, Granger DN. Equivalent pore modeling: vesicles and channels. Fed Proc. 1983;42:2440–5. [PubMed] [Google Scholar]
- 91.Dvorak HF, Nagy JA, Dvorak JT, Dvorak AM. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am J Pathol. 1988;133:95–109. [PMC free article] [PubMed] [Google Scholar]
- 92.Maeda H, Noguchi Y, Sato K, Akaike T. Enhanced vascular permeability in solid tumor is mediated by nitric oxide and inhibited by both new nitric oxide scavenger and nitric oxide synthase inhibitor. Jpn J Cancer Res. 1994;85:331–4. doi: 10.1111/j.1349-7006.1994.tb02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hon NK, Shaposhnik Z, Diebold ED, Tamanoi F, Jalali B. Tailoring the biodegradability of porous silicon nanoparticles. J Biomed Mater Res A. 2012 doi: 10.1002/jbm.a.34294. In press. [DOI] [PubMed] [Google Scholar]
- 94.De Angelis F, Pujia A, Falcone C, Iaccino E, Palmieri C, Liberale C, et al. Water soluble nanoporous nanoparticle for in vivo targeted drug delivery and controlled release in B cells tumor context. Nanoscale. 2010;2:2230–6. doi: 10.1039/c0nr00161a. [DOI] [PubMed] [Google Scholar]
- 95.Kim J, Kim HS, Lee N, Kim T, Kim H, Yu T, et al. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew Chem Int Ed Engl. 2008;47:8438–41. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]
- 96.Lee JE, Lee N, Kim H, Kim J, Choi SH, Kim JH, et al. Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. J Am Chem Soc. 2010;132:552–7. doi: 10.1021/ja905793q. [DOI] [PubMed] [Google Scholar]
- 97.Lu J, Li Z, Zink JI, Tamanoi F. In vivo tumor suppression efficacy of mesoporous silica nanoparticles-based drug-delivery system: enhanced efficacy by folate modification. Nanomedicine. 2012;8:212–20. doi: 10.1016/j.nano.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martin KR. The chemistry of silica and its potential health benefits. J Nutr Health Aging. 2007;11:94–7. [PubMed] [Google Scholar]
- 99.Steinbacher JL, Lathrop SA, Cheng K, Hillegass JM, Butnor KJ, Kauppinen RA, et al. Gd-labeled microparticles in MRI: in vivo imaging of microparticles after intraperitoneal injection. Small. 2010;6:2678–82. doi: 10.1002/smll.201001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang X, Li L, Liu T, Hao N, Liu H, Chen D, et al. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano. 2011;5:5390–9. doi: 10.1021/nn200365a. [DOI] [PubMed] [Google Scholar]
- 101.Lee C-H, Cheng S-H, Wang Y-J, Chen Y-C, Chen N-T, Souris J, et al. Near-Infrared Mesoporous Silica Nanoparticles for Optical Imaging: Characterization and In Vivo Biodistribution. Adv Funct Mater. 2009;19:215–22. doi: 10.1002/adfm.200800753. [DOI] [Google Scholar]
- 102.Souris JS, Lee CH, Cheng SH, Chen CT, Yang CS, Ho JA, et al. Surface charge-mediated rapid hepatobiliary excretion of mesoporous silica nanoparticles. Biomaterials. 2010;31:5564–74. doi: 10.1016/j.biomaterials.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.He Q, Zhang Z, Gao F, Li Y, Shi J. In vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: effects of particle size and PEGylation. Small. 2011;7:271–80. doi: 10.1002/smll.201001459. [DOI] [PubMed] [Google Scholar]
- 104.Park JH, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Micellar hybrid nanoparticles for simultaneous magnetofluorescent imaging and drug delivery. Angew Chem Int Ed Engl. 2008;47:7284–8. doi: 10.1002/anie.200801810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Schooneveld MM, Vucic E, Koole R, Zhou Y, Stocks J, Cormode DP, et al. Improved biocompatibility and pharmacokinetics of silica nanoparticles by means of a lipid coating: a multimodality investigation. Nano Lett. 2008;8:2517–25. doi: 10.1021/nl801596a. [DOI] [PubMed] [Google Scholar]
- 106.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 107.Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clinical cancer research: an official journal of the American Association for Cancer Research 2008; 14:1310-6. [DOI] [PubMed]
- 108.Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology applications in cancer. Annu Rev Biomed Eng. 2007;9:257–88. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- 109.Look M, Bandyopadhyay A, Blum JS, Fahmy TM. Application of nanotechnologies for improved immune response against infectious diseases in the developing world. Adv Drug Deliv Rev. 2010;62:378–93. doi: 10.1016/j.addr.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56:1649–59. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 111.Nabeshi H, Yoshikawa T, Matsuyama K, Nakazato Y, Arimori A, Isobe M, et al. Size-dependent cytotoxic effects of amorphous silica nanoparticles on Langerhans cells. Pharmazie. 2010;65:199–201. [PubMed] [Google Scholar]
- 112.Sudimack J, Lee RJ. Targeted drug delivery via the folate receptor. Adv Drug Deliv Rev. 2000;41:147–62. doi: 10.1016/S0169-409X(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 113.Gary-Bobo M, Hocine O, Brevet D, Maynadier M, Raehm L, Richeter S, et al. Cancer therapy improvement with mesoporous silica nanoparticles combining targeting, drug delivery and PDT. Int J Pharm. 2012;423:509–15. doi: 10.1016/j.ijpharm.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 114.Hamdy S, Haddadi A, Hung RW, Lavasanifar A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv Drug Deliv Rev. 2011;63:943–55. doi: 10.1016/j.addr.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 115.Hamdy S, Haddadi A, Ghotbi Z, Hung RW, Lavasanifar A. Part I: targeted particles for cancer immunotherapy. Curr Drug Deliv. 2011;8:261–73. doi: 10.2174/156720111795256101. [DOI] [PubMed] [Google Scholar]
- 116.Gu L, Ruff LE, Qin Z, Corr M, Hedrick SM, Sailor MJ. Multivalent porous silicon nanoparticles enhance the immune activation potency of agonistic CD40 antibody. Adv Mater. 2012;24:3981–7. doi: 10.1002/adma.201200776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Falahati M, Saboury AA, Ma’mani L, Shafiee A, Rafieepour HA. The effect of functionalization of mesoporous silica nanoparticles on the interaction and stability of confined enzyme. Int J Biol Macromol. 2012;50:1048–54. doi: 10.1016/j.ijbiomac.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 118.Méndez J, Monteagudo A, Griebenow K. Stimulus-responsive controlled release system by covalent immobilization of an enzyme into mesoporous silica nanoparticles. Bioconjug Chem. 2012;23:698–704. doi: 10.1021/bc200301a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lim JS, Lee K, Choi JN, Hwang YK, Yun MY, Kim HJ, et al. Intracellular protein delivery by hollow mesoporous silica capsules with a large surface hole. Nanotechnology. 2012;23:085101. doi: 10.1088/0957-4484/23/8/085101. [DOI] [PubMed] [Google Scholar]
- 120.Cheng K, Blumen SR, MacPherson MB, Steinbacher JL, Mossman BT, Landry CC. Enhanced uptake of porous silica microparticles by bifunctional surface modification with a targeting antibody and a biocompatible polymer. ACS Appl Mater Interfaces. 2010;2:2489–95. doi: 10.1021/am100530t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lei C, Liu P, Chen B, Mao Y, Engelmann H, Shin Y, et al. Local release of highly loaded antibodies from functionalized nanoporous support for cancer immunotherapy. J Am Chem Soc. 2010;132:6906–7. doi: 10.1021/ja102414t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meng H, Xue M, Xia T, Ji Z, Tarn DY, Zink JI, et al. Use of size and a copolymer design feature to improve the biodistribution and the enhanced permeability and retention effect of doxorubicin-loaded mesoporous silica nanoparticles in a murine xenograft tumor model. ACS Nano. 2011;5:4131–44. doi: 10.1021/nn200809t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–78. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 124.Giri S, Trewyn BG, Lin VS. Mesoporous silica nanomaterial-based biotechnological and biomedical delivery systems. Nanomedicine (Lond) 2007;2:99–111. doi: 10.2217/17435889.2.1.99. [DOI] [PubMed] [Google Scholar]
- 125.Chen AM, Zhang M, Wei D, Stueber D, Taratula O, Minko T, et al. Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small. 2009;5:2673–7. doi: 10.1002/smll.200900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Trewyn BG, Giri S, Slowing II, Lin VS. Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor systems. Chem Commun (Camb) 2007:3236–45. doi: 10.1039/b701744h. [DOI] [PubMed] [Google Scholar]
- 127.Mortera R, Fiorilli S, Garrone E, Vernão E, Onida B. Pores occlusion in MCM-41 spheres immersed in SBF and the effect on ibuprofen delivery kinetics: A quantitative model. Chem Eng J. 2009;156:184–92. doi: 10.1016/j.cej.2009.10.018. [DOI] [Google Scholar]
- 128.Zhao Y, Sun X, Zhang G, Trewyn BG, Slowing II, Lin VS. Interaction of mesoporous silica nanoparticles with human red blood cell membranes: size and surface effects. ACS Nano. 2011;5:1366–75. doi: 10.1021/nn103077k. [DOI] [PubMed] [Google Scholar]
- 129.Huang X, Zhuang J, Teng X, Li L, Chen D, Yan X, et al. The promotion of human malignant melanoma growth by mesoporous silica nanoparticles through decreased reactive oxygen species. Biomaterials. 2010;31:6142–53. doi: 10.1016/j.biomaterials.2010.04.055. [DOI] [PubMed] [Google Scholar]
- 130.Santos HA, Bimbo LM, Herranz B, Shahbazi MA, Hirvonen J, Salonen J. Nanostructured porous silicon in preclinical imaging: Moving from bench to bedside. J Mater Res. 2012 doi: 10.1557/jmr.2012.271. In Press. [DOI] [Google Scholar]
- 131.Santos HA, Hirvonen Nanostructured porous silicon materials: Potential candidates for improving drug delivery. Nanomedicine. 2012;7:1281–84. doi: 10.2217/nnm.12.106. [DOI] [PubMed] [Google Scholar]