Abstract
The first human isolate of Borrelia lusitaniae recovered from a Portuguese patient with suspected Lyme borreliosis is described. This isolate, from a chronic skin lesion, is also the first human isolate of Borrelia in Portugal. Different phenotypic and molecular methods are used to characterize it.
In Portugal, Lyme borreliosis has been known since 1989 (9) and became a notifiable disease in 1999. Although the causative agent has not been isolated from humans, several genospecies of Borrelia burgdorferi sensu lato have been detected in Ixodes ricinus ticks. They are B. garinii, B. afzelii, B. valaisiana, and B. lusitaniae (1, 6, 7). The last, which was isolated from ticks for the first time in 1993 (in Portugal) (10), appears to circulate mainly around the western Mediterranean basin (1, 3, 6, 7, 14). To date, the reservoir of B. lusitaniae has not been defined, and little is known about the ecology of this genospecies. B. lusitaniae is known to cause experimental disease in the C3H/HeN mouse model (13), suggesting that some strains of this genospecies could also be associated with human Lyme borreliosis.
The present study describes and characterizes the first human isolate of B. lusitaniae and the first human isolate of Borrelia in Portugal. A 46-year-old woman from the Lisbon area in Portugal presented with skin lesions on her left thigh that had persisted for approximately 10 years. These chronic skin lesions were characterized by two ill-defined erythematous macules associated with a local diffuse infiltration of the subcutaneous tissues.
Punch biopsy samples (5 mm3) were taken from the margin of the skin lesion and divided into two parts. One part was inoculated immediately into Barbour-Stoenner-Kelly H medium (Sigma) supplemented with gelatin, pH 7.6, and incubated at 32°C for 4 weeks. Cultures were examined weekly by dark-field microscopy for motile spirochetes. The second half of the skin biopsy sample was washed in physiological saline and then transferred to a dry tube and stored at −30°C until PCR was performed. The patient's blood was collected on the day of the biopsy and again 4 months later, and prepared sera were stored at −20°C.
For comparative phenotypic and genetic purposes, an additional 14 strains were evaluated (Table 1). The human isolate PoHL1 and the six Portuguese tick-derived strains PoTiBG4, PoTiBV6, PoTiBG20, PoTiBL37, PoTiBG86, and PoTiBG163 (1) were from low-passage cultures when analyzed. Whole-cell lysates of the spirochetes were centrifuged at 10,000 × g at 4°C for 30 min, washed twice in phosphate-buffered saline, resuspended in Tris-hydrochloride buffer, and stored at −20°C.
TABLE 1.
Strains and GenBank rrf-rrl nucleotide sequences used in this study
| Species | Strain or isolate | Origin | rrf-rrl accession no. |
|---|---|---|---|
| B. afzelii | PGaua | Human, Germany | NAb |
| PKo | Human, Germany | NA | |
| B. burgdorferi sensu stricto | B31 | I. scapularis, United States | L30127 |
| VS219 | Human, France | AY032919 | |
| IP3a | Human, France | NA | |
| B. garinii | Pbia | Human, Austria | Z77175 |
| PoTiBG4 | I. ricinus, Portugal | AY463164 | |
| PoTiBG20 | I. ricinus, Portugal | AY463166 | |
| PoTiBG86 | I. ricinus, Portugal | AY463168 | |
| PoTiBG163 | I. ricinus, Portugal | AY463169 | |
| B. lusitaniae | PoHL1 | Human, Portugal | AY209179 |
| PoTiBL37c | I. ricinus, Portugal | AY463167 | |
| B. valaisiana | PoTiBV6 | I. ricinus, Portugal | AY463165 |
| B. japonica | HO14 | I. ovatus, Japan | L30125 |
Strain cultured and used as a positive control.
NA, not applicable.
Sequence identical to that derived from GT058.
Genomic DNA from all strains and from the skin biopsy sample was extracted with the QIAamp DNA minikit (Qiagen) in accordance with the manufacturer's instructions. The tissue fragments were incubated overnight at 56°C in lysis buffer, concentrated by evaporation, and analyzed by PCR. A nested PCR targeting the 5S (rrf)-23S (rrl) intergenic spacer and the gene (ospA) encoding the outer surface protein A of B. burgdorferi sensu lato was performed as described previously (4, 5, 11).
Intergenic spacer amplicons were digested with MseI at 37°C for 2 h. Electrophoresis was carried out on a 16% acrylamide-bisacrylamide gel for 1 h at 120 V, the gels were stained with ethidium bromide, and the amplicons were visualized with UV transillumination.
The ospA gene and the intergenic spacer of PoHL1, the human isolate, were sequenced (3). In addition, spacer amplicons were subjected to DNA-DNA hybridization by the reverse-line blot assay (5, 11).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed according to the method of Laemmli (8). The patient's antibodies were detected in a two-step approach: an in-house indirect immunofluorescence assay with a polyvalent conjugate (2), followed by an in-house Western blot (12). Immunoblots were calibrated with the following B. burgdorferi monoclonal antibodies: anti-OspA (O31a), anti-OspB (H6831), antiflagellin (H9724), anti-OspC (CB625), anti-p72 (CB312), and anti-Hsp60 (O62a).
Borrelia was successfully isolated from the skin lesion of the human patient. The sequence of the intergenic spacer sequence of the B. lusitaniae human isolate (strain PoHL1) is shown in Fig. 1.
FIG. 1.
Sequence identification of the B. lusitaniae human isolate (PoHL1). GenBank accession number, AY209179.
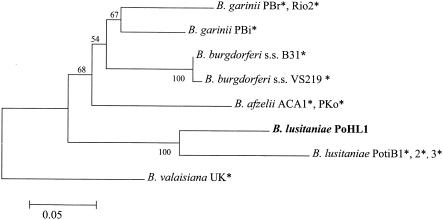
DNA-DNA hybridization by the reverse line blot assay showed that only the DNA probe specific for B. burgdorferi sensu lato hybridized to the human skin isolate, since no DNA probe specific for B. lusitaniae was available. Phylogenetic analysis of the partial ospA sequence revealed that the patient isolate clustered with B. lusitaniae (Fig. 2). The human isolate had an ospA allele that was not identical to those of previously described tick isolates of B. lusitaniae. A nucleotide similarity of 84.2% to alleles of tick isolates PotiB1, PotiB2, and PotiB3 was observed. At the rrf-rrl locus, the alleles of the human isolate and the B. lusitaniae tick isolate PoTiBL37 differed by only 1 nucleotide, leading to a sequence identity of 99.5%.
FIG. 2.
Midpoint-rooted neighbor-joining tree of partial ospA sequences from the isolate obtained in this study (boldface) and GenBank downloads (*). The Kimura two-parameter model was used to estimate the pairwise distances of the aligned nucleotide sequences (MEGA program, version 2.1). Bootstrap values (500 replicates) are given next to the nodes. The scale indicates the estimated number of nucleotide substitutions per sequence position. GenBank accession numbers: A24014 (ACA1), X14407 (B31), X80257 (PBi), X80256 (PBr), A33374 (PKo), Y10837 (PotiB1), Y10838 (PotiB2), Y10839 (PotiB3), AF227319 (Rio2), AF095941 (UK), and AF369948 (VS219).
The PCR-restriction fragment length polymorphism profile resulting from the MseI-digested 230-bp 5S-23S intergenic spacer showed four fragments (78, 69, 39, and 29 bp) for both the human and the tick B. lusitaniae isolates. The observed restriction patterns were indistinguishable from each other but different from those of the other members of the B. burgdorferi sensu lato complex (Fig. 3).
FIG. 3.
MseI restriction patterns of B. burgdorferi sensu lato rrf-rrl spacer nested-PCR products amplified from cultured strains. Lanes: 1, PoHL1, human isolate; 2, PoTiBG163, tick isolate, B. garinii; 3, PBi, B. garinii; 4 and 5, PKo and PGau, respectively, B. afzelii; 6, PoTiBV6, tick isolate, B. valaisiana; 7 to 9, B31, IP3, and Vs219, respectively, B. burgdorferi sensu stricto; 10, HO14, B. japonica.
Analysis of the whole-cell lysate protein profile of the human B. lusitaniae isolate with monoclonal antibodies revealed the presence of OspA, OspB, OspC, flagellin, p72, and Hsp60 proteins. The patient's serum specimens were analyzed for antibodies reactive with B. lusitaniae, B. garinii, and B. afzelii by immunoblotting. No immunoglobulin M (IgM) response was detected. However, a weak IgG reaction with proteins p72 and Hsp60 and a protein with a molecular size of approximately 14 kb was observed with the three studied strains. The immunofluorescence assay only showed a slight reactivity (1:32) in the second serum sample.
The results of the molecular analyses of the patient isolate allows the assignment of this strain to B. lusitaniae, a genospecies previously considered to be nonpathogenic in humans. This finding supports our recent genotyping of B. lusitaniae from directly amplified spirochetal DNA from skin biopsy samples from Portuguese patients with suspected Lyme disease. Besides being prevalent in Tunisia, B. lusitaniae is considered to be predominant in the south of Portugal (GrÂndola region), where it is the unique strain isolated so far from I. ricinus ticks or detected by DNA amplification in hard-tick species other than I. ricinus (1, 3). This predominance of B. lusitaniae in southern habitats, as well as its first isolation in a patient living near Lisbon, suggests an important transmission risk for this genospecies in these areas.
In conclusion, for this human case, the described weak serological response, which is present in a high percentage of our patients with unspecific and long-lasting skin manifestations, suggests a clinical pattern for B. lusitaniae different from those for other Borrelia spp. in the Portuguese population examined so far.
Nucleotide sequence accession number.
The intergenic spacer sequence of the B. lusitaniae human isolate (strain PoHL1) has been assigned GenBank accession number AY209179.
Acknowledgments
This work was partially supported by a grant of the Portuguese Society of Dermatology.
We are grateful to Bettina Wilske, Guy Baranton, Gerold Stanek, and Toshi Mazusawa for supplying Borrelia cultures and/or monoclonal antibodies.
REFERENCES
- 1.Baptista, S., A. Quaresma, T. Aires, K. Kurtenbach, M. Santos-Reis, M. Nicholson, and M. Collares-Pereira. Lyme borreliosis spirochetes in questing ticks from mainland Portugal. Int. J. Med. Microbiol., in press. [DOI] [PubMed]
- 2.Collares-Pereira, M., S. C. Santos, and M. L. Vieira. 2000. Valor diagnóstico da técnica de imunofluorescência indirecta utilizando diferentes preparações antigénicas no imunodiagnóstico da Borreliose de Lyme em Portugal. Supl. Trab. Soc. Port. Dermatol. Venereol. 58:97-105. [Google Scholar]
- 3.De Michelis, S., H.-S. Sewell, M. Collares-Pereira, M. Santos-Reis, L. M. Schouls, V. Benes, E. C. Holmes, and K. Kurtenbach. 2000. Genetic diversity of Borrelia burgdorferi sensu lato in ticks from mainland Portugal. J. Clin. Microbiol. 38:2128-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guy, E. C., and G. Stanek. 1991. Detection of Borrelia burgdorferi in patients with Lyme disease by the polymerase chain-reaction. J. Clin. Pathol. 44:610-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurtenbach, K., M. Peacey, S. G. T. Rijpkema, A. N. Hoodless, P. A. Nuttall, and S. E. Randolph. 1998. Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl. Environ. Microbiol. 64:1169-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurtenbach, K., S. De Michelis, H.-S. Sewell, S. Etti, M. Schäfer, R. Hails, M. Collares-Pereira, M. Santos-Reis, K. Haninçová, M. Labuda, A. Bormane, and M. Donaghy. 2001. Distinct combinations of Borrelia burgdorferi sensu lato genospecies found in individual questing ticks from Europe. Appl. Environ. Microbiol. 67:4926-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtenbach, K., S. De Michelis, H.-S. Sewell, S. Etti, S. M. Schäfer, E. Holmes, R. Hails, M. Collares-Pereira, M. Santos-Reis, K. Haninçová, M. Labuda, A. Bormane, and M. Donaghy. 2002. The key roles of selection and migration in the ecology of Lyme borreliosis. Int. J. Med. Microbiol. 291:152-154. [DOI] [PubMed] [Google Scholar]
- 8.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London). 227:680-685. [DOI] [PubMed] [Google Scholar]
- 9.Morais, J. A., J. Abranches, J. Parra, et al. 1994. Artrite de Lyme em Portugal. A propósito dos primeiros casos diagnosticados em Portugal. Rev. Port. Doenç. Infec. 17:183-195. [Google Scholar]
- 10.Núncio, M. S., O. Péter, M. J. Alves, F. Bacellar, and A. R. Filipe. 1993. Isolamento e caracterização de borrélias de Ixodes ricinus L. em Portugal. Rev. Port. Doenç. Infec. 16:175-179. [Google Scholar]
- 11.Rijpkema, S. G. T., M. J. C. H. Molkenboer, L. M. Schouls, F. Jongejan, and J. F. P. Schellekens. 1995. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J. Clin. Microbiol. 33:3091-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilske, B., V. Fingerle, V. Preac-Mursic, S. Jauris-Heipke, A. Hofmann, H. Loy, H.-W. Pfister, D. Rössler, and E. Soutschek. 1994. Immunoblot using recombinant antigens derived from different species of Borrelia burgdorferi sensu lato. Med. Microbiol. Immunol. 183:43-59. [DOI] [PubMed] [Google Scholar]
- 13.Zeidner, N. S., M. S. Núncio, B. S. Schneider, L. Gern, J. Piesman, O. Brandão, and A. R. Filipe. 2001. A Portuguese isolate of Borrelia lusitaniae induces disease in C3H/HeN mice. J. Med. Microbiol. 50:1055-1060. [DOI] [PubMed] [Google Scholar]
- 14.Zhioua, E., A. Bouattour, C. M. Hu, M. Gharbvi, A. Aeschlimann, H. S. Ginsberg, and L. Gern,. 1999. Infection of Ixodes ricinus (Acari: Ixodidae) by Borrelia burgdorferi sensu lato in North Africa. J. Med. Entomol. 36:216-218. [DOI] [PubMed] [Google Scholar]