Abstract
More than 110 million individuals will suffer from cognitive loss worldwide by the year 2050 with a majority of individuals presenting with Alzheimer’s disease (AD). Yet, successful treatments for etiologies that involve β-amyloid (Aβ) toxicity in AD remain elusive and await novel avenues for drug development. Here we show that Wnt1 inducible signaling pathway protein 1 (WISP1/CCN4) controls the post-translational phosphorylation of Akt1, p70S6K, and AMP activated protein kinase (AMPK) to the extent that tuberous sclerosis complex 2 (TSC2) (Ser1387) phosphorylation, a target of AMPK, is decreased and TSC2 (Thr1462) phosphorylation, a target of Akt1, is increased. The ability of WISP1 to limit TSC2 activity allows WISP1 to increase the activity of p70S6K, since gene silencing of TSC2 further enhances WISP1 phosphorylation of p70S6K. However, a minimal level of TSC2 activity is necessary to modulate WISP1 cytoprotection that may require modulation of mTOR activity, since gene knockdown of TSC2 impairs the ability of WISP1 to protect microglia against apoptotic membrane phosphatidylserine (PS) exposure, nuclear DNA degradation, mitochondrial membrane depolarization, and cytochrome c release during Aβ exposure.
Keywords: Alzheimer’s disease, amyloid, Akt, CCN4, microglia, mTOR, PI 3-K, p70S6K, TSC2, tuberin, WISP1
INTRODUCTION
By the year 2050, it is projected that more than 110 million individuals will suffer from cognitive loss worldwide [1,2]. The majority of individuals with dementia that currently number over 40 million are believed to have Alzheimer’s disease (AD) and care for these individuals translates into a global health care cost of greater than 600 billion US dollars. Deposition of β-amyloid (Aβ) in the brain is considered one primary component that leads to cell injury and clinical demise during AD. Yet, current strategies that focus on immunotherapy and the lessening of the cellular load of Aβ may have unproven or limited success in patients with long-standing AD [3]. Although such treatments may be better suited for individuals during the early stages of AD development, it is clear that other novel strategies should be pursued for this devastating neurodegenerative disorder.
In this respect, Wnt1 inducible signaling pathway protein 1 (WISP1) may offer an exciting prospect for the treatment of AD. WISP1 is a member of the CCN family and is also known as CCN4. The CCN protein family consists of six secreted extracellular matrix associated proteins with four cysteine-rich modular domains that include insulin-like growth factor-binding domain, von Willebrand factor type C module, thrombospondin domain, and C-terminal cysteine knot-like domain [4]. WISP1 has a cytoprotective role during bone repair [5], renal injury [6,7], cardiac cell injury [8], and lung epithelial cell damage [9]. Interestingly, WISP1 has been shown to be protective against oxidative stress in neurons [10–12] and to block amyloid toxicity in inflammatory microglia of the brain [13]. Protection of central nervous system microglia may foster immune mediated therapies to limit Aβ accumulation and sequester Aβ [14–19].
WISP1 promotes cytoprotection in multiple cell types through pathways that not only require phosphatidylinositol-3-kinase (PI 3-K) and protein kinase B (Akt) [7, 8, 10, 11, 20, 21], but also utilize pathways in microglia that involve the mammalian target of rapamycin (mTOR) and the proline rich Akt substrate 40 kDa (PRAS40) [13]. mTOR is the catalytic component of two mTOR complexes termed mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) [22, 23]. One pathway that can modulate mTOR activity is the tuberous sclerosis complex (TSC1, hamartin/ TSC2, tuberin) complex [24, 25]. The TSC1/TSC2 complex is a negative regulator of mTORC1 by controlling the activity of Ras homologue enriched in brain (Rheb). Rheb-GTP can directly interact with Raptor and activate mTORC1 complex. In particular, TSC2, also known as tuberous sclerosis protein 2, functions as a GTPase-activating protein (GAP) to convert active Rheb-GTP to the inactive GDP-bound form (Rheb-GDP) resulting in the inhibition of mTOR, the mTORC1 complex [26], and the mTOR signaling pathway p70S6K [27]. Subsequently, TSC2 can be inhibited by Akt phosphorylation that serves to destabilize TSC2 and disrupt its interaction with TSC1 [26, 28]. In contrast, activation of AMP activated protein kinase (AMPK), such as during decreased energy states, can block mTOR and mTORC1 activity by phosphorylating and activating TSC2 [29, 30]. As a result, TSC2 may function as a modulator of mTOR cellular activity that can influence cellular survival during normal physiology and during toxic cellular insults.
We therefore investigated whether WISP1 cytoprotection in inflammatory microglia during Aβ toxicity was dependent upon the presence and activity of TSC2. Here we show that WISP1 during Aβ exposure modulates the post-translational phosphorylation of several components of the mTOR pathway by enhancing phosphorylation of Akt1 and p70S6K, but limiting the phosphorylation of AMPK and TSC2 (Ser1387), a downstream target of AMPK. The ability of WISP1 to limit TSC2 (Ser1387) phosphorylation appears to allow WISP1 to increase the activity of p70S6K, since gene silencing of TSC2 further enhances WISP1 phosphorylation of p70S6K. Yet, WISP1 also appears to have a role in TSC2 activation since we illustrate that WISP1 increases phosphorylation of TSC2 (Thr1462), a target of Akt1. At least a minimal level of TSC2 activity is necessary to modulate WISP1 cytoprotection. Loss of TSC2 through gene silencing increases the activation and phosphorylation of Akt1 and p70S6K by WISP1. This increased activation of Akt1 and p70S6K during gene silencing of TSC2 may be detrimental to cell survival, since gene knockdown of TSC2 impairs the ability of WISP1 to protect microglia against apoptotic membrane phosphatidylserine (PS) exposure and nuclear DNA degradation during Aβ exposure. Furthermore, the capacity for WISP1 to block activation of the apoptotic cascade that leads to mitochondrial membrane depolarization and cytochrome c release in microglia requires the presence of TSC2, since loss of TSC2 prevent WISP1 from limiting these apoptotic pathways.
MATERIALS AND METHODS
Microglial Cell Cultures
Per our prior protocols, the microglial cell line EOC 2 was obtained from American Type Culture Collection (ATTC, Manassas, VA.) [13, 19]. Cells were maintained in Dulbecco’s modified Eagle medium (ATTC, Manassas, VA) and supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St Louis, MO), 50 µg/ml penicillin and streptomycin, and 20% media from the LADMAC cell line (ATCC, Manassas, VA) that contains colony stimulating factor-1 (CSF-1) secreted by the LADMAC cells. Cells were seeded onto 24-well plates or 35 mm culture dishes at a density of 1.5 × 106 cells per well or 4 × 106 cells per dish.
Experimental Treatments
Per our prior protocols, β-amyloid (Aβ1–42) (American Peptide Co., Sunnyvale, CA) was dissolved in PBS at a concentration of 100 µM [18, 19, 31, 32]. To allow for Aβ aggregation, Aβ was incubated at 37°C for a 7 day period and then directly applied to microglial cell cultures per the experimental protocols. For treatments applied prior to Aβ, human recombinant WISP1 protein (R&D Systems, Minneapolis, MN) was applied 1 hour prior to Aβ administration and the treatment was continuous.
Assessment of Cell Survival
Microglial injury was determined by bright field microscopy using a 0.4% trypan blue dye exclusion method 24 hours following treatment with Aβ per our previous protocols [13, 19, 32]. For each experimental condition, 8 × 35 mm2 dishes were used, and for each dish, the mean survival was determined by counting eight randomly selected non-overlapping fields with each containing approximately 20 cells (viable + non-viable). Each experiment was replicated 6 times with different cultures.
Assessment of DNA Fragmentation
Genomic DNA fragmentation was determined by the terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay [33–35]. Briefly, microglial cells were fixed in 4% paraformaldehyde/0.2% picric acid/0.05% glutaraldehyde. The 3’-hydroxy ends of DNA were labeled with biotinylated dUTP using the enzyme terminal deoxytransferase (Promega, Madison, WI) followed by streptavidin-peroxidase and visualized with 3,3’-diaminobenzidine (Vector Laboratories, Burlingame, CA).
Assessment of Membrane Phosphatidylserine (PS) Membrane Externalization
Externalization of membrane PS residues was determined by using Annexin V labeling [33, 36, 37]. A 30 µg/ml stock solution of Annexin V conjugated to phycoerythrin (PE) (R&D Systems, Minneapolis, MN) was diluted to 3 µg/ml in warmed calcium containing binding buffer (10 mmol/L Hepes, pH 7.5, 150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 1.8 mmol/L CaCl2). Plates were incubated with 500 µl of diluted Annexin V for 10 minutes. Images were acquired with "blinded" assessment with a Leitz DMIRB microscope (Leica, McHenry, IL) and a Fuji/Nikon Super CCD (6.1 megapixels) using transmitted light and fluorescent single excitation light at 490 nm and detected emission at 585 nm.
Transfection of TSC2 shRNA Construct
To silence TSC2 expression in microglia, transfection of short hairpin RNA (shRNA) was used against TSC2. Microglia were plated into 35 mm dishes or 24-well plates. The shRNA pool targeting the TSC2 mRNA plasmid was commercially obtained (Santa Cruz, CA). Transfection of shRNA was performed with Lipofectamine™ RNAiMAX reagent according to manufacturer guidelines (Life Technologies Corp, Carlsbad, CA). Experimental assays were performed 72 hours post-transfection. For each shRNA assay, scrambled shRNA and an empty plasmid were used as controls.
Expression of WISP1, AMPK, p70S6K, and TSC2 with Relevant Phosphorylated Moieties
Cells were homogenized and following protein determination, each sample (50 µg/lane) was then subjected to a gradient 4–20% SDS-polyacrylamide gel (NuSep, Bogart, GA). After transfer, the membranes were incubated with a rabbit antibody against (p = phosphorylated) phospho-TSC2 (p-TSC2, Thr1462 or Ser1387, 1:1000) and total TSC2 (Cell Signaling, Beverly, MA), a rabbit antibody against phospho-AMPK (p-AMPK, Thr172, 1:1000) and total AMPK (1:1000) (Cell signaling Technology, Beverly, MA), and a rabbit antibody against phospho-p70S6K (p-70S6K, Thr389, 1:1000) and total p70S6K (1:1000) (Cell signaling Technology, Beverly, MA). Following incubation, the membranes were incubated with a horseradish peroxidase (HRP) conjugated secondary antibody goat anti-rabbit IgG (goat anti-rabbit IgG, 1:5000) (Pierce, Rockford, IL). The antibody-reactive bands were revealed by chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) and band density was performed using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
Assessment of Mitochondrial Membrane Potential
Per our prior protocols, the fluorescent probe JC-1 (Molecular Probes, Eugene, OR), a cationic membrane potential indicator, was used to assess the mitochondrial membrane potential [37–39]. Microglia in 35 mm dishes were incubated with 2 µg/ml JC-1 in growth medium at 37 °C for 30 min. The cultures were washed three times using fresh growth medium. Subsequently, mitochondria were analyzed immediately under a Leitz DMIRB microscope (Leica, McHenry, IL, USA) with a dual emission fluorescence filter with 515–545 nm for green fluorescence and emission at 585–615 nm for red fluorescence.
Preparation of Mitochondria for the Analysis of Cytochrome c Release
Briefly, cells were harvested, homogenized, and the harvested supernatants were centrifuged at 10,000 g for 15 min at 4 °C [37–39]. The resulting pellet was re-suspended in isolation buffer (20 mM HEPES, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 phenylmethylsulfonylfluoride) containing 250 mM sucrose and used as the mitochondrial fraction. The supernatant was subjected to further ultra-centrifugation at 50,000 g for 1 h, with the resultant supernatant being used as the cytosolic fraction. Western blot for cytochrome c in both mitochondrial and cytosolic fractions was performed by using a rabbit antibody against cytochrome c (1:1000) (Cell signaling Technology, Beverly, MA).
Statistical Analysis
For each experiment, the mean and standard deviation (SD) was determined. Statistical differences among groups were assessed by means of analysis of variance (ANOVA) with the post-hoc Dunnett's test. Statistical significance was considered at P<0.05.
RESULTS
WISP1 Modulates the post-Translational Phosphorylation of Akt1, p70S6K, AMPK, and TSC2
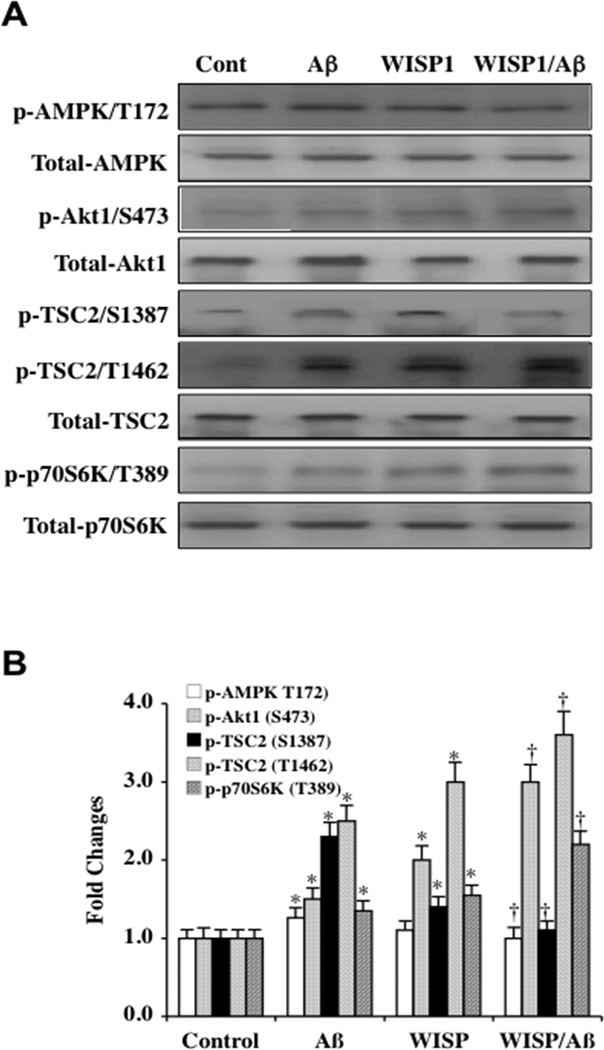
WISP1 has been demonstrated to rely upon the phosphorylation and activation of phosphatidylinositol-3-kinase (PI 3-K) and protein kinase B (Akt) [7, 8, 10, 11, 20, 21] as well as the mammalian target of rapamycin (mTOR) [13] to promote cellular protection. In addition, mTOR phosphorylates and activates p70S6K at threonine389 and is a marker of mTOR activity [40]. We therefore examined the ability of WISP1 to modulate the phosphorylation of AMP activated protein kinase (AMPK), which can inhibit mTOR activity [41, 42], tuberous sclerosis complex 2 (TSC2), that also blocks mTOR activity [26], and Akt1 and p70S6K. WISP1 (10 ng/ml) was applied to microglial cultures 1 hour prior to Aβ exposure (10 µM). Western blots for the expression of phosphorylated (p)-AMPK (Thr172, active form), p-Akt1 (Ser473, active form), p-TSC2 (Ser1387/Thr1462), and p-p70S6K (Thr389, active form) were determined 6 hours following Aβ exposure. Representative images (Fig. 1A) and quantitative results (Fig. 1B) demonstrate that Aβ exposure increased the expression of p-AMPK and p-TSC2 (Ser1387/Thr1462). Mild increases in the expression of p-Akt1 and p-p70S6K also occurred after Aβ exposure. WISP1 (10 ng/ml) application decreased the expression of p-AMPK and the expression of p-TSC2 (Ser1387), a downstream target of AMPK. In contrast, WISP1 significantly increased the expression of p-Akt1 and p-p70S6K during Aβ exposure when compared to cells exposed to Aβ alone. WISP1 also increased the expression of p-TSC2 (Thr1462) that is the target of Akt1.
Fig. (1). WISP1 modulates post-translational phosphorylation of AMPK, Akt1, TSC2, and p70S6K during Aβ exposure.
(A) Equal amounts of microglial protein extracts (50 µg/lane) were immunoblotted at 6 hours following Aβ exposure with total and anti–phospho p-AMPK (Thr172), p-Akt1 (Ser2448), p-TSC2 (Ser1387/Thr1462), and p-p70S6K (Thr389) antibodies. WISP1 (10 ng/ml) was applied to microglia cultures 1 hour prior to Aβ exposure (10 µM). During Aβ exposure, phosphorylation of AMPK, TSC2 Ser1387, TSC2 Thr1462 were increased. In contrast, WISP1 decreased phosphorylation of AMPK and TSC2 Ser1387 in the presence of Aβ. WISP1 increased phosphorylation of Akt1, TSC2 Thr1462, and p70S6K during Aβ exposure. Representative images for western blot analysis are illustrated. (B) Quantitative analysis of western analysis from 3 experiments was performed using the public domain NIH Image program (US National Institutes of Health at http://rsb.info.nih.gov/nih-image/) (*P < 0.01 vs. Control; †P<0.01 vs. Aβ treated alone). Control = untreated microglia. Each data point represents the mean and SD.
Phosphorylation and Activation of p70S6K by WISP1 during Aβ Exposure is Enhanced During Gene Silencing of TSC2
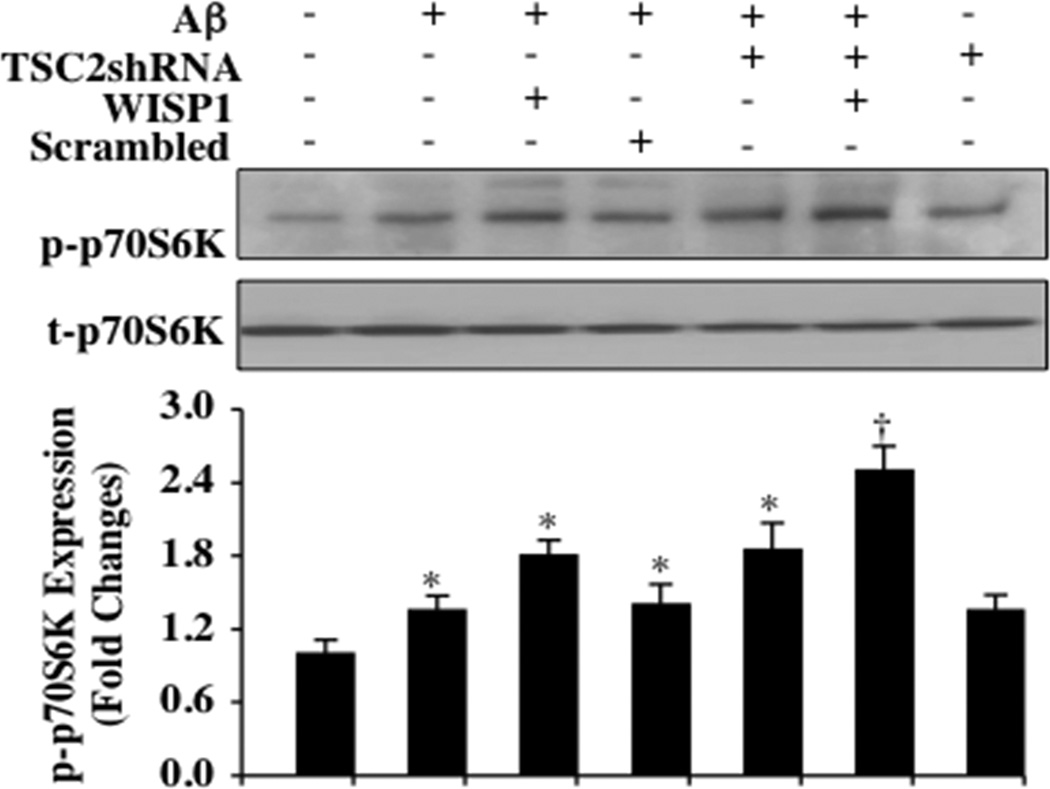
WISP1 (10 ng/ml) was applied to microglial cultures 1 hour prior to the administration of Aβ (10 µM) and western blot analysis for phosphorylated (p)-p70S6K (Thr389) was determined 6 hours following Aβ exposure. As shown in Fig. (2), representative western blot images and quantitative results demonstrate that Aβ exposure mildly increased the expression of p-p70S6K. WISP1 during Aβ exposure significantly increased the expression of p-p70S6K that was further enhanced during gene silencing of TSC2 with transfection of TSC2 shRNA. Gene silencing of TSC2 in untreated microglia and during Aβ exposure alone also significantly increased p-p70S6K expression but to a lesser extent than observed during gene silencing of TSC2 and combined WISP1 application, suggesting that loss of TSC2 fostered phosphorylation and activation of p70S6K that is consistent with increased mTOR activity [40]. Non-specific scrambled shRNA did not alter p-p70S6K expression during Aβ exposure.
Fig. (2). Loss of TSC2 promotes WISP1 phosphorylation and activation of p70S6K during Aβ exposure.
Western blot for phosphorylated p-p70S6K (Thr389) and total p70S6K (Thr389) was determined 6 hours following Aβ exposure. Representative images of Western blot and quantitative results demonstrate that Aβ exposure (10 µM) for 6 hours mildly increased the expression of p-p70S6K. WISP1 (10 ng/ml) administered 1 hour prior to Aβ exposure significantly increased p-p70S6K (Thr389) expression during Aβ exposure and during transfection of shRNA TSC2. Independently, transfection of shRNA TSC2 increased p-p70S6K (Thr389) expression during Aβ exposure and in untreated microglial cultures. Non-specific scrambled shRNA did not alter p-p70S6K expression during Aβ exposure. Quantitative results of western blot band density was obtained from three experiments using the public domain NIH Image program (using the public domain NIH Image program (US National Institutes of Health at http://rsb.info.nih.gov/nih-image/) (*P < 0.01 vs. Control; †P<0.01 vs. WISP1/Aβ). Each data point represents the mean and SD.
Loss of TSC2 Impairs the Ability of WISP1 to pryotect Microglia Against Apoptotic Injury During Aβ Exposure
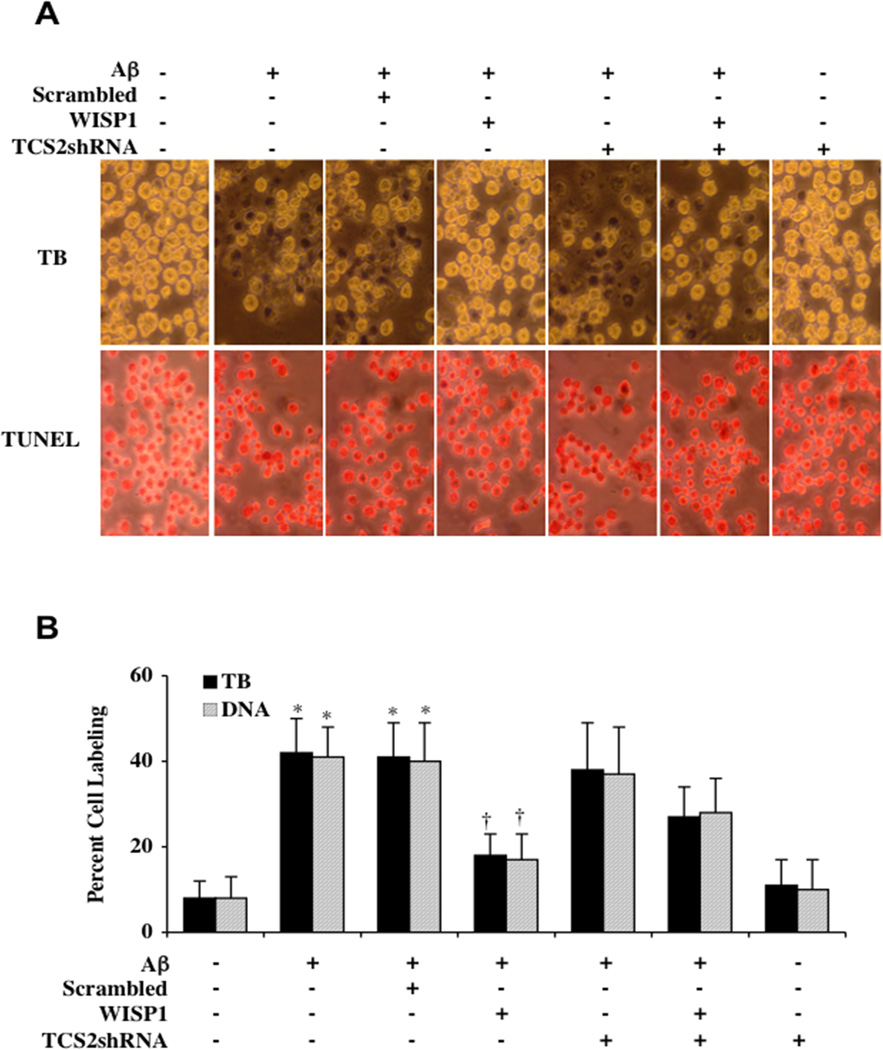
In Figs. (3A and 3B), Aβ (10 µM) was applied to microglial cultures and cell survival and apoptotic DNA fragmentation were determined through trypan blue dye exclusion and TUNEL staining respectively 24 hours following Aβ administration. Aβ exposure alone significantly increased trypan blue and TUNEL staining. WISP1 (10 ng/ml) administered 1 hour prior to Aβ significantly reduced trypan blue and TUNEL staining. However, transfection with TSC2 shRNA prior to Aβ exposure impaired the ability of WISP1 to protect against cell injury and apoptosis and resulted in increased trypan blue dye exclusion and TUNEL staining. Loss of TSC2 during Aβ exposure alone did not increase cell injury. In the absence of Aβ exposure, loss of TSC2 did not alter microglial cell survival. Quantitative results demonstrate that Aβ exposure significantly increased percent trypan blue and TUNEL labeling from 8 ± 4% and 8 ± 5% of untreated cultures to 42 ± 8% and 41 ± 7% respectively. In contrast, WISP1 (10 ng/ml) applied 1 hour prior to Aβ exposure significantly decreased percent trypan blue and TUNEL labeling to 18 ± 5% and 17 ± 6% respectively (Fig. 3B).
Fig. (3). Gene silencing of TSC2 limits WISP1 cytoprotection during Aβ exposure.
(A) Microglia were exposed to Aβ (10 µM) for 24 hours. Cell survival and apoptotic DNA fragmentation were determined by using trypan blue dye exclusion and TUNEL staining methods respectively. In representative images for trypan blue and TUNEL staining, Aβ exposure significantly increased trypan blue and TUNEL staining. In contrast, WISP1 (10 ng/ml) administered 1 hour prior to Aβ significantly reduced trypan blue and TUNEL staining. Transfection with TSC2 shRNA prior to Aβ exposure limited the ability of WISP1 to reduce cell injury during Aβ exposure, resulting in an increase in trypan blue and TUNEL labeling. Loss of TSC2 during Aβ exposure alone did not increase cell injury. In the absence of Aβ exposure, loss of TSC2 also did not alter microglial cell survival. (B) Quantitative results for trypan blue and TUNEL demonstrate that Aβ significantly increased percent trypan blue labeling and DNA fragmentation. WISP1 (10 ng/ml) administration 1 hour prior to Aβ exposure significantly blocked cell death and DNA fragmentation. Yet, combined transfection of the TSC2shRNA construct with WISP1 administration (10 ng/ml) resulted in an increase in the percentage of trypan blue and TUNEL labeling when compared to WISP1 treated alone during Aβ exposure. Loss of TSC2 did not alter microglial cell survival in the absence of Aβ exposure (*P <0.01 vs. untreated control; P <0.01 vs. Aβ). Non-specific scrambled shRNA did not affect trypan blue or TUNEL staining when compared with cells exposed to Aβ alone. Each data point represents the mean and SD from 4 experiments.
Transfection with TSC2 shRNA prior to Aβ exposure did not significantly change trypan blue staining and TUNEL labeling. However, loss of TSC2 significantly reduced the ability of WISP1 to protect microglia during Aβ exposure, resulting in an increase in percent trypan blue staining (27 ± 7%) and TUNEL labeling (28 ± 8%). Scrambled shRNA during Aβ exposure did not injury cells in normal cultures or alter cell injury during Aβ exposure when compared to Aβ exposure alone.
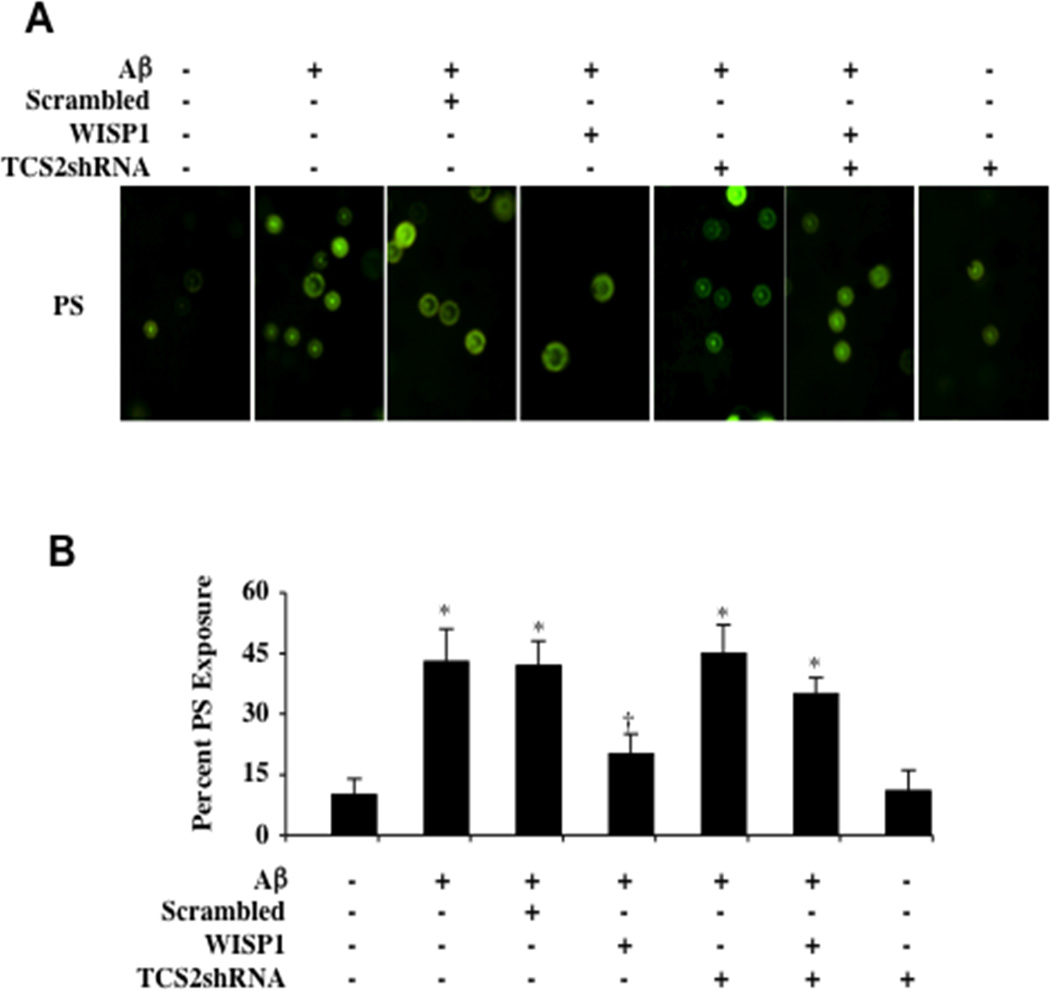
In Figs. (4A and 4B), WISP1 (10 ng/ml) was applied to microglial cultures 1 hour prior to Aβ (10 µM) administration and apoptotic PS externalization was determined by annexin V labeling method 24 hours following Aβ exposure. Representative images illustrate that Aβ exposure resulted in increased annexin V labeling. WISP1 (10 ng/ml) application significantly decreased apoptotic membrane PS externalization during Aβ exposure, but the ability of WISP1 to modulate membrane PS externalization was significantly limited during gene silencing of TSC2 with transfection of TSC2 shRNA. Loss of TSC2 in untreated cultures and during Aβ exposure alone did not increase membrane PS exposure. Quantitative results illustrate that Aβ exposure significantly increased percent PS exposure from 10 ± 4% in untreated microglial cultures to 43 ± 8%. In contrast, WISP1 (10 ng/ml) applied 1 hour prior to Aβ exposure significantly decreased percent PS exposure to 20 ± 5% (Fig. 4B). Transfection with TSC2 shRNA significantly impaired the ability of WISP1 to prevent membrane PS exposure in microglia during Aβ exposure, resulting in an increase in percent PS exposure (35 ± 4%). Scrambled shRNA during Aβ exposure did not increase PS exposure during Aβ exposure when compared to Aβ exposure alone.
Fig. (4). Control of apoptotic phosphatidylserine (PS) membrane exposure by WISP1 is lost with gene silencing of TSC2 during Aβ exposure.
(A) Microglia were exposed to Aβ (10 µM) for 24 hours and membrane PS externalization was assessed by annexin V phycoerythrin (green fluorescence) labeling. In representative images, Aβ significantly increased annexin V phycoerythrin staining. In contrast, WISP1 (10 ng/ml) administered 1 hour prior to Aβ exposure significantly reduces the presence of membrane PS exposure. Transfection with TSC2 shRNA prior to Aβ exposure markedly reduced the capacity of WISP1 to reduce annexin V labeling. Loss of TSC2 in untreated cultures and during Aβ exposure alone did not increase membrane PS exposure. (B) Quantitative results for annexin V phycoerythrin labeling demonstrate that Aβ significantly increases membrane PS exposure, but WISP1 (10 ng/ml) administration 1 hour prior to Aβ exposure almost eliminates microglial membrane PS exposure. Combined transfection of TSC2shRNA construct with WISP1 (10 ng/ml) administration results in a significant loss of WISP1 to decrease membrane PS exposure during Aβ exposure when compared to WISP1 administration alone during Aβ exposure (*P <0.01 vs. untreated control; P <0.01 vs. Aβ). Non-specific scrambled shRNA did not affect membrane PS exposure during Aβ exposure when compared to Aβ exposure alone. Each data point represents the mean and SD from 4 experiments.
WISP1 Relies Upon TSC2 to Prevent Mitochondrial Depolarization and Cytochrome c Release During Aβ Exposure
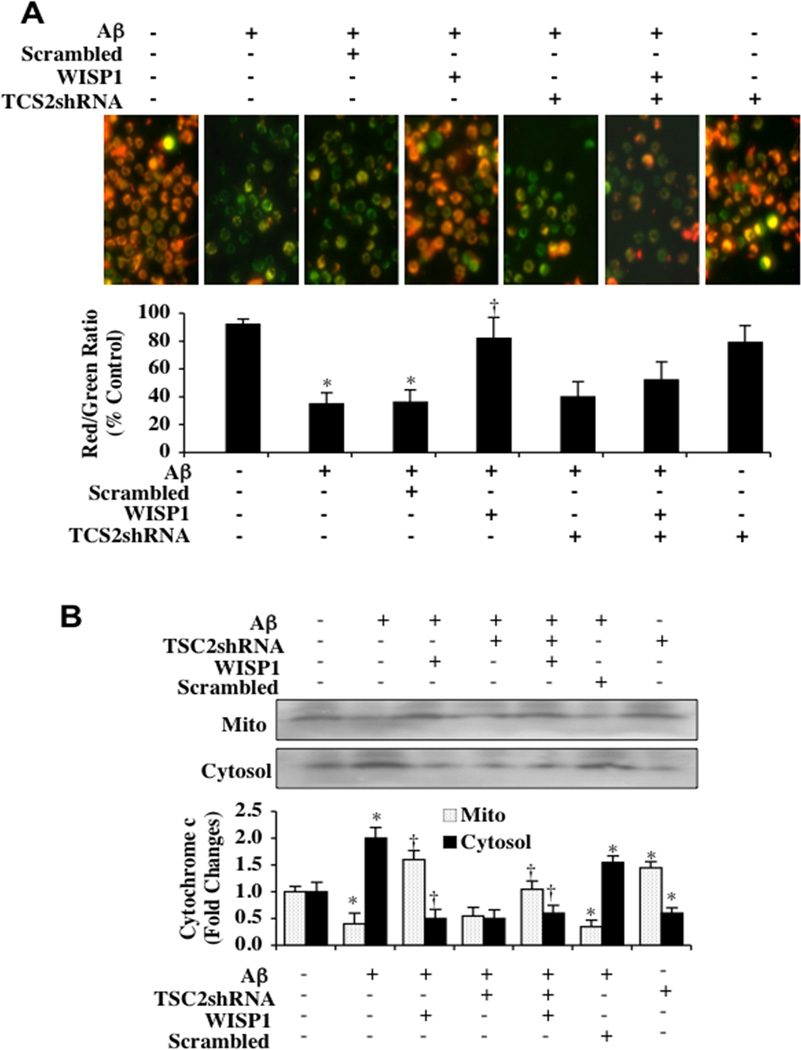
In Figs. (5A), Aβ (10 µM) was applied to microglia cultures and mitochondrial membrane depolarization was determined by using the cationic membrane potential indicator JC-1 six hours following Aβ exposure. As shown in Fig. (5A), Aβ exposure produced a significant decrease in the red/green fluorescence intensity ratio of mitochondria within 6 hours when compared with untreated control cultures, suggesting that Aβ leads to mitochondrial membrane depolarization. WISP (10 ng/ml) administrated 1 hour prior to Aβ exposure significantly increased the red/green fluorescence intensity of mitochondria in microglia and preserved mitochondrial membrane polarization. Gene silencing of TSC2 with transfection of TSC2 shRNA did not significantly alter the red/green fluorescence intensity ratio of mitochondria during Aβ exposure or in untreated control cultures. However, loss of TSC2 during WISP1 administration and Aβ exposure prevented WISP1 from maintaining mitochondrial polarization. Scrambled shRNA during Aβ exposure did not increase mitochondrial depolarization during Aβ exposure when compared to Aβ exposure alone.
Fig. (5). TSC2 is necessary for WISP1 to prevent mitochondrial depolarization and cytochrome c release during Aβ exposure.
(A) Aβ (10 µM) exposure produced a significant decrease in the red/green fluorescence intensity ratio of mitochondria within 6 hours using the cationic membrane potential indicator JC-1 when compared with untreated control cultures, demonstrating that Aβ results in mitochondrial membrane depolarization. WISP (10 ng/ml) administrated 1 hour prior to Aβ significantly increased the red/green fluorescence intensity of mitochondria in microglia, illustrating that mitochondrial membrane potential was restored. Loss of TSC2 with transfection of TSC2 shRNA significantly reduced the ability of WISP1 to prevent mitochondrial depolarization during Aβ exposure. Gene silencing of TSC2 did not alter mitochondrial membrane depolarization in the absence of Aβ exposure. The relative ratio of red/green fluorescent intensity of mitochondrial staining was measured in 3 independent experiments with analysis performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) (*P<0.01 vs. untreated control; †P <0.01 vs. Aβ). (B) Equal amounts of mitochondrial (Mito) or cytosol protein extracts (50 µg/lane) were immunoblotted demonstrating that WISP1 administration significantly prevented cytochrome c release from mitochondria during Aβ exposure (*P<0.01 vs. untreated control; †P <0.01 vs. Aβ). Transfection with TSC2 shRNA attenuated the ability of WISP1 to prevent mitochondrial release of cytochrome c. Gene silencing of TSC2 did not alter cytochrome c release in the absence of Aβ exposure. Non-specific scrambled shRNA did not affect mitochondrial depolarization or cytochrome c release during Aβ exposure when compared to Aβ exposure alone.. Each data point represents the mean and SD from 3 experiments.
In Fig. (5B), cytochrome c release from mitochondria was evaluated by western blot for cytochrome c expression in both mitochondrial and cytosol extractions at 6 hours following Aβ exposure. Aβ exposure yielded a significant increase in the release of cytochrome c from the mitochondria. WISP1 (10 ng/ml) administration prevented cytochrome c release and decreased the expression of cytochrome c in the cytosol during Aβ exposure. Yet, loss of TSC2 during WISP1 application and Aβ exposure resulted in the release of cytochrome c into the cytosol. Gene silencing of TSC2 with transfection of TSC2 shRNA did not significantly alter cytochrome c release in untreated cultures. Scrambled shRNA during Aβ exposure did not increase cytochrome c release during Aβ exposure when compared to Aβ exposure alone.
DISCUSSION
As a potential novel therapeutic target for AD, WISP1 has been shown to prevent neuronal death during oxidative stress [10, 11] and block Aβ toxicity in microglia (13). Although multiple cellular pathways may account for the cytoprotective capacity of WISP1 [7, 8, 10, 11, 20, 21], recent work suggests that mTOR may play a significant role in providing cellular protection with WISP1 [13]. Yet, cellular protection by mTOR may require a level of modulation since high mTOR activity has been associated with apoptotic cell death [43]. One pathway that can modulate and inhibit mTOR activity along with the downstream signaling pathway of p70S6K involves TSC2 [26, 27]. TSC2 can be activated by AMPK [29, 30] and inhibited by Akt [26, 28].
We demonstrate that during Aβ exposure, WISP1 significantly increases post-translational phosphorylation of Akt1 and p70S6K, a downstream pathway of mTOR and a marker of mTOR activity [40]. WISP1 has previously been shown to require PI 3-K and Akt1 to protect neurons against oxidative stress [10, 11] and microglia from Aβ toxicity [13]. In addition, WISP1 increases mTOR activity to promote cytoprotection [13]. However, we also show that WISP1 during Aβ exposure can decrease the phosphorylation and activation of both AMPK and TSC2 (Ser1387). WISP1 may be controlling TSC2 phosphorylation and activation through AMPK. AMPK can phosphorylate TSC2 on serine1387 to foster GAP activity and turn Rheb-GTP into Rheb-GDP to inhibit the activity of mTOR and the mTORC1 complex [29]. Yet, WISP1 also appears to have a role in TSC2 activation since we illustrate that WISP1 increases the phosphorylation of TSC2 (Thr1462), a target of Akt1.
The ability of WISP1 to limit TSC2 (Ser1387) phosphorylation appears to allow WISP1 to increase the activity of p70S6K. During Aβ exposure, WISP1 can significantly increase the phosphorylation of p70S6K that can be indicative of increased mTOR activity [40]. During Aβ exposure, WISP1 has been shown to phosphorylate mTOR, p70S6K and the eukaryotic initiation factor 4E-binding protein 1 (4EBP1) [13]. During gene silencing of TSC2, phosphorylation of p70S6K is further enhanced during Aβ exposure alone and in the presence of WISP1.
However, WISP1 remains dependent upon TSC2 to foster cytoprotection in microglia against Aβ toxicity. During periods of oxidative stress, loss of the TSC2 results in purkinje cell degeneration [44]. We demonstrate that loss of TSC2 through gene silencing in microglia limits the ability of WISP1 to provide cytoprotection and increases cell injury, apoptotic membrane PS exposure, and DNA degradation in microglia. Apoptosis in cells involves both an early phase consisting of the exposure of membrane PS residues and a late phase that involves the destruction of genomic DNA [23, 45–47]. The early phase is energy dependent and involves the externalization of PS residues on the surface of cells that can be a signal for inflammatory cells to engulf and dispose of injured cells [46, 48, 49]. The disposal of “tagged” cells may assist with the repair and regeneration of injured tissues to remove non-functional dying cells, but also at times may lead to the removal of otherwise functional cells if not kept in-check [50, 51]. The later apoptotic phase involving the cleavage of genomic DNA into fragments usually does not allow for the repair or recovery of cells [41, 52, 53]. As a result, at least a minimal presence of TSC2 appears necessary to promote the ability of WISP1 to prevent both early and late phases of apoptotic cell injury that may involve limiting mTOR activity. Some studies have suggested that inhibition of mTOR activity may lead to cognitive improvement in mouse models of AD [54].
Although the presence of TSC2 may foster cell protection with WISP1 through pathways that may limit excessive mTOR activity similar to observations with autophagic cell survival [43] and in models of AD [54], we show that TSC2 also is necessary for WISP1 to control the apoptotic cascade through the prevention of mitochondrial membrane depolarization and cytochrome c release. WISP1 during Aβ exposure significantly prevents mitochondrial membrane depolarization and cytochrome c release in microglia. However, loss of TSC2 through gene silencing during WISP1 administration and Aβ exposure blocks WISP1 from maintaining mitochondrial polarization and preventing the release of cytochrome c. In other cell systems such as with neurons, neurons without a functional TSC1/TSC2 complex have been shown to have increased vulnerability to endoplasmic reticulum stress-induced cell death through the activation of the mitochondrial death pathway [55]. The mechanisms that allow TSC2 to prevent mitochondrial membrane depolarization and cytochrome c release that either involve WISP1 or are independent of WISP1 are not entirely clear at this time, but some studies suggest that inhibition of the mTOR pathway in cancer cells may not adversely influence apoptosis through the mitochondrial pathway [56].
ACKNOWLEDGMENTS
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association (National), Bugher Foundation Award, LEARN Foundation Award, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
Footnotes
CONFLICTS OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Bajda M, Guzior N, Ignasik M, Malawska B. Multi-target-directed ligands in Alzheimer's disease treatment. Curr Med Chem. 2011 Nov 1;18(32):4949–4975. doi: 10.2174/092986711797535245. [DOI] [PubMed] [Google Scholar]
- 2.Maiese K, Chong ZZ, Hou J, Shang YC. New strategies for Alzheimer's disease and cognitive impairment. Oxid Med Cell Longev. 2009 Nov-Dec;2(5):279–289. doi: 10.4161/oxim.2.5.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delrieu J, Ousset PJ, Vellas B. Gantenerumab for the treatment of Alzheimer's disease. Expert Opin Biol Ther. 2012 May 15; doi: 10.1517/14712598.2012.688022. [DOI] [PubMed] [Google Scholar]
- 4.Yeger H, Perbal B. The CCN family of genes: a perspective on CCN biology and therapeutic potential. J Cell Commun Signal. 2007 Dec;1(3–4):159–164. doi: 10.1007/s12079-008-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French DM, Kaul RJ, D'Souza AL, Crowley CW, Bao M, Frantz GD, et al. WISP-1 is an osteoblastic regulator expressed during skeletal development and fracture repair. Am J Pathol. 2004 Sep;165(3):855–867. doi: 10.1016/S0002-9440(10)63348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hennemeier I, Humpf HU, Gekle M, Schwerdt G. The food contaminant and nephrotoxin ochratoxin A enhances Wnt1 inducible signaling protein 1 and tumor necrosis factor-alpha expression in human primary proximal tubule cells. Molecular nutrition & food research. 2012 Sep;56(9):1375–1384. doi: 10.1002/mnfr.201200164. [DOI] [PubMed] [Google Scholar]
- 7.Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002 Jan 1;16(1):46–57. doi: 10.1101/gad.942902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkatesan B, Prabhu SD, Venkatachalam K, Mummidi S, Valente AJ, Clark RA, et al. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal. 2010 May;22(5):809–820. doi: 10.1016/j.cellsig.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heise RL, Stober V, Cheluvaraju C, Hollingsworth JW, Garantziotis S. Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J Biol Chem. 2011 May 20;286(20):17435–17444. doi: 10.1074/jbc.M110.137273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Chong ZZ, Shang YC, Maiese K. Wnt1 inducible signaling pathway protein 1 (WISP1) blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL. Curr Neurovasc Res. 2012 Feb;9(1):20–31. doi: 10.2174/156720212799297137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Chong ZZ, Shang YC, Maiese K. WISP1 (CCN4) autoregulates its expression and nuclear trafficking of beta-catenin during oxidant stress with limited effects upon neuronal autophagy. Curr Neurovasc Res. 2012 Apr 4;9(2):89–99. doi: 10.2174/156720212800410858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Chong ZZ, Shang YC, Maiese K. WISP1 neuroprotection requires FoxO3a post-translational modulation with autoregulatory control of SIRT1. Curr Neurovasc Res. 2012 Nov 12; doi: 10.2174/156720213804805945. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang YC, Chong ZZ, Wang S, Maiese K. WNT1 Inducible Signaling Pathway Protein 1 (WISP1) Targets PRAS40 to Govern beta-Amyloid Apoptotic Injury of Microglia. Curr Neurovasc Res. 2012 Aug 6;9(4):239–249. doi: 10.2174/156720212803530618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bach JP, Mengel D, Wahle T, Kautz A, Balzer-Geldsetzer M, Al-Abed Y, et al. The Role of CNI-1493 in the Function of Primary Microglia with Respect to Amyloid-beta. J Alzheimers Dis. 2011 Jan 1;26(1):69–80. doi: 10.3233/JAD-2011-110179. [DOI] [PubMed] [Google Scholar]
- 15.Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, et al. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nat Neurosci. 2010 Mar 21; doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers J, Lue LF. Microglial chemotaxis, activation, and phagocytosis of amyloid beta-peptide as linked phenomena in Alzheimer's disease. Neurochem Int. 2001 Nov-Dec;39(5–6):333–340. doi: 10.1016/s0197-0186(01)00040-7. [DOI] [PubMed] [Google Scholar]
- 17.Salminen A, Kaarniranta K. Siglec receptors and hiding plaques in Alzheimer's disease. J Mol Med. 2009 Jul;87(7):697–701. doi: 10.1007/s00109-009-0472-1. [DOI] [PubMed] [Google Scholar]
- 18.Shang YC, Chong ZZ, Hou J, Maiese K. The forkhead transcription factor FoxO3a controls microglial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009 Feb;6(1):20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY) 2012 Mar 3;4(3):187–201. doi: 10.18632/aging.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colston JT, de la Rosa SD, Koehler M, Gonzales K, Mestril R, Freeman GL, et al. Wnt-induced secreted protein-1 is a prohypertrophic and profibrotic growth factor. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1839–H1846. doi: 10.1152/ajpheart.00428.2007. [DOI] [PubMed] [Google Scholar]
- 21.Reddy VS, Valente AJ, Delafontaine P, Chandrasekar B. Interleukin-18/WNT1-inducible signaling pathway protein-1 signaling mediates human saphenous vein smooth muscle cell proliferation. J Cell Physiol. 2011 Dec;226(12):3303–3315. doi: 10.1002/jcp.22676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chong ZZ, Shang YC, Wang S, Maiese K. A Critical Kinase Cascade in Neurological Disorders: PI 3-K, Akt, and mTOR. Future Neurol. 2012;7(6):733–748. doi: 10.2217/fnl.12.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maiese K, Chong ZZ, Wang S, Shang YC. Oxidant Stress and Signal Transduction in the Nervous System with the PI 3-K, Akt, and mTOR Cascade. International journal of molecular sciences. 2012;13(11):13830–13866. doi: 10.3390/ijms131113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chong ZZ, Maiese K. Mammalian Target of Rapamycin Signaling in Diabetic Cardiovascular Disease. Cardiovasc Diabetol. 2012;11(1):45. doi: 10.1186/1475-2840-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong ZZ, Shang YC, Maiese K. Cardiovascular Disease and mTOR Signaling. Trends Cardiovasc Med. 2011;21(5):151–155. doi: 10.1016/j.tcm.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4(9):648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 27.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10(1):151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 28.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4(9):658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 29.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. doi: 10.1016/s0092-8674(03)00929-2. 26. [DOI] [PubMed] [Google Scholar]
- 30.Sofer A, Lei K, Johannessen CM, Ellisen LW. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol Cell Biol. 2005;25(14):5834–5845. doi: 10.1128/MCB.25.14.5834-5845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005;2(5):387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007;19(6):1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106(23):2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 34.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003;138(6):1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010;7(2):95–112. doi: 10.2174/156720210791184899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou J, Wang S, Shang YC, Chong ZZ, Maiese K. Erythropoietin Employs Cell Longevity Pathways of SIRT1 to Foster Endothelial Vascular Integrity During Oxidant Stress. Curr Neurovasc Res. 2011;8(3):220–235. doi: 10.2174/156720211796558069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shang YC, Chong ZZ, Hou J, Maiese K. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal. 2010;22(9):1317–1329. doi: 10.1016/j.cellsig.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou J, Chong ZZ, Shang YC, Maiese K. FoxO3a governs early and late apoptotic endothelial programs during elevated glucose through mitochondrial and caspase signaling. Mol Cell Endocrinol. 2010;321(2):194–206. doi: 10.1016/j.mce.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 Govern Pathways of mTOR, Apaf-1, and XIAP in Inflammatory Microglia. Curr Neurovasc Res. 2011;8(4):270–285. doi: 10.2174/156720211798120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearson RB, Dennis PB, Han JW, Williamson NA, Kozma SC, Wettenhall RE, et al. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. Embo J. 1995;14(21):5279–5287. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol. 2012;99(2):128–148. doi: 10.1016/j.pneurobio.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng S, Wu YT, Chen B, Zhou J, Shen HM. Impaired autophagy due to constitutive mTOR activation sensitizes TSC2-null cells to cell death under stress. Autophagy. 2011;7(10):1173–1186. doi: 10.4161/auto.7.10.16681. [DOI] [PubMed] [Google Scholar]
- 44.Reith RM, Way S, McKenna J, 3rd, Haines K, Gambello MJ. Loss of the tuberous sclerosis complex protein tuberin causes Purkinje cell degeneration. Neurobiol Dis. 2011;43(1):113–122. doi: 10.1016/j.nbd.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75(3):207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008;14(5):219–227. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert opinion on therapeutic targets. 2012 doi: 10.1517/14728222.2012.719499. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bailey TJ, Fossum SL, Fimbel SM, Montgomery JE, Hyde DR. The inhibitor of phagocytosis, O-phospho-L-serine, suppresses Muller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp Eye Res. 2010;91(5):601–612. doi: 10.1016/j.exer.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schutters K, Reutelingsperger C. Phosphatidylserine targeting for diagnosis and treatment of human diseases. Apoptosis. 2010;15(9):1072–1082. doi: 10.1007/s10495-010-0503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koh PO. Nicotinamide attenuates the decrease of astrocytic phosphoprotein PEA-15 in focal cerebral ischemic injury. J Vet Med Sci. 2012;74(3):377–380. doi: 10.1292/jvms.11-0392. [DOI] [PubMed] [Google Scholar]
- 51.Maiese K, Chong ZZ, Shang YC. "Sly as a FOXO": New paths with Forkhead signaling in the brain. Curr Neurovasc Res. 2007;4(4):295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V. Role of Apoptosis in disease. Aging (Albany NY) 2012;4(5):330–349. doi: 10.18632/aging.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maiese K, Chong ZZ, Shang YC, Wang S. Novel directions for diabetes mellitus drug discovery. Expert opinion on drug discovery. 2012 doi: 10.1517/17460441.2013.736485. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5(4):e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Nardo A, Kramvis I, Cho N, Sadowski A, Meikle L, Kwiatkowski DJ, et al. Tuberous sclerosis complex activity is required to control neuronal stress responses in an mTOR-dependent manner. J Neurosci. 2009;29(18):5926–5937. doi: 10.1523/JNEUROSCI.0778-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gazitt Y, Kolaparthi V, Moncada K, Thomas C, Freeman J. Targeted therapy of human osteosarcoma with 17AAG or rapamycin: characterization of induced apoptosis and inhibition of mTOR and Akt/MAPK/Wnt pathways. Int J Oncol. 2009;34(2):551–561. [PubMed] [Google Scholar]