Abstract
Bone marrow mesenchymal stem cells (BMMSCs) are multipotent marrow stromal cells with the ability to differentiate into a variety of cell types required for tissue regeneration including osteoblasts and chondrocytes. Thus, they hold tremendous potential as powerful therapeutic strategies for the prevention and treatment of degenerative disorders including osteoporosis and osteoarthritis. The differentiation of BMMSCs into competing lineages such as osteoblasts and marrow adipocytes is regulated by various environmental cues and intrinsic signaling pathways. Here I highlight recent advances in the understanding of BMMSC function and regulation, including the interaction between BMMSCs with the hematopoietic/immune system, and the identification of novel modulators of BMMSC differentiation such as the metabolic hormone fibroblast growth factor 21 (FGF21). These new findings will further elucidate the dynamic regulation of BMMSCs in the pathophysiological control of skeletal homeostasis, and facilitate the clinical applications of BMMSCs in regenerative medicine.
Keywords: bone marrow mesenchymal stem cell, osteoblast, adipocyte, FGF21, PPARγ
Introduction
Bone marrow mesenchymal stem cells (BMMSCs) are marrow stromal cells that possess the capability of self-renewal and multipotency, allowing them to differentiate into several mesenchymal lineages upon cell type-specific stimulation. They exhibit a fibroblast-like morphology and are distinct from hematopoietic stem cells (HSCs) that give rise to all blood and immune lineages. MSCs are thought to be first identified in 1924 by Alexander Maximow (Stem Cell Handbook, Humana Press, 2004). In 1960s, Ernest McCulloch and James Till first revealed the clonal nature of BMMSCs. In 1970’s, Alexander Friedenstein reported an ex vivo assay for examining the clonogenic potential of BMMSCs: colony forming unit-fibroblast (CFU-f). Since 1980’s, plasticity of BMMSCs and how their differentiation outcome is determined by environmental cues has been intensively investigated.
Cell lineage and plasticity
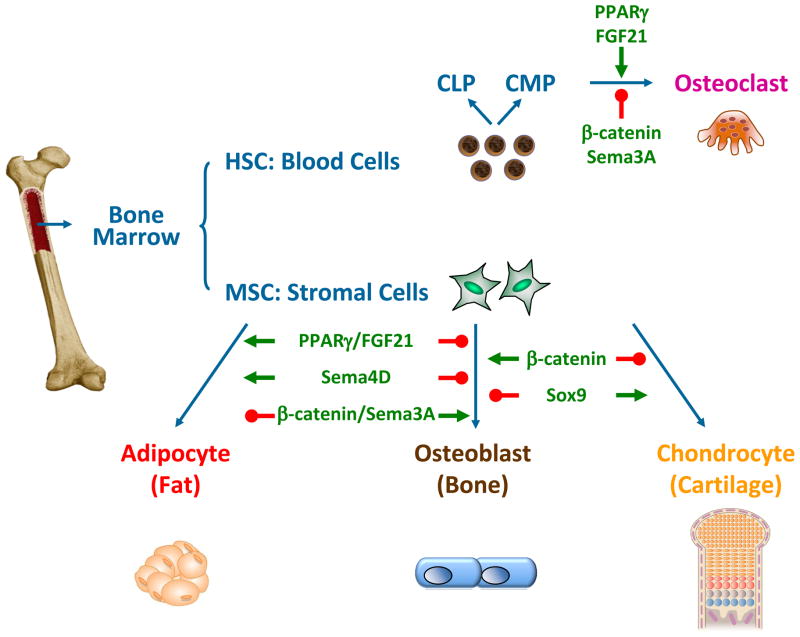
Upon exposure to different extracellular stimuli, different sets of signaling pathways and transcription factors are activated or inhibited in BMMSCs. This leads to distinct differentiation outcomes into different lineages such as osteoblasts (bone forming cells), chondrocytes (cartilage cells) or marrow adipocytes (fat cells) (Fig. 1). Similarly, HSCs can be differentiated into common lymphoid progenitors (CLPs), or common myeloid progenitors (CMPs) that serve as precursors for the bone-specific macrophage cell type osteoclasts (Fig. 1). Under physiological conditions, osteoblast-mediated bone formation couples with osteoclast-mediated bone resorption to maintain bone mass, bone quality and mineral density. Under pathological conditions such as osteopenia and osteoporosis, bone formation lags behind bone resorption, leading to bone loss, skeletal fragility and fractures. Thus, BMMSC differentiation into osteoblast is essential for normal skeletal homeostasis.
Figure 1. Regulation of BMMSC differentiation plasticity.
A simplified diagram for the differentiation of MSCs or HSCs into distinct lineages in bone and marrow; and the recently identified ligands, receptors and signaling molecules that modulate lineage allocation and fate choice. CLP, common lymphoid progenitor; CMP, common myeloid progenitor.
A variety of hormones and ligands have been shown to be able to shift the balance of BMMSC differentiation. For example, activation of the nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR-γ) by its synthetic ligand rosiglitazone, a widely used anti-diabetes drug in the thiazolidinedione (TZD) class, can promote adipocyte differentiation but inhibit osteoblast differentiation, which contributes to its bone loss side effect together with its ability to enhance osteoclastogenesis and bone resorption (Akune et al., 2004, Wan, 2010, Wan et al., 2007, Wei et al., 2010) (Fig. 1). On the other hand, activation of the Wnt/β-catenin signaling pathway can stimulate BMMSCs to differentiate into osteoblast but inhibit adipogenesis and chondrogenesis (Day et al., 2005, Hill et al., 2005), as well as suppressing osteoclast differentiation and bone resorption (Glass et al., 2005, Wei et al., 2011), both contributing to its bone-enhancing effects (Fig. 1). Moreover, a recent study shows that Sox9, a transcription factor previously known to be essential for early chondrogenesis, blocks osteoblast differentiation but direct chondrocyte hypertrophic maturation (Dy et al., 2012) (Fig. 1).
Our group has recently identified fibroblast growth factor 21 (FGF21) as a novel yet critical regulator of BMMSC differentiation (Wei et al., 2012). FGF21 is an atypical member of the FGF family that functions as an endocrine and paracrine hormone. It is a powerful regulator of glucose and lipid metabolism. Physiologically, FGF21 expression is induced both in the liver by prolonged fasting via PPARγ activation and in the white adipose tissue by feeding via PPARγ activation. Pharmacologically, administration of recombinant FGF21 to diabetic mice and rhesus monkeys strongly enhances insulin sensitivity, decreases plasma glucose and triglyceride, and reduces body weight (Canto and Auwerx, 2012, Potthoff et al., 2012). Hence, FGF21 is a potential new drug for the treatment of obesity and diabetes that is currently in clinical trials. In light of the already increased skeletal fragility in diabetic patients (Strotmeyer and Cauley, 2007) and the reported bone-loss side effects of the current anti-diabetes thiazolidinedione (TZD) drugs such as rosiglitazone (Home et al., 2009, Kahn et al., 2008, Zinman et al., 2010), we investigated the role of FGF21 in the regulation of bone homeostasis and BMMSC differentiation.
Both genetic gain-of-function as in the FGF21 transgenic mice and pharmacological gain-of-function as in the recombinant FGF21-treated mice result in a lower bone mass with increased marrow adipocytes but decreased osteoblasts and bone formation. Consistent with these findings, genetic loss-of-function as in the FGF21 knockout mice leads to a higher bone mass with less marrow adipocytes but more osteoblasts and accelerated bone formation. BMMSC differentiation assays reveal that FGF21 inhibits osteoblastogenesis but enhances marrow adipogenesis via potentiating the activation of PPARγ by rosiglitazone (Fig. 1). Mechanistic studies show that FGF21 forms a feed-forward loop with PPARγ: ligand activation of PPARγ increases the expression of FGF21 and its co-receptor β-Klotho in BMMSCs; in turn, FGF21 enhances PPARγ activity by attenuating its inhibitory sumoylation (Wei et al., 2012, Dutchak et al., 2012). Consequently, FGF21 deletion abolishes this feed-forward loop, leading to elevated osteoblastogenesis and reduced marrow adipogenesis, as well as resistance to rosiglitazone-induced bone loss (Wei et al., 2012). These findings uncover FGF21 as a physiologically relevant metabolic regulator of skeletal homeostasis and BMMSC differentiation, which underscores the importance of whole body energy metabolism in BMMSC lineage allocation. Importantly, this suggests that despite the beneficial effects of FGF21 in treating diabetes, long term FGF21 administration may cause skeletal fragility. Future studies are needed to determine whether FGF21 also promotes bone loss in diabetic patients.
Semaphorins are another family of signaling molecules that have been recently identified as key regulators of BMMSC differentiation and bone remodeling. Semaphorin 3A (Sema3A) binding to neuropilin-1 (Nrp1) stimulates osteoblast and inhibits adipocyte differentiation, as well as suppressing osteoclast differentiation (Fig. 1). Sema3A−/− mice and mice in which the Sema3A-binding site of Nrp1 had been genetically disrupted exhibit osteopenia. Intravenous Sema3A administration in mice increases bone volume and expedites bone regeneration. These findings highlight Sema3A as a promising new therapeutic agent in bone and joint diseases (Hayashi et al., 2012). In contrast, osteoclast-derived semaphorin 4D (Sema4D) potently inhibits osteoblastogenesis and bone formation by binding to its receptor Plexin-B1 (Fig. 1). It activates the small GTPase RhoA, which inhibits bone formation by suppressing insulin-like growth factor-1 signaling. Sema4d−/− mice, Plxnb1−/− mice and mice expressing a dominant-negative RhoA specifically in osteoblasts show an osteosclerotic phenotype due to augmented bone formation. Sema4D-specific antibody treatment markedly prevents bone loss in a model of postmenopausal osteoporosis. Thus, Sema4D has emerged as a new therapeutic target for bone degenerating diseases (Negishi-Koga et al., 2011).
Functions
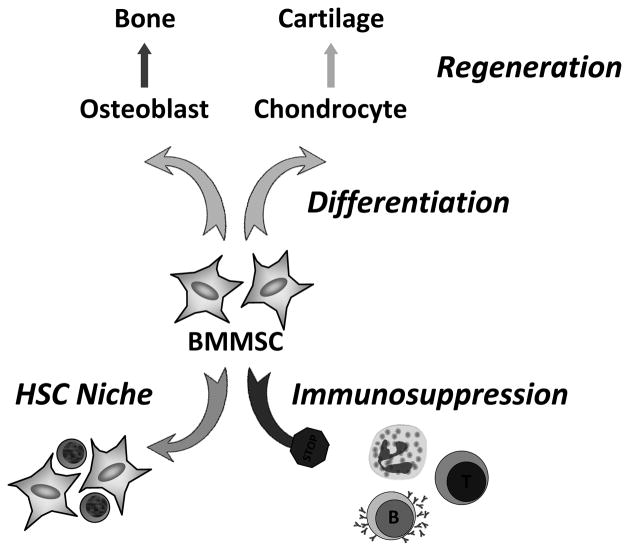
One of the key functions of BMMSCs is to maintain and regenerate bone by differentiating into various mesenchymal lineages upon demand, including osteoblasts in the bone and chondrocytes in the cartilage (Fig. 2). Osteoblast is required to regenerate bone tissue by depositing both bone matrix proteins such as type-I collagens, bone sialoprotein and osteocalcin; as well as minerals such as calcium phosphate. Chondrocyte is required to produce and maintain the cartilaginous matrix, which consists mainly of type-II collagen and proteoglycans. In contrast, the function and significance of marrow adipocytes are still unclear. The ability of BMMSCs to self-renew, expand and differentiate into a variety of connective tissue/cell types forms the basis of its clinical potential as regenerative therapeutics. In addition, recent studies have attributed several novel functions to BMMSCs, particularly the interaction of BMMSCs with HSCs and the immune system.
Figure 2. BMMSC functions.
A diagram illustrating three of the major functions of BMMSC: cell differentiation and tissue regeneration, HSC niche, and immunosuppression.
BMMSC has recently been proposed to be an essential component of the HSC niche (Mendez-Ferrer et al., 2010) (Fig. 2). HSC niche is a confined microenvironment that supports the self-renewal, expansion and activity of HSCs. It has been previously shown to include osteoblasts, endothelial and perivascular cells. In this study, BMMSCs are identified as nestin+ cells because they contain all the bone marrow CFU-f activity and can be propagated as non-adherent mesenspheres that can self-renew and expand in serial transplantations. Nestin+ BMMSCs are spatially associated with HSCs, and express HSC maintenance genes. These genes are down-regulated during enforced HSC mobilization. Furthermore, purified HSCs home near nestin+ BMMSCs in lethally irradiated mice, whereas nestin+ cell depletion rapidly reduces HSC number and significantly reduces HSC homing. These findings uncover a potential partnership between two distinct somatic stem-cell types and indicate a unique niche in the bone marrow comprised of heterotypic stem-cell pairs. A provocative yet underexplored question is that what regulates the location, dynamic and cellular constituents of the BMMSC niche.
BMMSCs have also been implicated to regulate both adaptive and innate immunity (Fig. 2). Human BMMSCs can inhibit the proliferation, maturation and activation of T cells, B cells, dendritic cells, neutrophils and natural killer cells in vitro, by secreting various factors such as transforming growth factor-β. BMMSC infusion in mice can ameliorate autoimmune disorders including experimental autoimmune encephalomyelitis, diabetes, inflammatory bowl disease and multiple sclerosis, as well as attenuate severe graft-versus-host diseases (GVHD). The clinical benefits of BMMSCs in autoimmunity and alloimmunity are being revealed by an increasing number of clinical trials and preclinical studies (Le Blanc and Mougiakakos, 2012). Nonetheless, the mechanisms underlying the immunosuppressive functions of BMMSCs are not well understood; the efficacy of BMMSC in preventing GVHD is inconsistent; and it is unclear whether endogenous BMMSCs (rather than transplanted BMMSCs) are also immunosuppressive under physiological or pathological conditions. Intriguingly, a recent study suggests that BMMSCs are an important component of the innate immune system that can directly sense the TLR ligands and promote the recruitment of immune cells (Shi et al., 2011). Thus, mechanisms may exist that can switch BMMSC functions between immunostimulation and immunosuppression. In future studies, genetic and pharmacological tools that allow BMMSC-specific gain- or loss-of-function manipulation would be crucial for further investigation of the physiological roles of BMMSCs. Moreover, another exciting uncharted area is whether and how the immune system regulates BMMSC abundance, differentiation and lineage specification.
Associated pathologies
The function and regulation of BMMSCs are crucial for the dynamic remodeling of the skeletal system, which rely on osteoblast-mediated bone formation. During aging, the number and fitness of BMMSCs gradually decrease, leading to reduced osteogenesis and bone formation (Stolzing et al., 2008). In osteoporosis and anorexia nervosa, osteoblast differentiation from BMMSCs is compromised, associated with increased marrow adipogenesis, leading to bone loss (Bredella et al., 2009, Justesen et al., 2001, Shen et al., 2007). In osteoarthritis, chondrocyte differentiation from BMMSCs is deregulated, contributing to the destruction of articular cartilage and eventually joint degeneration (Goldring and Goldring, 2010). Furthermore, long-term usage of insulin-sensitizing drugs such as rosiglitazone and possibly FGF21 may cause bone loss and fractures, in part due to a shift of BMMSC differentiation favoring marrow adipocytes at the expense of osteoblasts. Therefore, understanding the molecular mechanisms for BMMSC self-renewal and differentiation is critical to gain new insights to the etiology of these disorders.
BMMSCs hold tremendous potential in regenerative medicine because they are not only multipotent but also low immunogenic. Clinically, BMMSC transplantation, induction and/or activation provide a rational therapeutic strategy for bone or cartilage regeneration by promoting osteogenesis or chondrogenesis. This strategy can be applied to not only aging-related diseases such as osteoporosis and osteoarthritis, but also genetic disorders such as osteogenesis imperfecta in which osteoblasts produce defective type-I collagen. Recent exciting development of novel BMMSC application approaches, such as stem cell-to-bone delivery (Guan et al., 2012), anti-osteogenic T cell suppression (Liu et al., 2011), and chondrogenesis-inducing small molecules (Johnson et al., 2012), demonstrate the feasibility of harnessing BMMSCs for the prevention and treatment of degenerative bone diseases.
Cell Facts.
BMMSCs are multipotent marrow stromal cells that can differentiate into osteoblasts, chondrocytes and adipocytes.
BMMSC differentiation is regulated by ligands and receptors such as PPARγ and FGF21.
BMMSCs play a key role in tissue repair, and are clinically explored as regenerative therapeutics.
BMMSC deregulation contributes to degenerative diseases such as osteoporosis and osteoarthritis.
Acknowledgments
I thank all the investigators whose studies contributed to the understanding of BMMSCs but could not be cited here due to space limitation. Y. Wan is a Virginia Murchison Linthicum Scholar in Medical Research. This work was supported by the UT Southwestern Endowed Scholar Startup Fund, CPRIT (RP100841), NIH (RO1DK089113), March of Dimes (#5-FY10-1) and The Welch Foundation (I-1751).
Footnotes
I declare that I have no financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–2136. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Auwerx J. Cell biology. FGF21 takes a fat bite. Science. 2012;336:675–676. doi: 10.1126/science.1222646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, Kliewer SA. Fibroblast Growth Factor-21 Regulates PPARgamma Activity and the Antidiabetic Actions of Thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dy P, Wang W, Bhattaram P, Wang Q, Wang L, Ballock RT, Lefebvre V. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev Cell. 2012;22:597–609. doi: 10.1016/j.devcel.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- Guan M, Yao W, Liu R, Lam KS, Nolta J, Jia J, Panganiban B, Meng L, Zhou P, Shahnazari M, Ritchie RO, Lane NE. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med. 2012;18:456–462. doi: 10.1038/nm.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3A. Nature. 2012;485:69–74. doi: 10.1038/nature11000. [DOI] [PubMed] [Google Scholar]
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- Johnson K, Zhu S, Tremblay MS, Payette JN, Wang J, Bouchez LC, Meeusen S, Althage A, Cho CY, Wu X, Schultz PG. A stem cell-based approach to cartilage repair. Science. 2012;336:717–721. doi: 10.1126/science.1215157. [DOI] [PubMed] [Google Scholar]
- Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, Kravitz BG, Yu D, Heise MA, Aftring RP, Viberti G. Rosiglitazone-associated fractures in type 2 diabetes: an Analysis from A Diabetes Outcome Progression Trial (ADOPT) Diabetes Care. 2008;31:845–851. doi: 10.2337/dc07-2270. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-gamma and TNF-alpha. Nat Med. 2011;17:1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, Takayanagi H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011;17:1473–1480. doi: 10.1038/nm.2489. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18:641–647. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Strotmeyer ES, Cauley JA. Diabetes mellitus, bone mineral density, and fracture risk. Curr Opin Endocrinol Diabetes Obes. 2007;14:429–435. doi: 10.1097/MED.0b013e3282f1cba3. [DOI] [PubMed] [Google Scholar]
- Wan Y. PPARgamma in bone homeostasis. Trends Endocrinol Metab. 2010;21:722–728. doi: 10.1016/j.tem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007;13:1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- Wei W, Dutchak PA, Wang X, Ding X, Bookout AL, Goetz R, Mohammadi M, Gerard RD, Dechow PC, Mangelsdorf DJ, Kliewer SA, Wan Y. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci U S A. 2012;109:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Wang X, Yang M, Smith LC, Dechow PC, Wan Y. PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab. 2010;11:503–516. doi: 10.1016/j.cmet.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zeve D, Suh JM, Wang X, Du Y, Zerwekh JE, Dechow PC, Graff JM, Wan Y. Biphasic and dosage-dependent regulation of osteoclastogenesis by beta-catenin. Mol Cell Biol. 2011;31:4706–4719. doi: 10.1128/MCB.05980-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinman B, Haffner SM, Herman WH, Holman RR, Lachin JM, Kravitz BG, Paul G, Jones NP, Aftring RP, Viberti G, Kahn SE. Effect of rosiglitazone, metformin, and glyburide on bone biomarkers in patients with type 2 diabetes. J Clin Endocrinol Metab. 2010;95:134–142. doi: 10.1210/jc.2009-0572. [DOI] [PubMed] [Google Scholar]