Abstract
Purpose
To investigate the novel application of tissue microarray (TMA) technology to corneal disease and to report altered protein expression of senescence-associated cyclin-dependent kinase inhibitors p21 and p16 in Fuchs endothelial corneal dystrophy (FECD).
Methods
A TMA including 208 cores was generated from paraffin-embedded tissues including corneal buttons of 50 FECD and 5 keratoconus (KC) patients retrieved post penetrating keratoplasty, 10 autopsy globes with non-pathologic corneas, and non-ocular control specimens. TMA sections were immunolabeled for p21 and p16 and analyzed using a nine-grade scoring system (0–8). Result validation was performed by immunolabeling of individual whole tissue sections. Corneal endothelial p21 and p16 expression levels in FECD specimens compared to controls served as main outcome measures.
Results
TMA immunohistochemical analysis disclosed increased endothelial expression levels of nuclear p21 in FECD specimens (p<0.05) and an altered endothelial p16 expression pattern. Immunolabeling of whole tissue sections showed statistically significant endothelial overexpres-sion of both proteins (p21 and p16, p<0.05).
Conclusion
The present study introduces TMA technology as a valuable tool for molecular high-throughput profiling of corneal tissues. It demonstrates p21 and p16 overexpression in the corneal endothelium of genetically undifferentiated FECD patients supporting a role of cellular senescence in the pathogenesis of FECD.
Keywords: Fuchs dystrophy, tissue microarray, cyclin-dependent kinase inhibitor
INTRODUCTION
Fuchs endothelial corneal dystrophy (FECD) is characterized by a progressive decrease of endothelial cell density and a thickening of Descemet membrane with the formation of focal excrescences called guttae. In end-stage FECD corneas, the decreasing ability of the endothelium to act as a pump and a barrier is no longer sufficient to maintain corneal deturgescence resulting in vision impairing corneal edema.
Prolonged periods of subcytotoxic stress may cause “stress induced premature senescence” (SIPS), a state of permanent cell cycle arrest and decreased cellular function, in cells from various tissues.[1, 2] Senescent cells may negatively influence tissue homeo-stasis by exhibiting an altered gene expression and production of inappropriate molecules disturbing the cellular microenvironment.[3] SIPS occurs independently from senescence due to telomere shortening. Increased endoplasmic reticulum stress and oxidative stress have been identified as potential pathogenetic key factors in FECD. [4–8] This suggests that increased endothelial SIPS may also occur in FECD.
Human corneal endothelial cells (CECs) are arrested in G1-phase and do not normally undergo cell division in vivo.[9, 10] However, they may be propagated in vitro.[9, 10] The presence of an increased proportion of CECs with important features of premature senescence has been demonstrated in the central endothelium of normal corneas from older donors.[11] This finding was attributed to an accumulation of endothelial oxidative stress and DNA damage in the long-term stress exposed normal corneal tissue.[12, 13] In this context, it was also assumed that overexpression of the cyclin-dependent kinase inhibitors (CDKI) p16 and p21 may be at least in part responsible for the reduced proliferative response of senescent CECs in vitro.[11] P21 and p16 represent important bi-omarkers of cellular senescence (including SIPS) and aging.
Tissue microarray (TMA) technology, as first described by Kononen et al., is a powerful technique for high-throughput molecular-profiling of pre-fixed tissue samples.[14] Multiple cylindrical cores from different “donor” tissue blocks are united in a single “recipient” block and may be investigated simultaneously on one tissue section.[15] Thus a larger number of separate patient tissue samples can be experimentally processed as a single procedure, thereby greatly reducing time, resources, and experimental variability associated with processing samples individually. TMAs have been widely used for studying a variety of human cancers but also non-neoplastic entities like neurodegenerative or inflammatory disorders.[16] However, to our knowledge TMAs have not yet been applied to the study of corneal diseases.
The present study sought to apply for the first time a TMA approach to investigate an abundance of the cyclin-dependent kinase inhibitors (CDKIs) p21 and p16 in a large number of human FECD and control samples and to provide further evidence for a pathogenetic role of increased cellular senescence in FECD.
METHODS
Human Tissue
This study was approved by The Johns Hopkins University School of Medicine Institutional Review Board and adhered to the tenets of the Declaration of Helsinki.
Seventy-five formalin-fixed and paraffin-embedded corneal specimens originating from the years 2001 to 2003 were selected from the archives of the Wilmer Eye Pathology Service. These included corneal buttons post penetrating keratoplasty in FECD (n=55) or KC (n=5) patients and whole autopsy globes (n=15). Individual demographic and his-tological donor information was recorded from pathology reports (summarized in Table). Selection criteria for corneal specimens were the unequivocal histopathological diagnoses of FECD or KC and, for autopsy eyes, the presence of an unremarkable cornea and no history of corneal disease. Thirteen formalin-fixed and paraffin-embedded non-ocular control specimens (brain, urinary bladder, thyroid, liver, kidney, tonsil, placenta, skin, testis, myometrium, lung, breast) were obtained from the Johns Hopkins Hospital Autopsy Service.
TABLE.
Donor information for corneal specimens used for tissue microarray (TMA) production and validation (whole sections)
| Patient Cohort | n | Age | Gender (f:m) | Endothelial nuclei per hpf | Thickness (μm)DM | Death to Autopsy (d) | |
|---|---|---|---|---|---|---|---|
| TMA | FECD | 50 | 69.62 ± 1.2 | 35:15 | 5.6 ± 0.3 | 17.0 ± 1.0 | n/a |
| KC | 5 | 58.6 ± 8.6 | 3:2 | 15.6 ± 0.5 | n/a | n/a | |
| Autopsy | 10 | 63.9 ± 2.9 | 5:5 | 15.8 ± 1.4 | n/a | 1.0 ± 0.3 | |
| Whole Sections | FECD | 5 | 78.6 ± 2.8 | 3:2 | 5.3 ± 0.3 | 18.4 ± 4.1 | n/a |
| Autopsy | 5 | 79.4 ± 3.4 | 3:2 | n/a | n/a | 0.8 ± 0.4 |
TMA: tissue microarray; FECD: Fuchs endothelial corneal dystrophy; KC: Keratoconus; hpf: high power field; DM: Descemet membrane; d: days; data are means ± SEM
Hematoxylin and eosin (H&E) stained tissue sections were used to evaluate the individual tissue quality of each block. We excluded specimens with gross microscopic abnormalities. We also excluded FECD specimens with an endothelial cell count of less than three nuclei per high power field (HPF) in order to keep a significant number of evaluable endothelial cells on each core.
Tissue Microarray
A TMA was generated by the Johns Hopkins TMA Facility as previously described.[15] Beforehand, an electronic sample map containing the pertinent information for each core was created using the TMAJ software (The Johns Hopkins University, Baltimore, MD; http://tmaj.pathology.jhmi.edu/). The TMA included an array of 13×16 (height × width) 1 mm diameter cores with a 0.3 mm interspace (Figure 1). The corneal/autopsy donor blocks mentioned above were represented by triplicate adjacent cores from the corneal center, respectively. One core from each non-ocular control tissue was included (Figure 1).
Figure 1.
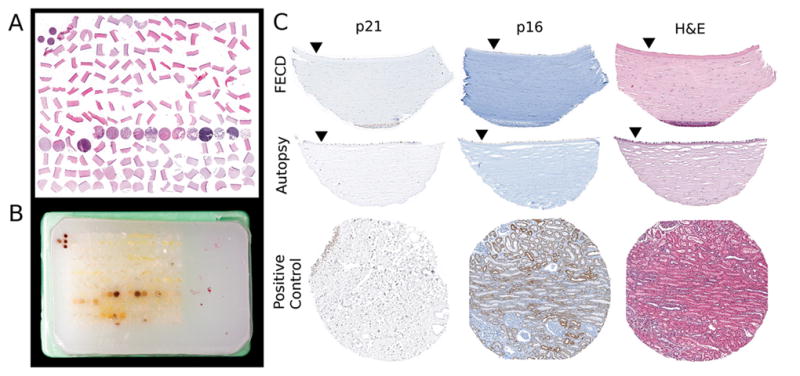
Corneal Tissue Microarray: A) Microscopic image of a hematoxylin & eosin (H&E) stained section from the TMA block of paraffin embedded tissue cores presented in B). The TMA paraffin block displayed in B) includes a total of 208 cylindrical tissue cores of 1 mm diameter and 0.3 mm interspace (individual cores from non-corneal controls show physiologic tissue-specific brown color). Four orientation-markers (non-corneal control tissue cores) are arranged in a “T”-shape on a pre-defined pole (top-left) of the microarray for facilitated microscopic navigation. C) Microscopic image of the same corneal core-sections from Fuchs Endothelial Corneal Dystrophy (FECD) and autopsy specimens after immunohistochemical staining for p21 and p16 or after H&E staining (closed triangles mark endothelial sides of sections). Cores of urinary bladder tissue (bottom left) and kidney tissue (bottom middle, and H&E stained bottom right) served as positive controls for p21 and p16 stainings, respectively. Original magnification of C) 200x.
Immunohistochemistry for p21
Four to 5 μm TMA sections were preheated at 65°C for 60 minutes before deparaffinizing and hydrating in xylene and graded ethanol. Slides were placed in dis- tilled H2O (dH2O) with 0.1% Tween-20 detergent for 1 minute. Heat-mediated antigen retrieval was performed for 50 minutes in Target Retrieval Solution (DAKO, Carpinteria, CA). Cooled sections were permeabilized in tris-buffered saline with Tween (TBST) for 5 minutes and blocked with dual enzyme block solution (DAKO) for 10 minutes. Mouse-anti human p21 IgG (Clone 6B6, BD Biosciences, Franklin Lakes, NJ) was applied at a concentration of 1:50 at 4°C overnight and detected by application of Power Vision Poly-HRP anti-Mouse IgG reagent (Leica Microsystems, Buffalo Grove, IL) for 30 minutes and developing in filtered diaminobenzidine (DAB) chromogen solution (Sigma Fast DAB tablet set, Sigma, St. Louis, MO). Nuclei were counterstained briefly (1:5 Mayers hematoxylin in dH2O, DAKO). After dehydrating in reversed ethanol gradients and xy- lene, sections were coverslipped using permanent mounting medium (Vector Laboratories, Burlingame, CA).
Immunohistochemistry for p16
Four to 5 μm TMA sections were preheated at 65°C for 60 minutes. Immunohistochem-istry, including deparaffinization, blocking-steps and antigen retrieval, was performed on a Benchmark XT automated tissue staining system (Ventana Medical Systems, Inc., Tucson, AZ) according to standardized autostainer protocols. A monoclonal mouse anti-p16 antibody (clone E6H4, prediluted, MTM Laboratories, Heidelberg, Germany) was used as the primary antibody. Tissue-bound antibody was detected using the iVIEW DAB Detection Kit (Ventana Medical Systems).
TMAJ-Software guided analysis of stained TMA sections
Stained TMA tissue sections (p21, p16, H&E) were scanned using a ScanScope CS (Aperio, Vista, CA) with a magnification of 200x. TMALab in Aperio’s Spectrum software (Aperio) was used to subdivide the image of the entire TMA section by an electronic raster into 208 individual images of single tissue cores. Individual images were uploaded into the TMAJ software and served as a roadmap for the evaluation of immunohistochemical TMA stains.
Immunostained TMA sections were evaluated by light microscopy using an 80x objective and a modified scoring system previously used by Bhutto et al. taking into account the nuclear staining for p21 and the nuclear and cytoplasmic staining for p16.[17] A score was assigned to each core according to its endothelial staining intensity and expression pattern as follows: 8) uniformly intense, 7) patchy and intense, 6) uniform and moderate, 5) patchy and moderate, 4) uniform and weak, 3) patchy and weak, 2) uniform and very weak, 1) patchy and very weak, 0) no reactivity. The mean score was calculated for each donor specimen and for the FECD, KC and autopsy groups, respectively. Tissue sections with less than four evaluable CECs were excluded from the analysis.
Validation of p21 and p16 Stainings
For validation of immunohistochemical stainings for p21 and p16 in TMA sections, 4μm thick whole tissue-sections were taken from formalin-fixed paraffin-embedded FECD corneas (n=5) or autopsy eyes with normal corneas (n=5) from age-matched donors (Table). Immunohistochemistry and scoring of the stained sections was performed as described above.
Statistics
PRISM4 software (Graphpad Software, La Jolla, CA) was used for statistical analyses of all data applying the Mann-Whitney test. P<0.05 was considered statistically significant.
RESULTS
Specificity of Antibodies
On the TMA sections, a specific positive reaction could be detected in non-corneal positive control cores for both the anti-p21 antibody (in epithelium of urinary bladder tissue; Figure 2) and the anti-p16 antibody (in cortical tubular cells of kidney tissue; Figure 3). The p21 antibody showed primarily a nuclear reactivity, whereas a nuclear and cytoplasmic reaction was found for the p16 antibody (Figure 2 and 3).
Figure 2.
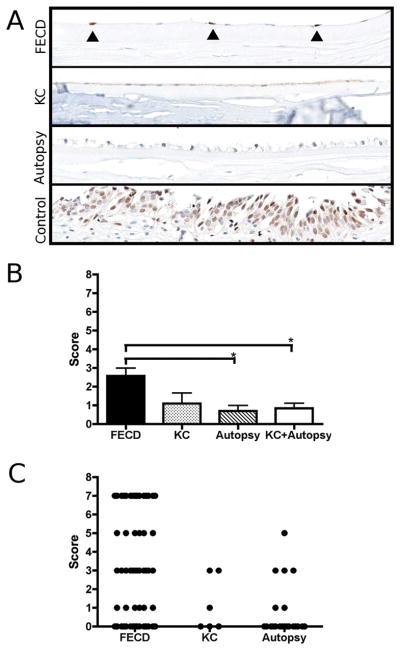
Immunohistochemical staining of p21 protein on tissue microarray (TMA) section: A) Microscopic image of corneal endothelium from Fuchs endothelial corneal dystrophy (FECD), keratoconus (KC) and ocular autopsy specimens (original magnification 200x). Nuclear p21 expression was found in scattered endothelial cells of FECD corneas (marked by closed triangles). Bottom image shows non-ocular positive control tissue (urinary bladder epithelium). B) Endothelial p21 expression was scored using a modified scoring system previously described by Bhutto et al.[17] The bar graph depicts mean values ± SEM (*p<0.05). C) Scatter plot indicating the individual score for each evalua- ble core on the TMA.
Figure 3.
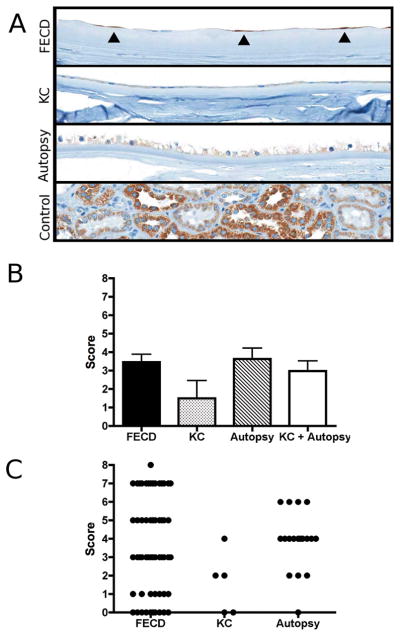
Immunohistochemical staining of p16 protein on tissue microarray (TMA) section: A) Microscopic image of corneal endothelium from Fuchs endothelial corneal dystrophy (FECD), keratoconus (KC) and ocular autopsy specimens (original magnification 200x). Scattered endothelial cells of several FECD specimens showed p16 expression (marked by closed triangles). P16 expression among endothelial cells from KC and autopsy corneas was fainter, though more uniform. Bottom image shows non-ocular positive control tissue (kidney). B) Endothelial p16 expression was scored using a modified scoring system previously described by Bhutto et al.[17] The bar diagram depicts mean values ± SEM. C) Scatter plot of the individual scores for each evaluable core on TMA.
P21 Expression in Corneal Tissue
Fifty-three of 65 (81.5%) donor specimens (FECD 39, KC 5, Autopsy 9) that were represented on the TMA could be informatively evaluated. The most common reason for a non-analyzable tissue sample was the partial or total absence of the core-section after the staining procedure. The mean values (± SEM) of the number of evaluable cores per primary individual donor block were 1.9±0.1 (FECD), 1.2±0.2 (KC) and 2.3±0.3 (Autopsy), respectively. The sex ratios (female: male) of the evaluable donor specimens from the three groups were 26:13 (FECD), KC 3:2 (KC) and 4:5 (Autopsy), respectively. The mean (± SEM) age of the evaluable specimens from each group was 68.5±1.3 (FECD), 58.6±8.6 (KC) and 62.2±2.6 (Autopsy), respectively.
The mean (± SEM) p21-scores according to the scoring system by Bhutto and coworkers were 2.6±0.4 (FECD), 1.1±0.6 (KC) and 0.7±0.3 (Autopsy), respectively.[17] The FECD group showed a statistically significant difference compared to autopsy controls (p=0.042, Figure 2) and to the combined group of KC and autopsy samples (p=0.01, Figure 2). According to the scoring system, “patchy” staining patterns received an odd score (1, 3, 5, 7) and “uniform” expression patterns received an even one (2, 4, 6, 8). Thus, the scatter plot in Figure 2 indicates that positive nuclear p21 staining in FECD and corneal control cores occurred generally not in a uniform distribution but rather in a “patchy” manner and in individual cells. Furthermore, staining intensities varied from cell to cell. Most FECD cores were scored as “patchy/intense” (7), “patchy/weak” (3) or “negative” (0). Cores from all corneal control cases with one exception from the autopsy group were scored as “patchy/weak” (3), “patchy/very weak”(1) or negative” (0) (Figure 2).
P16 Expression in Corneal Tissue
Forty-nine of 65 (75.4%) primary donor specimens (FECD 36, KC 4, Autopsy 9) could be informatively evaluated. The mean (±SEM) evaluable cores per donor specimen were 1.7±0.1 (FECD), 1.3±0.3 (KC) and 2.0±0.3 (Autopsy). Again, the majority of non-evaluable cores was due to the partial or complete absence of the tissue sections after the staining procedure. Sex ratios (female:male) of the evaluable donor specimen within each group were 23:13 (FECD), 2:2 (KC), 4:5 (Autopsy). The mean (± SEM) ages were 69.1±1.4 (FECD), 53.3±8.7 (KC) and 62.2±2.6 (Autopsy). The mean (± SEM) p16-scores were 3.5±0.4 (FECD), 1.5±1.0 (KC), 3.6±0.6 (Autopsy), respectively. There was no statistically significant difference between these three groups (p>0.05, Figure 3). The scatter-plot in Figure 3 demonstrates that a high variability of staining intensities for p16 was found among the FECD cores. FECD cores, if not considered negative, showed a patchy endothelial staining (as indicated by odd scores on scatter plot) with negative CECs in between the stained cells (Figure 3). The staining intensity usually exhibited some variation among the positive cells. Uniform (as indicated by even scores on scatter plot), though generally weak or very weak, p16 distribution was found in all positive corneal control cores (Figure 3).
Validation of Tissue Microarray Results
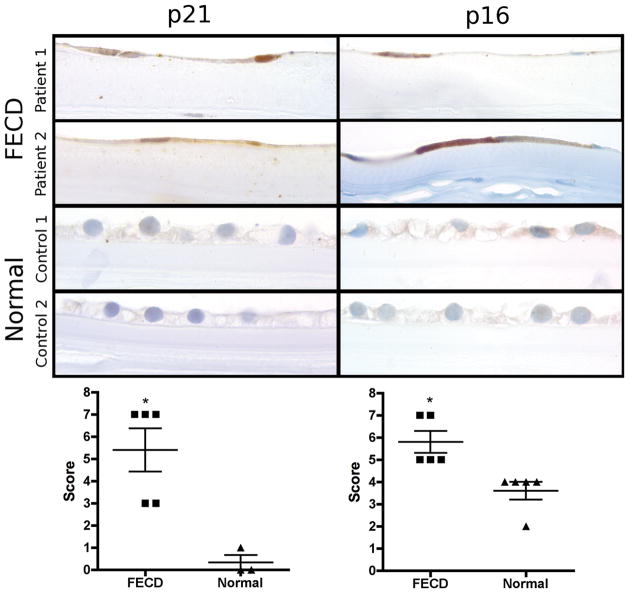
The mean (± SEM) endothelial p21-scores on evaluable tissue sections were 5.4±1.0 in FECD corneas and 0.3±0.3 in autopsy eyes indicating a statistically significant difference between both groups (p=0.018, Figure 4). As on the TMA sections, staining intensity showed some variability among the individual positive endothelial nuclei (Figure 4). The mean scores for p16 on whole tissue sections were 5.8±0.5 in FECD corneas and 3.6±0.4 in autopsy eyes. The difference between both groups was found to be statistically significant (p=0.004). As in the TMA sections, microscopic imaging showed patchy endothelial p16 expression of individual cells in FECD endothelium whereas uniform weaker endothelial p16 positivity was detected in CECs of normal corneas (Figure 4).
Figure 4.
Immunohistochemical staining of p21 and p16 protein on whole corneal tissue sections: Panel of microscopic images presents two examples of endothelial p21 and p16 expression in FECD and normal corneas, respectively (original magnification 1000x). Top and second row show FECD specific flattening of corneal endothelial cells and their nuclei due to cell loss with p21 positive (left column) and p16 positive (right column) cells. The normal corneal endothelium (third and bottom row) shows no or only (very) weak staining for p21 (left column) and p16 (right column). Scatter plots show p21 (left) and p16 (right) expression-levels of each individual specimen analyzed (scored according to Bhutto et al.[17]), horizontal lines indicate means ± SEM (*p<0.05)
DISCUSSION
Although CECs do not normally divide in vivo they may retain their ability to undergo mitosis and can be propagated in vitro.[18] It was found that CECs especially from the corneal center of old donors are prone to have a reduced proliferative capacity.[19] This finding was attributed to increased levels of oxidative stress and DNA damage causing stress induced premature senescence (SIPS).[12, 13, 19] SIPS is a condition of permanent cell cycle arrest that is independent of proliferation associated telomere shortening and may be caused by various forms of cellular stress in proliferating cells.[2] Two key molecular factors of senescence are the CDKI molecules p21 and p16. Recent studies demonstrated that cellular stress plays a central role in the pathogenesis of FECD.[4–8]. This suggests that SIPS of CECs may also occur in FECD.
TMAs facilitate the implementation of high-throughput histological investigations in tissue samples. In the present study we generated a TMA to investigate the differential expression of CDKIs p21 and p16 in the endothelium of a definitive number of FECD patients.
P21 is a CDKI of the CIP/KIP family.[20] It inhibits cyclin/cyclin-dependent kinase complexes leading to maintained hypophosphorylation of retinoblastoma protein (pRB).[21] This results in reduced release of E2F, a key regulator of the G1/S-Phase transition.[22] P21 plays a role in senescence of CECs: it was found to be transcriptionally increased in CECs undergoing replicative senescence, and at the protein level in CECs cultured from old donors.[23, 24] The results of the present study demonstrate in a large number of specimens that enhanced nuclear p21 expression can be found in the endothelium of FECD corneas. A study by Azizi et al. recently demonstrated that p53-protein is increased in the endothelium of FECD corneas.[25] P53 leads to p21 production and is a major common regulator of both apoptosis and senescence.[26]
P16 is a CDKI of the INK4 family.[27] In a similar fashion to p21, it maintains pRb in a hypophosphorylated state by formation of binary complexes with CDK4 and CDK6.[27] It was previously shown that p16 mRNA is increased in both cultured human CECs at high passages and donor corneas from old donors and that it was elevated at the protein level in cultured CECs from old donors.[23, 24, 28] In our study, intense p16-reactivity in individual scattered CECs among p16-negative CECs was a common finding on the stained FECD TMA core-sections (Figure 3). This stood in strong contrast to the uniform, generally weak endothelial p16 expression in most of the control corneas (Figure 3). The analysis of whole tissue sections enabled us to verify these qualitative differences and, furthermore, demonstrated a statistically significant endothelial overexpression in FECD corneas (Figure 4).
Corneas, with an approximate thickness of 500 μm, are relatively small for the production of TMAs. However, TMAs of small tissue samples, like needle-biopsies, have been described before.[29] A corneal cross-section can be well captured by a core diameter of 1 mm and the validation of our results for endothelial p21 expression in whole corneal tissue-sections from independent samples demonstrated good reproducibility of the results obtained on the TMA. TMA analysis of p16 expression allowed for the detection of a qualitative difference between FECD specimens and controls. However, additional assessment of whole tissue sections was needed to prove a statistically significant quantitative difference based on the applied scoring system. Thus, in some cases larger amounts of tissue per specimen may be needed for the detection of quantitative differences occurring in relatively few individual cells. For CECs, this may be achieved by investigating multiple non-overlapping TMA sections, whole tissue sections, or endothelial flatmounts.
We conclude that the TMA technique is a valuable tool for the high-throughput analysis of corneal specimens. The use of TMAs provides the advantages of a well-standardized tissue-, time- and cost-saving approach as compared to the previous method of immunohistochemical examination of individual slides or flatmounts, where each separate specimen needs to be prepared, stained and evaluated.[30] Its application for other ocular diseases should be considered. Our analyses demonstrate in a large FECD patient population increased endothelial levels of nuclear p21 and p16 proteins supporting the involvement of cellular senescence in the pathogenesis of FECD.
Acknowledgments
The authors thank Abdalhossein Ghafourian, MD, Helen Fedor, Marcela Southerland, Barbara Reiss, Zeny Martin and Kristen Lecksell for technical assistance
Funding/Support: Deutsche Forschungsgemeinschaft (DFG MA 5110/2–1 to M.M.), Richard Lindstrom/Eye Bank Association of America Research Grant (to M.M.), Fuchs Dystrophy Research Grant (Wilmer Eye Institute to M.M.), National Institutes of Health (NIH EY019874 to A.S.J.), Medical Illness Counseling Center (to A.S.J.), Research to Prevent Blindness (to Wilmer Eye Institute).
References
- 1.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37(5):961–76. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Campisi J, di Fagagna FD. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Bio. 2007;8(9):729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 3.Muller M. Cellular senescence: molecular mechanisms, in vivo significance, and redox considerations. Antioxid Redox Signal. 2009;11(1):59–98. doi: 10.1089/ars.2008.2104. [DOI] [PubMed] [Google Scholar]
- 4.Jun AS, Meng H, Ramanan N, et al. An alpha 2 collagen VIII transgenic knock-in mouse model of Fuchs endothelial corneal dystrophy shows early endothelial cell unfolded protein response and apoptosis. Hum Mol Genet. 2012;21(2):384–93. doi: 10.1093/hmg/ddr473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engler C, Kelliher C, Spitze AR, et al. Unfolded protein response in fuchs endothelial corneal dystrophy: a unifying pathogenic pathway? Am J Ophthalmol. 2010;149(2):194–202. e2. doi: 10.1016/j.ajo.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Handa JT, Green WR, et al. Advanced glycation end products and receptors in Fuchs’ dystrophy corneas undergoing Descemet’s stripping with endothelial keratoplasty. Ophthalmology. 2007;114(8):1453–60. doi: 10.1016/j.ophtha.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 7.Jurkunas UV, Bitar MS, Funaki T, et al. Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy. Am J Pathol. 2010;177(5):2278–89. doi: 10.2353/ajpath.2010.100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buddi R, Lin B, Atilano SR, et al. Evidence of oxidative stress in human corneal diseases. J Histochem Cytochem. 2002;50(3):341–51. doi: 10.1177/002215540205000306. [DOI] [PubMed] [Google Scholar]
- 9.Joyce NC. Cell cycle status in human corneal endothelium. Exp Eye Res. 2005;81(6):629–38. doi: 10.1016/j.exer.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Joyce NC. Proliferative capacity of corneal endothelial cells. Experimental Eye Research. 2012;95(1):16–23. doi: 10.1016/j.exer.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mimura T, Joyce NC. Replication competence and senescence in central and peripheral human corneal endothelium. Invest Ophthalmol Vis Sci. 2006;47(4):1387–96. doi: 10.1167/iovs.05-1199. [DOI] [PubMed] [Google Scholar]
- 12.Konomi K, Joyce NC. Age and topographical comparison of telomere lengths in human corneal endothelial cells. Mol Vis. 2007;13:1251–8. [PubMed] [Google Scholar]
- 13.Joyce NC, Zhu CC, Harris DL. Relationship among Oxidative Stress, DNA Damage, and Proliferative Capacity in Human Corneal Endothelium. Invest Ophth Vis Sci. 2009;50(5):2116–22. doi: 10.1167/iovs.08-3007. [DOI] [PubMed] [Google Scholar]
- 14.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4(7):844–7. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 15.Fedor HL, De Marzo AM. Practical methods for tissue microarray construction. Methods Mol Med. 2005;103:89–101. doi: 10.1385/1-59259-780-7:089. [DOI] [PubMed] [Google Scholar]
- 16.Simon R. Applications of tissue microarray technology. Methods Mol Biol. 2010;664:1–16. doi: 10.1007/978-1-60761-806-5_1. [DOI] [PubMed] [Google Scholar]
- 17.Bhutto IA, Kim SY, McLeod DS, et al. Localization of collagen XVIII and the endostatin portion of collagen XVIII in aged human control eyes and eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45(5):1544–52. doi: 10.1167/iovs.03-0862. [DOI] [PubMed] [Google Scholar]
- 18.Engelmann K, Bohnke M, Friedl P. Isolation and long-term cultivation of human corneal endothelial cells. Invest Ophthalmol Vis Sci. 1988;29(11):1656–62. [PubMed] [Google Scholar]
- 19.Konomi K, Zhu C, Harris D, et al. Comparison of the proliferative capacity of human corneal endothelial cells from the central and peripheral areas. Invest Ophthalmol Vis Sci. 2005;46(11):4086–91. doi: 10.1167/iovs.05-0245. [DOI] [PubMed] [Google Scholar]
- 20.Jung YS, Qian Y, Chen X. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell Signal. 2010;22(7):1003–12. doi: 10.1016/j.cellsig.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harper JW, Adami GR, Wei N, et al. The P21 Cdk-Interacting Protein Cip1 Is a Potent Inhibitor of G1 Cyclin-Dependent Kinases. Cell. 1993;75(4):805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 22.DeGregori J, Leone G, Miron A, et al. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc Natl Acad Sci U S A. 1997;94(14):7245–50. doi: 10.1073/pnas.94.14.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheerin AN, Smith SK, Jennert-Burston K, et al. Characterization of cellular senescence mechanisms in human corneal endothelial cells. Aging Cell. 2012;11(2):234–40. doi: 10.1111/j.1474-9726.2011.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enomoto K, Mimura T, Harris DL, et al. Age differences in cyclin-dependent kinase inhibitor expression and rb hyperphosphorylation in human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2006;47(10):4330–40. doi: 10.1167/iovs.05-1581. [DOI] [PubMed] [Google Scholar]
- 25.Azizi B, Ziaei A, Fuchsluger T, et al. p53-regulated increase in oxidative-stress--induced apoptosis in Fuchs endothelial corneal dystrophy: a native tissue model. Invest Ophthalmol Vis Sci. 2011;52(13):9291–7. doi: 10.1167/iovs.11-8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seluanov A, Gorbunova V, Falcovitz A, et al. Change of the death pathway in senescent human fibroblasts in response to DNA damage is caused by an inability to stabilize p53. Mol Cell Biol. 2001;21(5):1552–64. doi: 10.1128/MCB.21.5.1552-1564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pei XH, Xiong Y. Biochemical and cellular mechanisms of mammalian CDK inhibitors: a few unresolved issues. Oncogene. 2005;24(17):2787–95. doi: 10.1038/sj.onc.1208611. [DOI] [PubMed] [Google Scholar]
- 28.Song Z, Wang Y, Xie L, et al. Expression of senescence-related genes in human corneal endothelial cells. Mol Vis. 2008;14:161–70. [PMC free article] [PubMed] [Google Scholar]
- 29.Datta MW, Kahler A, Macias V, et al. A simple inexpensive method for the production of tissue microarrays from needle biopsy specimens - Examples with prostate cancer. Appl Immunohisto M M. 2005;13(1):96–103. doi: 10.1097/00129039-200503000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Camp RL, Neumeister V, Rimm DL. A decade of tissue microarrays: progress in the discovery and validation of cancer biomarkers. J Clin Oncol. 2008;26(34):5630–7. doi: 10.1200/JCO.2008.17.3567. [DOI] [PubMed] [Google Scholar]