Abstract
Prostaglandin E2 (PGE2) is a bioactive lipid that elicits a wide range of biological effects associated with inflammation and cancer. PGE2 exerts diverse effects on cell proliferation, apoptosis, angiogenesis, inflammation and immune surveillance. This review concentrates primarily on gastrointestinal cancers, where the actions of PGE2 are most prominent, most likely due to the constant exposure to dietary and environmental insults and the intrinsic role of PGE2 in tissue homeostasis. A discussion of recent efforts to elucidate the complex and interconnected pathways that link PGE2 signaling with inflammation and cancer is provided, supported by the abundant literature showing a protective effect of NSAIDs and the therapeutic efficacy of targeting mPGES-1 or EP receptors for cancer prevention. However, suppressing PGE2 formation as a means of providing chemoprotection against all cancers may not ultimately be tenable, undoubtedly the situation for patients with inflammatory bowel disease. Future studies to fully understand the complex role of PGE2 in both inflammation and cancer will be required to develop novel strategies for cancer prevention that are both effective and safe.
Keywords: PGE2, inflammation, gastrointestinal cancer, NSAIDs, COX-2, mPGES-1
1. Introduction and overview
Prostaglandin E2 (PGE2) is a bioactive lipid that can elicit a wide range of biological effects associated with inflammation and cancer. PGE2 belongs to the prostanoid family of lipids, which is a subclass of eicosanoids produced by oxidation of 20-carbon essential fatty acids (EFAs) that are commonly incorporated within membrane phospholipids. Prostanoids including PGE2, PGF2α, PGD2, PGI2 and thromboxane A2 (TXA2) are synthesized by the sequential actions of a panel of highly specific enzymes. Their synthesis is initiated by phospholipases (PLAs), a family of enzymes that catalyze the hydrolysis of membrane phospholipids at the sn-2 position, liberating free fatty acids, including arachidonic acid (AA), from membrane lipids. PLA2s are grouped according to their structure and enzymatic characteristics, and are comprised of both secretory and intracellular forms. cPLA2α is the best characterized isoform and the only one that is regulated by Ca2+ binding and phosphorylation by mitogen-activated protein kinase (MAPK). In addition, its expression is altered in cancer cells, suggesting an important role in disease development [1–3].
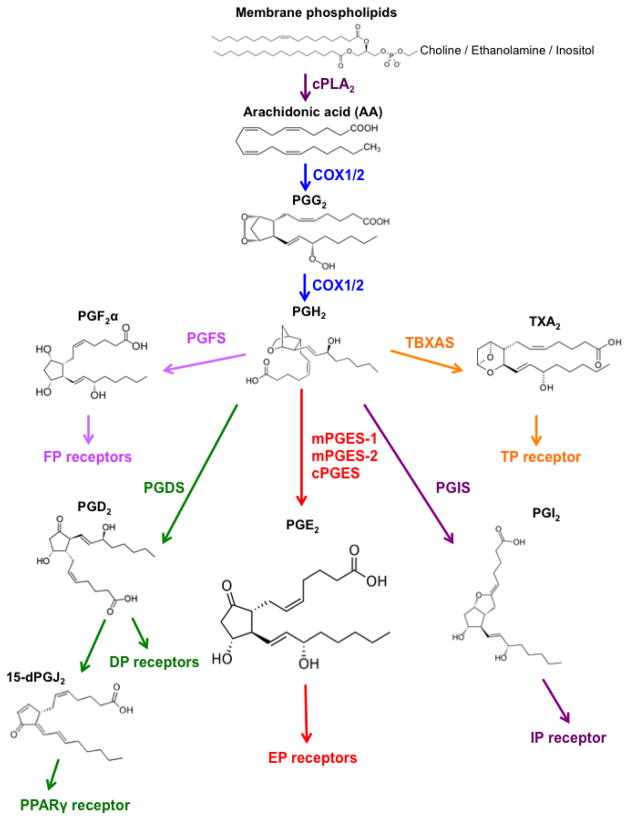
Membrane-released AA is rapidly oxidized into the relatively unstable metabolite, PGG2, which is subsequently reduced to PGH2, both steps sequentially catalyzed by the cyclooxygenase (COXs) enzymes. There are two major COX isoforms; COX-1 is constitutively active and present within most cells in the body, whereas constitutive COX-2 expression is largely restricted to the kidney as well as areas of the central nervous system. However, COX-2 levels are highly inducible in many tissues by pro-inflammatory and mitogenic stimuli, including cytokines and growth factors [4]. Once synthesized, PGH2 is rapidly converted into prostanoids by a panel of terminal synthases. The metabolic steps in the formation of the PGs are summarized in Figure 1.
Fig. 1.
PGE2 biosynthetic pathway
Three distinct synthases contribute to PGE2 synthesis [5–7]. These terminal synthases are comprised of three isoforms that are tightly regulated under various conditions and include microsomal PGE synthase-1 (mPGES-1), mPGES-2 and cytosolic PGE synthase (cPGES) [8]. mPGES-1 is frequently induced concomitantly with COX-2 by various pro-inflammatory stimuli to generate a transient spike in PGE2 levels [9]. On the other hand, mPGES-2 and cPGES are constitutively expressed and functionally coupled with COX-1 to maintain basal levels of PGE2 [9]. While mPGES-1 is glutathione (GSH)-dependent, mPGES-2 and cPGES do not require co-factors for their biosynthetic activity [8].
The levels of PGE2 can also be regulated by its metabolic turnover. The activation of two key catabolic enzymes, 15-hydroxyprostaglandin dehydrogenase (15-PGDH) and 15-keto-prostaglandin- 13-reductase (13-PGR) can essentially eliminate the biological activity of PGE2 [10]. In particular, 15-PGDH may play a prominent role in colon carcinogenesis; in many human colorectal cancer (CRC), there is a significant reduction in the expression of 15-PGDH, suggesting a likely tumor suppressor role for this protein [11–13]. Under some conditions, 15-PGDH expression can be directly activated by TGF-β signaling [11]. Importantly, using a mouse knockout model, the Markowitz laboratory [14] has identified a role for 15-PGDH in the resistance that may develop to celecoxib during chemoprevention treatment of colon tumors. Consistent with these findings in the mouse, Yan et al. [14] also reported that human subjects who develop new adenomas during the course of celecoxib treatment had very low levels of 15-PGDH expression. Further mechanistic studies showed that the absence of 15-PGDH activity significantly increased intestinal tumorigenesis in ApcMin mice and sensitized normally resistant C57BL/6J mice to azoxymethane (AOM) induced colon carcinogenesis [15]. Finally, Backlund et al. [16] examined the epigenetic regulation of 15-PGDH by histone deacetylases and reported that HDACs interact with Snail at the 15-PGDH promoter, contributing to its repression. Interestingly, treatment of colon cancer cells with HDAC inhibitors such as sodium butyrate and valproic acid can reactivate its gene expression [16]. Overall, these findings in animal models and human tissues reinforce the central role of PGE2 in colon cancer development.
The physiological activity of PGE2 and related prostanoids are mediated by the activation of a diverse group of downstream signaling cascades via seven transmembrane G-protein coupled receptors (GPCR), referred to as the EP, FP, DP, IP and TP receptors [17]. These receptors are highly selective for individual prostanoid substrates, including PGE2 PGF2α, PGD2, PGI2 and TxA2, respectively [17]. Each receptor has a cell type-specific expression pattern that enables tight control over their distinct but occasionally overlapping physiological functions [5]. PGE2 binds to members of the EP family of receptors that consist of four isoforms (EP1-4) and play a major role during inflammation [5]. The EP receptors are coupled to Gα proteins that contain stimulatory (GαS) or inhibitory (Gαi) subunits that can modulate the levels of Ca2+, cyclic AMP (cAMP) and inositol phosphate, activating divergent downstream signaling pathways [18]. EP receptors are ubiquitously expressed within most organ systems. Coupled with the ubiquitous formation of PGE2, EP receptor signaling accounts for the pleiotropic ability of PGE2 to potently activate diverse biological effects, including cell proliferation, apoptosis, angiogenesis, inflammation and immune surveillance in different cell types within a wide range of tissues [7, 19, 20].
In this review, we focus on the role of PGE2 in inflammation and cancer. PGE2 clearly provides a pivotal connection between a number of chronic inflammatory signaling cascades and cancer pathogenesis. We will concentrate primarily on gastrointestinal (GI) cancers, where the actions of PGE2 are most prominent, most likely due to the constant exposure to dietary and environmental insults and the intrinsic role of PGE2 in tissue homeostasis. We will provide an overview of recent efforts to elucidate the complex and interconnected pathways that link PGE2 signaling, inflammation and cancer.
2. Multifaceted roles of PGE2 in inflammation
The inflammatory response is comprised of a finely orchestrated set of interconnected processes, involving a diversity of cell types and inflammatory mediators. PGE2 plays a critical role in guiding and governing various aspects of the inflammatory response. The role of PGE2 in driving acute inflammation is well established. However, PGE2 also elicits powerful immunosuppressive properties that contribute to the resolution phase of acute inflammation, facilitating tissue regeneration and the return to homeostasis. These multifaceted properties of PGE2 are both cell type and context specific. A number of comprehensive reviews focused on the regulation of the immune response by PGE2 are available [21, 22]. In this section, we provide a brief overview of how PGE2 impacts the inflammatory response and discuss more recent data concerning how PGE2 intimately links chronic inflammation with cancer.
Pro-inflammatory effects of PGE2
During the initial phase of the inflammatory response, PGE2 and related prostanoids such as PGI2, act as vasodilators to facilitate the tissue influx of neutrophils, macrophages and mast cells from the bloodstream leading to swelling and edema at the site of infection or tissue injury [23]. Furthermore, PGE2 stimulates sensory nerves to increase the pain response and acts on neurons in the preoptic area to promote pyrogenic effects [23]. The contribution of PGE2 to inflammation has been evaluated in a number of disease models, which has been facilitated by the generation of the mPGES-1 knockout (KO) mouse [24]. The mPGES-1 KO mice are generally protected against a variety of inflammatory disease phenotypes, including collagen-induced arthritis, LPS-induced bone loss and antigen-induced paw edema (reviewed by [25]). In a study employing a collagen-induced arthritis model, reduced inflammation in the mPGES-1 KO mice was associated with a failure to produce antibody against type II collagen, suggesting a role for mPGES-1 in the development of a humoral immune response [26]. Moreover, mPGES-1 KO mice displayed significantly reduced accumulation of exudate and impaired leukocyte migration into the pleural cavity during carrageenan-induced paw edema formation, confirming earlier observations that PGE2 regulates vascular permeability during acute inflammation [25]. It is important to note that genetic deletion of mPGES-1 in mice does not adversely affect cardiovascular function (reviewed by [27]). Furthermore, mPGES-1 deletion increases tissue levels of PGI2, which may compensate for the suppression of PGE2 synthesis [27]. These results in pre-clinical mouse models strongly suggest the possibility that pharmacologic targeting of mPGES-1 may ultimately prove to less toxic and perhaps more effective than the traditional non-steroidal anti-inflammatory drugs (NSAIDs) for controlling acute inflammatory diseases. New drug candidates that have recently been developed for targeting mPGES-1 are discussed later in this review.
An additional pro-inflammatory effect of PGE2 has recently been underscore by its role in promoting the activation of TH17 cells, a subset of CD4+ helper T cells that are characterized by the production of interleukin-17 (IL-17). The IL-17 family of cytokines represents a potent set of pro-inflammatory mediators that recruit monocytes and neutrophils to the site of inflammation. This has been shown to occur during the course of disease progression in several models of autoimmunity and infection (reviewed by [28]). The maturation and activation of TH17 cells is initiated by the binding of IL-23 to its receptor, IL-23R, present on naïve CD4+ T cells, which subsequently drives the expression of the retinoic acid receptor-related orphan receptor (ROR)- t that is required for the production of IL-17 [29]. PGE2 induces both the production of IL-23 in dendritic cells (DCs) via EP4 receptor signaling, and also promotes the expression of the IL-23R in naïve CD4+ T cells via the EP2/EP4 receptors [30]. PGE2-mediated production of IL-17 has been shown to contribute to the development of a variety of inflammatory diseases, including collagen-induced arthritis and inflammatory bowel disease (IBD) in mice [31, 32].
Anti-inflammatory activities of PGE2
Somewhat paradoxically, PGE2 also exerts control over a number of mechanisms that lead to the resolution of inflammation and subsequent tissue repair. Indeed, pharmacological inhibition of COX-2 during the latter phases of an inflammatory response has been shown to interfere with complete tissue recovery in the liver, lung and colon [33–36]. Among the large group of prostanoid metabolites that have been studied, PGD2 and its metabolite PGJ2 have received considerable interest regarding their potent anti-inflammatory properties. However, PGE2 has also been clearly established as a key component of anti-inflammatory processes [37]. PGE2-mediated immunosuppressive activities are associated in part with the expression of specific cytokines and chemokines, as well as their cognate receptors present on immune, stromal and epithelial cells. One important effect of PGE2 is its ability to directly inhibit the synthesis of IL-2 and the expression of the IL-2 receptor in T cells. As reviewed by Kalinski [21], the suppression of IL-2 signaling contributes to the inhibition of effector T cell proliferation and activation. Moreover, PGE2 suppresses the cytotoxic activities of natural killer (NK) cells, -T cells and CD8+ cytotoxic T lymphocytes (CTLs), in part by down-regulating cytokine receptor expression [38–41].
In monocytes and DCs, PGE2 has an inhibitory effect on the production of CCL19, a key chemokine for attracting naïve T cells, which interferes with the activation of effector T cells [42]. Moreover, PGE2 has been shown to suppress the formation of an additional T cell stimulating factor, IL-12, and to induce IL-12p40 expression, a competitive inhibitor of the IL-12 receptor [21]. Most importantly, the suppression of IL-2 by PGE2 promotes a change in the immune response from a TH1 to a TH2 response [43, 44]. The TH1-type response promotes cellular immunity by stimulating the production of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), which enhances the cytotoxic activities of macrophages and CTLs. On the other hand, the TH2-type response is generally less tissue-destructive with cytokine profiles featuring IL-4 and IL-13.
Role of PGE2 in the wound repair process
The TH-type switch highlights the critical role of PGE2 in the process of tissue repair, the final phase of the inflammatory response. As noted by Allen et al. [45], TH2-type cytokines have been postulated to promote localized wound healing by enhancing M2-type macrophage activity that facilitates the production of proteins associated with accelerated tissue repair. The direct involvement of PGE2 in wound healing has been demonstrated by Ae et al. [46], where mPGES-1 deficient mice exhibit delayed healing following acetic acid-induced gastric ulceration. Furthermore, the absence of inducible mPGES-1 caused an enhanced sensitivity to dextran sodium sulfate (DSS) treatment, with the development of a more severe ulcerative colitis phenotype in the KOs compared to wild-type mice [25]. A similar exacerbation of intestinal injury and ulceration has been found in EP4-deficient mice following exposure to DSS [47]. In our laboratory, we recently reported the presence of spontaneous, localized colonic ulcerations in strain A mice harboring a genetic deletion of mPGES-1 [48]. The presence of this spontaneous tissue damage provides direct evidence for the role of inducible PGE2 synthesis in mucosal homeostasis [48]. In addition, we have found that mPGES-1 KO mice display impaired tissue recovery in response to DSS-induced mucosal injury (unpublished results), underscoring the critical role of inducible PGE2 synthesis in epithelial repair.
Epithelial cells play a key role in maintaining mucosal homeostasis within the gut, a tissue that is under a constant threat of inflammatory insult. Following acute injury, the tissue repair process is orchestrated by a plethora of mediators produced by a variety of cell types [49]. The inducible formation of PGE2 is critical for maintaining epithelial barrier function within the GI tract, especially under conditions of increased stress [50]. PGE2 has been shown to play an essential role in epithelial regeneration and reconstitution following tissue injury [51]. As part of the mechanism of tissue repair, PGE2 directly induces epithelial cell proliferation via the activation of several key signaling pathways, including PI3K/Akt and the Wnt cascade [52]. In addition, PGE2 can activate the MAPK and JNK pathways via transactivation of epidermal growth factor receptor (EGFR) [53]. The potent growth promoting effects of PGE2 are discussed in detail below under 'The role of PGE2 in cancer'.
Stromal cells also play an important role in intestinal tissue homeostasis and repair, and PGE2 can directly affect several of these critical cellular processes. For example, recent studies have shown that PGE2 can stimulate the expression of vascular endothelial growth factor (VEGF) in lung and stomach fibroblasts, promoting angiogenesis [54, 55]. Additional evidence for the pro-angiogenic effects of PGE2 was demonstrated by Zhang et al. [56], in which PGE2 induced in vitro tube formation of human microvascular endothelial cells, ex vivo vessel outgrowth of aortic rings and an angiogenic response via EP4-PKA signaling. The PGE2-EP4 axis has also been shown to control the differentiation of endothelial cells from bone marrow-derived cells via the activation of AMP-activated protein kinase (AMPK) [57]. In addition, PGE2 can affect endothelial cell migration via activation of the ERK signaling pathway [58].
Myofibroblasts represent a population of differentiated mesenchymal cells residing within the stroma that contribute to the coordination of tissue regeneration by secreting TGF- , epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), pro-inflammatory cytokines, and basement membrane components [59]. PGE2 has been shown to inhibit myofibroblast differentiation and limit their collagen secretion during pulmonary fibrosis [60], in lung allografts [61] and in bleomycin-induced skin fibrosis [62]. Although the inhibitory effects of PGE2 on myofibroblasts are protective against excessive fibrotic scar formation in the lung and skin, PGE2 may elicit distinct effects at other sites of tissue injury. For example, Iwanage et al. [63] have recently shown that EP2/3/4 receptor signaling can induce the migration of intestinal sub-epithelial myofibroblasts (ISMFs) during wound closure. Moreover, PGE2 is also capable of promoting the proliferation of cardiac fibroblasts via EP1/EP3 signaling [64], and inducing liver contraction via activation of the EP3 and FP receptors [65].
In summary, the results of these studies highlight the complex and context-dependent role of PGE2 in contributing to epithelial homeostasis and wound healing.
3. The role of PGE2 in cancer
Within the context of cancer, PGE2 is generally considered to possess potent tumor-promoting activity. This inference is based on a substantial body of evidence obtained from rodent studies, as well as several decades of clinical research on the effects of NSAIDs on cancer risk [66]. In several early case-report studies, Waddell and Loughry [67, 68] showed that treatment of a small number of Gardner's syndrome patients with sulindac resulted in an almost complete regression of polyps. Epidemiological studies also demonstrated that regular NSAID use was associated with a 50 percent reduction in risk for colon and rectal cancers [69]. In a large prospective study by Thun et al. [70], regular aspirin use at low doses was associated with a significantly reduced risk of fatal colon cancer.
The protective effects of aspirin and other NSAIDs on tumor formation are most likely due to inhibition of the COX enzymes with reduced synthesis of the prostanoid metabolites, specifically PGE2. A direct role for PGE2 in tumorigenesis has been demonstrated in a number of animal models as well as in in vitro studies. These studies are summarized in Table 1. For example, Kawamori et al. [71] showed that weekly i.p. administration of PGE2 significantly increased the incidence and multiplicity of intestinal adenomas in F344 rats. In a subsequent mechanistic study, the Dubois laboratory [72] showed that gavage treatment of ApcMin mice with PGE2 increased epithelial cell proliferation and COX-2 expression, effects that were mediated in part by the activation of the Ras-MAPK signaling cascade. In striking contrast to these studies, administration of the stable PGE2 analogue, 16,16-dimethyl-PGE2, for 8 weeks to ApcMin mice resulted in a surprising decrease in the size and number of tumors throughout the intestine, prompting speculation that PGE2 may also have tumor suppressive properties [73]. Tumor suppression occurred despite increased in cell turnover demonstrated by elevated thymidine incorporation. While intriguing, these latter findings have not been reproduced by other laboratories, raising the possibility that the effect may have been environmentally influenced or perhaps the result of genetic changes occurring within the ApcMin mouse colony under study.
Table 1.
Overview of studies that have targeted PGE2 biosynthesis
| Organ | Target gene | Model | Effects | Reference |
|---|---|---|---|---|
| Intestine | cPLA2α KO | ApcMin | SI tumor ↓ (83%) | [139] |
| cPLA2α KO | AOM | Colon tumor ↑ (5.6-fold) | [3] | |
| COX-1 KO | ApcMin | SI tumor ↓ (77%) | [140] | |
| COX-1 KO | AOM/DSS | Tumor incidence ↓ | [141] | |
| COX-2 KO | ApcMin | SI tumor ↓ (84%) | [140] | |
| COX-2 KO | ApcΔ716 | SI tumor ↓ (86%) | [142] | |
| COX-2 KO | AOM/DSS | Tumor Incidence ↑ | [141] | |
| mPGES-1 KO | ApcΔ14 | SI tumor ↓ (66%) Colon tumor ↓ (51%) |
[74] | |
| mPGES-1 KO | ApcMin | SI tumor ↑ (48%) | [143] | |
| mPGES-1 KO | AOM | ACF ↓ (40%) Colon tumor ↓ (85%) |
[48] | |
| mPGES-1 KO | AOM | ACF ↓ Colon tumor ↓ |
[144] | |
| mPGES-1 Tg | AOM | ACF ↑ | [144] | |
| EP1 KO | AOM | ACF ↓ (60%) | [89] | |
| EP1 |
ApcMin
ONO-8711 (antagonist) |
SI tumor ↓ (57%) | [89] | |
| EP2 KO | ApcΔ716 | SI tumor ↓ | [92] | |
| EP3 KO | AOM | Tumor incidence ↑ Colon tumor ↑ |
[90] | |
| EP4 KO | AOM | ACF ↓ (56%) | [85] | |
| EP4 | AOM ONO-AE2-227 (antagonist) |
ACF ↓ (67%) | [85] | |
| EP4 |
ApcMin
ONO-AE2-227 (antagonist) |
SI tumor ↓ (69%) | [85] | |
| Esophagus | EP2 | Barrett's metaplasia, intraepithelial neoplasia, adenocarcinoma | Expression ↑ | [93] |
| EP4 | Adenocarcinoma | Expression ↑ | [93] | |
| Liver | EP1 | HepG2 cells ONO-D1-004 (agonist) ONO-8711 (antagonist) |
Growth and migration ↓ Growth and migration ↓ |
[94] |
| Stomach | COX-2/mPGES-1 Tg in epithelial cells |
K19-C2mE | Gastric hyperplasia and tumorous growth ↑ | [81] |
| EP2/4 | MKN-7 MKN-28 MKN45 AGS ONO-AE1-259-01 ONO-AE1-329 (agonists) |
Cell proliferation ↓ | [145] | |
| Breast | COX-2 Tg COX-2 KO HER2/neu Tg |
MMTV MMTV / NDL |
Tumor incidence ↑ Tumors ↓ (50%) |
[146] [147] |
| Floxed COX-2 in mammary epithelial cells | MPA / DMBA | Delayed onset | [80] | |
| Skin | EP2 KO | DMBA / TPA | Tumors ↓ | [148] |
| EP2 Tg | DMBA / TPA | Tumors ↑ | [148] |
ACF, aberrant crypt foci; AOM, azoxymethane; DMBA, 7,12-Dimethylbenz(a)anthracene, KO, knockout; MMTV, mouse mammary tumor virus; MPA, medroxyprogesterone acetate; NDL, neu deletion mutant; SI, small intestine; Tg, transgenic; TPA, 12-O-tetradecanoylphorbol-13-acetate
Further evidence supporting a role for PGE2 in tumor promotion comes from recent studies focused on mPGES-1, the terminal synthase in the formation of inducible PGE2. Our laboratory has recently shown that genetic deletion of mPGES-1 reduces the synthesis of inducible PGE2 and markedly suppresses (up to 70%) intestinal tumor formation in ApcΔ14 mice [74]. Although neither cell turnover nor β-catenin expression was significantly affected by mPGES-1 status, the potent tumor suppressive properties are associated with impaired neovessel formation within the adenomas, consistent with a previous study of human CRC [75]. In a follow-up study to test the possibility that the potent tumor suppression in the small intestine may be extended to the colon, Nakanishi et al. [48, 74] backcrossed the mPGES-1 gene KO onto strain A mice that are exquisitely sensitive to colon tumorigenesis by AOM [76–78]. Consistent with the previous study in ApcΔ14 mice, genetic deletion of mPGES-1 resulted in an even more dramatic (~95%) suppression in tumor size within the distal colon [48]. A role for PGE2 in cancer has been demonstrated in other organ systems as well. For example, over-expression of COX-2 in mammary tissue by the transgenic mammary tumor virus (MMTV) was sufficient to induce breast cancer development, which was reportedly dependent on PGE2-EP2 receptor signaling [79]. Further support for mammary tumor promotion by COX-2 was elegantly demonstrated by Smyth and colleagues [80] using mice that lack COX-2 expression selectively within mammary epithelial cells. Interestingly, breast carcinogenesis induced by medroxyprogesterone acetate and dimethylbenzanthracene (DMBA) was markedly reduced in these mice, an effect that was accompanied by a shift towards an anti-tumorigenic TH1 type immune response [80], a finding that illustrates the complex role that PGE2 can play in cancer promotion. In a model for gastric cancer, Oshima et al. [81] generated K19-C2mE transgenic mice that express both COX-2 and mPGES-1 in gastric epithelial cells. The K19-C2mE mice develop hyperplastic lesions with mucous cells in the glandular stomach, similar to H. pylori-induced precancerous lesions [81]. Interestingly, when the K19-C2mE mice were further engineered to express proteins that induce gastric epithelial cell proliferation (Wnt1 or Noggin), the compound mutant mice developed gastric adenocarcinomas [82, 83]. These observations demonstrate that gastric epithelial cells transformed by alterations in Wnt or Noggin signaling can be further driven to develop tumors in the presence of elevated levels of PGE2, an outcome that may be induced by co-infection of mice with H. pylori. [84].
4. PGE2 receptor-mediated signaling and cancer
In combination with stimulation of PGE2 formation, EP receptors are aberrantly expressed in multiple GI cancers. In CRC, for example, EP4 is the most abundantly expressed subtype of the EP receptors, and its levels are often up-regulated during colon carcinogenesis. This was initially shown experimentally in mice by Mutoh et al. [85], and then in human colon cancer cell lines by Chell et al. [86] and later confirmed [87]. As recently demonstrated by Chandramouli et al. [88], EP4 is negatively regulated in human cancer cells by miR-101, a microRNA that also inhibits COX-2 expression, raising the possibility that EP4 may ultimately provide a viable chemoprevention target. To assess the functional role of the specific EP receptor subtypes in intestinal cancer, a series of studies using genetic mouse models were performed more than a decade ago. In the first study of its kind, Watanabe et al. [89] examined the role of EP1 and EP3 in colon carcinogenesis. The formation of carcinogen-induced colonic aberrant crypt foci (ACF) was reduced by ~ 60%, an effect that occurred only in the EP1 KO mice. On the other hand, the EP3 receptor may play an important role in later stages of colon carcinogenesis. As observed by Shoji et al. [90], EP3 expression was significantly reduced within the AOM-induced tumors and there was an increase in tumor incidence and multiplicity in EP3-deficient mice. Interestingly, treatment of colon cancer cells with 5-aza-2'-deoxycytidine (5-aza-dC) restored EP3 receptor expression, providing evidence that aberrant DNA methylation may contribute to the down-regulation of EP3 expression in colon cancer cells [90]. In line with this observation, Xia et al. [91] have recently demonstrated in both in vitro and in vivo models that PGE2 promotes intestinal tumor growth by altering the expression of certain tumor-suppressor and DNA repair genes via epigenetic silencing. Exact mechanisms by which PGE2 affects DNA methylation must be further addressed in future studies.
In an important study by the Taketo laboratory [92], additional proof for the key role of the EP2 receptor in small intestinal tumorigenesis was obtained. Homozygous deletion of the EP2 receptor in ApcΔ716 compound mutant mice resulted in significant protection against intestinal cancer (tumor size and numbers), an effect that partially phenocopied the COX-2 KO mouse model. Genetic deletion of EP1 and EP3 were only slightly protective, whereas perhaps surprisingly, no protection was afforded to the intestine by the genetic deletion of the EP4 receptor. It was further proposed that increased cellular cAMP levels involving PGE2–EP2 receptor signaling amplified the actions of COX-2, possibly activating the expression of VEGF within the tumor microenvironment. Finally, the Wakabayashi laboratory [85] further assessed the role of the prostanoid receptors in colon carcinogenesis using six KO mouse lines (EP2, EP4, DP, FP, IP and TP). After treatment with AOM, ACF formation was suppressed only in the EP4 KO mice to levels that were 56% of wild-type controls. The lack of protection afforded by genetic deletion of the EP2 receptor was surprising, based on the previous findings in ApcΔ716 mice. This may be a result of tissue-specific actions of the EP receptors within different regions of the intestinal epithelium, or perhaps underlying differences in the initiating events that drive cancer in these two mouse models.
Several recent studies have addressed the potential role of the COX-2-PGE2-EP signaling axis in other GI cancers. A recent study by Jimenez et al. [93] examined human esophageal cancers and found elevated levels of COX-2 and EP2 during the course of disease progression from Barrett's metaplasia to intra-epithelial neoplasia, and finally to adenocarcinoma formation. While the expression of the EP4 receptor was increased in esophageal adenocarcinomas, the expression levels of COX-1 and the EP3 receptor were actually decreased during disease progression. In a liver cancer cell line (HepG2), treatment with the EP1 receptor agonist, ONO-DI-004, increased their viability and migration [94]. This effect was reversed by the EP1 receptor antagonist, ONO-8711, as well as by treatment with epigallocatechin gallate (EGCG), suggesting a novel mechanism for the chemopreventive efficacy of EGCG within the context of PGE2 signaling. It is important to note that EP receptors exhibit highly tissue-specific functional activities. For example, EP3 has been shown to induce matrix metalloproteinase-9 (MMP-9) and vascular endothelial growth factor (VEGF) expression in Lewis lung carcinoma (LLC) cells, suggesting its involvement in angiogenesis and tumor metastasis [95].
EP receptor agonist and antagonist studies
In addition to the aforementioned genetic studies related to EP receptor functional activity and their impact on GI cancers, several pharmacological studies using EP receptor agonists and antagonists have also been conducted. In one of the earliest studies, administration of the selective EP1 receptor antagonist, ONO-8711, caused a dose-dependent suppression of colon ACF in response to AOM treatment [89]. The protection was extended to ApcMin mice given 500 ppm ONO-8711 in the diet. Mutoh et al. [85] used an EP4-selective antagonist, ONO-AE2-227, to confirm the protection against AOM-induced ACF reported in the EP4 KO mice. A dose of 400 ppm ONO-AE2-227 moderately reduced the numbers of ACF, as well as the number of intestinal polyps in ApcMin mice by 31% [85]. The overall reduction in ACF numbers, however, was somewhat disappointing, especially in light of the fact that the drug regimen was initiated at the start of AOM treatment and was maintained throughout the entire experimental period. Perhaps the modest response is related to pharmacokinetic factors that need to be optimized.
5. The contribution of PGE2 to the tumor microenvironment
The tumor microenvironment is comprised of a complex array of cells, extracellular matrix (ECM) components and signaling molecules. The tumor microenvironment is established by the altered communication between stromal and epithelial cells through growth factors, cytokines and chemokines [96]. As tumors expand in size, they increasingly elicit diverse factors that can alter the host immune response, in part by exploiting the immuno-modulatory properties of PGE2. For example, Holt et al. [97] have shown that in tumor-bearing mice, PGE2 suppresses the cytotoxicity and cytokine production of natural killer (NK) cells via EP4 signaling. Furthermore, the polarization of tumor-associated macrophages (TAMs) towards tumor-promoting M2 macrophages is also influenced by PGE2 in lung carcinoma cells [98]. Interestingly, Liu et al. [98] also reported that IL-17 is important in recruiting macrophages to the tumor microenvironment prior to their polarization, demonstrating a cooperative effect between IL-17 and PGE2.
Fibroblasts, especially the myofibroblast cells, play a major role in the tumor microenvironment by providing oncogenic signals to facilitate tumorigenic events, including angiogenesis, cell migration and invasion [96]. However, the direct effects of PGE2 on myofibroblasts have not yet been clearly defined. The interaction of PGE2 with myofibroblasts appears to be context- and tissue-specific, especially during the wound-healing process (described above). Using transgenic mice that overexpress COX-2, PGE2 and Wnt1 in stomach, Guo et al. [55] have shown that myofibroblasts associated with gastric tumors express high levels of the angiogenic factor, VEGF-A, suggesting a positive effect of PGE2 signaling on a key function of myofibroblasts. However, VEGF-A expression was found to be regulated by other tumor-derived factors, suggesting that PGE2 may not contribute a direct role in this angiogenic process [55]. Regardless, additional studies are warranted that may more clearly define the role of PGE2 with respect to myofibroblasts.
Cancer-associated stromal reactions that contribute to the evasion of host-related immune response are the regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs). The accumulation of these potent immunosuppressive cells in tumors has been well-documented both in patients and in experimental animal models [99–101]. As reviewed by Fehervari and Sakaguchi [102], the primary role of the Tregs and MDSCs is to suppress the immune response by inducing T cell anergy and by restraining CTL proliferation and functional activity. PGE2 plays a critical role in controlling anti-tumor immunity in part by regulating the activation and expansion of both the Tregs and MDSC.
During the past decade, a number of studies have shown that PGE2 can modulate the proliferative capacity and effector functions of Tregs. Baratelli et al. [103] were the first to report that PGE2, derived from the supernatants of lung cancer cells, can potently induce Foxp3 expression in naïve T cells, a transcription factor that is necessary for the development of Treg-associated immunosuppressive properties. Additionally, Sharma et al. [104] showed that tumor-reactive T cells accumulate in lung cancer tissue, but frequently fail to respond effectively to the tumor because of the large proportion of immunosuppressive Tregs that are present within the microenvironment. This study showed that PGE2 alone was capable of inducing Foxp3 expression in vitro, and importantly, they further demonstrated that treatment of mice with PGE2 increased Foxp3 expression in splenocytes [104]. In addition, COX-2 KO mice showed a reduced activity of Tregs and suppressed the growth of tumors in vivo [104]. In gastric cancers, Yuan et al. [105] showed that elevated Foxp3 levels in tumor infiltrating Tregs can suppress T-cell function via a Cox-2/PGE2 mediated mechanism. In patients with prostate cancer, Tregs in the peripheral blood had much greater suppressive activity in comparison to Tregs harvested from healthy donors [106]. Importantly, there was a direct correlation between serum PGE2 levels and Treg functionality in the prostate cancer patients. Lee et al. [107] showed that celecoxib treatment of tumor-bearing mice harboring Lewis lung (3LL) carcinomas had reduced levels of Tregs, as well as reduced expression of COX-2, indoleamine 2,3-dioxygenase (IDO) and Foxp3, a finding that was correlated with suppressed tumor growth and metastasis. These findings prompted the speculation that COX-2 inhibitors might provide a useful therapeutic strategy for overcoming Treg-induced tumor immune tolerance. In a more recent study, Mandapathil et al. [108] concluded that targeting COX-2 activity in combination with adenosine might provide a novel approach for improving the outcome of immune-based cancer therapies by suppressing adaptive Tregs (Tr1) cells. A study by Soontrape et al. [109] recently demonstrated that PGE2 signaling, in collaboration with other immunosuppressive mediators, increases the number of Foxp3+ Tregs as a consequence of EP4 receptor signaling occurring during UV-induced immunosuppression. A novel mechanism has been described by Pinchuk et al. [110], in which colonic myofibroblasts induce the expansion of Foxp3-expressing Tregs through both cell-contact–mediated interactions (MHC class II-TCR signaling) and stimulation of PGE2 formation.
As discussed above, our laboratory recently examined the impact of inducible PGE2 synthesis on colon carcinogenesis. Despite the dramatic tumor suppression associated with mPGES-1 deletion, it was also found that mPGES-1 KO mice develop spontaneous, localized colonic ulcerations within 10 weeks of age [48]. We further investigated the immunoregulatory mechanisms that may underlie this mucosal inflammation in the mPGES-1 KO mice, focusing on CD4-Foxp3 double-positive cells. Despite the active, ongoing inflammation that is present within the colon, the levels of Tregs within the mesenteric lymph nodes of the mPGES-1 KO mice were reduced by almost 50% compared to the control mice. Importantly, this effect was not systemic as the population of Tregs within the spleen was unaffected by the mPGES-1 genotype. These results highlight the importance of inducible PGE2 formation in the expansion of Tregs in vivo and emphasize the critical role of inducible mPGES-1 in mediating this tumor promoting effect [48].
PGE2 may also dampen anti-tumor immunity by triggering the functional activation of MDSC. As extensively reviewed [111, 112], MDSC represent a subgroup of immature myeloid cells that are comprised of hematopoietic progenitor cells as well as precursors of macrophages, dendritic cells, and granulocytes. A component of normal hematopoiesis, MDSC numbers can greatly expand under a variety of pathological conditions, including cancer [113]. Upon their expansion, MDSC exert powerful immunosuppressive effects on both innate and adaptive immunity [111]. MDSC are highly active in the suppression of T-cell responses. They express high levels of a panel of immunosuppressive factors, including IDO, IL-10, arginase, nitric oxide (NO), nitric dioxide (NO2) and reactive oxygen species (ROS) to suppress T-cell responses [112, 114].
Kalinski and co-workers [114] have recently shown that PGE2 derived from COX-2 is a critical factor for redirecting DC development toward functionally stable MDSC. A positive feedback loop has been established between PGE2 and COX-2 in immature monocytes within the tumor microenvironment that facilitates the redirection of DCs to MDSC [114]. This same group has shown that even short-term inhibition of COX-2 can profoundly affect the immunosuppressive activity of mature MDSC isolated from cancer patients, further demonstrating the critical role of PGE2 in the development of functionally stable MDSC [115]. In a tumor explant study using spontaneously metastatic BALB/c-derived 4T1 mammary carcinomas, Sinha et al. [116] demonstrated that MDSC express EP receptors, and that receptor agonists, including PGE2, induce the differentiation of MDSC from bone marrow stem cells. Further support for an essential role of PGE2 in the differentiation of MDSC was obtained from a tumor explant study conducted in EP2 receptor KO mice, in which tumor growth and the accumulation of MDSC was reduced compared to wild-type mice.
Finally, the Ochoa laboratory [117] has examined the affects of PGE2 on arginase activity in MDSC. They report that arginase production by MDSC depletes arginine from the tumor microenvironment, thereby impairing the functional activation of T cells. Focusing on T cell activity in patients with renal cell carcinomas, Ochoa and colleagues [117] found that arginase activity was markedly reduced in the peripheral blood mononuclear cells of cancer patients, associated with reduced arginine levels. It was also demonstrated that tumor-derived PGE2 may induce arginase expression in MDSC, providing an important link between the COX-2/PGE2 pathway and MDSC function.
6. Drug targeting of mPGES-1 for cancer suppression
While genetic inhibition of inducible mPGES-1 activity offers a reasonable approach for studying its functional role in inflammation and cancer, the development of high affinity pharmacologic agents is critical for establishing novel therapeutic approaches. In this section, we review some of the most promising studies that have attempted to uncover new inhibitors of inducible PGE2 formation. Li et al. [118] have recently identified over-expression of mPGES-1 in human acute myeloid leukemia cells. As noted by these investigators, treatment of HL-60 cells with MK886 inhibited proliferation and induced apoptosis, accompanied by up-regulation of BAX expression and caspase-3 activity and reduced Bcl-2 and p-AKT. Deckmann et al. [119, 120] treated human cervical cancer cells (HeLa) with dimethylcelecoxib, a non-COX-2 inhibiting derivative of celecoxib. HeLa cell treatment with dimethylcoxib leads to an enhanced formation of a complex consisting of NF-κB and HDAC1 that binds to the EGR1 promoter, resulting in the down-regulation of EGR1 expression, an important mechanism for inhibition of mPGES-1 expression. Koeberle et al. [121] also showed that the anti-inflammatory drug licofelone suppressed PGE2 formation by inhibiting mPGES-1 without targeting COX-2 activity. Cote et al. [122] identified by high-throughput screening a lead compound, phenanthrene imidzaole (MF63), as a potent, selective and orally active mPGES-1 inhibitor with both in vitro and in vivo activity. The drug shows good selectivity towards mPGES-2, with an IC50 = 0.42 uM in A549 whole cells. In addition, several specific modifications to this chemical structure have been reported by Giroux et al. [123] that enhance oral bioavailability and improve the pharmacokinetic profile. More recently, as part of a highly comprehensive study, Chini et al. [124] have reported promising results with respect to the design and synthesis of a new generation of drugs based on the triazole scaffold that provide dual inhibition of both mPGES-1 and 5-lipoxygenase, offering the promise of safer and more effective anti-inflammatory agents. Finally, Beales and Ogunwobi [125] demonstrated with the use of either RNA interference or a small molecule inhibitor (CAY10526) the inhibition of esophogeal adenocarcinoma growth in cell culture. Many of these novel chemical structures have recently been reviewed by Chang and Meuillet [126].
Several natural compounds have also been evaluated for their ability to inhibit mPGES-1 activity. Moon et al. [127] showed that curcumin (diferuloylmethane) suppresses IL-1 -induced PGE2 formation in A549 human lung epithelial cells. Interestingly, mPGES-1 inhibition actually caused a metabolite shift from PGE2 to PGF2 and 6-keto-PGF. The curcumin-mediated inhibition of IL-1 -induced mPGES-1 expression is mediated by suppression of the transcription factor, EGR1, with an additional role played by NF- B and JNK1/2. Koeberle et al. [128] also showed that curcumin blocks PGE2 formation by direct inhibition of mPGES-1 in IL-1 stimulated A549 lung carcinoma cells with an IC50 = 0.2 M. The group also tested the ability of the chemopreventive agent, EGCG isolated from green tea (Camellia sinensis), to inhibit PGE2 biosynthesis [129]. ECGC was relatively effective (IC50=1.8 M) as an mPGES-1 inhibitor, an efficacy that was attained in the absence of inhibition of other pathway enzymes, including cPLA2 or COX-2. ECGC was also effective in blocking PGE2 synthesis in LPS-stimulated human whole blood cells [129]. Finally, Koeberle et al. [130] showed that myrtucommulone, a naturally occurring acylphloroglucinol derived from Myrtus communis, could efficiently suppress PGE2 synthesis in both A549 cells and LPS-stimulated human whole blood cells by inhibiting mPGES-1. This mPGES-1 inhibition occurred independently of COX inhibition. It should be noted, however, that the majority of these pre-clincial studies, have yet to establish the therapeutic efficacy of these agents in protecting against tumorigenesis. Thus the critical and exciting studies that will further validate many of these promising therapeutic agents remain to be performed.
7. Perspectives on PGE2 and inflammation-associated cancer
Emerging evidence places chronic inflammation directly within the pathogenetic pathway of many human cancers, particularly those affecting the GI tract [131, 132]. The notion that prolonged tissue damage contributes to cancer development has been confirmed in the case of long-standing IBD, which is a significant risk factor for CRC [133, 134]. In a recent study conducted in California (1998–2010), the incidence of CRC in patients with IBD was 60% higher than the general population [135]. In addition, the risk for developing CRC increases at a rate of approximately 0.5 – 1.0% per year in individuals with at least seven years of active disease [136]. Similar to sporadic CRC, the progression of IBD-related cancer occurs in a step-wise manner, driven to a varying extent by chromosomal instability (CIN), microsatellite instability (MSI), and mutations in key tumor-related genes, including p53, KRAS, and APC [136]. However, the timing of these molecular events differs with respect to the etiologies of sporadic and inflammation-associated cancers (reviewed by [136]).
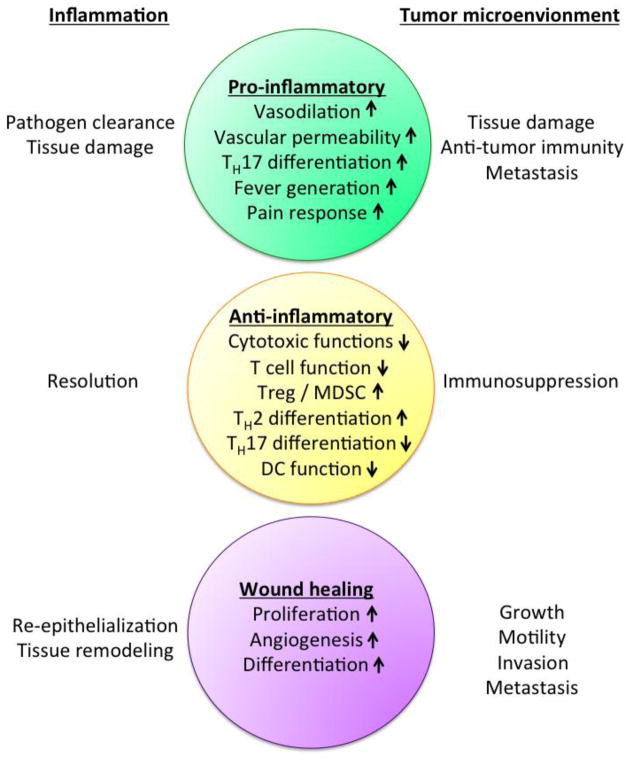
As evident from this review, accumulating data suggests that PGE2 plays an important role in the growth and progression of not only sporadic but also inflammation-related intestinal cancers. This conclusion is supported by an abundant literature showing colon cancer protection by NSAIDs, the overwhelmingly positive results associated with COX-2 or mPGES-1 deletion in pre-clinical mouse tumor models, and the efficacy of targeting EP receptors for cancer prevention. Considering the bipartite functions of PGE2 in inflammation and cancer (Figure 2), what role, if any, does PGE2 play in the timing of the molecular events that occur during inflammation-associated cancer? There are few studies that have attempted to clarify the potential role of PGE2 in the pathogenesis of IBD-related cancer. This is because the indiscriminate application of NSAIDs to IBD patients with active disease is strongly contraindicated, most likely attributable to the critical role of PGE2 in maintaining GI epithelial barrier function. In addition, suppressing PGE2 formation with the use of NSAIDs may further interrupt the wound-healing process, thereby exacerbating the severity of the disease. Thus, targeting PGE2 formation within the context of IBD-related cancers presents an important clinical challenge and underscores a key paradox for successful clinical management. As postulated by Dvorak, "tumors are wounds that never heal" [137], thus underscoring the fundamental similarities that exist between tumor development and the wound healing process [138]. Ultimately, what is needed is a way to block the tumor-enhancing properties of PGE2 without affecting its critical role in mucosal homeostasis and wound repair.
Fig. 2.
Bipartite functions of PGE2 in inflammation and cancer
Footnotes
This article is published as part of the Special Issue on Inflammation and Cancer [35:2]
References
- 1.Dong M, Guda K, Nambiar PR, Rezaie A, Belinsky GS, et al. Inverse association between phospholipase A2 and COX-2 expression during mouse colon tumorigenesis. Carcinogenesis. 2003;24(2):307–15. doi: 10.1093/carcin/24.2.307. [DOI] [PubMed] [Google Scholar]
- 2.Dong M, Rezaie A, Nakanishi M, Guda K, Nambiar PR, et al. Disparate cPLA2 and COX-2 expression may be associated with human colon tumorigenesis. Digestive Disease Week; 2004; New Orleans. 2004. p. 782. [Google Scholar]
- 3.Ilsley JN, Nakanishi M, Flynn C, Belinsky GS, De Guise S, et al. Cytoplasmic phospholipase A2 deletion enhances colon tumorigenesis. Cancer Res. 2005;65(7):2636–43. doi: 10.1158/0008-5472.CAN-04-3446. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55(1):115–22. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 6.Smith WL. The eicosanoids and their biochemical mechanisms of action. Biochem J. 1989;259(2):315–24. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Mann JR, DuBois RN. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology. 2005;128(5):1445–61. doi: 10.1053/j.gastro.2004.09.080. [DOI] [PubMed] [Google Scholar]
- 8.Jakobsson PJ, Thoren S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Natl Acad Sci U S A. 1999;96(13):7220–5. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murakami M, Naraba H, Tanioka T, Semmyo N, Nakatani Y, et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem. 2000;275(42):32783–92. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- 10.Tai HH, Cho H, Tong M, Ding Y. NAD+-linked 15-hydroxyprostaglandin dehydrogenase: structure and biological functions. Curr Pharm Des. 2006;12(8):955–62. doi: 10.2174/138161206776055958. [DOI] [PubMed] [Google Scholar]
- 11.Yan M, Rerko RM, Platzer P, Dawson D, Willis J, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(50):17468–73. doi: 10.1073/pnas.0406142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Backlund MG, Mann JR, Holla VR, Buchanan FG, Tai HH, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. The Journal of biological chemistry. 2005;280(5):3217–23. doi: 10.1074/jbc.M411221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding Y, Tong M, Liu S, Moscow JA, Tai HH. NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15–PGDH) behaves as a tumor suppressor in lung cancer. Carcinogenesis. 2005;26(1):65–72. doi: 10.1093/carcin/bgh277. [DOI] [PubMed] [Google Scholar]
- 14.Yan M, Myung SJ, Fink SP, Lawrence E, Lutterbaugh J, et al. 15-Hydroxyprostaglandin dehydrogenase inactivation as a mechanism of resistance to celecoxib chemoprevention of colon tumors. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(23):9409–13. doi: 10.1073/pnas.0902367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(32):12098–102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backlund MG, Mann JR, Holla VR, Shi Q, Daikoku T, et al. Repression of 15-hydroxyprostaglandin dehydrogenase involves histone deacetylase 2 and snail in colorectal cancer. Cancer research. 2008;68(22):9331–7. doi: 10.1158/0008-5472.CAN-08-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacological reviews. 1994;46(2):205–29. [PubMed] [Google Scholar]
- 18.Sugimoto Y, Narumiya S. Prostaglandin E receptors. The Journal of biological chemistry. 2007;282(16):11613–7. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 19.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001:41661–90. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 20.Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev. 2007;59(3):207–24. doi: 10.1124/pr.59.3.1. [DOI] [PubMed] [Google Scholar]
- 21.Kalinski P. Regulation of immune responses by prostaglandin e2. J Immunol. 2012;188(1):21–8. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakata D, Yao C, Narumiya S. Prostaglandin E2, an immunoactivator. Journal of pharmacological sciences. 2010;112(1):1–5. doi: 10.1254/jphs.09r03cp. [DOI] [PubMed] [Google Scholar]
- 23.Wallace JL. Prostaglandin biology in inflammatory bowel disease. Gastroenterology clinics of North America. 2001;30(4):971–80. doi: 10.1016/s0889-8553(05)70223-5. [DOI] [PubMed] [Google Scholar]
- 24.Uematsu S, Matsumoto M, Takeda K, Akira S. Lipopolysaccharide-dependent prostaglandin E(2) production is regulated by the glutathione-dependent prostaglandin E(2) synthase gene induced by the Toll-like receptor 4/MyD88/NF-IL6 pathway. J Immunol. 2002;168(11):5811–6. doi: 10.4049/jimmunol.168.11.5811. [DOI] [PubMed] [Google Scholar]
- 25.Hara S, Kamei D, Sasaki Y, Tanemoto A, Nakatani Y, et al. Prostaglandin E synthases: Understanding their pathophysiological roles through mouse genetic models. Biochimie. 2010;92(6):651–9. doi: 10.1016/j.biochi.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Kojima F, Kapoor M, Yang L, Fleishaker EL, Ward MR, et al. Defective generation of a humoral immune response is associated with a reduced incidence and severity of collagen-induced arthritis in microsomal prostaglandin E synthase-1 null mice. Journal of immunology. 2008;180(12):8361–8. doi: 10.4049/jimmunol.180.12.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Song WL, Cheng Y, Fitzgerald GA. Microsomal prostaglandin E synthase-1 inhibition in cardiovascular inflammatory disease. Journal of internal medicine. 2008;263(5):500–5. doi: 10.1111/j.1365-2796.2008.01938.x. [DOI] [PubMed] [Google Scholar]
- 28.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34(2):149–62. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature immunology. 2007;8(9):950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 30.Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. The Journal of experimental medicine. 2009;206(3):535–48. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheibanie AF, Khayrullina T, Safadi FF, Ganea D. Prostaglandin E2 exacerbates collagen-induced arthritis in mice through the inflammatory interleukin-23/interleukin-17 axis. Arthritis Rheum. 2007;56(8):2608–19. doi: 10.1002/art.22794. [DOI] [PubMed] [Google Scholar]
- 32.Sheibanie AF, Yen JH, Khayrullina T, Emig F, Zhang M, et al. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23-->IL-17 axis. Journal of immunology. 2007;178(12):8138–47. doi: 10.4049/jimmunol.178.12.8138. [DOI] [PubMed] [Google Scholar]
- 33.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. Journal of immunology. 2005;174(8):5033–9. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 34.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nature medicine. 1999;5(6):698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 35.Wallace JL. COX-2: a pivotal enzyme in mucosal protection and resolution of inflammation. Scientific World Journal. 2006:6577–88. doi: 10.1100/tsw.2006.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin H, Cheng L, Langenbach R, Ju C. Prostaglandin I(2) and E(2) mediate the protective effects of cyclooxygenase-2 in a mouse model of immune-mediated liver injury. Hepatology. 2007;45(1):159–69. doi: 10.1002/hep.21493. [DOI] [PubMed] [Google Scholar]
- 37.Scher JU, Pillinger MH. The anti-inflammatory effects of prostaglandins. J Investig Med. 2009;57(6):703–8. doi: 10.2310/JIM.0b013e31819aaa76. [DOI] [PubMed] [Google Scholar]
- 38.Joshi PC, Zhou X, Cuchens M, Jones Q. Prostaglandin E2 suppressed IL-15-mediated human NK cell function through down-regulation of common gamma-chain. Journal of immunology. 2001;166(2):885–91. doi: 10.4049/jimmunol.166.2.885. [DOI] [PubMed] [Google Scholar]
- 39.Linnemeyer PA, Pollack SB. Prostaglandin E2-induced changes in the phenotype, morphology, and lytic activity of IL-2-activated natural killer cells. Journal of immunology. 1993;150(9):3747–54. [PubMed] [Google Scholar]
- 40.Martinet L, Jean C, Dietrich G, Fournie JJ, Poupot R. PGE2 inhibits natural killer and gamma delta T cell cytotoxicity triggered by NKR and TCR through a cAMP-mediated PKA type I-dependent signaling. Biochemical pharmacology. 2010;80(6):838–45. doi: 10.1016/j.bcp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Yakar I, Melamed R, Shakhar G, Shakhar K, Rosenne E, et al. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol. 2003;10(4):469–79. doi: 10.1245/aso.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 42.Muthuswamy R, Mueller-Berghaus J, Haberkorn U, Reinhart TA, Schadendorf D, et al. PGE(2) transiently enhances DC expression of CCR7 but inhibits the ability of DCs to produce CCL19 and attract naive T cells. Blood. 2010;116(9):1454–9. doi: 10.1182/blood-2009-12-258038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. Journal of immunology. 1991;146(1):108–13. [PubMed] [Google Scholar]
- 44.Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. Journal of immunology. 1993;150(12):5321–9. [PubMed] [Google Scholar]
- 45.Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7(5):e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ae T, Ohno T, Hattori Y, Suzuki T, Hosono K, et al. Role of microsomal prostaglandin E synthase-1 in the facilitation of angiogenesis and the healing of gastric ulcers. American journal of physiology Gastrointestinal and liver physiology. 2010;299(5):G1139–46. doi: 10.1152/ajpgi.00013.2010. [DOI] [PubMed] [Google Scholar]
- 47.Kabashima K, Saji T, Murata T, Nagamachi M, Matsuoka T, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002;109(7):883–93. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakanishi M, Menoret A, Tanaka T, Miyamoto S, Montrose DC, et al. Selective PGE(2) suppression inhibits colon carcinogenesis and modifies local mucosal immunity. Cancer Prev Res (Phila) 2011;4(8):1198–208. doi: 10.1158/1940-6207.CAPR-11-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 50.Montrose DC, Kadaveru K, Ilsley JN, Root SH, Rajan TV, et al. cPLA2 is protective against COX inhibitor-induced intestinal damage. Toxicol Sci. 2010;117(1):122–32. doi: 10.1093/toxsci/kfq184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iizuka M, Konno S. Wound healing of intestinal epithelial cells. World journal of gastroenterology : WJG. 2011;17(17):2161–71. doi: 10.3748/wjg.v17.i17.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310(5753):1504–10. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 53.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. The Journal of biological chemistry. 2003;278(37):35451–7. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- 54.Nakanishi M, Sato T, Li Y, Nelson AJ, Farid M, et al. Prostaglandin E2 stimulates the production of vascular endothelial growth factor through the E-prostanoid-2 receptor in cultured human lung fibroblasts. American journal of respiratory cell and molecular biology. 2012;46(2):217–23. doi: 10.1165/rcmb.2010-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo X, Oshima H, Kitmura T, Taketo MM, Oshima M. Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. The Journal of biological chemistry. 2008;283(28):19864–71. doi: 10.1074/jbc.M800798200. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Daaka Y. PGE2 promotes angiogenesis through EP4 and PKA Cgamma pathway. Blood. 2011;118(19):5355–64. doi: 10.1182/blood-2011-04-350587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu Z, Fu C, Li X, Song Y, Li C, et al. Prostaglandin E2 promotes endothelial differentiation from bone marrow-derived cells through AMPK activation. PLoS One. 2011;6(8):e23554. doi: 10.1371/journal.pone.0023554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rao R, Redha R, Macias-Perez I, Su Y, Hao C, et al. Prostaglandin E2-EP4 receptor promotes endothelial cell migration via ERK activation and angiogenesis in vivo. The Journal of biological chemistry. 2007;282(23):16959–68. doi: 10.1074/jbc.M701214200. [DOI] [PubMed] [Google Scholar]
- 59.Sipos F, Valcz G, Molnar B. Physiological and pathological role of local and immigrating colonic stem cells. World journal of gastroenterology : WJG. 2012;18(4):295–301. doi: 10.3748/wjg.v18.i4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, et al. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. American journal of respiratory cell and molecular biology. 2003;29(5):537–44. doi: 10.1165/rcmb.2002-0243OC. [DOI] [PubMed] [Google Scholar]
- 61.Walker NM, Badri LN, Wadhwa A, Wettlaufer S, Peters-Golden M, et al. Prostaglandin E2 as an inhibitory modulator of fibrogenesis in human lung allografts. Am J Respir Crit Care Med. 2012;185(1):77–84. doi: 10.1164/rccm.201105-0834OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCann MR, Monemdjou R, Ghassemi-Kakroodi P, Fahmi H, Perez G, et al. mPGES-1 null mice are resistant to bleomycin-induced skin fibrosis. Arthritis Res Ther. 2011;13(1):R6. doi: 10.1186/ar3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iwanaga K, Okada M, Murata T, Hori M, Ozaki H. Prostaglandin E2 promotes wound-induced migration of intestinal subepithelial myofibroblasts via EP2, EP3, and EP4 prostanoid receptor activation. The Journal of pharmacology and experimental therapeutics. 2012;340(3):604–11. doi: 10.1124/jpet.111.189845. [DOI] [PubMed] [Google Scholar]
- 64.Harding P, LaPointe MC. Prostaglandin E2 increases cardiac fibroblast proliferation and increases cyclin D expression via EP1 receptor. Prostaglandins, leukotrienes, and essential fatty acids. 2011;84(5–6):147–52. doi: 10.1016/j.plefa.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ayabe S, Murata T, Maruyama T, Hori M, Ozaki H. Prostaglandin E2 induces contraction of liver myofibroblasts by activating EP3 and FP prostanoid receptors. British journal of pharmacology. 2009;156(5):835–45. doi: 10.1111/j.1476-5381.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fischer SM, Hawk ET, Lubet RA. Coxibs and other nonsteroidal anti-inflammatory drugs in animal models of cancer chemoprevention. Cancer prevention research. 2011;4(11):1728–35. doi: 10.1158/1940-6207.CAPR-11-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waddell WR, Ganser GF, Cerise EJ, Loughry RW. Sulindac for polyposis of the colon. Am J Surg. 1989;157(1):175–9. doi: 10.1016/0002-9610(89)90442-x. [DOI] [PubMed] [Google Scholar]
- 68.Waddell WR, Loughry RW. Sulindac for polyposis of the colon. Journal of surgical oncology. 1983;24(1):83–7. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- 69.Rosenberg L, Palmer JR, Zauber AG, Warshauer ME, Stolley PD, et al. A hypothesis: nonsteroidal anti-inflammatory drugs reduce the incidence of large-bowel cancer. Journal of the National Cancer Institute. 1991;83(5):355–8. doi: 10.1093/jnci/83.5.355. [DOI] [PubMed] [Google Scholar]
- 70.Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325(23):1593–6. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 71.Kawamori T, Uchiya N, Sugimura T, Wakabayashi K. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003;24(5):985–90. doi: 10.1093/carcin/bgg033. [DOI] [PubMed] [Google Scholar]
- 72.Wang D, Buchanan FG, Wang H, Dey SK, DuBois RN. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 2005;65(5):1822–9. doi: 10.1158/0008-5472.CAN-04-3671. [DOI] [PubMed] [Google Scholar]
- 73.Wilson JW, Potten CS. The effect of exogenous prostaglandin administration on tumor size and yield in Min/+ mice. Cancer research. 2000;60(16):4645–53. [PubMed] [Google Scholar]
- 74.Nakanishi M, Montrose DC, Clark P, Nambiar PR, Belinsky GS, et al. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 2008;68(9):3251–9. doi: 10.1158/0008-5472.CAN-07-6100. [DOI] [PubMed] [Google Scholar]
- 75.Cianchi F, Cortesini C, Bechi P, Fantappie O, Messerini L, et al. Up-regulation of cyclooxygenase 2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology. 2001;121(6):1339–47. doi: 10.1053/gast.2001.29691. [DOI] [PubMed] [Google Scholar]
- 76.Guda K, Upender MB, Belinsky G, Flynn C, Nakanishi M, et al. Carcinogen-induced colon tumors in mice are chromosomally stable and are characterized by low-level microsatellite instability. Oncogene. 2004;23(21):3813–21. doi: 10.1038/sj.onc.1207489. [DOI] [PubMed] [Google Scholar]
- 77.Nambiar PR, Nakanishi M, Gupta R, Cheung E, Firouzi A, et al. Genetic signatures of high- and low-risk aberrant crypt foci in a mouse model of sporadic colon cancer. Cancer Res. 2004;64(18):6394–401. doi: 10.1158/0008-5472.CAN-04-0933. [DOI] [PubMed] [Google Scholar]
- 78.Papanikolaou A, Wang QS, Papanikolaou D, Whiteley HE, Rosenberg DW. Sequential and morphological analyses of aberrant crypt foci formation in mice of differing susceptibility to azoxymethane-induced colon carcinogenesis. Carcinogenesis. 2000;21(8):1567–72. [PubMed] [Google Scholar]
- 79.Chen EP, Smyth EM. COX-2 and PGE2-dependent immunomodulation in breast cancer. Prostaglandins & other lipid mediators. 2011;96(1–4):14–20. doi: 10.1016/j.prostaglandins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Markosyan N, Chen EP, Ndong VN, Yao Y, Sterner CJ, et al. Deletion of cyclooxygenase 2 in mouse mammary epithelial cells delays breast cancer onset through augmentation of type 1 immune responses in tumors. Carcinogenesis. 2011;32(10):1441–9. doi: 10.1093/carcin/bgr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oshima H, Oshima M, Inaba K, Taketo MM. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. Embo J. 2004;23(7):1669–78. doi: 10.1038/sj.emboj.7600170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Itadani H, Oshima H, Oshima M, Kotani H. Mouse gastric tumor models with prostaglandin E2 pathway activation show similar gene expression profiles to intestinal-type human gastric cancer. BMC Genomics. 2009:10615. doi: 10.1186/1471-2164-10-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, et al. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131(4):1086–95. doi: 10.1053/j.gastro.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 84.Oshima H, Oguma K, Du YC, Oshima M. Prostaglandin E2, Wnt, and BMP in gastric tumor mouse models. Cancer science. 2009;100(10):1779–85. doi: 10.1111/j.1349-7006.2009.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mutoh M, Watanabe K, Kitamura T, Shoji Y, Takahashi M, et al. Involvement of prostaglandin E receptor subtype EP(4) in colon carcinogenesis. Cancer research. 2002;62(1):28–32. [PubMed] [Google Scholar]
- 86.Chell SD, Witherden IR, Dobson RR, Moorghen M, Herman AA, et al. Increased EP4 receptor expression in colorectal cancer progression promotes cell growth and anchorage independence. Cancer research. 2006;66(6):3106–13. doi: 10.1158/0008-5472.CAN-05-3702. [DOI] [PubMed] [Google Scholar]
- 87.Doherty GA, Byrne SM, Molloy ES, Malhotra V, Austin SC, et al. Proneoplastic effects of PGE2 mediated by EP4 receptor in colorectal cancer. BMC Cancer. 2009:9207. doi: 10.1186/1471-2407-9-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chandramouli A, Onyeagucha BC, Mercado-Pimentel ME, Stankova L, Shahin NA, et al. MicroRNA-101 (miR-101) post-transcriptionally regulates the expression of EP4 receptor in colon cancers. Cancer biology & therapy. 2012;13(3):175–83. doi: 10.4161/cbt.13.3.18874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Watanabe K, Kawamori T, Nakatsugi S, Ohta T, Ohuchida S, et al. Role of the prostaglandin E receptor subtype EP1 in colon carcinogenesis. Cancer research. 1999;59(20):5093–6. [PubMed] [Google Scholar]
- 90.Shoji Y, Takahashi M, Kitamura T, Watanabe K, Kawamori T, et al. Downregulation of prostaglandin E receptor subtype EP3 during colon cancer development. Gut. 2004;53(8):1151–8. doi: 10.1136/gut.2003.028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xia D, Wang D, Kim SH, Katoh H, DuBois RN. Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nature medicine. 2012;18(2):224–6. doi: 10.1038/nm.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nature medicine. 2001;7(9):1048–51. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- 93.Jimenez P, Piazuelo E, Cebrian C, Ortego J, Strunk M, et al. Prostaglandin EP2 receptor expression is increased in Barrett's oesophagus and oesophageal adenocarcinoma. Alimentary pharmacology & therapeutics. 2010;31(3):440–51. doi: 10.1111/j.1365-2036.2009.04172.x. [DOI] [PubMed] [Google Scholar]
- 94.Jin J, Chang Y, Wei W, He YF, Hu SS, et al. Prostanoid EP1 receptor as the target of (-)-epigallocatechin-3-gallate in suppressing hepatocellular carcinoma cells in vitro. Acta Pharmacol Sin. 2012;33(5):701–9. doi: 10.1038/aps.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Amano H, Ito Y, Suzuki T, Kato S, Matsui Y, et al. Roles of a prostaglandin E-type receptor, EP3, in upregulation of matrix metalloproteinase-9 and vascular endothelial growth factor during enhancement of tumor metastasis. Cancer science. 2009;100(12):2318–24. doi: 10.1111/j.1349-7006.2009.01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature reviews Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 97.Holt DM, Ma X, Kundu N, Collin PD, Fulton AM. Modulation of host natural killer cell functions in breast cancer via prostaglandin E2 receptors EP2 and EP4. Journal of immunotherapy. 2012;35(2):179–88. doi: 10.1097/CJI.0b013e318247a5e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu L, Ge D, Ma L, Mei J, Liu S, et al. Interleukin-17 and prostaglandin E2 are involved in formation of an M2 macrophage-dominant microenvironment in lung cancer. J Thorac Oncol. 2012;7(7):1091–100. doi: 10.1097/JTO.0b013e3182542752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Betts GJ, Clarke SL, Richards HE, Godkin AJ, Gallimore AM. Regulating the immune response to tumours. Adv Drug Deliv Rev. 2006;58(8):948–61. doi: 10.1016/j.addr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 100.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. Journal of immunology. 2009;182(8):4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009:27313–38. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 102.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. The Journal of clinical investigation. 2004;114(9):1209–17. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc'h N, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175(3):1483–90. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 104.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer research. 2005;65(12):5211–20. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 105.Yuan XL, Chen L, Li MX, Dong P, Xue J, et al. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. 2010;134(3):277–88. doi: 10.1016/j.clim.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 106.Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, et al. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(4):1032–40. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 107.Lee SY, Choi HK, Lee KJ, Jung JY, Hur GY, et al. The immune tolerance of cancer is mediated by IDO that is inhibited by COX-2 inhibitors through regulatory T cells. Journal of immunotherapy. 2009;32(1):22–8. doi: 10.1097/CJI.0b013e31818ac2f7. [DOI] [PubMed] [Google Scholar]
- 108.Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Jackson EK, et al. Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. The Journal of biological chemistry. 2010;285(36):27571–80. doi: 10.1074/jbc.M110.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Soontrapa K, Honda T, Sakata D, Yao C, Hirata T, et al. Prostaglandin E2-prostaglandin E receptor subtype 4 (EP4) signaling mediates UV irradiation-induced systemic immunosuppression. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(16):6668–73. doi: 10.1073/pnas.1018625108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pinchuk IV, Beswick EJ, Saada JI, Boya G, Schmitt D, et al. Human colonic myofibroblasts promote expansion of CD4+ CD25high Foxp3+ regulatory T cells. Gastroenterology. 2011;140(7):2019–30. doi: 10.1053/j.gastro.2011.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lu T, Gabrilovich DI. Molecular Pathways : Tumor Infiltrating Myeloid Cells and Reactive Oxygen Species in Regulation of Tumor Microenvironment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 doi: 10.1158/1078-0432.CCR-11-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nature reviews Immunology. 2012;12(4):253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118(20):5498–505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer research. 2011;71(24):7463–70. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer research. 2007;67(9):4507–13. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 117.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer research. 2009;69(4):1553–60. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Y, Yin S, Nie D, Xie S, Ma L, et al. MK886 inhibits the proliferation of HL-60 leukemia cells by suppressing the expression of mPGES-1 and reducing prostaglandin E2 synthesis. Int J Hematol. 2011;94(5):472–8. doi: 10.1007/s12185-011-0954-0. [DOI] [PubMed] [Google Scholar]
- 119.Deckmann K, Rorsch F, Geisslinger G, Grosch S. Dimethylcelecoxib induces an inhibitory complex consisting of HDAC1/NF-kappaB(p65)RelA leading to transcriptional downregulation of mPGES-1 and EGR1. Cell Signal. 2012;24(2):460–7. doi: 10.1016/j.cellsig.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 120.Deckmann K, Rorsch F, Steri R, Schubert-Zsilavecz M, Geisslinger G, et al. Dimethylcelecoxib inhibits mPGES-1 promoter activity by influencing EGR1 and NF-kappaB. Biochemical pharmacology. 2010;80(9):1365–72. doi: 10.1016/j.bcp.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 121.Koeberle A, Siemoneit U, Buhring U, Northoff H, Laufer S, et al. Licofelone suppresses prostaglandin E2 formation by interference with the inducible microsomal prostaglandin E2 synthase-1. The Journal of pharmacology and experimental therapeutics. 2008;326(3):975–82. doi: 10.1124/jpet.108.139444. [DOI] [PubMed] [Google Scholar]
- 122.Cote B, Boulet L, Brideau C, Claveau D, Ethier D, et al. Substituted phenanthrene imidazoles as potent, selective, and orally active mPGES-1 inhibitors. Bioorg Med Chem Lett. 2007;17(24):6816–20. doi: 10.1016/j.bmcl.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 123.Giroux A, Boulet L, Brideau C, Chau A, Claveau D, et al. Discovery of disubstituted phenanthrene imidazoles as potent, selective and orally active mPGES-1 inhibitors. Bioorganic & medicinal chemistry letters. 2009;19(20):5837–41. doi: 10.1016/j.bmcl.2009.08.085. [DOI] [PubMed] [Google Scholar]
- 124.Chini MG, De Simone R, Bruno I, Riccio R, Dehm F, et al. Design and synthesis of a second series of triazole-based compounds as potent dual mPGES-1 and 5-lipoxygenase inhibitors. Eur J Med Chem. 2012:54311–23. doi: 10.1016/j.ejmech.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 125.Beales IL, Ogunwobi OO. Microsomal prostaglandin E synthase-1 inhibition blocks proliferation and enhances apoptosis in oesophageal adenocarcinoma cells without affecting endothelial prostacyclin production. International journal of cancer Journal international du cancer. 2010;126(9):2247–55. doi: 10.1002/ijc.24875. [DOI] [PubMed] [Google Scholar]
- 126.Chang HH, Meuillet EJ. Identification and development of mPGES-1 inhibitors: where we are at? Future Med Chem. 2011;3(15):1909–34. doi: 10.4155/fmc.11.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moon Y, Glasgow WC, Eling TE. Curcumin suppresses interleukin 1beta-mediated microsomal prostaglandin E synthase 1 by altering early growth response gene 1 and other signaling pathways. The Journal of pharmacology and experimental therapeutics. 2005;315(2):788–95. doi: 10.1124/jpet.105.084434. [DOI] [PubMed] [Google Scholar]
- 128.Koeberle A, Northoff H, Werz O. Curcumin blocks prostaglandin E2 biosynthesis through direct inhibition of the microsomal prostaglandin E2 synthase- 1. Mol Cancer Ther. 2009;8(8):2348–55. doi: 10.1158/1535-7163.MCT-09-0290. [DOI] [PubMed] [Google Scholar]
- 129.Koeberle A, Bauer J, Verhoff M, Hoffmann M, Northoff H, et al. Green tea epigallocatechin-3-gallate inhibits microsomal prostaglandin E(2) synthase-1. Biochem Biophys Res Commun. 2009;388(2):350–4. doi: 10.1016/j.bbrc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 130.Koeberle A, Pollastro F, Northoff H, Werz O. Myrtucommulone, a natural acylphloroglucinol, inhibits microsomal prostaglandin E(2) synthase-1. Br J Pharmacol. 2009;156(6):952–61. doi: 10.1111/j.1476-5381.2009.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 132.Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Current opinion in pharmacology. 2009;9(4):351–69. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Waldner MJ, Neurath MF. Colitis-associated cancer: the role of T cells in tumor development. Seminars in immunopathology. 2009;31(2):249–56. doi: 10.1007/s00281-009-0161-8. [DOI] [PubMed] [Google Scholar]
- 134.Lewis JD, Deren JJ, Lichtenstein GR. Cancer risk in patients with inflammatory bowel disease. Gastroenterology clinics of North America. 1999;28(2):459–77. x. doi: 10.1016/s0889-8553(05)70065-0. [DOI] [PubMed] [Google Scholar]
- 135.Herrinton LJ, Liu L, Levin TR, Allison JE, Lewis JD, et al. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012;143(2):382–9. doi: 10.1053/j.gastro.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 136.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140(6):1807–16. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 137.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. The New England journal of medicine. 1986;315(26):1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 138.Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9(8):628–38. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 139.Hong KH, Bonventre JC, O'Leary E, Bonventre JV, Lander ES. Deletion of cytosolic phospholipase A(2) suppresses Apc(Min)-induced tumorigenesis. Proc Natl Acad Sci U S A. 2001;98(7):3935–9. doi: 10.1073/pnas.051635898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, et al. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res. 2000;60(17):4705–8. [PubMed] [Google Scholar]
- 141.Ishikawa TO, Herschman HR. Tumor formation in a mouse model of colitis-associated colon cancer does not require COX-1 or COX-2 expression. Carcinogenesis. 2010;31(4):729–36. doi: 10.1093/carcin/bgq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87(5):803–9. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 143.Elander N, Ungerback J, Olsson H, Uematsu S, Akira S, et al. Genetic deletion of mPGES-1 accelerates intestinal tumorigenesis in APC(Min/+) mice. Biochem Biophys Res Commun. 2008;372(1):249–53. doi: 10.1016/j.bbrc.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 144.Sasaki Y, Kamei D, Ishikawa Y, Ishii T, Uematsu S, et al. Microsomal prostaglandin E synthase-1 is involved in multiple steps of colon carcinogenesis. Oncogene. 2012;31(24):2943–52. doi: 10.1038/onc.2011.472. [DOI] [PubMed] [Google Scholar]
- 145.Okuyama T, Ishihara S, Sato H, Rumi MA, Kawashima K, et al. Activation of prostaglandin E2-receptor EP2 and EP4 pathways induces growth inhibition in human gastric carcinoma cell lines. The Journal of laboratory and clinical medicine. 2002;140(2):92–102. doi: 10.1067/mlc.2002.125784. [DOI] [PubMed] [Google Scholar]
- 146.Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. The Journal of biological chemistry. 2001;276(21):18563–9. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 147.Howe LR, Chang SH, Tolle KC, Dillon R, Young LJ, et al. HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer research. 2005;65(21):10113–9. doi: 10.1158/0008-5472.CAN-05-1524. [DOI] [PubMed] [Google Scholar]
- 148.Sung YM, He G, Fischer SM. Lack of expression of the EP2 but not EP3 receptor for prostaglandin E2 results in suppression of skin tumor development. Cancer research. 2005;65(20):9304–11. doi: 10.1158/0008-5472.CAN-05-1015. [DOI] [PubMed] [Google Scholar]