Abstract
Caenorhabditis elegansCPB-1 (cytoplasmic polyadenylation element binding protein) and FBF (fem-3 mRNA binding factor) are evolutionary conserved regulators of mRNA translation that belong to the CPEB (cytoplasmic polyadenylation element binding) and PUF (Pumilio and FBF) protein families, respectively. In hermaphrodite worms CPB-1 and FBF control key steps during germline development, including stem cell maintenance and sex determination. While CPB-1 and FBF are known to interact, the molecular basis and function of the CPB-1•FBF complex are not known. The surface of CPB-1 that interacts with FBF was localized using in vivo and in vitro methods to a ten-residue region at the N-terminus of the protein and these residues are present in the FBF-binding protein GLD-3 (germline development defective). PUF proteins are characterized by the presence of eight α-helical repeats (PUF repeats) arranged side by side in an elongated structure. Critical residues for CPB-1 binding are found in the extended loop that connects PUF repeats seven and eight. The same FBF residues also mediate binding to GLD-3, indicating a conserved binding mode between different protein partners. CPB-1 binding was competitive with GLD-3, suggestive of mutual exclusivity in vivo. RNA binding measurements demonstrated that CPB-1 alters the affinity of FBF for specific RNA sequences, implying a functional model where the coregulatory protein CPB-1 modulates FBF target selection.
Keywords: Pumilio, PUF protein, FBF, protein-protein interactions, germline development
Introduction
Post-transcriptional regulation of gene expression is vital for many diverse biological processes, including stem cell maintenance and differentiation1; 2, neuronal synaptic plasticity3 and cellular senescence4. The fate of the mRNA is often determined by the length of the poly(A) tail, which controls its localization, stability and translational efficiency. Many mRNAs are stored with a short poly(A) tail and their translation is activated by polyadenylation5. Shortening of the poly(A) tail triggers mRNA degradation6. The regulation of poly(A) tail length is often mediated by specific proteins that bind to elements located in the 3′untranslated region (UTR) of the mRNA and nucleate formation of large multi-protein complexes.
Caenorhabditis elegans CPB-1 (cytoplasmic polyadenylation element binding protein homolog)7 and FBF (fem-3 mRNA binding factor)7 are evolutionary conserved 3′ UTR regulatory proteins that control key steps in germline development. CPB-1 is required for spermatognonia to progress from first to second meiosis7. CPB-1 belongs to the cytoplasmic polyadenylation element binding (CPEB) family of proteins, that is found in mammals and invertebrates4. CPEB proteins contain two RNA recognition motifs (RRMs) followed by a zinc finger domain (Figure 1A), that are required for the interaction with the cytoplasmic polyadenylation element (CPE) consensus sequence in the 3′UTR of target mRNAs8. In Xenopus oocytes, CPEB regulates both mRNA activation and repression5; 9. Both processes require the dynamic assembly of a complex of proteins at the CPE. During oocyte maturation, cytoplasmic polyadenylation involves, in addition to CPEB, the scaffolding protein symplekin, the poly(A) polymerase GLD-2 (germline development factor-2) and the multisubunit cleavage and polyadenylation specificity factor CSPF10. CPEB-mediated translational repression involves the poly(A) ribonuclease, PARN11.
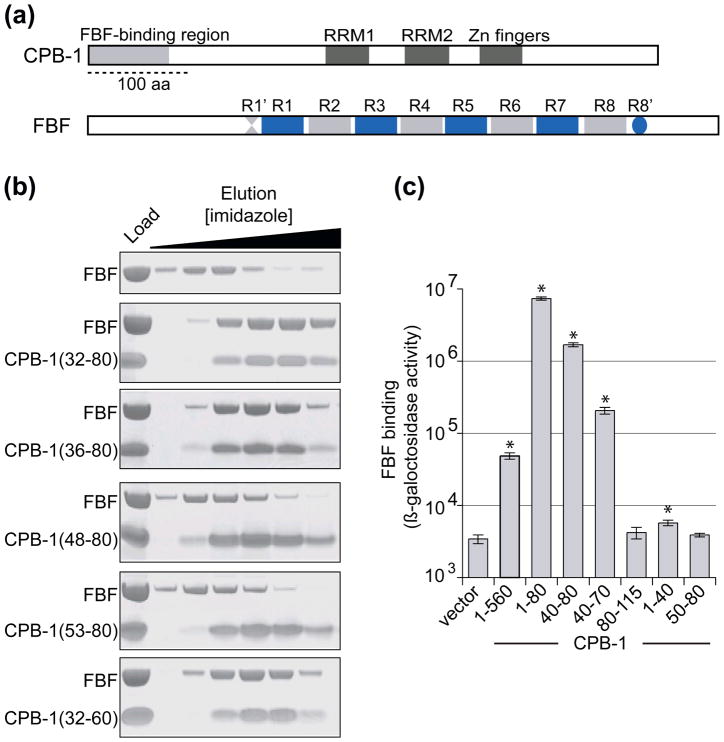
Figure 1. Mapping of the FBF-binding site within CPB-1.
A) Domain organization of CPB-1 and FBF. CPB-1 contains two RRM domains and a zinc finger domain. The previously identified FBF-binding region (amino acids 1–80) is indicated. FBF contains 8 PUF repeats (R1 to R8) flanked by conserved regions Csp1a and Csp2 indicated as R1′ and R8′. The bar represents 100 amino acids. B) In vitro binding assay of CPB-1 deletion fragments to FBF. His-tagged CPB-1 fragments were mixed with FBF and applied onto NiNTA resin. The resin was washed with buffer containing increasing concentrations of imidazole and the eluate was analyzed by SDS PAGE. Load indicates the samples that were used in the binding assay. FBF was eluted from the NiNTA resin with low imidazole concentration (upper panel) and was retained on the resin only when bound to the His-tagged CPB-1. C) Yeast two-hybrid analysis of the interaction of CPB-1 fragments with FBF. N- and C- terminal CPB-1 deletion mutants fused to the LexA DNA-binding domain were tested with FBF fused to the Gal4 transcriptional activation domain. CPB-1 (1-560) indicates full-length protein, vector indicates no CPB-1. The shortest CPB-1 fragment that strongly activated the lacZ reporter consisted of residues 40–70. Taken together, the in vitro and in vivo experiments indicate that the FBF-binding region of CPB-1 is contained within residues 40–60. Statistical analysis was performed using a two-tailed Student’s t-test, P-values were computed relative to the no CPB-1 control. P-values less than 0.05 were considered statistically significant and are indicated with an asterisk (* P-value < 0.05). Results are representative of three independent experiments, all the P-values are reported in Supplementary Table 1.
In many species, including Xenopus and C. elegans, CPEB proteins interact with relatives of Drosophila PUM and C. elegans FBF, termed PUF proteins12,9,13. PUF proteins recruit the conserved deadenylase complex CCR4–Pop2–Not14; 15, Argonaute (Ago)16, and the translational repressor Nanos5; 17. In C. elegans FBF controls sex determination by interacting with GLD-3 (germline development defective)18.
FBF belongs to the Pumilio and FBF (PUF) family of RNA-binding proteins found in Drosophila, C. elegans, humans and yeast. FBF plays an important role in germline stem cell maintenance2, spermatogenesis7 and in the spermatogenesis to oogenesis switch19. PUF proteins are characterized by the presence of eight adjacent repeats (PUF repeats) arranged side by side in an elongated structure20; 21. Each repeat consists of three α-helices and recognizes a single RNA base20; 22; 23. In addition to binding RNA, the PUF repeats also mediate the interaction with protein partners16; 23; 24. PUF proteins repress2 or activate mRNA translation1; 3 depending on the mRNA target and the repertoire of associated proteins. PUF protein–RNA interactions have been extensively characterized in recent years20; 21; 23; 25; 26; 27; 28; 29, but less is known about mechanisms of molecular recognition of protein partners. An additional open question about PUF mediated regulation of gene expression is if PUF proteins simply function as a scaffold for the recruitment of protein cofactors to the mRNA or if the interacting partners modulate their RNA binding activity24; 30; 31.
In this study the specificity of the interaction between CPB-1 and FBF was investigated, as well as cooperative binding of the multiprotein complex to RNA. Using a combination of in vitro studies with recombinant proteins and the yeast two-hybrid approach, the FBF-binding region of CPB-1 was narrowed down to a short stretch of amino acids and key residues were identified, whose mutation had a deleterious effect on the interaction. Residues in the FBF loop between repeats seven and eight were also found to be important for the interaction. Residues in the CPB-1/FBF interface are also found in the GLD-3•FBF complex31, indicating a conserved mode of interaction between different protein partners. Finally, the FBF-binding region of CPB-1 enhances binding of FBF to a putative mRNA target of the CPB-1•FBF complex both in vitro and in vivo, supporting a mechanicistic model where cooperative RNA-protein complex formation can modulate FBF’s target selection.
Results
Mapping the FBF-binding site within CPB-1 indicates that the interaction is mediated by a short stretch of amino acids
Yeast two-hybrid experiments previously showed that CPB-1 binds to FBF-1 through the first 80 N-terminal amino acids7. This interaction was confirmed in vitro with a pulldown assay with the RNA-binding domain of FBF-1 and additionally with the same domain of the protein FBF-219 (not shown). Since these two proteins are almost identical in sequence, have overlapping function in vivo19 and yielded the same results in the binding assays with CPB-1, they will collectively be referred to as FBF.
Analysis of the CPB-1(1-80) peptide by circular dichroism (CD) showed that the FBF-binding region of CPB-1 is largely unordered (Supplementary Figure 2A), in agreement with results from secondary structure prediction alogrithms (http://bioinf.cs.ucl.ac.uk/psipred/). Moreover, the observation that CPB-1 was sensitive to low concentrations of protease further supported the absence of a compact structure (see below).
Many protein interactions are mediated by short peptide segments that bind to a globular domain32; 33. Such peptide motifs often lie within disordered regions33. The observation that CPB-1(1-80) is mostly unfolded led us to investigate whether a shorter motif within this segment was sufficient to bind FBF. Two approaches were used in parallel to accurately define the FBF-binding region: limited proteolysis experiments with recombinant proteins and yeast two-hybrid assays with CPB-1 deletion mutants. Limited proteolysis with trypsin was performed on free CPB-1 and CPB-1 that had been chemically cross-linked to FBF. Comparison of the digestion pattern of CPB-1 alone and CPB-1 cross-linked to FBF identified regions of the protein that were no longer accessible to the protease upon binding to FBF. FBF was refractory to mild proteolysis with trypsin, which facilitated the analysis of the CPB-1 fragments. This crude approach identified CPB-1 residues 26–63 as the shortest fragment protected from proteolysis when bound to FBF (Supplementary Figure 1). At the same time, a complex of FBF with the longest recombinant CPB-1 construct that could be expressed and purified (residues 1–363) was digested with trypsin. The CPB-1 fragments bound to FBF were isolated and identified by mass spectrometry (not shown). The CPB-1 fragments were then produced recombinantly as hexahistidine (His)-tagged proteins and tested for binding to FBF with a pulldown binding assay (Figure 1B). In the pulldown assay the His-tagged protein is immobilized on NiNTA resin and eluted with buffer containing high concentration of imidazole. The second protein, the putative interacting partner, is produced without the affinity tag and is therefore not retained by the NiNTA resin. When protein-protein interaction occurs both proteins are retained on the resin and co-elute with high imidazole concentration. With this approach, the FBF-binding region within CPB-1 was narrowed to amino acid residues 32–60 (lower panel in Figure 1B), although CPB-1 deletion fragments that did not contain residues 32–47 did not bind to FBF. In parallel, a number of CPB-1 deletion mutants were tested for binding with the yeast two-hybrid method. The smallest CPB-1 fragment that still showed high β-galactosidase activity consisted of residues 40–70, in good agreement with the in vitro data (Figure 1C). Removal of the 40–50 stretch of amino acids abolished binding. A summary of all the constructs tested for binding in vitro and with the yeast two-hybrid method is shown in Supplementary Figure 2B. Taken together, the data indicate that the minimal FBF-binding region is contained within the 40–60 stretch of amino acids.
Upon narrowing down the FBF-binding region of CPB-1 we proceeded to investigate the binding mode of the CPB-1/FBF system. Upon binding to their specific target some disordered protein segments may transition to regular secondary or tertiary structures, inducing long-range structural rearrangements, while others remain ordered coils in their bound form. To gain insights into the binding mode of CPB-1, heteronuclear single quantum coherence [15N,1H]-HSQC spectra were acquired using 15N-labeled CPB-1(32-80). The spectra were recorded in the presence and absence of the FBF•RNA complex. In the absence of RNA, FBF tended to precipitate out of solution under NMR conditions at high concentration for an extended period of time at room temperature. CPB-1 and RNA form a stable ternary complex on FBF as discussed below. Free CPB-1(32-80) is flexibly disordered in solution, as indicated by the low chemical shift dispersion in the [15N,1H]-HSQC spectrum shown in Supplementary Figure 2C. The spectrum is characterized by a crowded set of backbone amide moieties located between the 7.5 to 8.5 ppm proton-frequency range, which is typically populated by residues found in flexibly disordered polypeptide segments. In the presence of FBF the [15N,1H]-HSQC spectrum of CPB-1 exhibits a similar pattern with a reduced number of peaks. The conserved pattern indicates that no major structural rearrangement occurs for the residues observed in these conditions (Supplementary Figure 2C). Such residues can be confidently mapped in the protein sequence to the regions 32 to 37 and 64 to 80 using the characteristic backbone HN Gly, and the side-chain H2N Gln and Asn peaks as guides. The missing peaks in the [15N,1H]-HSQC of the CPB-1•FBF complex most likely correspond to residues bound to FBF or proximal to the binding site, mapped to residues 38 to 63. Although the current data do not allow us to rule out a structural rearrangement in the segment 38 to 63, they indicate that FBF does not induce long-range conformational changes in other regions of the CPB-1 sequence employed.
Molecular underpinnings of the CPB-1•FBF complex
FBF interacts with GLD-324,31. Previous experiments demonstrated that loss of the FBF loop (residues 479–485) joining PUF repeats seven and eight disrupts binding to both GLD-3 and CPB-1, suggesting that the two proteins share a common FBF-binding site34,31. If CPB-1 and GLD-3 bind to the same site on FBF this would prevent formation of a ternary complex and binding of the two proteins may be competitive. In order to test this hypothesis, CPB-1 and GLD-3 were bound to FBF varying the order of addition. Upon complex formation the proteins were covalently cross-linked in order to allow their identification by denaturing protein gel electrophoresis (SDS PAGE). The chemically cross-linked GLD-3•FBF and CPB-1•FBF complexes showed different migration patters on the gel (Figure 2A). GLD-3 bound to free FBF but not to FBF pre-incubated with CPB-1. When CPB-1 was added to FBF a complex with a 1:1 stoichiometry was formed. When added to the GLD-3•FBF complex, which also has a 1:1 stoichiometry, CPB-1 replaced GLD-3 on FBF. No ternary complex formation was observed, independent of the order of protein addition. CPB-1 and GLD-3 may therefore bind to the same site, with CPB-1 binding with a greater affinity.
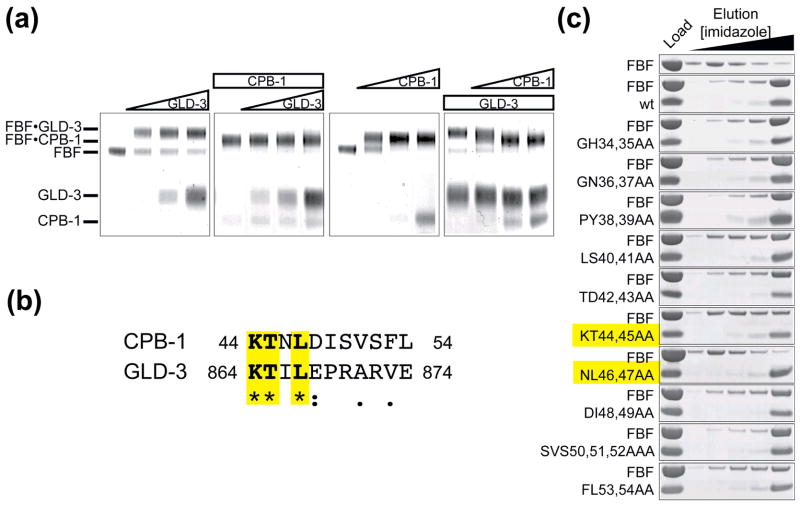
Figure 2. Sequence alignment with GLD-3 shows sequence conservation between the FBF-binding region of GLD-3 and CPB-1. CPB-1 and GLD-3 do not co-exist on FBF.
(A) CPB-1 and GLD-3 do not form a ternary complex with FBF. Protein complexes were covalently linked before analysis by SDS PAGE. The in-gel mobility of proteins alone and in complex is indicated on the left. The first panel from the left shows GLD-3 titration into a constant amount of FBF (increasing GLD-3 concentration is indicated with a triangle above the gel). The second panel shows GLD-3 titration to FBF pre-incubated with CPB-1 (constant amounts of protein are indicated with a rectangle). In the last two panels the order of addition of CPB-1 and GLD-3 to FBF was reversed. Increasing amounts of CPB-1 were added to FBF (third panel) and finally increasing amounts of CPB-1 were added to FBF pre-incubated with GLD-3. In the last experiment CPB-1 replaced GLD-3 on FBF. (B) Sequence alignment of CPB-1 with the FBF-binding region of GLD-3 (residues 864–874). Conserved residues are highlighted. The alignment was performed with ClustalW46, asterisks indicate positions that have a fully conserved residue, colons indicates conservation between groups of strongly similar properties and periods indicates conservation between groups of weakly similar properties. (C) Pulldown assay with FBF and CPB-1 double alanine mutants. Residues that most affected binding to FBF were located in the conserved 40–47 region.
The sequence alignment of the FBF-binding region of GLD-3 with CPB-1 indicated that the most important residues in the GLD-3 FBF-binding region are also present in CPB-131 and are found within the newly identified FBF-binding region (Figure 2B). Mapping of the residues in CPB-1 involved in binding to FBF was done by substituting pairs of amino acids with alanines. The CPB-1 double alanine mutants were produced recombinantly as His-tagged proteins, and subjected to a pulldown assay with FBF. The residues with the greatest effect on binding to FBF were located in the 40–47 region (Figure 2C). The effect of each residue in this region was then quantified by systematic single alanine scanning mutagenesis. The relative affinity of the CPB-1 alanine mutants for FBF was measured by competition titration experiments using fluorescence polarization assay (Figure 3A). In the competition-binding assay FBF was bound to fluorescently labeled wild-type CPB-1(19-80) (fl-CPB-1) and increasing concentrations of unlabeled competitor CPB-1 (wild-type or mutant) were titrated into the complex. The plot of the normalized polarization as a function of competitor CPB-1 concentration was used to derive the half-maximal inhibitory concentration (IC50) (Figure 3B). The IC50 of self-competitor (wild-type) CPB-1 was 1.15 ± 0.05 μM. The reduction in competition efficiency relatively to wild-type CPB-1 ranged from approximately three- to forty-fold for the most significant single alanine mutants. Some double alanine mutants were also tested for loss of binding and the effects were additive (not shown). Similar effects were observed when the alanine-scanning strategy was used in the yeast two-hybrid assay (Figure 3C). Residues whose mutations caused the greatest loss of binding were Leu 40, Lys 44, Thr 45, Leu 47 and Ile 49.
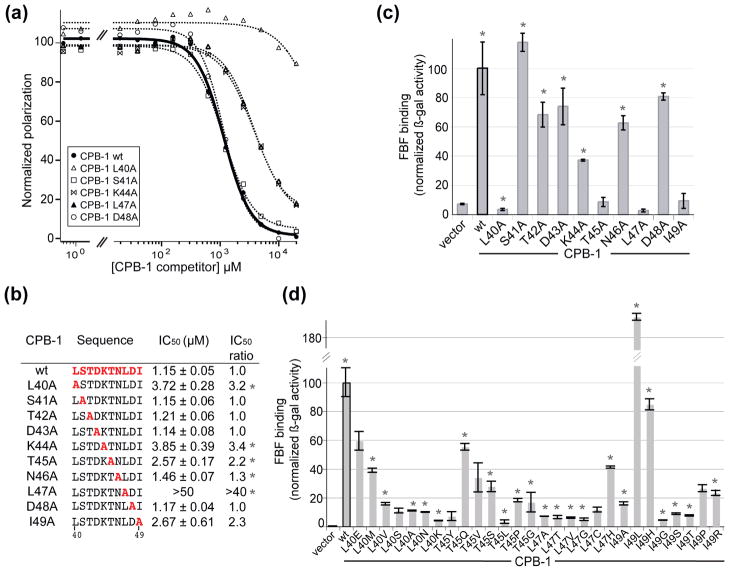
Figure 3. Alanine scan of the minimal FBF-binding region of CPB-1 identifies key residues.
(A) Determination of CPB-1/FBF binding specificity by competition-binding assay. The relative affinity of CPB-1 alanine mutants for FBF was measured by competition-binding assay using fluorescently-labeled CPB-1 (fl-CPB-1). Fl-CPB-1 was bound to FBF in order to achieve 90% complex formation and was competed off with increasing concentrations of competitor CPB-1. The plot of the normalized polarization as a function of competitor CPB-1 concentration is shown for wild-type CPB-1 (wt) self-competition and representative CPB-1 alanine mutants. (B) Fluorescence polarization data were fit to a Hill equation to determine the apparent IC50. The relative IC50 of each mutant relative to wild-type CPB-1 is shown. Experiments were performed at least in triplicate, asterisks indicate statistically significant P-values computed versus wt CPB-1 (* P-value <0.05). (C) The same alanine mutants were also tested for binding to FBF in the yeast two-hybrid system. (D) Additional mutations of the key CPB-1 residues were screened in the yeast-two hybrid assay for interaction with FBF. Asterisks indicate statistically significant P-values (* P-value <0.05) computed versus the vector control (no CPB-1).
To test if additional amino acid substitutions at key sites in CPB-1 compromised binding to FBF, additional mutagenesis was conducted. Random mutants were introduced into four key sites in CPB-1 using mutagenesis primers containing three random oligonucleotides (Figure 3D). The resulting mutants were transformed into yeast and their relative interactions quantified using a yeast-two hybrid assay. The identity of each mutant was then inferred based on the DNA composition obtained by Sanger sequencing35. The majority of mutations at positions 40, 45, and 47 reduce binding to FBF. However, multiple mutations of Ile 49 were permissible. A small gain in apparent binding activity was observed for a conservative Ile to Leu mutation. We conclude that the majority of mutations at key sites in CPB-1 are deleterious for binding of FBF.
Identification of key FBF residues for CPB-1 binding
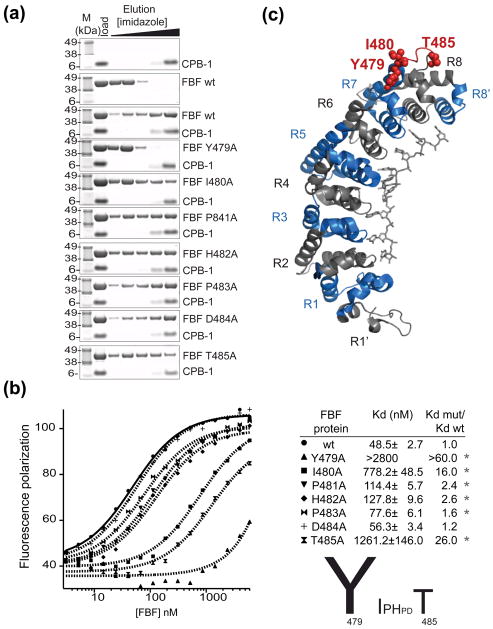
Removal of the long loop connecting PUF repeats seven and eight of FBF disrupts binding to CPB-136. In order to investigate the contribution of each residue in the loop to CPB-1 binding, a mutational analysis was performed in vitro with recombinant proteins (Figure 4A). To ensure that the reduced affinity between CPB-1 and mutant FBF was due to genuine loss of binding contacts at the protein–protein interface rather than being caused by protein misfolding, the FBF mutants were tested for folding defects using RNA binding as a readout. Electrophoretic mobility shift assay (EMSA) experiments showed the mutants were properly folded and active in binding RNA (not shown). Pulldown assays indicated that mutation of Tyr 479 to Ala completely disrupted binding to CPB-1 (Figure 4A). This qualitative study also indicated that Ile 480 and Thr 485 play an important role in binding (Figure 4A). The effects of the alanine mutations were quantified with the fluorescence polarization assay by direct titration into fl-CPB-1 (Figure 4B). Fl-CPB-1 bound to FBF with a Kd=49±3 nM, residues that most significantly affected binding to CPB-1 when mutated to alanines were Tyr 479 (which showed > 60 fold increase in Kd), Ile 480, with a Kd=778±49 nM, and Thr 485, with a Kd=1261±146 nM, in agreement with the pulldown assay. Important residues for the interaction with CPB-1 are mapped onto the three-dimensional structure of FBF-2 in complex with the protein binding element of fem-3 (UGUGUCAUU) 29 in Figure 4C (PDB accession code=3K64).
Figure 4. Identification of FBF-2 amino acids that are necessary for the interaction with CPB-1.
(A) Single alanine FBF mutants were tested for biding to CPB-1 with the NiNTA pulldown assay. The first two panels show the controls with CPB-1 or FBF only, mutation of FBF Tyr 479 to Ala completely disrupted the interaction with CPB-1 (forth panel). (B) The affinity of fl-CPB-1 for FBF wild-type and FBF alanine mutants was determined by fluorescence polarization assays. Fluorescence polarization is plotted as a function of FBF concentration and fitted to the Hill equation to determine the apparent equilibrium dissociation constant Kd,(shown in the table; the Hill coefficient was set to 1). The significance (* P-value <0.05) was determined by Student-t-test versus wild type FBF. A graphical summary of the effect of single alanine mutations in the Y479-T485 loop is shown. (C) FBF residues that affect binding to CPB-1 are highlighted in the crystal structure of FBF-2 in complex with PME RNA29. The 8 PUF repeats (R1–R8) are colored blue and grey, the loop connecting repeats seven and eight encompassing residues 479–485 is in red, key residues for the interaction with CPB-1 are shown as spheres.
CPB-1 alters the affinity of FBF for cyb-1 mRNA
EMSA experiments were performed to investigate binding of FBF to RNA in the presence of CPB-1. The mRNA target for the CPB-1•FBF complex is unknown. However, in Xenopus, CPEB and PUF families act together to regulate expression of cyclin B9. Since cyclin B mRNA is a putative conserved target of FBF37, we used cyclin B of C. elegans (cyb-1) in our in vitro studies. FBF bound to cyb-1 RNA with a Kd=24 nM (Hill=1.5) but in the presence of CPB-1 binding was about 4 times tighter (Kd=5.8 nM, Hill=1.0). (Figure 5A and 5B). The effect of CPB-1 on FBF binding to cyb-1 RNA in vitro is modest but suggestive that CPB-1 binding and RNA binding to FBF may be cooperative.
Figure 5. CPB-1 alters the affinity of FBF for specific RNAs.
(A) Representative EMSA gel determining FBF association with cyb-1 RNA in the absence (left) or presence of CPB-1 (right). In gel migration of free RNA, binary and ternary complexes is indicate on the left with a black line, purple diamond and blue square respectively. (B) Quantification of gel shifts shown on the left. The fraction of bound RNA was plotted as a function of FBF concentration and fitted to the Hill equation to determine Kd. (C) Schematic representation of the modified yeast three-hybrid assay. (D) Cell based assays confirm the cooperative effects observed for the cyb-1 RNA. Four additional RNAs were examined for stimulated binding in the presence of CPB-1. The “none” construct lacks an insert, an additional negative control, PME U9C, is included. The RNA sequences are shown in the table as is the fold activation determined by dividing binding activity in the presence of CPB-1 by the corresponding negative control experiment conducted in the absence of CPB-1. All of the values are normalized to cell density. Asterisks indicate P-values < 0.05.
To corroborate these results and examine the effects of CPB-1 binding on other RNA targets, we utilized a modified yeast-three hybrid assay (Figure 5C and D). In these experiments, binding of FBF to candidate RNAs is measured in the presence or absence of CPB-1 fused to an SV40 NLS. Thus, the effects of complex formation on the affinity of FBF for a given RNA can be determined in a cell-based assay. CPB-1 appears to stimulate binding to the NRE (nanos response element), cyb-1 and fog-1a (feminization of germline) RNAs. Based on comparison of the various RNAs, we conclude that there is a sequence dependence to the observed cooperativity. Intriguingly, expression of CPB-1 appeared to consistently enhance binding to the target RNA consistent with previous results on synthetic targets34,38.
Discussion
CPEB and PUF proteins are key regulators of post-transcriptional gene expression and are conserved from mammals to invertebrates4; 39. Generally, post-transcriptional regulators control translation of multiple mRNA targets by nucleating the assembly of dynamic protein complexes. In this study the specificity determinants of the interaction between the C. elegans member of the CPEB family CPB-1 and PUF protein FBF were determined. CPEB proteins contain an N-terminal sequence of no conserved structural motif and two RRMs and zinc finger motives that mediate their binding to mRNA40. PUF proteins bind target mRNAs as well as protein partners through their PUF repeats. In recent years a wealth of biochemical and structural information on the mRNA binding specificity of PUF proteins has been published27, but less is known about how they interact with their protein partners. Biochemical analysis of the CPB-1 and FBF surface allowed the identification of key residues for the interaction. CPB-1 binds FBF through a short stretch of amino acids located at the N-terminus of the protein that has no regular secondary structure free in solution, and undergoes no major structural rearrangement upon binding to FBF. In vitro analysis of recombinant proteins and yeast two-hybrid assays showed that CPB-1 residues important for the interaction with FBF are found within the 10-mer peptide LSTDKTNLDI encompassing residues 40–49. Amino acids whose mutation to alanine mostly disrupted binding to FBF were Leu 40, Lys 44, Thr 45, Leu 47 and Ile 49. In particular, the Leu 47 to Ala mutation had a deleterious effect on the interaction.
This newly identified FBF-binding region of CPB-1 is similar in sequence to the FBF-binding region of GLD-331. In GLD-3 residues important for binding are also contained in a short stretch of amino acids and include Lys 864, Thr 865 and most importantly Leu 867, whose mutation to Ala completely disrupts binding to FBF31. The conserved KTXL (X= any residue) motif plays a key role in both the GLD-3 and CPB-1 interaction with FBF. Although a KTXL sequence is important for both proteins to bind to FBF, CPB-1 and GLD-3 have different affinities for FBF, with CPB-1 binding with a Kd in the low nanomolar range and GLD-3 showing a Kd about 20 times higher31. Binding of both proteins to FBF is mediated by the loop between PUF repeats seven and eight and mutation of Tyr 479, Ile 480 and and Thr 485 to Ala significantly impacts the affinity of FBF for both proteins31, showing a conserved mode of binding.
In vitro, CPB-1 and GLD-3 compete with one another for binding to the same region of FBF. The in vivo consequences of their binding to the same site in FBF are unclear, and a key area for future analysis. CPB-1 and GLD-3 might antagonize one another in controlling FBF activity in vivo; alternatively, they could act additively on FBF, or in different cells and times. CPB-1 and GLD-3 are both expressed in early stage spermatocytes7,41, but their co-localization with one another, and with FBF, has not been analyzed. Similarly, loss of either CPB-1 or GLD-3 leads to the same spermatogenesis defect, arrest as primary spermatocytes7; 18, but further genetic analysis is required to understand their regulatory relationships and the mechanisms leading to that arrest. In particular, similar mutant phenotypes could arise from triggering a common checkpoint, or from additional, as yet unexplored roles of the two proteins. Use of the mutations described here as C. elegans transgenes should provide precise probes for the biological roles of the interactions between FBF and its partners. The CPB-1/FBF interaction is also disrupted or considerably reduced by point mutations in FBF (Tyr 479 to Ala and Thr 485 to Ala), although these mutations will certainly affect FBF’s interaction with multiple proteins34,31 and therefore will not be the ideal candidate for in vivo studies.
FBF physically associates with ~7% of C. elegans transcriptome37, interacts with a number of proteins including CPB-1, GLD-3, NOS-317 (Nanos-related), CCF-1 (the C. elegans CAF-1)1 and forms a ternary complex with EF1A and CSR-1 (a translation initiation factor and Ago family member, respectively)16. The key FBF residues for the interaction with CPB-1 are contained within the loop connecting PUF repeats seven and eight. These results indicate that relatively small surfaces are involved in binding, consistent with the fact these are transient interactions. Mutation of single residues on either one of the protein interaction surfaces has a deleterious effect on their interaction. Protruding loops on the concave surface of PUF proteins mediate a plethora of interactions34,31. For instance Drosophila Brain Tumor (Brat) protein is recruited by Pumilio to repress translation of hunchback mRNA42. The structural model of the Pumilio•Brat complex showed that an extended loop in PUF repeat eight fits in the entrance to the central channel of the β-propeller formed by Brat’s NHL domain23. Flexible loops on the outer concave surface of PUF proteins bind specifically to both structured proteins like Brat and disordered proteins such as CPB-1 or GLD-323.
The direct, physical interaction of CPEB and PUF proteins is broadly conserved from humans to C. elegans 12,36. On many 3′UTRs in vivo, PUF and CPEB proteins are likely to be bound to their own elements, and physically interact with one another as well. Our data show that CPEB also can influence FBF specificity directly, independent of binding RNA. Two key observations support his hypothesis. First, the affinity of a PUF protein, FBF-2, for specific RNA sequences is influenced by a fragment of CPEB that lacks RNA recognition domains and possesses no specificity for RNA itself36. Second, different RNAs vary in their sensitivity to CPEB’s effects on PUF specificity: for example, CPB-1 increases the affinity of FBF for the binding element in fog-1 but not gld-1 RNAs. In a parallel study, we examined the global specificity for RNA of FBF-2 alone, CPB-1 alone, and the protein complex30. In those studies, the short segment of CPB-1 broadened the specificity of FBF-2. We suggest that CPEB facilitates binding of PUF proteins to RNAs that lack the highest affinity PUF binding elements. Indeed, sub-optimal sites appear to be common among the targets of FBF-2 and human Pumilio43,44. We propose that on some mRNAs, CPEB acts and influences PUF protein activity without that CPEB molecule binding to the RNA. Such mRNA targets would lack CPEs, but nonetheless respond to the protein.
PUF proteins bind RNA though a conserved mechanism. The PUF domain forms a curved, elongated structure where each of the 8 PUF repeats recognizes a single nucleotide20; 21; 29 (Figure 4C). Despite the conserved binding mode, the length of the consensus sequence is protein specific. For instance, human Pumilio binds 8 nucleotide RNA sequences, while the target recognized by FBF is 9 nucleotides in length. The flatter curvature of FBF relative to human Pumilio requires an additional spacer nucleotide, whose base flips out and points away from the protein. The RNA specificity is therefore obtained through perturbations of the curvature of the PUF domain45. By binding to the outer surface of FBF, opposite the concave RNA binding surface, CPB-1 may alter the curvature of the protein, causing a tighter binding to specific RNA sequences. This result suggests a functional model where the FBF target selection is modulated by the coregulatory factor CPB-1, consistent with the observation that the FBF-binding region of CPB-1 is sufficient for promoting translational repression in the presence of FBF34. Structural analysis of the FBF•CPB-1•RNA complex is needed to understand how CPB-1 modulates FBF’s RNA binding activity, and is now an important goal.
Materials and Methods
Protein expression and purification
CPB-1(1-80) and CPB-1(19-80) constructs were cloned into pET-22b(+) (Novagen) between restriction sites NdeI-XhoI in order to introduce a C-terminal heahistidine (His) tag. Strains used for cloning and expression of recombinant proteins were E. coli DH5α (Invitrogen) and E. coli BL21-Gold(DE3) (Stratagene) respectively. Cells were grown at 37°C in Luria Bertani medium supplemented with 50 μg/ml ampicillin (LB/Amp) and protein expression was induced with 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) for 3 hours. Cell pellets were re-suspended in lysis buffer [20 mM Tris-HCl (pH 7.0), 300 mM NaCl, 20 mM imidazole, 5% glycerol) supplemented with Complete, EDTA-free Protease Inhibitor Cocktail Tablets (Roche) and subjected to mild sonication followed by 30 minute incubation at 4°C with 0.5X FastBreak Cell Lysis Reagent (Promega). The cleared lysate was loaded onto TALON Metal Affinity Resin (Clontech). The resin was washed with lysis buffer supplemented with 1 M NaCl and the protein was eluted with lysis buffer containing 150 mM imidazole (pH 7.0). Only fractions where protein purity was >95% were pooled and concentrated in Amicon Ultra-15 Centrifugal Filter Units (Millipore).
The CPB-1(19-80) construct used in the EMSA experiments and the CPB-1(1-363) construct contained an N-terminal maltose binding protein (MBP) tag. The proteins were cloned into a modified pMAL-c2x vector (New England Biolabs) in which a tobacco etch virus (TEV) cleavage site had been introduced between the MBP tag and the multiple cloning site. A C-terminal His tag was introduced by PCR. Protein expression was carried out in E. coli BL21-Gold(DE3) cells as described for the previous proteins. Cell pellets were resuspended in lysis buffer [0.5M NaCl, 20 mM Tris HCl (pH 8.5), 20 mM imidazole, 5 mM betamercaptoethanol (βME)] supplemented with Complete, EDTA-free Protease Inhibitor Cocktail Tablets and lysed by sonication. The cleared lysate was mixed with NiNTA Superflow resin (Qiagen) and the protein was purified with the batch-binding procedure. The resin was washed with lysis buffer containing 1 M NaCl, equilibrated again with lysis buffer and finally eluted with 20 mM Tris HCl (pH 8.5), 300 mM imidazole, 250 mM NaCl, 10 mM βME. The eluate was applied to amylose resin (New England Biolabs) equilibrated with lysis buffer. The resin was washed with 20 mM Tris HCl (pH 8.5), 200 mM NaCl, 10 mM βME and the protein was eluted with the same buffer supplemented with 10 mM maltose. The buffer was exchanged to 20 mM Tris HCl (pH 8.5), 100 mM NaCl, 5 mM DTT by dialysis and the protein was stored at −80°C.
Double and single alanine mutations were introduced in the CPB-1(19-80) construct by PCR mutagenesis and proteins were purified as wild-type CPB-1(19-80).
CPB-1 deletion mutants shorter than the CPB-1(19-80) construct were also expressed as N-terminal, TEV cleavable, MBP fusions and with a C-terminal His tag. The proteins were purified over NiNTA and amylose resins, all the buffers were supplemented with 10% glycerol. The MBP tag was removed by overnight digestion at 4°C with recombinant His-tagged TEV protease. The MBP tag was separated from CPB-1 by centrifugation in Amicon Ultra-15 Centrifugal Filter Units with a molecular weight cut off of 30kDA (Millipore).
FBF-1 (164-566) and FBF-2 (164-566) were cloned into a modified pETDuet-1 expression plasmid (Novagen) that encoded for an N-terminal, TEV cleavable, His tag. Protein expression was carried out in E. coli BL21(DE3) cells (Stratagene) for 12 hours at 18°C upon induction with 0.5 mM IPTG. Cell pellets were resuspendend in lysis buffer [20 mM Tris HCl (pH 8.6), 200 mM NaCl, 10% glycerol, 10 mM βME, 5 mM imidazole. 0.5 M UREA, Complete, EDTA-free Protease Inhibitor Cocktail Tablets] and disrupted by sonication. The proteins were purified by NiNTA affinity chromatography and the His tag removed overnight at 4°C with recombinant His tagged TEV protease. A second NiNTA purification step was performed to remove the released tag and the TEV protease, followed by size exclusion chromatography (Superdex 200, GE Healthcare) in storage buffer [20 mM Tris HCl (pH 8.6), 200 mM NaCl, 5 mM DTT, 10% glycerol]. FBF-2 construct encompassing residues 164–575 was overexpressed and purified as described in Wu et al31.
Qualitative protein binding assay (NiNTA pulldown)
Protein complexes were reconstituted as follows: 10 to 20 μM FBF was incubated for 3 hours with 1.5–2.0 molar excess CPB-1 in reconstitution buffer [20 mM Tris HCl (pH 7.5 or pH 8.6), 100 mM NaCl, 10 mM βME, 5% glycerol]. Pulldown assays were performed by applying the reconstituted protein-protein complexes or the individual proteins as controls, to His SpinTrap columns (GE Healthcare) pre-equilibrated in reconstitution buffer. Columns were washed three times with 0.6 ml of buffer and the proteins were eluted with 0.4 ml of buffer containing increasing concentrations of imidazole (up to 300 mM). The content of each elution fraction was visualized by SDS PAGE. Wild-type FBF-1 and FBF-2 (residues 164–566) were used for pulldown experiments with the His tagged CPB-1 deletion mutants. Wild-type FBF-2 (residues 164–575) was used for pulldown assays with the single and double alanine mutant CPB-1 constructs. Single alanine mutations were introduced in the FBF-2 (164-575) construct. The reason for using slightly different FBF constructs is that for initial experiments we designed the FBF-1 (164-566) and FBF-2 (164-566) constructs based on sequence alignments with other PUF proteins. Since in parallel we were also pursuing crystallization attempts, when the crystal structure of FBF-2 (164-575) was published29 we switched to this slightly longer construct and for consistency we also used it for the biochemical experiments. It is worth noticing that the last 8 amino acids (residues 568–575) are not included in the crystal structure because of poor electron density29 and do not affect binding to CPB-1.
Yeast two and three-hybrid assays
Point mutants were generated using site directed mutagenesis (Invitrogen). Mixed base oligonucleotides were obtained by machine mixed random codons (IDT). In the yeast two-hybrid assays, CPB-1(1-80) was cloned into pBTM116 and FBF-2 (121-C-term) into pACT2 and expressed in strain L40U−. Modified yeast three-hybrid experiments were conducted as described (34; 38). Yeast strain YBZ-1 was transformed with an additional plasmid encoding p414TEF CPB-1 (40-80) fused to an SV40 nuclear localization signal. Measurements were determined in triplicate from three individual yeast transformants. Luminescence data were collected using the β-Glo reagent (Promega).
Tryptic digestion of CPB-1
11 μM His tagged FBF-1(164-566) was incubated on ice for 2 hours with 15 μM CPB-1(1-80) in binding buffer [20 mM Na-phosphate (pH 8.6), 100 mM NaCl,10 mM βME]. A freshly prepared solution of ethylene glycol bis[succinimidylsuccinate] (EGS) in DMSO was added to a final concentraton of 1.25 mM to half of the reaction mix and the sample was incubated on ice for 5 minutes. The crosslinking reaction was quenched by adding 50 mM Tris HCl pH 8.6. Trypsin was added to both samples to a final concentration of 0.5 ng/μl and the reactions were incubated at room temperature for 50 minutes. The tryptic digestion was stopped by adding 3% formic acid. The trypsinised fragments were identified by matrix-assisted laser desorption/ionization time-of-flight (MALDI TOF) mass spectrometry. The CPB-1 fragments that were protected upon binding to FBF were identified by comparison of the digestion pattern of the crosslinked FBF•CPB-1 sample with the non cross-linked sample.
Protein cross-linking
Cross-linking experiments were performed in parallel with both FBF-1(164-566) and FBF-2(164-566) and yielded the same results regardless of the protein used. A constant concentration of FBF (7.5 μM) was equilibrated with a 2 fold molar excess of GLD-3(860-949) in 20 mM sodium phosphate (pH 8.5), 200 mM NaCl, 10 mM βME in 20 μl total volume. Increasing concentrations of CPB-1(1-80) were added to a final concentration of 3.8 μM, 15.0 μM and 30.0 μM. 0.5 μl of freshly prepared 40 mM solution of EGS in DMSO was added and the cross-linking reaction was allowed to proceed for 1 minute at room temperature. The reaction was quenched by adding 1 μl of 100 mM Tris HCl (pH 8.6) and the samples were analyzed by SDS PAGE. The same type of experiment was repeated with FBF incubated with constant amount of CPB-1 and where GLD-3 was added in a 0.5, 2 and 4 fold molar excess prior to EGS crosslinking.
Labeling of CPB-1 with Alexa Fluor 488
A cysteine residue was introduced by PCR mutagenesis between residues Ser 35 and Phe 36 in the CPB-1(19-80) construct. Protein expression was performed as for wild-type protein. The cell pellet was re-suspended in lysis buffer supplemented with 2 mM DTT and lysed as described for wild-type CPB-1. The cleared lysate was loaded onto NiNTA resin. The resin was washed with labeling buffer [20 mM Hepes (pH 7.0), 150 mM NaCl, 2% glycerol] to remove the reducing agent. Precautions were taken to avoid cysteine oxidation (all the buffers were thoroughly degassed and the resin was flushed with nitrogen). The protein was incubated with a 5-fold molar excess of AlexaFluor 488 C5 maleimide (Invitrogen) while on the resin. Labeled CPB-1 was eluted and dialysed in 20 mM Tris HCl (pH 7.0), 50 mM NaCl, 10% glycerol, 2 mM DTT. The protein was loaded onto Q column (GE Healthcare) equilibrated with the same buffer. CPB-1(19-80) did not bind to the column in these conditions but was present in the flow through >95% free of contaminants.
Quantitative analysis of protein-protein interactions by fluorescence polarization
Fluorescence polarization experiments were carried out in 96-well opaque fluotrak 200 μl plates plates (Greiner), the polarization was determined using an Envision plate reader (Perkin Elmer). In the direct titration experiments 6 nM fl-CPB-1 was incubated for at least 1hour at room temperature with increasing concentrations of wild-type or mutant FBF-2(164-575) in a total volume of 100 μl. The equilibration buffer contained 20 mM Tris HCl (pH 8.6), 100 mM NaCl, 2 mM DTT, 0.10 mM EDTA, 0.10 mg/ml tRNA, 0.05 mg/ml BSA, 5% glycerol. The experiments were performed at least in triplicate and data were fitted with the Hill equation using IGOR (Wavemetric) to determine the Kd. For the binding-competition experiments 6 nM fl-CPB-1 was mixed with 200 nM wild-type FBF-2(164-575) in the same buffer as for the direct titration experiments and increasing concentrations of unlabelled competitor CPB-1 were added. IC50 values were calculated by fitting the data to the Hill equation.
RNA binding assay
A constant concentration of 75 pM 32P labeled cyb-1 RNA (5′ CGAAAUAAACAUUUUGUACCAUUCAGUC 3′) was incubated for 1 hour at room temperature with increasing concentrations of FBF-2(164-575) alone or pre-incubated for 2 hours with 6 μM MBP-CPB-1(1-80) in 10 mM Tris HCl (pH 7.5), 0.5 mM EDTA, 50 mM NaCl, 2 mM DTT, 0.1 mg/ml BSA, 0.02% Tween, 0.2 mg/ml tRNA. Samples were loaded onto native polyacrylamide gel, visualized and analyzed essentially as described in Wu et al31. The FBF Y479A point mutant and the FBF Δ(479-485) deletion mutant were tested for binding to 32P labeled FBE RNA in the same conditions.
NMR spectroscopy
15N labeled CPB-1(32-80) was expressed in M9 minimal medium containing 1 g/l (15NH4)2 SO4 (Cambridge Isotope Laboratories) as the source of nitrogen. The protein was purified as described above and buffer exchanged into 10 mM Tris HCl (pH 6.0), 50 mM NaCl, 5 mM DTT. For complex reconstitution 15N labeled CPB-1(32-80) was mixed with 1.5 molar excess of FBF-2(164-575)/FBE RNA (5′-UGUGCCAUA-3′) and the samples were concentrated to approximately 0.7 mM. NMR samples were prepared by adding 15%(v/v) D2O. The 2D [15N,1H]-HSQC spectra were recorded at 298K and 700 MHz on a Bruker DRX spectrometer.
Circular dichroism measurements
The CD spectrum of CPB-1(1-80) was recorded on a JASCO J-815 CD spectropolarimeter at protein concentration of 47 μM in 20 mM sodium phosphate (pH 8.0), 20 mM NaCl at 20°C in a 0.1 cm cuvette.
Supplementary Material
(A) The FBF•CPB-1 complex (lane 1) and the chemically cross-linked FBF•CPB-1 complex (lane 2) were treated with trypsin (lanes 3 and 4 respectively) and visualized on SDS PAGE. The proteolytic fragments were analyzed by mass spectrometry (B and C) and the CPB-1 fragments that were protected from cleavage upon binding to FBF (sample M2, lane 4) were identified by comparison with the digestion pattern of the non cross-linked sample in lane 3 (sample M1).
(A) CD analysis of CPB-1(1-80). The CD spectrum, recorded in 20 mM sodium phosphate (pH 8.0), 20 mM NaCl at 20°C, shows a large negative peak around 200 mm typical of natively unfolded proteins. (B) Summary of all the CPB-1 constructs tested in vitro with recombinant proteins and with the yeast two-hybrid assay for binding to FBF. The domain structure of CPB-1 is shown. The FBF-binding region is the previously identified 1–80 N-terminal stretch of residues. Solid lines indicate fragments that interacted with FBF in the yeast two-hybrid assay or in vitro, dotted lines show fragments that were not able to bind. (C) Superposition of the [15N,1H]-HSQC spectra of 15N-labeled CPB-1(32-80) alone (black peaks) and in complex with FBF-2(164-575)•RNA (orange peaks). Green and yellow boxes indicate respectively the typical Gly backbone HN and Asn and Gln H2N side-chain regions. The sequence of CPB-1(32-80) is shown below the spectra, residues in italics were introduced by the cloning strategy. In the [15N,1H]-HSQC spectrum of CPB-1 48 peaks are observed, including all 5 side chain H2N Gln and Asn peaks (residues in red in the sequence) as well as all the Gly. In the FBF-bound CPB-1 spectrum only 25 peaks are visible. In this case no changes in the backbone HN Gly region are observed but only 3 side chain H2N Gln and Asn peaks are present.
EMSA of FBF (Y479A) point mutant and FBF Δ(479-485) deletion mutant showed that such mutations do not impair binding to RNA.
Statistical analysis was performed using a two-tailed Student’s t-test. Experiments were performed at least three times with similar results obtained. Asterisks indicate statistically significant differences (* P-value <0.05).
Highlights.
C. elegans proteins CPB-1 and FBF act together to regulate mRNA translation.
The protein/protein interface is conserved with the FBF interacting partner GLD-3.
CPB-1 competes with GLD-3 for binding to FBF.
CPB-1 modulates the affinity of FBF for its putative mRNA target.
Acknowledgments
We wish to thank Pedro Serrano Navarro for help with [15N,1H]-HSQC data acquisition and useful discussions, Christine Beuck and John Hammond for comments on the manuscript. This work was supported by the National Institutes of Health grants F32 GM095169 (Z.T.C.), GM53320 (J.R.W), GM031892 and GM050942 (M.W.).
Abbreviations
- PUF
Pumilio and FBF
- FBF
fem-3 binding factor
- CPEB
cytoplasmic polyadenylation element binding
- CPB-1
cytoplasmic polyadenylation element binding protein homolog-1
- UTR
untranslated region
- GLD-3
germline development defective-3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suh N, Crittenden SL, Goldstrohm A, Hook B, Thompson B, Wickens M, Kimble J. FBF and its dual control of gld-1 expression in the Caenorhabditis elegans germline. Genetics. 2009;181:1249–60. doi: 10.1534/genetics.108.099440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crittenden SL, Bernstein DS, Bachorik JL, Thompson BE, Gallegos M, Petcherski AG, Moulder G, Barstead R, Wickens M, Kimble J. A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature. 2002;417:660–3. doi: 10.1038/nature754. [DOI] [PubMed] [Google Scholar]
- 3.Kaye JA, Rose NC, Goldsworthy B, Goga A, L’Etoile ND. A 3′UTR pumilio-binding element directs translational activation in olfactory sensory neurons. Neuron. 2009;61:57–70. doi: 10.1016/j.neuron.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richter JD. CPEB: a life in translation. Trends Biochem Sci. 2007;32:279–85. doi: 10.1016/j.tibs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Radford HE, Meijer HA, de Moor CH. Translational control by cytoplasmic polyadenylation in Xenopus oocytes. Biochim Biophys Acta. 2008;1779:217–29. doi: 10.1016/j.bbagrm.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstrohm AC, Wickens M. Multifunctional deadenylase complexes diversify mRNA control. Nat Rev Mol Cell Biol. 2008;9:337–44. doi: 10.1038/nrm2370. [DOI] [PubMed] [Google Scholar]
- 7.Luitjens C, Gallegos M, Kraemer B, Kimble J, Wickens M. CPEB proteins control two key steps in spermatogenesis in C. elegans. Genes Dev. 2000;14:2596–609. doi: 10.1101/gad.831700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hake LE, Mendez R, Richter JD. Specificity of RNA binding by CPEB: requirement for RNA recognition motifs and a novel zinc finger. Mol Cell Biol. 1998;18:685–93. doi: 10.1128/mcb.18.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pique M, Lopez JM, Foissac S, Guigo R, Mendez R. A combinatorial code for CPE-mediated translational control. Cell. 2008;132:434–48. doi: 10.1016/j.cell.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 10.Barnard DC, Ryan K, Manley JL, Richter JD. Symplekin and xGLD-2 are required for CPEB-mediated cytoplasmic polyadenylation. Cell. 2004;119:641–51. doi: 10.1016/j.cell.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Richter JD. Opposing polymerase-deadenylase activities regulate cytoplasmic polyadenylation. Mol Cell. 2006;24:173–83. doi: 10.1016/j.molcel.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Nakahata S, Katsu Y, Mita K, Inoue K, Nagahama Y, Yamashita M. Biochemical identification of Xenopus Pumilio as a sequence-specific cyclin B1 mRNA-binding protein that physically interacts with a Nanos homolog, Xcat-2, and a cytoplasmic polyadenylation element-binding protein. J Biol Chem. 2001;276:20945–53. doi: 10.1074/jbc.M010528200. [DOI] [PubMed] [Google Scholar]
- 13.Nakahata S, Kotani T, Mita K, Kawasaki T, Katsu Y, Nagahama Y, Yamashita M. Involvement of Xenopus Pumilio in the translational regulation that is specific to cyclin B1 mRNA during oocyte maturation. Mech Dev. 2003;120:865–80. doi: 10.1016/s0925-4773(03)00160-6. [DOI] [PubMed] [Google Scholar]
- 14.Goldstrohm AC, Seay DJ, Hook BA, Wickens M. PUF protein-mediated deadenylation is catalyzed by Ccr4p. J Biol Chem. 2006 doi: 10.1074/jbc.M609413200. [DOI] [PubMed] [Google Scholar]
- 15.Goldstrohm AC, Hook BA, Seay DJ, Wickens M. PUF proteins bind Pop2p to regulate messenger RNAs. Nat Struct Mol Biol. 2006;13:533–9. doi: 10.1038/nsmb1100. [DOI] [PubMed] [Google Scholar]
- 16.Friend K, Campbell ZT, Cooke A, Kroll-Conner P, Wickens MP, Kimble J. A conserved PUF-Ago-eEF1A complex attenuates translation elongation. Nat Struct Mol Biol. 2012;19:176–83. doi: 10.1038/nsmb.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraemer B, Crittenden S, Gallegos M, Moulder G, Barstead R, Kimble J, Wickens M. NANOS-3 and FBF proteins physically interact to control the sperm-oocyte switch in Caenorhabditis elegans. Curr Biol. 1999;9:1009–18. doi: 10.1016/s0960-9822(99)80449-7. [DOI] [PubMed] [Google Scholar]
- 18.Eckmann CR, Crittenden SL, Suh N, Kimble J. GLD-3 and control of the mitosis/meiosis decision in the germline of Caenorhabditis elegans. Genetics. 2004;168:147–60. doi: 10.1534/genetics.104.029264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang B, Gallegos M, Puoti A, Durkin E, Fields S, Kimble J, Wickens MP. A conserved RNA-binding protein that regulates sexual fates in the C. elegans hermaphrodite germ line. Nature. 1997;390:477–84. doi: 10.1038/37297. [DOI] [PubMed] [Google Scholar]
- 20.Edwards TA, Pyle SE, Wharton RP, Aggarwal AK. Structure of Pumilio reveals similarity between RNA and peptide binding motifs. Cell. 2001;105:281–9. doi: 10.1016/s0092-8674(01)00318-x. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Zamore PD, Hall TM. Crystal structure of a Pumilio homology domain. Mol Cell. 2001;7:855–65. doi: 10.1016/s1097-2765(01)00229-5. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, McLachlan J, Zamore PD, Hall TM. Modular recognition of RNA by a human pumilio-homology domain. Cell. 2002;110:501–12. doi: 10.1016/s0092-8674(02)00873-5. [DOI] [PubMed] [Google Scholar]
- 23.Edwards TA, Wilkinson BD, Wharton RP, Aggarwal AK. Model of the brain tumor-Pumilio translation repressor complex. Genes Dev. 2003;17:2508–13. doi: 10.1101/gad.1119403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckmann CR, Kraemer B, Wickens M, Kimble J. GLD-3, a bicaudal-C homolog that inhibits FBF to control germline sex determination in C. elegans. Dev Cell. 2002;3:697–710. doi: 10.1016/s1534-5807(02)00322-2. [DOI] [PubMed] [Google Scholar]
- 25.Dong S, Wang Y, Cassidy-Amstutz C, Lu G, Bigler R, Jezyk MR, Li C, Hall TM, Wang Z. Specific and modular binding code for cytosine recognition in Pumilio/FBF (PUF) RNA-binding domains. J Biol Chem. 2011;286:26732–42. doi: 10.1074/jbc.M111.244889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filipovska A, Razif MF, Nygard KK, Rackham O. A universal code for RNA recognition by PUF proteins. Nat Chem Biol. 2011;7:425–7. doi: 10.1038/nchembio.577. [DOI] [PubMed] [Google Scholar]
- 27.Kaymak E, Wee LM, Ryder SP. Structure and function of nematode RNA-binding proteins. Curr Opin Struct Biol. 2010;20:305–12. doi: 10.1016/j.sbi.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernstein D, Hook B, Hajarnavis A, Opperman L, Wickens M. Binding specificity and mRNA targets of a C. elegans PUF protein, FBF-1. Rna. 2005;11:447–58. doi: 10.1261/rna.7255805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Opperman L, Wickens M, Hall TM. Structural basis for specific recognition of multiple mRNA targets by a PUF regulatory protein. Proc Natl Acad Sci U S A. 2009;106:20186–91. doi: 10.1073/pnas.0812076106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell ZT, Bhimsaria D, Valley CT, Rodriguez-Martinez JA, Menichelli E, Williamson JR, Ansari AZ, Wickens M. Cooperativity in RNA-Protein Interactions: Global Analysis of RNA Binding Specificity. Cell Rep. 2012;1:570–581. doi: 10.1016/j.celrep.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J, Campbell ZT, Menichelli E, Wickens M, Williamson JR. Protein•Protein Interaction Sequences Specifying the Recruitment of GLD-3 to the FBF•fem-3 mRNA Complex. doi: 10.1016/j.jmb.2012.11.013. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 33.Petsalaki E, Russell RB. Peptide-mediated interactions in biological systems: new discoveries and applications. Curr Opin Biotechnol. 2008;19:344–50. doi: 10.1016/j.copbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Campbell ZT, Menichelli E, Friend K, Wu J, Kimble J, Williamson JR, Wickens M. A conserved interface between PUF and CPEB proteins. J Biol Chem. 2012 doi: 10.1074/jbc.M112.352815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74:5463–7. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell ZT, Menichelli E, Friend K, Wu J, Kimble J, Williamson JR, Wickens M. Identification of a Conserved Interface between PUF and CPEB Proteins. J Biol Chem. 2012;287:18854–62. doi: 10.1074/jbc.M112.352815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kershner AM, Kimble J. Genome-wide analysis of mRNA targets for Caenorhabditis elegans FBF, a conserved stem cell regulator. Proc Natl Acad Sci U S A. 2010;107:3936–41. doi: 10.1073/pnas.1000495107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell ZT, Bhimsaria Devesh, Valley Cary T, Rodriguez-Martinez Jose A, Menichelli Elena, Williamson James R, Ansari Aseem Z, Wickens M. Cooperativity in RNA-Protein Interactions: Global Analysis of RNA Binding Specificity. Cell Reports. 2012 doi: 10.1016/j.celrep.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zamore PD, Williamson JR, Lehmann R. The Pumilio protein binds RNA through a conserved domain that defines a new class of RNA-binding proteins. Rna. 1997;3:1421–33. [PMC free article] [PubMed] [Google Scholar]
- 40.Walker J, Minshall N, Hake L, Richter J, Standart N. The clam 3′ UTR masking element-binding protein p82 is a member of the CPEB family. Rna. 1999;5:14–26. doi: 10.1017/s1355838299981219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Eckmann CR, Kadyk LC, Wickens M, Kimble J. A regulatory cytoplasmic poly(A) polymerase in Caenorhabditis elegans. Nature. 2002;419:312–6. doi: 10.1038/nature01039. [DOI] [PubMed] [Google Scholar]
- 42.Sonoda J, Wharton RP. Drosophila Brain Tumor is a translational repressor. Genes Dev. 2001;15:762–73. doi: 10.1101/gad.870801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kershner AM, Kimble J. Genome-wide analysis of mRNA targets for Caenorhabditis elegans FBF, a conserved stem cell regulator. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3936–41. doi: 10.1073/pnas.1000495107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galgano A, Forrer M, Jaskiewicz L, Kanitz A, Zavolan M, Gerber AP. Comparative analysis of mRNA targets for human PUF-family proteins suggests extensive interaction with the miRNA regulatory system. PloS one. 2008;3:e3164. doi: 10.1371/journal.pone.0003164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooke A, Prigge A, Opperman L, Wickens M. Targeted translational regulation using the PUF protein family scaffold. Proc Natl Acad Sci U S A. 2011;108:15870–5. doi: 10.1073/pnas.1105151108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The FBF•CPB-1 complex (lane 1) and the chemically cross-linked FBF•CPB-1 complex (lane 2) were treated with trypsin (lanes 3 and 4 respectively) and visualized on SDS PAGE. The proteolytic fragments were analyzed by mass spectrometry (B and C) and the CPB-1 fragments that were protected from cleavage upon binding to FBF (sample M2, lane 4) were identified by comparison with the digestion pattern of the non cross-linked sample in lane 3 (sample M1).
(A) CD analysis of CPB-1(1-80). The CD spectrum, recorded in 20 mM sodium phosphate (pH 8.0), 20 mM NaCl at 20°C, shows a large negative peak around 200 mm typical of natively unfolded proteins. (B) Summary of all the CPB-1 constructs tested in vitro with recombinant proteins and with the yeast two-hybrid assay for binding to FBF. The domain structure of CPB-1 is shown. The FBF-binding region is the previously identified 1–80 N-terminal stretch of residues. Solid lines indicate fragments that interacted with FBF in the yeast two-hybrid assay or in vitro, dotted lines show fragments that were not able to bind. (C) Superposition of the [15N,1H]-HSQC spectra of 15N-labeled CPB-1(32-80) alone (black peaks) and in complex with FBF-2(164-575)•RNA (orange peaks). Green and yellow boxes indicate respectively the typical Gly backbone HN and Asn and Gln H2N side-chain regions. The sequence of CPB-1(32-80) is shown below the spectra, residues in italics were introduced by the cloning strategy. In the [15N,1H]-HSQC spectrum of CPB-1 48 peaks are observed, including all 5 side chain H2N Gln and Asn peaks (residues in red in the sequence) as well as all the Gly. In the FBF-bound CPB-1 spectrum only 25 peaks are visible. In this case no changes in the backbone HN Gly region are observed but only 3 side chain H2N Gln and Asn peaks are present.
EMSA of FBF (Y479A) point mutant and FBF Δ(479-485) deletion mutant showed that such mutations do not impair binding to RNA.
Statistical analysis was performed using a two-tailed Student’s t-test. Experiments were performed at least three times with similar results obtained. Asterisks indicate statistically significant differences (* P-value <0.05).