Abstract
We have previously reported neuroprotective properties of Ginkgo biloba/EGb 761® (EGb 761) in transient and permanent mouse models of brain ischemia. In a quest to extend our studies on EGB 761 and its constituents further, we used a model of transient global ischemia induced delayed hippocampal neuronal death and inflammation. Mice pretreated with different test drugs for 7 days were subjected to eight-minute bilateral common carotid artery occlusion (tBCCAO) at day 8. After 7 days of reperfusion, mice brains were dissected out for TUNEL assay and immunohistochemistry. In-situ detection of fragmented DNA (TUNEL staining) showed that out of all test drugs, only EGb 761 (13.6% ± 3.2) pretreatment protected neurons in the hippocampus against global ischemia (vs. vehicle, 85.1% ± 9.9; p < 0.05). Immunofluorescence-based studies demonstrated that pretreatment with EGb 761 upregulated the expression levels of heme oxygenase 1 (HO1), nuclear factor erythroid 2-related factor 2 (Nrf2), and vascular endothelial growth factor (VEGF) as compared to the vehicle group. In addition, increased number of activated astrocytes and microglia in the vehicle group was observed to be significantly lower in the EGb 761 pretreated group. Together, these results suggest that EGb 761 is a multifunctional neuroprotective agent, and the protection is in part associated with activation of the HO1/Nrf2 pathway, upregulation of VEGF and downregulation of inflammatory mediators such as astrocytes and microglia.
Keywords: global ischemia, heme oxygenase 1, Nrf2, VEGF, astrocytes, microglia
1 Introduction
Stroke causes immense disabilities worldwide. Brain ischemia is an acute neurological injury that results from interruption of blood supply to the brain. Conditions like cardiac arrest and cardio respiratory failure lead to reduction of blood flow to the brain, ultimately resulting in global cerebral ischemia (Chiarugi, 2005, Kaundal et al., 2009). There are approximately 200,000 cases of cardiac arrest reported annually in the United States, and this number may be increasing (Nichol et al., 2008, Merchant et al., 2011). For many decades, researchers have struggled to treat ischemia through neuroprotection and have aimed to develop target molecules with anti-apoptotic, anti-calcium, anti-inflammatory, and antioxidative properties (Fan and Yang, 2007).
The cellular mechanism of ischemic cell death has been studied extensively in the model of transient global ischemia induced by bilateral common carotid artery occlusion model (tBCCAO) in rats (Pulsinelli et al., 1982, Smith et al., 1984), mice (Smith et al., 1984, Zhen and Dore, 2007) and gerbils (Kirino, 1982). Gerbils provide a better option for studying global ischemia due to the absence of posterior communicating arteries (PcomA). Similarly, C57BL/6 mice lack or have low patency of carrying PcomA, thus also making them suitable for the tBCCAO ischemia studies (Kitagawa et al., 1998, Olsson et al., 2003). tBCCAO results in delayed neuronal death in the CA1 subfield of hippocampus and is reported to be the most sensitive region to ischemic insults (Smith et al., 1984, Zhen and Dore, 2007). CA1 and CA2 pyramidal neurons are more sensitive to damage during hypoxia, whereas CA3 and dentate gyrus neurons appear more resistant to damage (Kreisman et al., 2000). In contrast to the permanent and transient focal middle cerebral artery occlusion models, tBCCAO is considered an ideal model to study exclusively the delayed hippocampal neuronal death and reperfusion mediated inflammatory response. The increase in the number of cases of cardiac arrest and the evidence of beneficial effects of EGb 761 in other stroke models warrant further testing of this novel neuroprotective agent in the global model of ischemia.
Standardized extract of Ginkgo biloba/EGb 761® (EGb 761) is now used widely in Europe and China for the treatment of various conditions including dizziness, headache, depression, anxiety, memory and concentration problems (Chan et al., 2007, Shah et al., 2011). In the United States, EGb 761 is one of the most commonly used over the counter neutraceuticals for memory enhancement and overall wellbeing. It contains 24% ginkgo-flavanol glycosides; 6% terpene lactones such as ginkgolides A, B, C, J, and bilobalide; 5–10% organic acids; and >0.5% proanthocyanidins, defined as flavonoid-based polymers (van Beek, 2002). Numerous studies, including ours, have shown the neuroprotective effects of EGb 761 and its constituents in different models of ischemia, and the mechanism of action has been attributed to hemeoxygenase 1 (HO1), Nrf2, vascular endothelial growth factor (VEGF) and collapsin response mediator protein 2 (CRMP2) (Bastianetto et al., 2000, Shah et al., 2011, Nada and Shah, 2012a). However, very few studies have shown the protective mechanism of EGb 761 in tBCCAO induced delayed hippocampal neuronal death. After the transient global ischemia, reperfusion process brings a surge in inflammatory markers, leading to activation of microglial and astrocytic cells in addition to enhanced oxidative stress in the hippocampus (Morioka et al., 1991, Ordy et al., 1993, Chan et al., 1998). Of late, targeting these inflammatory markers in conjunction with oxidative stress has become a major focus of therapeutic strategies (Gage et al., 1990).
In this study, we hypothesized that pretreatment with EGb 761 and its bioactive compounds [bilobalide (BB); Ginkgolide A (GA); Ginkgolide B (GB); Terpene Free material (TFM)] could protect against delayed neuronal cell death in the hippocampus after 8-minutes of tBCCAO. Furthermore, we investigated for the first time the molecular mechanism of protection offered by EGb 761 in hippocampus of mice by studying the expression pattern of HO1, Nrf-2, VEGF, and other inflammatory markers.
2 Material and Methods
2.1. Animals
All animal protocols were approved by The University of Toledo Institutional Animal Care and Use Committee, and the guidelines of the National Institutes of Health were followed throughout the study. C57BL/6 male mice, 8-10 weeks old and weighing about 20-25 grams, were procured from Charles River Laboratories (Ann Arbor, MI). Animals were housed at 22 ± 1 °C with 12 h light and 12 h dark cycle; water and food were available ad libitum.
2.2. Drug treatment
EGb 761 and its components were kindly provided by Dr. Willmar Schwabe Pharmaceuticals (Karlsruhe, Germany). Animals were divided into 7 groups: one sham (naïve group) and 6 treatment groups with surgeries [vehicle (30% polyethylene glycol); EGb 761 (100 mg/kg); BB (6 mg/kg); GA (6 mg/kg); GB (6 mg/kg); TFM (10 mg/kg)]. Test drugs and vehicle were administered orally for 7 days prior to tBCCAO, and mice survived for 7 days after ischemia. Selection of the concentration of test drugs was based on concentration of individual components in the whole extract of the EGB 761 and on our previous studies that successfully reported the neuroprotective effects of EGb 761 and its components using the same concentration in permanent (Shah et al., 2011) and transient (Nada and Shah, 2012b) middle cerebral artery occlusion models.
2.3. Bilateral common carotid artery occlusion
The method of Zhen and Dore, 2007 (Zhen and Dore, 2007) was followed, with minor modifications. Briefly, mice were anesthetized initially by 2-4% isofluorane (Baxter, Deefield, IL) and placed on a mini-vent (Harvard Apparatus, Holliston, MA) with a tidal volume of 0.14 ml and 150 strokes per min using 24 gauge catheter; anesthesia was maintained at 1.5% throughout the surgery. A midline incision was made in the region between the neck and sternum, and the trachea was exposed. Both common carotid arteries were located lateral to sternocleidomastoid and freed from surrounding tissue, and the vagus nerve was separated. tBCCAO was initiated by occluding both right and left common carotid arteries with silk strings. After eight minutes of ischemia, the strings were removed from both the arteries to allow the recirculation of blood. The incision was sutured using 6.0 absorbent silk surgical sutures. During surgery, rectal temperature was measured and maintained at 37 °C with a heating pad. After completion of the surgical procedure, animals were shifted to an automatic temperature-controlled chamber, which was set at 32 °C to maintain 37 °C body temperature; animals were then moved to home cages after 2 hours.
Seven days after ischemia, the animals were anesthetized with sodium pentobarbital and transcardially perfused with saline and 4% buffered paraformaldehyde. Mice brains were removed and stored for at least 24 h in 4% paraformaldehyde before dehydration and embedding in paraffin. The coronal brain sections were cut into 4 μm sections 3-4 mm from bregma and prepared for TUNEL and immunofluorescence assays accordingly.
2.4. In-situ detection of DNA fragmentation (TUNEL staining)
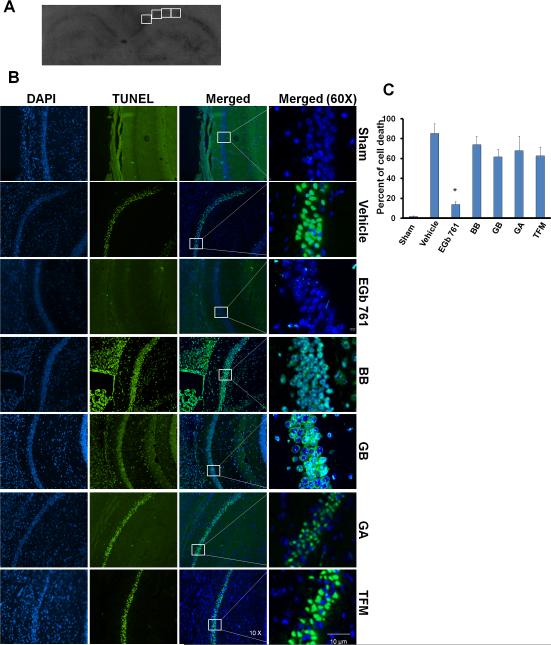
Quantitative analysis of the changes in the nuclear chromatin of hippocampal neurons in CA1and CA2 regions were performed by in-situ detection of DNA fragmentation using TUNEL staining and were detected under immunofluorescent microscopy. Sections were de-paraffinized in xylene and in graded series of ethanol; incubated with 20 μg/ml of proteinase K (Promega Co., Madison, WI) in 0.05M PBS for 10-15 min; incubated with equilibrium buffer (Promega Co.) for 10 min and then incubated in the mixture of terminal deoxynucleotidyl transferase (TrdT) and equilibration buffer in a humidified chamber at 37 °C for 1 h. 2X Saline-sodium citrate (SSC) buffer was used to stop the reaction after 1 h, followed by rinsing (4 times) and washing the slides in 1X PBS for 10 min. Slides were then mounted in VECTA SHIELD+4’,6-diamidino-2-phenylindole (DAPI) (Vector labs, Burlingame, CA) to stain nuclei, and a cover slip was mounted onto the slides. Analysis of dead neurons for TUNEL assay was quantified by counting the green fluorescent cells in four (570 μm × 680 μm) boxes (as shown in figure 1A), applied approximately at the center of CA-1 subregion. Counting of 4 boxes was averaged for each mouse as percentage of cell death (number of dead neurons divided by total number of neurons and multiplied by 100) and expressed as mean ± SEM (4-7 mice/group). Counting was performed by an investigator blinded to the study design.
Fig. 1.
EGb 761 pretreatment protects hippocampal neurons. Mice were pretreated with test drugs for 7 days and subjected to eight minutes of global ischemia at day 8. Mice brains isolated after 7 days of global ischemia were fixed in 4% paraformaldehyde and paraffin fixed for TUNEL assay. (A) Coronal hippocampal sections (4μm) showing 4 boxes (570 μm × 680 μm each/mice) in CA1 subregion were selected for counting TUNEL positive cells (B) TUNEL positive cells are stained fluorescent green, and the nuclei are stained blue (DAPI). The box in the merged photographs represents the area selected for magnified view (60X). Sham (n = 4), no death of neurons; vehicle (n = 6), significantly higher neuronal death was observed; EGb 761 (n = 7) pretreated group, significantly restored neuronal survival. Other components BB (n = 5), GA (n = 4), GB (n = 5) and TFM (n = 4), no protection was observed. (C) Quantitative analysis of CA-1 subregions showed that only EGb 761 significantly protected hippocampal neurons. Data are expressed as means ± SEM; 10X magnification for DAPI, TUNEL and merged photographs; 60X for magnified view; # vs. Sham; * vs. vehicle; p < 0.05.
2.5. Immunofluorescence
Hippocampal sections from different mice were de-paraffinized in xylene and in graded series of ethanol and incubated with 20 μg/ml of proteinase K (Promega Co.) in 0.05M PBS for 10-15 min, fixing in paraformaldehyde for 15-20 min. Non-specific binding sites were blocked by incubating brain sections in 3% bovine serum albumin for 1 h, followed by incubation with primary anti body HO1 (1:100 rabbit polyclonal, Santa Cruz Biotechnology Inc., Santa Cruz, CA); Nrf2 (1:100 rabbit polyclonal, Santa Cruz); Glial fibrillary acidic protein (GFAP) (1:1000, rabbit polyclonal, Abcam, Cambridge, MA) and VEGF (1:100, rabbit polyclonal, Santa Cruz) overnight at 4 °C. Antigen retrieval was performed to obtain better immunohistochemistry results with CD11b antibody, as suggested by the company. Sections were heated in a solution containing sodium citrate buffer (10 mmol/L, pH 6.0), EDTA (mM/L, pH 8.0) and Tris EDTA buffer (pH 9.0). Briefly, deparaffinized sections were heated in a pressure cooker for 3-4 min and allowed to cool for 10 min at room temperature. Immunohistochemistry was carried out by incubating sections with CD11b primary antibody (1:200, mouse monoclonal, Abcam). Sections were washed with PBS for 15 min and incubated for two hours at room temperature with anti-rabbit IgG. Images used for analysis by Image J software were captured with the same microscopic settings (exposure time, intensity, contrast and magnification) for all the groups. The intensity of the florescence in each photographed area (60X) was calculated by subtracting the background intensity from the total intensity and the values were divided by total selected area to give corrected florescence intensity for HO1, Nrf2, and VEGF (averaged in each treatment group). Activated astrocytes and microglia were counted in the entire 60X field/mice, averaged in each group and expressed as the number of activated cells/field. Counting was performed by an investigator blinded to the study design.
2.6. Double staining
Sections were first incubated in bovine serum albumin to block non-specific binding and then with primary antibodies to CD-31 (1:100, endothelial cell marker, mouse monoclonal, Millipore, Temecula CA); VEGF and HO1 overnight at 4 °C. Cells were washed in PBS and incubated in anti mouse IgG and anti rabbit IgG for 2 h at room temperature and then stained with DAPI for nucleus visualization.
2.7. Statistical analysis
The data were analyzed by one-way ANOVA with Tukey post hoc test for comparison between different treatment groups. Data are presented as mean ± SEM. A value of p < 0.05 was considered to be statistically significant.
3 Results
3.1. EGb 761 prevents delayed hippocampal cell death in transient global ischemia
In the TUNEL assay, dead neurons were stained green due to the binding of dUTP enzyme to their fragmented DNA. All mice survived in the sham group, and the morphology of the CA1 and CA2 regions did not show any changes in terms of neuronal death. In some of the vehicle-treated mice, neuronal injury was observed in the CA2 region, but consistent injury was prevalent in the CA1 region only. Mice pretreated for 7 days with EGb 761 showed preservation of the CA1 region, with significantly higher surviving neurons in both hemispheres (Fig. 1B). Pretreatment for 7 days with BB, GA, GB, and TFM did not show any protection against eight minutes of transient global ischemia induced hippocampal neuronal death. Quantitative analysis was carried out by counting dead cells manually in 4 selected boxes (570 μm × 680 μm each/mice) in the CA1 region of all treated groups (Fig. 1A). Compared to vehicle (85.1% ± 9.9), EGb 761 significantly protected (13.6% ± 3.2) hippocampal neurons against transient global ischemia (p < 0.05) (Fig. 1B and C). To further delve into the possible mechanism of action, only the EGb 761 pretreated group was pursued to study the changes in the expression of critical proteins.
3.2. EGb 761 induces HO1 in the hippocampus
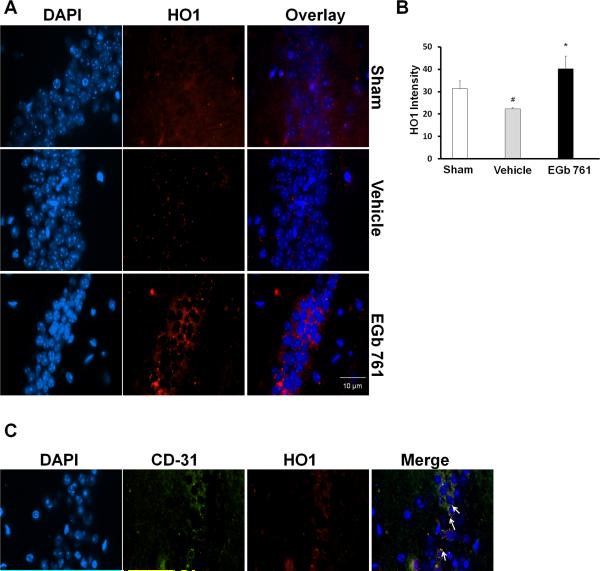
We further sought to know whether HO1 has a role to play in modulating delayed hippocampal neuronal death and subsequent EGb 761 neuroprotection. In vehicle-treated mice, 7 days after eight-minutes of transient global ischemia, a minimal level of HO1 expression or immunofluorescence was observed, and it was significantly reduced as compared to basal levels of the sham group. However, EGb 761 pretreated mice observed to have increased neuronal survival exhibited intense immunofluorescence or expression of HO1 protein (red), which suggests that upregulated HO1 may have played an important role in the observed neuroprotection (Fig. 2A and B). To determine the cellular location of HO1 in the hippocampus, we performed double staining of HO1 and endothelial cell marker (CD31), and the results show HO1 localization in the endothelial cells (Fig. 2C). Negative control experiments did not show non-specific binding of antibodies in the CA1 region of the hippocampus (data not shown).
Fig. 2.
EGb 761upregulates HO1 in the CA-1 region of the hippocampus. Hippocampal sections (paraffin) of mice pretreated with EGb 761 for 7 days and subjected to eight-minutes of global ischemia were used in this immunofluorescence assay. (A) HO1 expression was visualized by immunofluorescent staining with specific rabbit polyclonal antibody for HO1, followed by secondary anti rabbit IgG antibody (red). DNA was stained blue (DAPI). (B) Robust upregulation of HO1 expression was observed in the hippocampus of mice pretreated with EGb 761 compared to vehicle. (C) Localization of HO1 was evaluated by double staining with CD-31 (endothelial cell marker) monoclonal antibody followed by secondary anti rat IgG (green). The arrows in the figure show overlapping of CD-31(green) on HO1 (red), thereby giving out yellow fluorescence. HO1 was observed to be expressed by endothelial cells. Magnified view, 60X; # vs. Sham; * vs. vehicle; p < 0.05.
3.3. Role of Nrf2/HO1 pathway
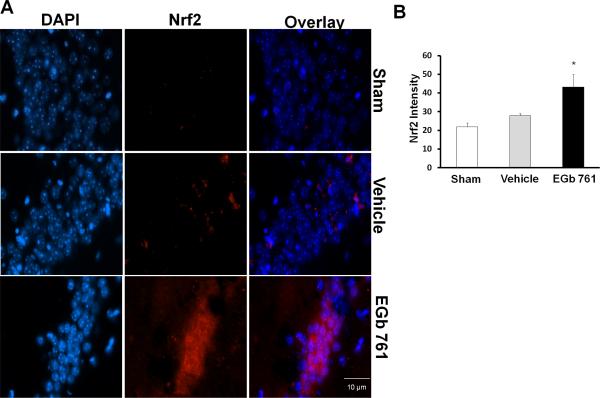
To elucidate the mechanism by which EGb 761 modulates the Nrf2/HO1 pathway, we investigated the events upstream to HO1. Considerable evidence suggests that Nrf2 translocation to the nucleus initiates ARE binding to the HO1 promotor region, resulting in HO1 upregulation (Satoh et al., 2006). Our results revealed that Nrf2 expression levels remained similar in the sham and vehicle treated groups but EGb 761 pretreatment for 7 days induced nuclear Nrf2 (red) in the mouse hippocampus, which in turn might have led to the HO1 induction (Fig.3A and B). These results suggest that Nrf2/HO1 pathway activation is important for EGb 761 and is at least partly associated with its neuroprotective mechanisms.
Fig.3.
EGb 761 upregulates Nrf2 in the CA-1 region of the hippocampus. (A) Hippocampal sections (paraffin) of mice pretreated with EGb 761 for 7 days and subjected to 8-minutes of global ischemia were used in this immunofluorescence assay. Nrf2 expression was visualized by immunofluorescent staining by a specific rabbit polyclonal antibody for Nrf2, followed by secondary anti rabbit IgG (red). DNA was stained blue (DAPI). (B) Nrf2 expression was observed to be increased in the hippocampus of mice pretreated with EGb 761 but not in vehicle group (B). Magnified view, 60X; * vs. vehicle; p < 0.05.
3.4. EGb 761 pretreatment induces VEGF in the hippocampus
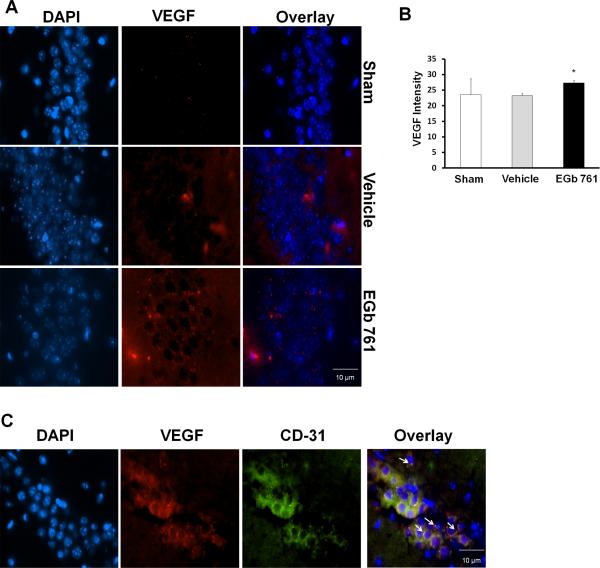
During cerebral ischemia, stimulation of VEGF in the brain is vital, as it leads to the development of new cerebral blood vessels (Hai et al., 2003, Pichiule et al., 2003). This led us to hypothesize that VEGF may also play an important part in the EGb 761 neuroprotection in global ischemia. Immunostaining was carried out on brain sections from sham, vehicle and EGb 761 pretreated groups. Mice pretreated with EGb 761 for 7 days showed robust expression of hippocampal VEGF compared to sham and vehicle treated group, suggesting that EGb 761 mediated formation of new microvessels in the hippocampus of mice subjected to global ischemia (Fig. 4A and B). To further ascertain the localization of the VEGF, we used CD-31 as an endothelial cell marker and found localization of VEGF in endothelial cells of the hippocampus (Fig. 4C). Negative control experiments did not show non-specific binding of antibodies in the CA1 region of the hippocampus (data not shown).
Fig. 4.
EGb 761 upregulates VEGF expression in the CA-1 region of the hippocampus. Hippocampal sections (paraffin) of mice pretreated with EGb 761 for 7 days and subjected to 8-minutes of global ischemia were used in this immunofluorescence assay. (A) VEGF expression was visualized by immunofluorescent staining with a specific rabbit polyclonal antibody followed by secondary anti rabbit IgG (red), and DNA was stained blue (DAPI). (B) VEGF expression was increased in EGb 761 pretreated mice as compared to vehicle group. (C) Double staining for VEGF localization was visualized by a specific anti rabbit polyclonal antibody followed by secondary anti rabbit IgG (red) and CD-31 mouse monoclonal antibody for endothelial cell marker followed by secondary anti-rat IgG (green). The arrows in the figure show overlapping of CD-31 (green) on HO1 (red), thereby giving out yellow fluorescence. VEGF was observed to be expressed in endothelial cells. Magnified view, 60X; * vs. vehicle; p < 0.05.
3.5. Effect of EGb 761 on inflammatory markers following global ischemia
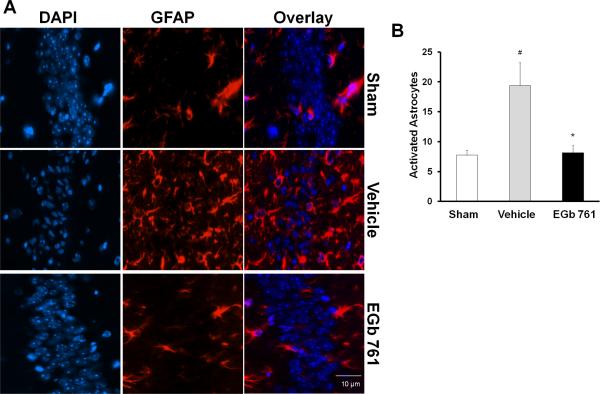
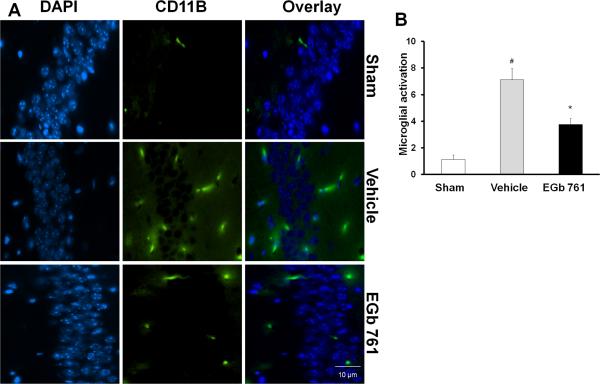
To test whether EGb 761 abates inflammation within the hippocampus of mice brains following eight-minutes of global ischemia, we examined the activation of microglia and astrocytes as markers of inflammation. Activation of microglia leads to increased expression of the integrin CD11b and “amoeboid” cell morphology with retracted, thickened processes and enlarged soma. Similarly, reactive or activated astrocytes increase expression of GFAP and exhibit retraction of processes and enlarged soma (d'Avila et al., 2012). After 7 days, the number of GFAP positive cells and activated microglia (counted in a 60X field) in the hippocampus were significantly higher in the vehicle-treated group than the basal levels observed in the sham group (Fig. 5A and B; 6A and B). Pretreatment with EGb 761 showed a reduced number of GFAP positive and activated microglia following tBCCAO, suggesting the anti-inflammatory role of EGb 761.
Fig. 5.
Effect of EGb 761 on astrocyte activation. (A and B) Activated astrocytes (stained with rabbit polyclonal antibody directed against astrocytic activation (GFAP) were higher in the hippocampus of the vehicle-treated group compared to basal levels in the sham group. EGb 761 pretreated group followed by global ischemia showed fewer activated astrocytes compared to vehicle group. Counting of active astrocytes was performed in a 60X field and expressed as activated cells/field. Magnified view, 60X; # vs. Sham; * vs. vehicle; p < 0.05
Fig. 6.
Effect of EGb 761 on microglial activation. (A and B) Activated microglial cells (stained with mouse monoclonal antibody against microglial activation (Cd11b) were higher in the hippocampus of the vehicle-treated group compared to basal levels in the sham group. EGb 761 pretreated group followed by global ischemia showed fewer number of activated microglia cells compared to vehicle group. Counting of active microglial cells were performed in a 60X magnified view and expressed as activated cells/field. Magnified view, 60X; # vs. Sham; * vs. vehicle; p < 0.05.
4 Discussion
In the present study, we have shown that 7 days of pretreatment with EGb 761 prevents delayed neuronal death induced by eight minutes of global ischemia. However, other components of EGb 761 (BB, GA, GB and TFM) were not observed to protect against hippocampal neuronal death. We also demonstrated that the hippocampal neurons of EGb 761 pretreated mice showed higher expressions of HO1, Nrf2 and VEGF. Interestingly, we have for the first time shown that HO1 is co-localized in endothelial cells of the hippocampus. In this study, we also extended our investigations to further delineate the role of inflammatory cells and their activation during global ischemia and the role of EGB 761 as an anti-inflammatory agent. Both microglia and astrocytes were observed to be activated during global ischemia, and the EGb 761 pretreated mice showed fewer activated cells in the hippocampus. Together, we have for the first time shown the different signaling targets involved in EGB 761 neuroprotective mechanism using an in vivo eight-minute global ischemia model.
EGb 761 has been used in traditional medicine for years and is the highest selling dietary supplement in the United States, but its use as a drug or ingredient in dietary products such as teas and beverages has not been thus far approved as “generally recognized as safe (GRAS)” by the U.S. Food and Drug Administration (USFDA). Nonetheless, a number of clinical trials on EGb 761 have vehemently reported its efficacy and safety in human subjects (Herrschaft et al., 2012). Previously, our group has shown that EGb 761 and some of its bioactive constituents have neuroprotective properties in permanent distal middle cerebral artery occlusion (pMCAO) (Shah et al., 2011) as well as in middle cerebral artery occlusion (MCAO) (Saleem et al., 2008, Shah et al., 2011, Nada and Shah, 2012a). In this study, we hypothesized that EGb 761 and its bioactive components (BB, GA, GB, and TFM) can provide protection to hippocampal neurons against global ischemia induced by tBCCAO. Hippocampal neuronal death was assessed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and, interestingly, we found that EGb 761 offers a significant amount of protection in the CA1 region of the hippocampus when compared to the vehicle group. Our studies are in agreement with our previous findings in which not all components of EGb 761 were found to be protective in pMCAO and tMCAO paradigms. In a quest to understand which component of EGb 761 can become a better drug molecule and work in different models of ischemia, it is again assumed that the whole extract of EGb 761 is better than any other individual components in providing neuroprotection. This is in contrast to the study by Chandrasekaran et al, 2003 (Chandrasekaran et al., 2003) in which both EGb 761 and BB were found to be protective in gerbils after 5-min global ischemia followed by 7 days of survival. These discrepancies may be attributed to the differences in animal species and the duration of the ischemia.
During ischemia, many anti-oxidant enzymes in the mitochondria and endoplasmic reticulum presumably undergo turnover (Saleem et al., 2008). The most important pathway activated during ischemic damage involves the transcriptional activation of phase-2 genes through a cis-acting element called ARE. By examining the role of the anti-oxidant proteins HO1 and Nrf2, we found that EGb 761 pretreated mice that have lower amounts of neuronal death showed higher expressions of HO1 and Nrf2. Expression of HO1 in neurons is usually detected in response to stimuli such as ischemia or oxidative stress (Geddes et al., 1996, Matsuoka et al., 1998). Evidence suggests that EGb 761 mediates induction of phase-2 enzymes through the HO1/Nrf2 pathway (Qureshi, 1996, Gilman, 2006, Shah et al., 2010). Our results are in consonance with other findings that polyphenolic compounds exhibit anti-oxidant properties on binding to ARE's of antioxidant proteins through Nrf2 activation and provide protection against oxidative stress. Since this is the first report of HO1 expression in the mouse hippocampus, we wanted to further investigate its localization. Our immunofluorescence studies have shown that HO1 is expressed in the endothelial cells. A recent study in cellular localization of HO1 on intracerebral hemorrhage showed that HO1 is expressed in endothelial cells (Wang and Dore, 2007).
The other important target molecule in ischemia is VEGF, which was observed to be unchanged in the vehicle group as compared to the sham mice. Mice pretreated with EGb 761 showed higher expression levels of VEGF in the hippocampus, suggesting that VEGF partially plays an important role in ischemia. Our results are in agreement with our previous findings in which EGb 761 has also been shown to increase cerebral blood flow in mice (Saleem et al., 2008), possibly through the upregulation of cortical VEGF (Shah et al., 2011). Our results are supported by the previous study that suggests that HO1 upregulation is linked to VEGF induction (Morita et al., 2009).
In addition, we wanted to investigate inflammatory responses in global ischemia and anti-inflammatory effects modulated by EGb 761. It has been shown unequivocally that ischemia leads to inflammatory responses, causing migration of leukocytes to the brain and thus activating microglia and astrocytes (Harukuni and Bhardwaj, 2006). Microglial cells are present within the normal brain in resting state, but an activated microglial response can contribute to neuronal death through excitotoxic mechanisms (Morioka et al., 1991, Lin et al., 1998). We have observed higher activation of CD11b (microglial marker) and glial fibrillary acidic protein (astrocyte marker) in the vehicle group as compared to the sham group; however, pretreatment of EGb 761 prevented the inflammatory response, as evidenced by fewer activated CD11b and GFAP cells in the hippocampus. In the hippocampus, GFAP expression increases post-ischemia and peaks at day 7 (Yasuda et al., 2011). Astrocytic and microglial activation lead to release of cytokines and negative growth regulators, which may suppress normal cell growth (Kuboyama et al., 2011). These results suggest that EGb 761 not only has antioxidant properties but also has anti-inflammatory properties.
To conclude, we investigated the neuroprotective effect of EGb 761 and its components in transient global ischemia and reperfusion model, and it was observed that EGb 761 prevents hippocampal neuronal death as a whole, as opposed to its individual components. We also showed higher expression levels of HO1, Nrf-2, and VEGF, and fewer activated microglia and astrocytes in the EGb 761 pretreatment group. Together, we believe that all the proteins investigated in this study are crucial in ischemic cascade and provide novel targets for drug development and discovery. Lastly, EGb 761 has evolved as a multifunctional extract that has provided a protective role in almost all models of cerebral ischemia through multiple pathways.
The solubility of CO2, CH4 and C2H6 in [emim][EtSO4] is measured with a magnetic suspension balance
New data and literature results have been modeled with a group contribution equation of state
A specific group definition is required to model data of ionic liquids with a [MeSO4] anion
Deviations between model and experiments are lower than 10 % in most cases
Deviations of 34% are observed in the case of the solubility of ethane in the ionic liquid
Acknowledgments
This work was supported by a grant from the National Institutes of Health-National Center for Complimentary and Alternative Medicine (R00AT004197) to ZAS. The authors would like to thank Charisse N. Montgomery for her assistance in the manuscript editing and M.R. Barazi for his help with blinded analysis.
Abbreviations
- BB
bilobalide
- EGb 761
Ginkgo biloba/EGb 761®
- GA
ginkgolide A
- GB
ginkgolide B
- HO1
heme oxygenase 1
- BCCAO
bilateral common carotid artery occlusion
- TFM
terpene free material
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bastianetto S, Ramassamy C, Dore S, Christen Y, Poirier J, Quirion R. The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. Eur J Neurosci. 2000;12:1882–1890. doi: 10.1046/j.1460-9568.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- Chan PC, Xia Q, Fu PP. Ginkgo biloba leave extract: biological, medicinal, and toxicological effects. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25:211–244. doi: 10.1080/10590500701569414. [DOI] [PubMed] [Google Scholar]
- Chan SA, Reid KH, Schurr A, Miller JJ, Iyer V, Tseng MT. Fosphenytoin reduces hippocampal neuronal damage in rat following transient global ischemia. Acta Neurochir (Wien) 1998;140:175–180. doi: 10.1007/s007010050080. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran K, Mehrabian Z, Spinnewyn B, Chinopoulos C, Drieu K, Fiskum G. Neuroprotective effects of bilobalide, a component of Ginkgo biloba extract (EGb 761) in global brain ischemia and in excitotoxicity-induced neuronal death. Pharmacopsychiatry 36 Suppl. 2003;1:S89–94. doi: 10.1055/s-2003-40447. [DOI] [PubMed] [Google Scholar]
- Chiarugi A. Poly(ADP-ribosyl)ation and stroke. Pharmacol Res. 2005;52:15–24. doi: 10.1016/j.phrs.2005.02.018. [DOI] [PubMed] [Google Scholar]
- d'Avila JC, Lam TI, Bingham D, Shi J, Won SJ, Kauppinen TM, Massa S, Liu J, Swanson RA. Microglial activation induced by brain trauma is suppressed by post-injury treatment with a PARP inhibitor. J Neuroinflammation. 2012;9:31. doi: 10.1186/1742-2094-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Yang GY. Therapeutic angiogenesis for brain ischemia: a brief review. J Neuroimmune Pharmacol. 2007;2:284–289. doi: 10.1007/s11481-007-9073-3. [DOI] [PubMed] [Google Scholar]
- Gage FH, Buzsaki G, Armstrong DM. NGF-dependent sprouting and regeneration in the hippocampus. Prog Brain Res. 1990;83:357–370. doi: 10.1016/s0079-6123(08)61262-5. [DOI] [PubMed] [Google Scholar]
- Geddes JW, Pettigrew LC, Holtz ML, Craddock SD, Maines MD. Permanent focal and transient global cerebral ischemia increase glial and neuronal expression of heme oxygenase-1, but not heme oxygenase-2, protein in rat brain. Neurosci Lett. 1996;210:205–208. doi: 10.1016/0304-3940(96)12703-8. [DOI] [PubMed] [Google Scholar]
- Gilman S. Pharmacologic management of ischemic stroke: relevance to stem cell therapy. Exp Neurol. 2006;199:28–36. doi: 10.1016/j.expneurol.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Hai J, Li ST, Lin Q, Pan QG, Gao F, Ding MX. Vascular endothelial growth factor expression and angiogenesis induced by chronic cerebral hypoperfusion in rat brain. Neurosurgery. 2003;53:963–970. doi: 10.1227/01.neu.0000083594.10117.7a. discussion 970-962. [DOI] [PubMed] [Google Scholar]
- Harukuni I, Bhardwaj A. Mechanisms of brain injury after global cerebral ischemia. Neurol Clin. 2006;24:1–21. doi: 10.1016/j.ncl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Herrschaft H, Nacu A, Likhachev S, Sholomov I, Hoerr R, Schlaefke S. Ginkgo biloba extract EGb 761(R) in dementia with neuropsychiatric features: a randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J Psychiatr Res. 2012;46:716–723. doi: 10.1016/j.jpsychires.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Kaundal RK, Iyer S, Kumar A, Sharma SS. Protective effects of pioglitazone against global cerebral ischemic-reperfusion injury in gerbils. J Pharmacol Sci. 2009;109:361–367. doi: 10.1254/jphs.08246fp. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, Yanagihara T. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 1998;18:570–579. doi: 10.1097/00004647-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Kreisman NR, Soliman S, Gozal D. Regional differences in hypoxic depolarization and swelling in hippocampal slices. J Neurophysiol. 2000;83:1031–1038. doi: 10.1152/jn.2000.83.2.1031. [DOI] [PubMed] [Google Scholar]
- Kuboyama K, Harada H, Tozaki-Saitoh H, Tsuda M, Ushijima K, Inoue K. Astrocytic P2Y(1) receptor is involved in the regulation of cytokine/chemokine transcription and cerebral damage in a rat model of cerebral ischemia. J Cereb Blood Flow Metab. 2011;31:1930–1941. doi: 10.1038/jcbfm.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Ginsberg MD, Busto R, Dietrich WD. Sequential analysis of subacute and chronic neuronal, astrocytic and microglial alterations after transient global ischemia in rats. Acta Neuropathol. 1998;95:511–523. doi: 10.1007/s004010050832. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Kitamura Y, Okazaki M, Sakata M, Tsukahara T, Taniguchi T. Induction of heme oxygenase-1 and major histocompatibility complex antigens in transient forebrain ischemia. J Cereb Blood Flow Metab. 1998;18:824–832. doi: 10.1097/00004647-199808000-00002. [DOI] [PubMed] [Google Scholar]
- Merchant RM, Yang L, Becker LB, Berg RA, Nadkarni V, Nichol G, Carr BG, Mitra N, Bradley SM, Abella BS, Groeneveld PW. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39:2401–2406. doi: 10.1097/CCM.0b013e3182257459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka T, Kalehua AN, Streit WJ. The microglial reaction in the rat dorsal hippocampus following transient forebrain ischemia. J Cereb Blood Flow Metab. 1991;11:966–973. doi: 10.1038/jcbfm.1991.162. [DOI] [PubMed] [Google Scholar]
- Morita K, Lee MS, Her S. Possible relation of hemin-induced HO-1 expression to the upregulation of VEGF and BDNF mRNA levels in rat C6 glioma cells. J Mol Neurosci. 2009;38:31–40. doi: 10.1007/s12031-008-9156-5. [DOI] [PubMed] [Google Scholar]
- Nada SE, Shah ZA. Preconditioning with Ginkgo biloba (EGb 761(R)) provides neuroprotection through HO1 and CRMP2. Neurobiol Dis. 2012b;46:180–189. doi: 10.1016/j.nbd.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson T, Wieloch T, Smith ML. Brain damage in a mouse model of global cerebral ischemia. Effect of NMDA receptor blockade. Brain Res. 2003;982:260–269. doi: 10.1016/s0006-8993(03)03014-2. [DOI] [PubMed] [Google Scholar]
- Ordy JM, Wengenack TM, Bialobok P, Coleman PD, Rodier P, Baggs RB, Dunlap WP, Kates B. Selective vulnerability and early progression of hippocampal CA1 pyramidal cell degeneration and GFAP-positive astrocyte reactivity in the rat four-vessel occlusion model of transient global ischemia. Exp Neurol. 1993;119:128–139. doi: 10.1006/exnr.1993.1014. [DOI] [PubMed] [Google Scholar]
- Pichiule P, Agani F, Chavez JC, Xu K, LaManna JC. HIF-1 alpha and VEGF expression after transient global cerebral ischemia. Adv Exp Med Biol. 2003;530:611–617. doi: 10.1007/978-1-4615-0075-9_60. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Qureshi N. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1996;334:1406. [PubMed] [Google Scholar]
- Saleem S, Zhuang H, Biswal S, Christen Y, Dore S. Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury. Stroke. 2008;39:3389–3396. doi: 10.1161/STROKEAHA.108.523480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Okamoto SI, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers. Proc Natl Acad Sci U S A. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZA, Li RC, Ahmad AS, Kensler TW, Yamamoto M, Biswal S, Dore S. The flavanol (-)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J Cereb Blood Flow Metab. 2010;30:1951–1961. doi: 10.1038/jcbfm.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZA, Nada SE, Dore S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience. 2011;180:248–255. doi: 10.1016/j.neuroscience.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Auer RN, Siesjo BK. The density and distribution of ischemic brain injury in the rat following 2-10 min of forebrain ischemia. Acta Neuropathol. 1984;64:319–332. doi: 10.1007/BF00690397. [DOI] [PubMed] [Google Scholar]
- van Beek TA. Chemical analysis of Ginkgo biloba leaves and extracts. J Chromatogr A. 2002;967:21–55. doi: 10.1016/s0021-9673(02)00172-3. [DOI] [PubMed] [Google Scholar]
- Wang J, Dore S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130:1643–1652. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda Y, Shimoda T, Uno K, Tateishi N, Furuya S, Tsuchihashi Y, Kawai Y, Naruse S, Fujita S. Temporal and sequential changes of glial cells and cytokine expression during neuronal degeneration after transient global ischemia in rats. J Neuroinflammation. 2011;8:70. doi: 10.1186/1742-2094-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen G, Dore S. Optimized protocol to reduce variable outcomes for the bilateral common carotid artery occlusion model in mice. J Neurosci Methods. 2007;166:73–80. doi: 10.1016/j.jneumeth.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]