Abstract
Interferon regulatory factor-3 (IRF-3) is a master transcription factor that drives the host intracellular innate immune response to virus infection. The importance of IRF-3 in innate immune responses is highlighted by the fact that pathogenic viruses have developed strategies for antagonism of IRF-3. Several tools exist for evaluation of viral regulation of IRF-3 activation and function, but high-quality monoclonal antibodies that mark the differential activation states of human IRF-3 are lacking. To study IRF-3 activation, turnover, and depletion in a high-throughput manner in the context of virus infection, we have developed two new monoclonal antibodies to human IRF-3. These antibodies detect IRF-3 in virus-infected cells in a wide variety of assays and provide a new tool to study virus-host interactions and innate immune signaling.
Keywords: Innate immunity, IRF-3, antibody development, flow cytometry
1. Introduction
IRF-3 is a transcription factor critical to the intracellular immune response to microbial infection [1]. Microbial infection of human cells is sensed by pattern recognition receptors (PRRs) that recognize microbial pathogen-associated molecular patterns (PAMPs). The expression of PRRs varies among cell types, but a full complement of these receptors includes those that sense microbial DNA, RNA, or cell wall components (reviewed in [2]). Microbial DNA is sensed in endosomes (by certain Toll-like receptors, or TLRs) or in the cytoplasm, such as through the Interferon-stimulating DNA (ISD) pathway [3]. While the molecules directly involved in sensing of ISD in human cells have not been fully defined, the cytosolic proteins IFI16 [4] and AIM2 [5] have been implicated, and transcription of DNA into RNA in the cytoplasm by RNA polymerase III can trigger RNA sensors [6, 7]. Pathogen-associated RNA in turn is sensed in endosomes (by certain TLRs) or the cytoplasm (by RIG-I-like receptors, or RLRs).
Downstream of PRRs, signaling cascades lead to the transcription of genes that mediate an antimicrobial state. In response to infection by RNA viruses, TLR3 and the RLRs have been shown to recognize double-stranded RNA, while TLR7 recognizes single-stranded RNA. TLR3 and the RLRs activate an antiviral state within the cell by initiating signaling cascades that require the adaptor proteins TRIF and MAVS, respectively, leading to the activation of IRF-3. It should be noted that direct sensing of ISD, as can occur during DNA virus or bacterial infection, also results in IRF-3 activation [3, 8]. When activated, IRF-3 directly drives the transcription of several genes with key antiviral functions. These genes include antiviral effectors such as viperin, ISG54, and the IFITM family of proteins, as well as pro-inflammatory cytokines like interferon-β (IFN-β) and CCL-5/RANTES [9]. Signaling by IFN-β, both in an autocrine and paracrine manner, leads to the expression of hundreds of interferon-stimulated genes (ISGs) [10]. ISG products inhibit viral replication through a diverse set of mechanisms, but most ISGs have yet to be characterized. In order to support replication, viruses have developed mechanisms to block the induction of the innate antiviral response, the function of specific ISGs, or both. Viral strategies that block IRF-3-dependent gene induction modify the normal expression, activation state, or function of IRF-3, and are linked to pathogenic outcome of infection.
IRF-3 is constitutively expressed in virtually all cells [11]. The IRF-3 mRNA encodes a ~55 kDa protein that contains a DNA-binding domain, nuclear export signal, IRF-interacting domain, and a C-terminal serine-rich region [12, 13]. This serine-rich region contains several phosphorylation sites. While some of these serine residues are phosphorylated in the resting state, activation of IRF-3 is characterized by additional phosphorylation, specifically at residues S385, S386, T390, and S396 [14]. Activation-specific phosphorylation depends on the actions of the protein kinases TBK1 and IKKε [15]. After phosphorylation by either protein kinase, IRF-3 dimerizes and translocates to the nucleus, driving IRF-3-dependent gene transcription. Phosphorylation at S385 or S386 is required for IRF-3 dimerization, while phosphorylation at S396 is required for IRF-3 association with CREB/p300, a cofactor necessary for IRF-3 transcriptional activity [16, 17]. Ubiquitination of IRF-3, which requires Pin1 and RBCK1, leads to proteasome-dependent degradation of IRF-3 thus ending IRF-3-dependent signaling [18, 19]. The IRF-3 activation cycle is depicted in Figure 1A.
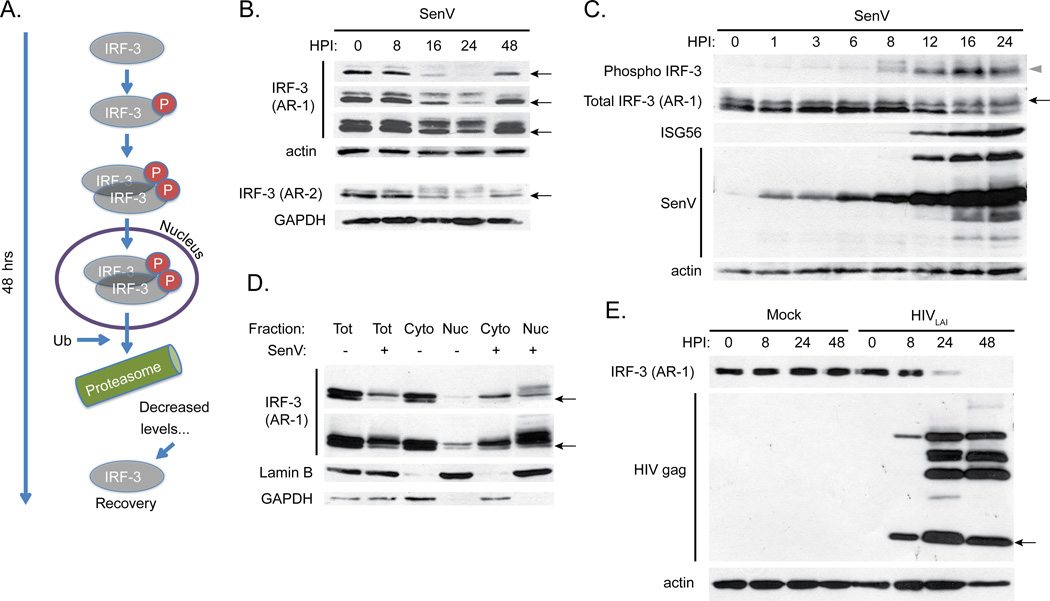
Figure 1.
A. Schematic of IRF-3 phosphorylation, dimerization, nuclear translocation, ubiquitination (designated by “Ub”) and degradation that occurs during normal activation over a 48-hour time course.
B. SDS-PAGE followed by immunoblot analysis of IRF-3 in SupT1 cells infected with SenV over the indicated time course. Three film exposures are shown for the AR-1 mAb (top), ordered from shortest (first) to longest time of exposure (third). Protein samples were re-run on a separate gel for probing with the AR-2 mAb (bottom). Actin and GAPDH are loading controls. For IRF-3, the indicated bands mark resting IRF-3 (black arrows).
C. SDS-PAGE followed by immunoblot analysis of phospho-S396 IRF-3, total IRF-3, ISG56, and SenV proteins in infected 293T cells at the indicated timepoints. For IRF-3, an upper band (black arrow) corresponds to phosphorylated IRF-3 (grey triangle).
D. Nuclear/cytoplasmic fractionation followed by SDS-PAGE and immunoblot analysis of IRF-3 in 293T cells infected with SenV for 18 hrs. Short (top panel) and long (bottom panel) film exposures are shown. Lamin B (nuclear) and GAPDH (cytoplasmic) markers are shown.
E. SDS-PAGE followed by immunoblot analysis of IRF-3 and HIV gag in SupT1 cells infected with HIVLAI (MOI 1) for the indicated timepoints. Black arrow indicates the mature form of HIV gag protein (p24).
Given the central role that IRF-3 plays in the stimulation and promotion of the antiviral state, a variety of tools have been developed to examine aspects of the normal IRF-3 activation cycle. Phosphorylation of IRF-3 can be detected by phospho-specific antibodies, while dimerization can be observed directly using native gel electrophoresis [20]. Upon IRF-3 activation, nuclear translocation can be reliably detected using nuclear/cytoplasmic fractionation analysis [21] or by immunostaining of cells and microscopic imaging [22]. Degradation of IRF-3 can be assessed by measuring ubiquitination of IRF-3 [19] and by assessing IRF-3 levels during viral infection in cells treated with proteasome or lysosome inhibitors [23]. As noted above, when activated and present in the nucleus, IRF-3 drives gene transcription, and this transcriptional activity can be observed in cells through the use of luciferase (luc)-reporter constructs containing IRF-3-target gene promoters. Promoters used in luc-reporter constructs include the ISG56/IFIT1 promoter, the full IFN-β promoter, the interferon-stimulated response element (ISRE), or the PRDIII sequence (the IRF-3 binding site encoded within the IFN-β promoter) [23, 24]. IRF-3 dependent gene transcription can also be monitored directly by measuring the transcriptional activity of known IRF-3 responsive genes [9] or by direct detection of protein abundance changes through immunoblot assays.
The above methods used to analyze IRF-3 function have all been used to evaluate viral antagonism of IRF-3 in a variety of virus infection systems, both in vivo and in vitro. Similar to other viruses, human immunodeficiency virus-1 (HIV-1) replication is inhibited by signaling-competent IRF-3 in T cells and macrophages [25]. To counter these antiviral actions of IRF-3, HIV-1 directs the virus-induced degradation of IRF-3 [25, 26], which occurs in a viral protease-independent process but independently of IRF-3 activation. Interestingly, this degradation of IRF-3 suppresses the innate immune effector function of the infected cell, thus supporting viral replication and spread while enhancing permissiveness to secondary viral challenge and superinfection. HIV-1 degradation of IRF-3 and suppression of innate immune defenses therefore supports HIV persistence and may contribute to the development of AIDS [25, 26]. Other examples of direct inhibition of IRF-3 have been observed: rotavirus directs the proteasome-dependent degradation of IRF-3 through the action of rotavirus nonstructural protein 1, resulting in decreased IRF-3 dimer formation, decreased nuclear translocation, and decreased IRF-3-dependent gene transcription [27], while the bovine viral diarrhea virus protein Npro targets IRF-3 for ubiquitination [28]. A variety of viruses promote the indirect inhibition of IRF-3 by affecting other molecules involved in the upstream signaling cascades required for activation. In hepatitis C virus-infected cells, IRF-3 activation is indirectly inhibited [24], due to cleavage of the upstream RLR adaptor protein MAVS by the viral protease NS3/4A [29–31]. Additionally, Borna disease virus phosphoprotein P competes with IRF-3 for TBK1 binding, thus preventing IRF-3 phosphorylation [32], while Kaposi’s sarcoma herpes virus encodes a protein that sequesters IRF-3 binding partners thus preventing transcriptional complex formation [33].
Tools used to evaluate IRF-3 function can be applied to examine both direct and indirect viral antagonism of innate immunity. To evaluate the downstream effects of IRF-3 antagonism on IRF-3-dependent gene induction in a high-throughput manner, plasmid constructs encoding viral proteins suspected of being IRF-3 antagonists can be expressed in transfected cells along with luc reporter constructs employing IRF-3-target gene promoters. Cells can then be treated with an IRF-3 activator, such as the RNA mimetic poly:I:C or the mouse paramyxovirus Sendai virus, allowing evaluation of the relative strength of IRF-3 activation via promoter-luciferase signal in the presence and absence of the suspected IRF-3 antagonist [24]. As a result of this and other approaches, several viral antagonists of IRF-3, including the aforementioned viral regulators, have been revealed [34].
Recent advances in specialized applications of microscopy (Cellomics, [35]) and flow cytometry (Amnis ImageStream, [36]) allow for the quantification of nuclear translocation of transcription factors (and therefore activation) in populations of cells. However, a conventional flow cytometry assay that allows for analysis of IRF-3 activation and degradation has not been described. Such an assay would provide a method for study of heterogeneous infected cell populations like human peripheral blood cells or mucosal lymphocytes using widely available instrumentation. For example, the relative degree of IRF-3 depletion in HIV-infected patient cells is unknown, and may correlate with viral pathogenesis. Additionally, small molecules that activate IRF-3 may enhance antiviral immunity in patients, and such molecules could be screened on human immune cells in a high-throughput manner. Several antibodies specific to human IRF-3 are commercially available, and we tested many of these prior to initiating the study. Problematically, we found that a limited subset of commercial anti-IRF-3 antibodies is actually suitable for flow cytometric analysis of total intracellular IRF-3 levels in the multiple cell types examined. Of these commercially-available antibodies, none have been reported to detect activated IRF-3 by flow cytometry. Furthermore, for high-throughput analyses of IRF-3 depletion or activation, acquisition of large quantities of commercial antibodies is prohibitively expensive. To these ends, we have developed two monoclonal antibodies against human IRF-3 that now facilitate a novel flow cytometric assay of IRF-3 expression and activation status, and will provide new tools for conventional analyses of IRF-3 in virus-infected cells.
2. Material and methods
2.1. Cells, transfections, and fractionation
SupT1 cells, THP-1 cells, CEM-ss cells, and PBMCs were cultured in cRPMI: RPMI 1640 media supplemented with 10% heat-inactivated FBS, L-glutamine, sodium pyruvate, and antibiotics, as described previously [25]. TZM-bl [37] and Vero cells were cultured in cDMEM: DMEM supplemented with 10% heat-inactivated FBS, L-glutamine, HEPES, sodium pyruvate, MEM non-essential amino acids, and antibiotics. GHOST [38] R3/X4/R5 cells were cultured in cDMEM supplemented with 500 µg/mL G418, 100 µg/mL hygromycin, and 1 µg/mL puromycin. Transfection of cells was performed using the calcium phosphate method or using Fugene6 transfection reagent (Roche) according to the manufacturer’s suggested protocol. Plasmids pBL-IRF-3 and pBL-IRF-3ΔN have been described previously [23]. SupT1 cells stably expressing lentiviral vectors (Sigma) containing shRNA to IRF-3 or nontargeting vector were generated according to the manufacturer’s suggested protocol. Nuclear and cytoplasmic fractionation was performed using standard methods previously described [21].
2.2. Viral stocks and infections
HIV-1LAI was propagated using CEM-ss cells grown in cRPMI and standard procedures as described previously. Mock infections represent addition of CEM-ss cell conditioned media. HIV-1 was titered on TZM-bl and GHOST cells to determine concentration of infectious virus. Sendai virus (SenV) strain Cantell was obtained from Charles River Laboratory.
2.3. Monoclonal antibody production and screening
Mice were immunized with His-tag purified IRF-3 and splenocytes were fused with the FOX-NY myeloma cell line and cultured under hybridoma selection using cRPMI plus AAT (adenine/aminopterin/thymidine, Sigma). Hybridoma supernatants were screened against input protein by ELISA, then by overexpressed and endogenous human IRF-3 by SDS-PAGE and immunoblot, and strong candidates were selected for confirmatory testing by immunofluorescence on TZM-bl cells infected with 200 hemagglutination units (HAU)/mL SenV. For antibody purification, hybridoma cells were weaned off of drug selection into serum-free media (Gibco), inoculated into a CELLine bioreactor (BD Biosciences) and antibody was purified from the supernatant by thiophilic adsorption (Pierce). Purified antibody was subtyped using an IsoStrip mAb typing kit (Roche).
2.4. Immunoblot analysis and fluorescent microscopy
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis were performed using standard procedures as described previously [22]. Native PAGE was performed using buffers and procedures adapted from [39] to use pre-cast TGX gels (Bio-Rad). Native gels were run for 30 min at 30 mA. The following antibodies were used in the study: Rabbit (Rb) α ISG56 (a gift from G. Sen), Mouse (m) α HIV-1 p24, Goat (Gt) α B-actin (Santa Cruz), Rb total α IRF-3 (a gift from M. David, [40]), Rb α IRF-3-p (Cell Signaling), Rb α Lamin B (Abcam), Rb α GAPDH (Santa Cruz), Rb α SenV (a gift from I. Julkunen). For immunoblot detection the appropriate HRP-conjugated secondary antibody was used (Jackson ImmunoResearch) followed by treatment of the membrane with ECL Plus reagent (GE Amersham) and imaging on X-ray film. For immunofluorescence imaging, TZM-bl cells were seeded in 8-well chamber slides prior to infection. Prior to staining, cells were washed with PBS, fixed in 3% formaldehyde, incubated in 0.1% Triton X-100, and blocked with 10% fetal bovine serum. The slides were then sequentially stained with the appropriate dilutions of primary antibodies, followed by incubation with AlexaFluor488-conjugated secondary antibody (Jackson ImmunoResearch) along with DAPI before mounting with ProLong Gold (Invitrogen). Samples were imaged on a Nikon TE2000-E microscope and images were processed with NIS Elements software.
2.5. Flow cytometry
For Amnis Imagestream analysis, SupT1 cells were fixed and stained using procedures adapted from Amnis. Cells were fixed in 3% formaldehyde and stained with primary antibody in 0.1% Triton X-100/1% BSA followed by secondary staining with DyLight 488-conjugated secondary antibody (Jackson ImmunoResearch). For quantitation of colocalization, ~2,500 cells per condition were analyzed. For conventional flow cytometry, antibody was directly conjugated to fluorophore using a Molecular Probes protein labeling kit (Invitrogen).
3. Results and discussion
We identified several hybridoma clones producing anti-IRF-3 antibodies that recognized both recombinant IRF-3 and endogenous IRF-3 within SupT1 and 293T cells. Monoclonal antibody (mAb) to IRF-3 was purified from a clone that exhibited high reactivity to IRF-3 and was designated AR-1. SenV is a potent viral agonist of IRF-3 that induces RIG-I signaling in human cells, and we used SenV infection as an activator of IRF-3. SDS-PAGE and immunoblot analysis using the AR-1 mAb demonstrates IRF-3 activation, turnover, and recovery over a 48-hour timecourse in SupT1 cells infected with 200 HAU/mL SenV (Figure 1B, top). The AR-1 mAb detects resting IRF-3 (indicated by the arrows; Fig 1B), which migrates more quickly compared to active IRF-3 on an SDS-PAGE gel. This IRF-3 isoform and the other IRF-3 bands visible at 0 hrs of infection have been phosphorylated heterogeneously [14] at non-activation-specific serine residues. The kinetics of IRF-3 activation during SenV infection reveal the appearance of more slowly-migrating AR-1 mAb-reactive bands, or “laddering,” which indicates phosphorylation of IRF-3 at various activation-specific residues. Such phospho-IRF-3 species are most apparent at 16 and 24 hrs post SenV infection. As a result of being modified by phosphorylation in response to SenV infection, the amount of the resting IRF-3 isoform (arrows; Fig 1B) decreases through 24 hrs and then recovers by 48 hrs post infection. The depletion and recovery of IRF-3 are mediated by degradation of activated IRF-3 and de novo synthesis of the pool of resting IRF-3, respectively. Though IRF-3 activation can be driven by non-viral stimuli, all upstream events that signal to IRF-3 impart IRF-3 phosphorylation at activation-specific residues, leading to activation of its transcriptional activity. Indeed, we found that the AR-1 mAb could detect activated IRF-3 by SDS-PAGE and immunoblot analysis of extracts from THP-1 cells (a human monocyte cell line that displays a macrophage-like phenotype upon differentiation with phorbol esters) that were stimulated by treatment with ISD, polyI:C, or HCV PAMP RNA [41] (data not shown).
To determine whether the appearance of IRF-3 laddering represents IRF-3 activation-induced phosphorylation detected by the AR-1 mAb, we conducted anti-IRF-3 phospho-S-396-specific antibody immunoblot assay of IRF-3. For this analysis we assessed 293T cells infected with SenV and compared the resulting pattern of IRF-3 abundance with that detected by the AR-1 mAb when used to probe the same blot. Our results show that the AR-1 mAb detects both non-activated and activated/phosphorylated IRF-3 isoforms (Figure 1C). The strongest-appearing S-396-phospho-IRF-3 bands (denoted by the grey triangle in Figure 1C) correspond to the slowest mobility bands visible on the AR-1 blot and are indicative of active IRF-3 (black arrow, Figure 1C). ISG56 is an IRF-3-dependent gene product, and is expressed after IRF-3 activation or IFN treatment of cells (Figure 1C). Immunoblot assay of nuclear/cytoplasmic fractions of 293T cells infected with SenV for 18 hrs demonstrates translocation of activated IRF-3 to the nucleus as detected by the AR-1 mAb (Figure 1D), as well as the presence of the resting IRF-3 isoform (black arrow) in the cytoplasm. Moreover, AR-1 mAb immunoblot analysis revealed the presence of several high mass/putative phosphorylated IRF-3 species in the nuclear fraction of infected cells. Thus, the AR-1 mAb provides a sensitive and specific immunoreagent for assessing IRF-3 abundance and activation and can detect both resting and active isoforms of IRF-3 by immunoblot assay of denatured protein.
We previously reported that HIV-1 directs a robust blockade of IRF-3 function through the direct targeting and destruction of IRF-3 [25]. Infection of SupT1 T cells with HIVLAI and analysis by immunoblot with the AR-1 mAb recapitulates this phenotype. We probed infected cell lysate with the AR-1 mAb and demonstrated HIV-1-mediated degradation of IRF-3 by 24 and 48 hours post infection, which is concomitant with viral replication and viral protein accumulation (Figure 1E). Importantly, no laddering of IRF-3 was observed upon HIV-1 infection of cells (Figure 1E), consistent with previous reports that HIV-1-induced degradation of IRF-3 occurs independently of IRF-3 activation and is not mediated by IRF-3 activation-induced turnover [25, 26]. Thus, the AR-1 mAb can effectively measure HIV-1 suppression of IRF-3 in T cells.
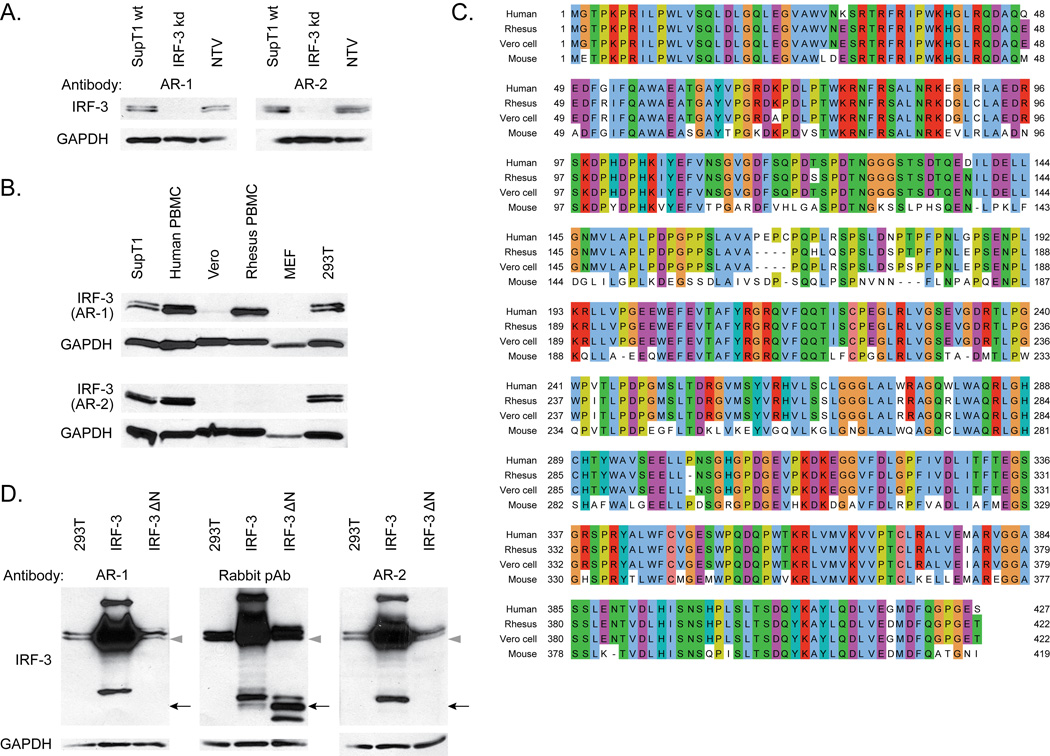
To further characterize the AR-1 mAb, we measured AR-1 immunoreactivity on extracts prepared from SupT1 cells stably expressing an shRNA that specifically knocks down IRF-3 expression (Figure 2A). We found that the AR-1 mAb could detect IRF-3 in control cells harboring nontargeting shRNA but had no reactivity to cell extracts prepared from cells with specific IRF-3 knockdown. Moreover, we examined the species-specific reactivity of the AR-1 mAb by conducting immunoblot analysis of various mouse, human, and non-human primate cell extracts. We found that the AR-1 mAb reacts to human and rhesus macaque but not mouse IRF-3 (Figure 2B). The AR-1 mAb also reacts to IRF-3 from Vero cells, an African green monkey-derived cell line (Figure 2B), although with lower signal strength compared to the human and rhesus macaque bands. Full-length protein sequence analysis using ClustalW [42] and Jalview [43] of human, macaque, Vero cell, and mouse IRF-3 (Figure 2C) showed that the N-terminal half of IRF-3 contains regions of dissimilarity between the human and mouse sequences but regions of high similarity between the human, macaque, and Vero cell sequences, suggesting that the N-terminus of IRF-3 might determine the antibody specificity between species. We therefore assessed AR-1 detection of human IRF-3 truncation mutants. We found that while the AR-1 mAb reacts efficiently to full-length IRF-3, it fails to detect recombinant IRF-3ΔN (Figure 2D) lacking amino acids 9–133. Amino acids 9–133 contain the DNA-binding domain of IRF-3 [23]; thus the AR-1 mAb recognizes an epitope within the DNA-binding domain of IRF-3. Since human, macaque, and Vero cell IRF-3 sequences of amino acids 9–133 are nearly identical, the reduced reactivity of the AR-1 mAb to Vero cells IRF-3 relative to the human and macaque IRF-3 (Figure 2B) is likely due to the relative lower abundance of IRF-3 in Vero cells compared to human and macaque cells, as has been previously reported [44].
Figure 2.
A. SDS-PAGE followed by immunoblot analysis of extracts of SupT1 cells, wildtype (wt) or stably expressing shRNA to IRF-3 (IRF-3 kd) or non-targeting vector (NTV).
B. SDS-PAGE followed by immunoblot analysis of IRF-3 in human (SupT1, human PBMC, and 293T), African green monkey (Vero), rhesus macaque, and mouse, using the indicated antibodies.
C. Sequence alignment using ClustalW of reference protein sequences for IRF-3 from human (NCBI Reference Sequence NP_001562.1), rhesus macaque (NP_001129269.1), Vero cell ([44]) and mouse (NP_058545.1), visualized in Jalview. Identical amino acids between at least two species are colored.
D. SDS-PAGE followed by immunoblot analysis of IRF-3 in 293Ts that were mock transfected or transiently transfected for 36 hrs with BL-CMV-IRF-3 or BL-CMV-IRF-3ΔN. For IRF-3, the band corresponding to resting full-length IRF-3 (grey triangle) and the three bands corresponding to IRF-3ΔN (black arrow) are indicated.
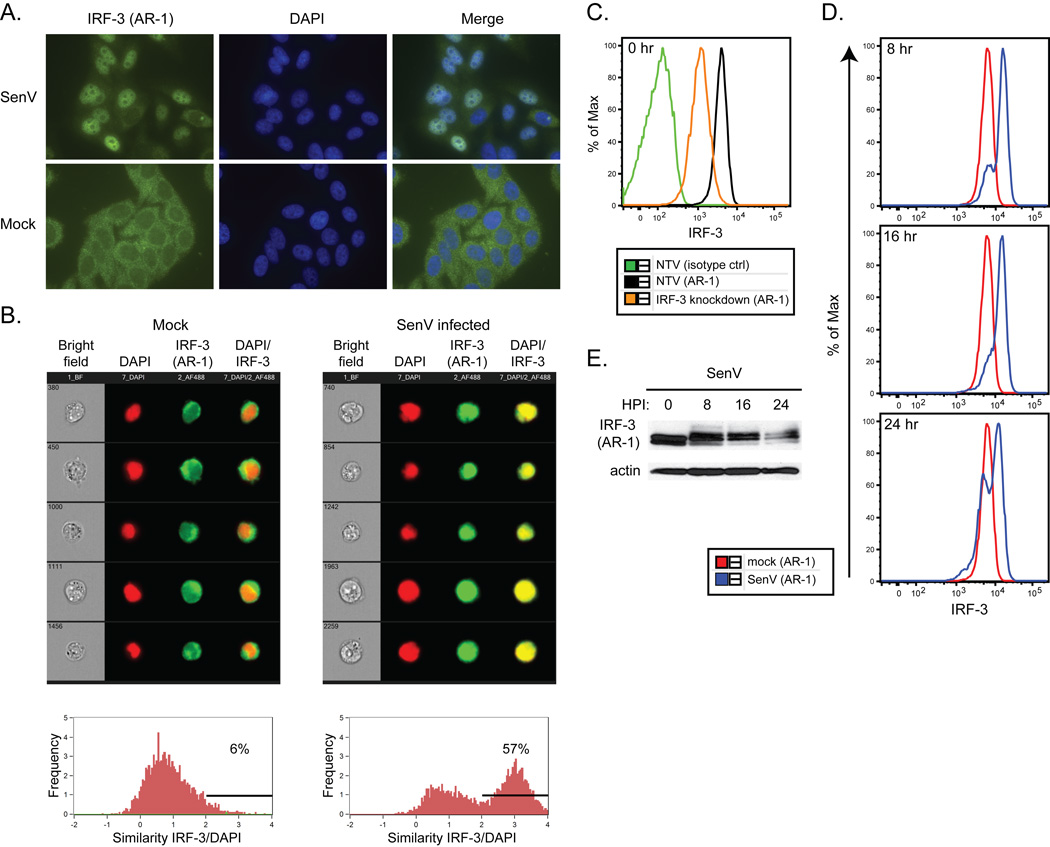
Our data indicate that the AR-1 mAb can detect denatured endogenous and recombinant human IRF-3, and is therefore useful as a reagent for immunoblot assay. To determine if AR-1 can detect IRF-3 protein in its native, three-dimensional conformation, we tested the ability of the AR-1 mAb to detect native, non-denatured IRF-3 in situ by immunofluorescence microscopy. When used as a primary antibody to stain uninfected TZM-bl cells (a HeLa-derived cell line), the AR-1 mAb detected the cytosolic/resting form of IRF-3 (Figure 3A, bottom). In cells that had been infected with 200 HAU/mL SenV for 18 hours, the AR-1 mAb detected the nuclear/active forms of IRF-3 (Figure 3A, top, and [45]). Importantly, however, the AR-1 mAb provided a much stronger signal of IRF-3 staining above background in infected cells in which IRF-3 had translocated to the nucleus, suggesting either that IRF-3 signal intensity increases when the antigen is concentrated in a smaller area of the cell, or that AR-1 could specifically detect native, active IRF-3 more efficiently than it detects the resting IRF-3 isoform.
Figure 3.
A. Immunofluorescence analysis of IRF-3 (AR-1 mAb, green) and nuclei (DAPI, blue) in TZM-bl cells infected with SenV for 18 hrs.
B. ImageStream analysis of IRF-3 (AR-1 mAb, green) and nuclei (DAPI, red) in SupT1 cells mock or SenV-infected for 18 hrs. Representative brightfield, red, green, and red/green merge images are shown (top). Analysis of IRF-3/DAPI colocalization on the whole population is quantified (bottom). The percentage of cells with similarity values above an arbitrary value of 2 is shown.
C. Flow cytometry analysis of SupT1 cells using isotype control antibody or AR-1 mAb (IRF-3).
D. Flow cytometry analysis of IRF-3 in SupT1 cells infected with SenV for the indicated timepoints. Mock refers to 0 hrs of infection.
E. SDS-PAGE followed by immunoblot analysis of IRF-3 in the same cells used in D.
To further characterize the ability of the AR-1 mAb to detect native IRF-3, we assessed IRF-3 localization in a highly sensitive flow cytometry assay developed in our laboratory. This assay allows high-throughput analysis of IRF-3 protein levels and activation state on a per-cell and quantitative basis. We permeabilized cells and used the Amnis Imagestream instrument for assessment of IRF-3 by AR-1 mAb immunostaining. We examined IRF-3 in uninfected SupT1 cells and in cells infected for 16 hrs with SenV. Colocalization of IRF-3 and the nuclear stain DAPI was apparent in SupT1 cells infected with SenV (Figure 3B, bottom). Analysis of infected cultures revealed the presence of cells harboring resting/cytoplasmic IRF-3 as well as those harboring nuclear/active IRF-3 responding to the SenV infection (Figure 3B, top). For further analysis using conventional flow cytometry, we directly conjugated the AR-1 mAb to AlexaFluor647 fluorophore to facilitate direct immunostaining of cells. An antibody concentration sensitive to IRF-3 knock-down in SupT1 cells was determined by titration analysis, and was applied to our assay (Figure 3C). Upon performing flow cytometry analysis on SenV-infected SupT1 cells, we found that virusinfected cells displayed an overall increase in IRF-3 fluorescence (Figure 3D), and this occurred concomitant with the appearance of activated IRF-3 isoforms as assessed in parallel by denaturing immunoblot assay of extracts from the same cells (Figure 3E). Moreover, the typical depletion and recovery of IRF-3 that occurs after virus infection (Figure 1B) was not detected by flow cytometry over a 24-hour SenV timecourse, suggesting that the AR-1 mAb is specific to the activated but not the resting IRF-3 isoform in the context of native, non-denaturing assay conditions.
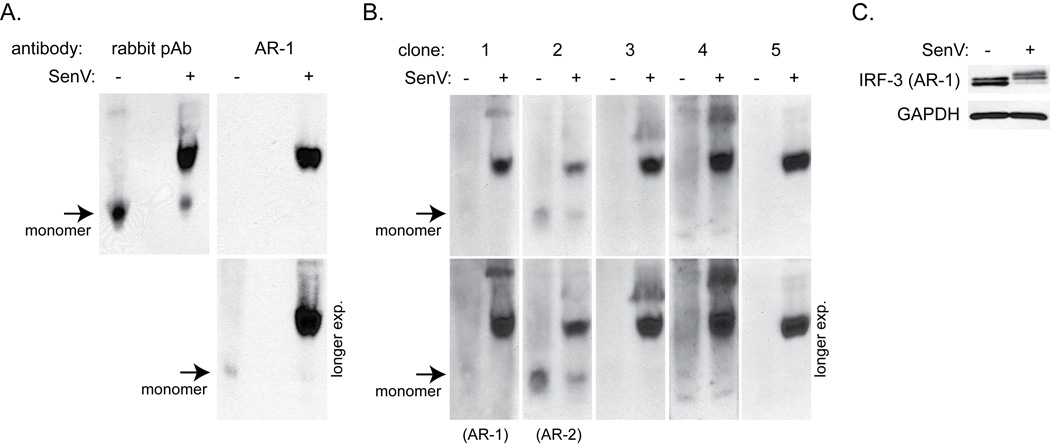
To confirm whether or not the AR-1 mAb preferentially detected active IRF-3 under native conditions, we compared the relative sensitivity of the AR-1 mAb for resting vs. active IRF-3 by native PAGE and immunoblot analysis. In the SenV-infected SupT1 cell lysate, the AR-1 mAb was highly specific to the active/dimeric IRF-3 isoform but did not react to the resting/monomeric isoform by native PAGE, though both isoforms were detected by SDS-PAGE and immunoblot analysis (Figure 4A and 4C). To facilitate a specific flow cytometry assay of total IRF-3 protein, including both active and resting IRF-3 isoforms, we sought to develop a mAb that could bind to native resting/monomeric IRF-3 as well reacting to IRF-3 in its active/dimeric isoform. We therefore tested supernatants from additional anti-IRF-3 mAb-producing hybridoma clones from our original screen in order to identify a novel mAb that could recognize IRF-3 isoforms under native conditions. This testing revealed an additional clone producing antibody that reacted well to the resting/monomeric IRF-3 isoform (clone 2, Figure 4B), as well as other clones (clones 3–5) that shared the AR-1 mAb specificity for dimeric IRF-3 in native conditions. Antibody from the newly-identified clone #2, designated AR-2, detected IRF-3 activation, turnover, and recovery within lysates from SenV-infected cells (see Figure 1B, bottom panel). The AR-2 mAb was specific for IRF-3, as it did not detect IRF-3 in lysate from cells with a stable knock-down of IRF-3 mRNA expression (Figure 2A). Similar to the AR-1 mAb, the AR-2 mAb detected full-length IRF-3 but not IRF-3 ΔN protein (Figure 2D). However, the AR-2 mAb was found to be human-specific and did not recognize non-human primate IRF-3 (Figure 2B). Thus, the AR-2 mAb offers a novel reagent for immunodetection of both resting and active IRF-3 isoforms, while the AR-1 mAb provides a novel mAb reagent for the specific detection of native, active IRF-3 and is amenable to high throughput flow cytometric assessment of IRF-3 activation state.
Figure 4.
A. Native PAGE followed by immunoblot analysis for IRF-3 in SupT1 cells infected with SenV for 18 hrs. A rabbit polyclonal antibody or AR-1 mAb was used to detect IRF-3. Arrows indicate monomeric IRF-3. Short (top) and long (bottom) film exposures are shown.
B. Native PAGE followed by immunoblot analysis for IRF-3 using supernatants from five hybridoma clones. Clone number 1 represents AR-1 mAb, while clone number 2 represents AR-2 mAb. Short and long film exposures of each immunoblot assay are shown as in 4A.
C. SDS-PAGE followed by immunoblot analysis for IRF-3 (AR-1) for loading control.
4. Conclusions
We have described two novel mouse monoclonal antibodies to human IRF-3, designated AR-1 and AR-2, and we have developed a novel flow cytometric assay using the AR-1 mAb for high throughput assessment of IRF-3 activation state. In analyses of native IRF-3 protein, the AR-1 mAb was found to specifically recognize active/dimeric IRF-3, while the AR-2 mAb can detect both resting/monomeric IRF-3 and active/dimeric IRF-3. The AR-1 mAb detects human and nonhuman primate IRF-3, while AR-2 mAb detection of IRF-3 is restricted to human. The AR-1 mAb is therefore a useful reagent for the detection of activated human IRF-3 by intracellular staining and flow cytometry. A summary of the characteristic properties of the two new reagents is depicted in Table 1. The AR-2 mAb may prove useful for high-throughput assessment of resting and active IRF-3 levels in a variety of cells types. These mAbs applied to our flow cytometry assay and other conventional assessments of IRF-3 status can provide insights into virus activation and regulation of human and macaque IRF-3 protein during infection. In clinical and research specimens, these reagents will allow for rapid screening of IRF-3 agonists for antiviral and immunomodulatory drug development.
Table 1.
Summary of new antibody properties. TBD, to be determined.
| Antibody | Detects total IRF-3 after SDS- PAGE |
Detects monomeric IRF- 3 after native PAGE |
Cross reacts with non-human primate |
Detects epitope in DNA-binding domain |
Detects IRF-3 activation by flow cytometry |
|---|---|---|---|---|---|
| AR-1 | Yes | Very weakly | Yes | Yes | Yes |
| AR-2 | Yes | Yes | No | Yes | TBD |
Acknowledgments
We thank the Antibody Development Facility and John P. McNevin at the Fred Hutchinson Cancer Research Center for technical assistance, and Dr. Stacy M. Horner for critical reading of the manuscript. The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: TZM-bl from Dr. John C. Kappes, Dr. Xiaoyun Wu and Tranzyme, Inc; GHOST R3/X4/R5 from Dr. Vineet N. KewalRamani and Dr. Dan R. Littman; mAb to HIV-1 p24 (AG3.0) from Jonathan Allan; CEM-ss cells from Peter L. Nara. This work was supported by grants from the UW Medical Scientist Training Program (5T32GM007266-34) and the UW STD/AIDS Training Grant Program (T32 AI07140-32) to A.R. and by NIH grants R01DA024563, R01AI060389, and U19AI08319 to M.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Arjun Rustagi, Email: arustagi@uw.edu.
Brian P. Doehle, Email: doehle@uw.edu.
M. Juliana McElrath, Email: jmcelrat@fhcrc.org.
Michael Gale, Jr., Email: mgale@uw.edu.
References
- 1.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi O, Akira S. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Stetson DB, Medzhitov R. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald KA, Paludan SR, Bowie AG. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, Hornung V. Nat Immunol. 2009;10:1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu YH, Macmillan JB, Chen ZJ. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen J, VanScoy S, Cheng TF, Gomez D, Reich N. Genes and immunity. 2008;9:168–243. doi: 10.1038/sj.gene.6364449. [DOI] [PubMed] [Google Scholar]
- 10.Der SD, Zhou A, Williams BR, Silverman RH. Proc Natl Acad Sci U S A. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Au WC, Moore PA, Lowther W, Juang YT, Pitha PM. Proc Natl Acad Sci U S A. 1995;92:11657–11661. doi: 10.1073/pnas.92.25.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin B, Liu C, Lam S, Srinath H, Delston R, Correia J, Derynck R, Lin K. Nature structural biology. 2003;10:913–934. doi: 10.1038/nsb1002. [DOI] [PubMed] [Google Scholar]
- 13.Lin RT, Mamane Y, Hiscott J. Molecular and Cellular Biology. 1999;19:2465–2474. doi: 10.1128/mcb.19.4.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergstroem B, Johnsen IB, Nguyen TT, Hagen L, Slupphaug G, Thommesen L, Anthonsen MW. J Biol Chem. 2010;285:24904–24914. doi: 10.1074/jbc.M109.084822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 16.Panne D, McWhirter SM, Maniatis T, Harrison SC. J Biol Chem. 2007;282:22816–22822. doi: 10.1074/jbc.M703019200. [DOI] [PubMed] [Google Scholar]
- 17.Yoneyama M, Suhara W, Fukuhara Y, Fukuda M, Nishida E, Fujita T. EMBO J. 1998;17:1087–1095. doi: 10.1093/emboj/17.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saitoh T, Tun-Kyi A, Ryo A, Yamamoto M, Finn G, Fujita T, Akira S, Yamamoto N, Lu KP, Yamaoka S. Nat Immunol. 2006;7:598–605. doi: 10.1038/ni1347. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Tian Y, Wang RP, Gao D, Zhang Y, Diao FC, Chen DY, Zhai ZH, Shu HB. Cell Res. 2008;18:1096–1104. doi: 10.1038/cr.2008.277. [DOI] [PubMed] [Google Scholar]
- 20.Sumpter R, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, Lemon SM, Gale M. J Virol. 2005;79:2689–2699. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin R, Heylbroeck C, Genin P, Pitha PM, Hiscott J. Mol Cell Biol. 1999;19:959–966. doi: 10.1128/mcb.19.2.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, Akira S, Gill MA, García-Sastre A, Katze MG, Gale M. J Virol. 2008;82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin RT, Heylbroeck C, Pitha PM, Hiscott J. Molecular and Cellular Biology. 1998;18:2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foy E, Li K, Wang C, Sumpter R, Ikeda M, Lemon SM, Gale M. Science. 2003;300:1145–1148. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- 25.Doehle BP, Hladik F, McNevin JP, McElrath MJ, Gale M. J Virol. 2009;83:10395–10405. doi: 10.1128/JVI.00849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okumura A, Alce T, Lubyova B, Ezelle H, Strebel K, Pitha PM. Virology. 2008;373:85–97. doi: 10.1016/j.virol.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barro M, Patton JT. Proc Natl Acad Sci U S A. 2005;102:4114–4119. doi: 10.1073/pnas.0408376102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilton L, Moganeradj K, Zhang G, Chen YH, Randall RE, McCauley JW, Goodbourn S. J Virol. 2006;80:11723–11732. doi: 10.1128/JVI.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loo YM, Owen DM, Li K, Erickson AK, Johnson CL, Fish PM, Carney DS, Wang T, Ishida H, Yoneyama M, Fujita T, Saito T, Lee WM, Hagedorn CH, Lau DT, Weinman SA, Lemon SM, Gale M. Proc Natl Acad Sci U S A. 2006;103:6001–6006. doi: 10.1073/pnas.0601523103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin RT, Lacoste J, Nakhaei P, Sun Q, Yang L, Paz S, Wilkinson P, Julkunen I, Vitour D, Meurs E, Hiscott J. Journal of Virology. 2006;80:6072–6083. doi: 10.1128/JVI.02495-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Nature. 2005;437:1167–1239. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 32.Unterstab G, Ludwig S, Anton A, Planz O, Dauber B, Krappmann D, Heins G, Ehrhardt C, Wolff T. Proc Natl Acad Sci U S A. 2005;102:13640–13645. doi: 10.1073/pnas.0502883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin R, Genin P, Mamane Y, Sgarbanti M, Battistini A, Harrington WJ, Barber GN, Hiscott J. Oncogene. 2001;20:800–811. doi: 10.1038/sj.onc.1204163. [DOI] [PubMed] [Google Scholar]
- 34.Yoneyama M, Fujita T. J Biol Chem. 2007;282:15315–15318. doi: 10.1074/jbc.R700007200. [DOI] [PubMed] [Google Scholar]
- 35.Yim HC, Li JC, Lau JS, Lau AS. AIDS. 2009;23:1473–1484. doi: 10.1097/QAD.0b013e32832d7abe. [DOI] [PubMed] [Google Scholar]
- 36.Lepelley A, Louis S, Sourisseau M, Law HK, Pothlichet J, Schilte C, Chaperot L, Plumas J, Randall RE, Si-Tahar M, Mammano F, Albert ML, Schwartz O. PLoS Pathog. 2011;7:e1001284. doi: 10.1371/journal.ppat.1001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. J Virol. 2000;74:8358–8367. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mörner A, Björndal A, Albert J, Kewalramani VN, Littman DR, Inoue R, Thorstensson R, Fenyö EM, Björling E. J Virol. 1999;73:2343–2349. doi: 10.1128/jvi.73.3.2343-2349.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwamura T, Yoneyama M, Yamaguchi K, Suhara W, Mori W, Shiota K, Okabe Y, Namiki H, Fujita T. Genes to cells : devoted to molecular & cellular mechanisms. 2001;6:375–463. doi: 10.1046/j.1365-2443.2001.00426.x. [DOI] [PubMed] [Google Scholar]
- 40.Navarro L, David M. J Biol Chem. 1999;274:35535–35538. doi: 10.1074/jbc.274.50.35535. [DOI] [PubMed] [Google Scholar]
- 41.Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M. Nature. 2008;454:523–527. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 43.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chew T, Noyce R, Collins SE, Hancock MH, Mossman KL. Mol Immunol. 2009;46:393–399. doi: 10.1016/j.molimm.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Harman AN, Lai J, Turville S, Samarajiwa S, Gray L, Marsden V, Mercier S, Jones K, Nasr N, Rustagi A, Cumming H, Donaghy H, Mak J, Gale M, Churchill M, Hertzog P, Cunningham AL. Blood. 2011;118:298–308. doi: 10.1182/blood-2010-07-297721. [DOI] [PMC free article] [PubMed] [Google Scholar]