Abstract
Chronic kidney disease (CKD) significantly increases cardiovascular morbidity and mortality. Chronic kidney disease remains an under-represented population in cardiovascular clinical trials, and cardiovascular disease is an under-treated entity in CKD. Traditional cardiovascular risk factors in conjunction with uremia-related complications often progress to myocardial dysfunction. Such uremic cardiomyopathy leads to over-activation of neurohormonal pathways with detrimental effects. Management of the reno-cardiac syndrome (RCS) requires the targeting of these multiple facets. In this article we discuss the relevant pathophysiology of RCS, and present the clinical data related to its management.
Keywords: Cardiorenal syndrome, Renocardiac syndrome, management, renin-angiotensin-aldosterone system, sympathetic nervous system, hypervolemia
INTRODUCTION
Bidirectional interactions between the cardiac and renal systems are mediated through hemodynamic and neurohormonal pathways. In pathologic conditions, this organ cross talk culminates into the vicious cycle often loosely defined as cardiorenal syndrome (CRS). Renocardiac syndrome sometimes referred to as CRS-type 4 (1) has been defined as CKD leading to progressive secondary cardiac dysfunction, which may include structural abnormalities (such as fibrosis, left ventricular hypertrophy (LVH), low capillary density), as well as functional changes (such as ischemia, arrhythmia and systolic/diastolic dysfunction). There is no single biomarker or imaging modality that can diagnose RCS, which complicates our ongoing efforts to better understand the potential treatment strategies. Hence, the most common inclusion criteria are the combination of underlying CKD or end-stage renal disease (ESRD) with concomitant cardiac pathology.
Patients with RCS have higher rates of cardiac complications and all-cause mortality, (2) with cardiovascular disease accounting for more than 50 % of deaths in ESRD. (3) Cardiovascular events and mortality increase in proportion to worsening glomerular filtration rate (GFR) (4). Moreover, cardiac complications that develop in CKD portend worse outcomes (4). This review will discuss our current clinical understanding of RCS, providing the evidence for its pharmacologic management.
PATHOPHYSIOLOGY
Although an extensive discussion of the underlying pathophysiology of RCS is not the focus of this review, a brief overview may highlight the therapeutic targets of RCS that will be discussed. Despite higher prevalence of traditional risk factors in patients with CKD, including diabetes mellitus, hypertension, and hyperlipidemia, (5) the burden of cardiovascular disease in this population remains disproportionate (6). Non-conventional risk factors resulting from the uremic state likely play an important role, although the precise causative factors for accelerated cardiovascular disease are often unclear. These include hemodynamic factors such as volume and pressure-overload, and non-hemodynamic factors such as anemia, abnormal calciumphosphorous metabolism, uremic toxins (e.g., homocysteine, indoxyl sulfate), cardiotonic steroids, increased inflammatory markers, elevated lipoprotein levels, endothelial dysfunction, and oxidative stress (7). The negative effect of the hostile uremic milieu on cardiac status is highlighted in findings from the Frequent Hemodialysis Network study that demonstrated favorable outcomes on left ventricular mass and cardiac death in a frequent dialysis group (six times weekly, 1.5–2.5 hours/treatment) as compared to a conventional dialysis group (8). Moreover, the reversible nature of heart failure with kidney transplantation further highlights the direct contribution of uremia in myocardial performance (9).
As cardiac dysfunction ensues in the setting of progressive renal failure, the vicious cycle of CRS is created. Previously thought to be the predominant result of an impaired circulatory state, the heart and the kidneys are now believed to be a complex interaction of multiple factors. The compensatory activation of the sympathetic nervous system (SNS) and renin-angiotensinaldosterone system (RAAS) becomes maladaptive as kidney function deteriorates. Sympathetic overactivity leads to adverse consequences as it: 1) reduces the myocardial β-adrenergic receptor density; 2) induces insulin resistance and dyslipidemia leading to accelerated atherosclerosis; 3) potentiates vasoconstriction; and 4) induces abnormal renal sodium handling (10). Meanwhile, excess activation of RAAS (potentiated in an overactive sympathetic state) can lead to a sodium-avid state and adverse ventricular remodeling. Progressive volume overload as a result of RCS may also contribute to such neurohormonal overactivation. In fact, decades ago Winton demonstrated the superior influence of elevated renal venous pressure over reduced renal arterial pressure on worsening kidney function in an animal model (11). This concept formed the basis for our understanding of the integral role of renal venous, central venous and intra-abdominal pressures in CRS (12). (12
TREATMENT
Management of RCS necessitates a comprehensive approach that takes into account its proposed pathophysiology. Conventional risk factors, as well as factors related to the uremic milieu must be addressed in conjunction with SNS, RAAS, and congestion. In clinical practice cardiovascular disease remains an under-treated entity in CKD, with patients less likely to receive medications that interrupt neurohormonal pathways (13). This is due to a paucity of data derived from randomized controlled trials in the CKD population (see table 1) (14). There is also reluctance on the part of healthcare providers in prescribing these medications due to their perceived negative impact in CKD. Increasing physician awareness with the available data is paramount in changing practice patterns and improving outcomes.
Table 1.
Selected randomized controlled trials using cardiovascular medications in CKD
| Description | End-Points | Result | Reference |
|---|---|---|---|
| Carvedilol vs. placebo in HD patients with dilated CM, n=114 |
Changes in LVEDP, LVESV, LVEF, symptoms | Improvement | (77) |
| Carvedilol vs. placebo in HD patients with dilated CM, n=114 |
All-cause mortality and hospital admissions, CV mortality and hospital admissions |
Improvement | (78) |
| Telmisartan vs. placebo in HD patients with CHF, n=332 |
All-cause mortality, CV mortality, hospital admission for CHF |
Improvement | (64) |
| Fosinopril vs. placebo in HD patients with LVH, n=397 |
Composite end-point of fatal and non-fatal major CV events |
No significant improvement |
(95) |
| Spironolactone vs. placebo in CKD stage 2 and 3, n=112 |
Changes in LV mass and arterial stiffness | Improvement | (68) |
| Simvastatin + ezetimibe vs. placebo in CKD patients, n=9270 |
First major atherosclerotic event | Improvement | (28) |
| Atorvastatin vs. placebo in HD patients with diabetes, n=1255 |
CV mortality, non-fatal MI, stroke | No significant improvement |
(31) |
| Rosuvastatin vs. placebo in HD patients, n=2776 |
CV mortality, non-fatal MI, non-fatal stroke | No significant improvement |
(32) |
CKD, chronic kidney disease; HD, hemodialysis; CM, cardiomyopathy; LVEDP, left ventricular end-diastolic pressure; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; CV, cardiovascular; CHF, chronic heart failure; LV, left ventricular; MI, myocardial infarction.
Modifying Conventional Risk Factors
Aspirin
Patients with CKD including those with ESRD are less likely to be prescribed aspirin following a myocardial infarction, (15) likely due to bleeding concerns related to the uremic state (16). Nevertheless in the chronic care setting, a subgroup analysis of the Hypertension Optimal Treatment (HOT) study compared aspirin (75 mg) to placebo, and stratified patients according to estimated GFR (eGFR) (17). The investigators observed a reduction in major cardiovascular events, ranging from 9–66 %, with the higher end of improvement in those with an eGFR <45 mL/min. This protective effect was significantly greater than in those without CKD. Although an increased bleeding risk was expectedly noted, there was a tendency towards a more favorable benefit-risk ratio with a decline in GFR. Meanwhile, the data supporting the routine use of other anti-platelet agents in the management of RCS has been less robust. In fact, outcomes in clopidogrel-treated patients with CKD were not shown to be superior to placebo, (18) and clopidogrel may even be harmful in patients with significant diabetic nephropathy (19). The risk-benefit considerations for aspirin and other anti-platelet agents in RCS warrant further investigations.
Managing Hypertension
The presence of severe LVH and diastolic dysfunction in RCS is often attributed to poorly controlled hypertension. A well-defined correlation between hypertension and cardiovascular events has been established in the general population, (20) and can be extrapolated to the CKD population. A study attempted to delineate the potential cardiovascular benefit of lowering blood pressure (BP) in CKD patients with a history of cerebrovascular disease (21). The BPlowering therapy with perindopril reduced the risk of major vascular events with a 1.7-fold greater effect in those with CKD. Similar results were seen in a post-hoc analysis with ramipril (22). However, the relationship between hypertension and mortality in the dialysis population has demonstrated a “U”-shaped curve with worse outcomes at either end of the BP spectrum. (23) Lower BP may hinder the adequacy of dialysis sessions to attain a euvolemic and nonuremic state, and may pose challenges for maintaining drug therapy such as neurohormonal antagonists that have the propensity to lower BP. It may also be a case of reverse causality, wherein poor myocardial structure and function can be associated with lower baseline BP. While the optimal BP for CKD and ESRD patients is unknown, avoiding significant decreases in BP with medical therapy or hemodialysis is critical to prevent cardiovascular events. The 24-hour ambulatory monitor may provide important clinical data to guide therapy in these settings.
Managing Hyperlipidemia and Statin Therapy
Recent evidence suggests that by lowering cholesterol, statins have the potential to stimulate transforming growth factor-β, (24) a cytokine that mediates progression of renal fibrosis (25). Such an effect has not been evident in clinical trials, and statins may even offer renoprotective effects in CKD by reducing proteinuria (26). In a subgroup analysis, pravastatin was associated with a reduction in the primary end-point of death from coronary disease or symptomatic nonfatal myocardial infarction in those with mild renal insufficiency (27). The Study of Heart and Renal Protection (SHARP) trial demonstrated the safe reduction of major atherosclerotic events in patients with CKD with a reduction in LDL cholesterol using simvastatin plus ezetimibe (28). The results of this study may seem contradictory to those performed in dialysis patients that show increased mortality with lower cholesterol levels (29). This may be related to the lower cholesterol levels being a marker of an inflammatory and malnourished state associated with decreased survival, a concept that was highlighted in a study in dialysis patients (30). Other studies that assessed statins in ESRD failed to show a significant improvement in cardiovascular events, despite reductions in LDL cholesterol (31, 32). However, a post-hoc analysis of one such trial, the 4D study, demonstrated a significant reduction in cardiac events and mortality with the use of atorvastatin in those with baseline LDL >145 mg/dL (33).
Managing Diabetes Mellitus
Diabetes mellitus remains the most common cause of ESRD. In this setting it amplifies the cardiovascular risk of CKD thus representing a major challenge. Microalbuminuria, a common complication of diabetes, is a harbinger of diabetic nephropathy and a marker of cardiovascular morbidity and mortality independent of renal function (34). Evidence suggests that microalbuminuria at levels less than the conventional definition is associated with cardiovascular events, and therapy aimed at reducing albuminuria decreases these events and slows the 8 annual decline of GFR (35, 36). The risk of microvascular complications can be reduced with intensive glycemic control with a goal HbA1c of 7 % (37). Despite HbA1c correlating with cardiovascular disease, (38) clinical trials have failed to show clear benefit of strict glycemic control in reducing cardiovascular events (39, 40). However, during a mean 17 year follow-up of the Diabetes Control and Complications Trial (DCCT), beneficial effects on macrovascular disease were noted with intensive glucose-lowering therapy (41).
Modifying Uremic Complications
Anemia
The physiologic response to anemia is a hemodynamic compensatory high-output state. Chronically this leads to LVH and arterial remodeling, factors that contribute to left ventricular wall stress and impaired coronary perfusion (42). The relationship between anemia and cardiovascular disease has been well established in CKD, (43) with evidence suggesting improvement of heart failure and cardiac function by correcting anemia with erythropoietin and intravenous iron (44, 45).
Ideal target hemoglobin remains a controversial issue. The Normal Hematocrit Cardiac Trial (NHCT) was one of the earliest randomized trials to assess erythropoietin in patients on hemodialysis (46). The study involved 1,233 patients with underlying ischemic heart disease or congestive heart failure randomized to a goal hematocrit of 30 % or 42 %, with the former group yielding better results, albeit not statistically significant. The results led to early termination of the study.
A meta-analysis of randomized controlled trials that studied the targeting of different hemoglobin concentrations with erythropoietin therapy for anemia of CKD concluded that targeting higher hemoglobin increases all-cause mortality (risk ratio 1.17, 95 % CI 1.01–1.35; p=0.031) (47). A subsequent meta-analysis revealed a significantly increased risk of stroke, hypertension, and vascular access thrombosis when targeting higher hemoglobin (48). The increased morbidity and mortality resulting from attempts to normalize hemoglobin does not justify the possible benefits of improved quality of life and reduced transfusion requirement. Current evidence recommends against targeting hemoglobin levels above 12 mg/dL.
Calcium-Phosphorous Metabolism
Mineral homeostasis maintained through interactions between calcium, phosphorous, parathyroid hormone (PTH) and vitamin D, is disturbed in CKD. A rise in phosphorous is seen with declining renal function, ultimately leading to hypocalcemia and secondary hyperparathyroidism. Alternatively, elevated PTH can be a consequence of aggressive volume management with diuretic therapy and subsequent development of secondary hyperaldosteronism, particularly in the setting of RCS. The primary adverse consequence of such changes is manifested in the vascular system through vascular calcification, leading to arterial stiffness with downstream effects resulting in LVH and diminished sub-endocardial coronary perfusion (49).
Hemodialysis patients with lower serum phosphorous have improved survival (50). Lowering phosphorous may be utilized as a strategy to preserve mineral homeostasis and prevent secondary hyperparathyroidism with its associated cardiovascular and bone mineral disease. The use of calcium-containing phosphate binders and vitamin D analogues to inhibit the cascade that leads to secondary hyperparathyroidism may inadvertently worsen the calciumphosphate product. One study showed that the calcium score in coronary arteries and the aorta was increased with calcium-containing phosphate binders but not with sevelamer, a non calcium-containing phosphate binder (51). Suppressing PTH using cinacalcet may theoretically provide an additional area of target. Preliminary results from the Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE) trial however, failed to reveal positive outcomes (52). Current evidence suggests that interventions on mineral disorders in CKD may have favorable effects on surrogate markers, e.g., vascular calcification or biochemical mineral improvements. However, this may not necessarily translate into clinically improved cardiovascular outcomes (53).
Homocysteine
Homocysteine, an independent risk factor for cardiovascular disease (54) is elevated in CKD in proportion to the decline in GFR (55). Homocysteine fulfills the criteria for a host of protein-bound and circulating factors known as “uremic toxins” that exert adverse metabolic consequences in the setting of RCS. Although meta-analyses of observational studies demonstrate an inverse relationship between cardiovascular disease and homocysteine levels, (56) prospective trials have failed to show a protective effect with folic acid, vitamin B6, and vitamin B12, despite a reduction in homocysteine (57). Similar negative results were seen in those with ESRD (58). These findings are intriguing since homocysteine is an established mediator of atherothrombosis through endothelial injury and oxidative stress, (59) and not merely a surrogate marker of cardiovascular disease. The metabolic pathways involving homocysteine, and the interaction of vitamins and minerals with these pathways may be more complex than our current understanding.
Modifying Cardiorenal Interactions
Modulating Renin-Angiotensin-Aldosterone System
Uremic cardiomyopathy ultimately results in the uncontrolled activation of RAAS forming a primary target for therapy. The importance of RAAS in the context of heart failure is highlighted in a study that identified patients who did not tolerate ACE-inhibitors as a group at higher mortality risk (60). Targeting RAAS may be at the expense of worsening renal function. An analysis of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) showed that creatinine levels stabilized after an initial slight increase, with enalapril being tolerated in the majority of patients (61). Of note, hypotension was the strongest factor associated with increased creatinine in this study, a point that must be taken into consideration when diuresing patients in an attempt to attain euvolemia. Interestingly, despite early worsening of renal function, those that continued to take enalapril in the Studies of Left Ventricular Dysfunction (SOLVD) trial had improved survival, whereas worsening creatinine in the placebo group was a more ominous sign (62).
In the setting of RCS, a recent study looking at patients with CKD demonstrated consistent mortality risk reduction with benazepril, although its impact on myocardial structure and function is unclear (63). A double-blinded randomized controlled trial in ESRD patients with a left ventricular ejection fraction (LVEF) <40 % and NYHA class II or III symptoms resulted in a significant improvement in survival and cardiovascular morbidity and mortality with the addition of telmisartan to standard therapy with ACE-inhibitor (64). In a 2-week preliminary run-in phase, 19 of the 351 enrolled patients were excluded, of which seven were for symptomatic hypotension (2.0 %). During the 3-year maintenance period, hypotension resulted in 18 exclusions in the telmisartan arm (10.9 %), and seven exclusions in the placebo arm (4.2 %). The risk of hypotension, although present, does not seem prohibitive. Moreover, the trial used an aggressive regimen including maximum dose telmisartan (76 % of patients reached the target dose of 80mg), an ACE-inhibitor, and carvedilol in 60.3 % of patients in the telmisartan arm. The role of angiotensin blockade in ESRD is not limited to those with heart failure. A randomized controlled trial of candesartan in hemodialysis patients with no clinical evidence of underlying cardiac dysfunction demonstrated favorable cardiovascular outcomes (65). In hypertensive patients, angiotensin receptor blockers (ARB) may provide a cardioprotective effect greater than their BP-lowering effect. In one study, losartan produced greater regression of LVH in hemodialysis patients as compared to enalapril and amlodipine, despite similar reductions in mean BP (66). A similar effect on LVH was seen with valsartan in patients on continuous ambulatory peritoneal dialysis (CAPD), and this was associated with a reduction in arterial stiffness (67).
Aldosterone inhibition provides a further means of intervention. Edwards et al., studied the use of spironolactone in early CKD in two randomized controlled trials, demonstrating regression in LV mass and improved arterial stiffness, (68) in addition to improved markers of regional systolic and diastolic function (69).
In addition to their cardioprotective effects, RAAS-inhibitors possess renoprotective properties. This has been proven in clinical trials in which ARBs were shown to yield beneficial renal effects in type 2 diabetes (70). Such effects include reduction in the level of microalbuminuria, and halting progression to overt nephropathy, ESRD, or death, independent or out of proportion to their BP-lowering property. Aldosterone levels correlate with proteinuria, an important indicator of progression of kidney disease (71). Spironolactone may play an adjunctive role with ACEinhibitors and/or ARBs to offer a greater reduction in proteinuria (72).
Following the Randomized Aldactone Evaluation Study (RALES), the prescription of spironolactone considerably increased, along with associated hyperkalemia and its complications (73). Navaneethan et al., described the increased risk of hyperkalemia noted with the addition of a non-specific aldosterone antagonist to ACE-inhibitor and/or ARB in a systematic review of 11 trials (72). Therefore, these agents should be used with caution in CKD especially with concomitant ACE-inhibitors and/or ARBs. Multiple small studies with either low-dose spironolactone or eplerenone have indicated relative safety of these medications in the setting of ESRD (74). A mild, and for the most, clinically insignificant rise in potassium was seen in some patients. This slight risk is clearly offset by the benefits of preventing vascular injury and improving cardiac function and geometry. Meanwhile, a novel potassium binder, RLY5016, has been shown to safely prevent hyperkalemia in patients with heart failure receiving standard therapy and spironolactone (75). This may provide a future strategy that allows safe inhibition of RAAS in patients with CKD.
Modulating Sympathetic Nervous System
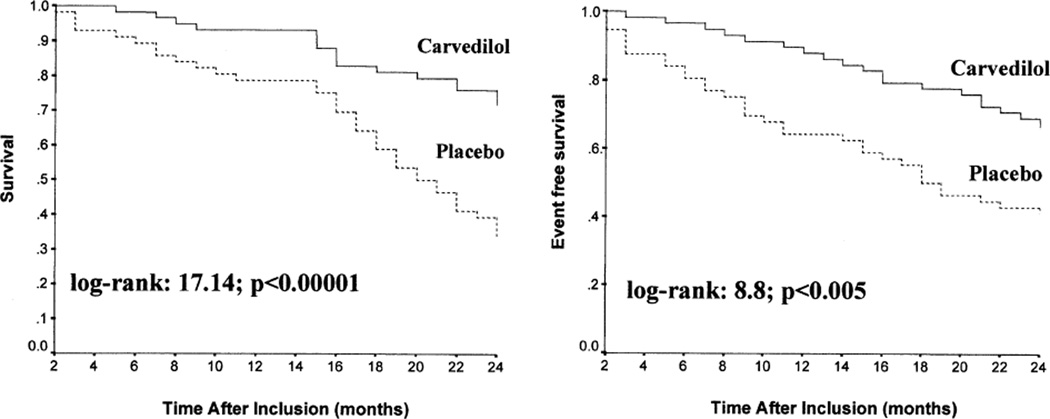
CKD leads to a state of sympathetic overactivity with potential to worsen cardiovascular disease and lead to progression of renal disease. In a post-hoc analysis of the Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF), mortality and hospitalization for heart failure were effectively reduced even in those with an eGFR <45 mL/min (76). Carvedilol possesses the strongest evidence for use in ESRD patients with heart failure. An open-label randomized controlled trial of 114 patients with LVEF <35 % and NYHA class II to III symptoms demonstrated an improvement in cardiac geometry and function in addition to symptomatic benefits with carvedilol as compared to baseline measurement and the placebo group (77). The tolerability of carvedilol was assessed in this trial in a 2-week preliminary run-in phase. Eighteen of the 132 patients (13.6 %) that entered this phase were excluded, of which three were due to hypotension (2.3 %). During the maintenance phase, hypotension resulted in only one drop out in the carvedilol group (1.7 %). It is evident that after initially tolerating the medication, most patients can be maintained on carvedilol with few side effects. Following the initial study, an additional 12-month follow-up was designed to assess cardiovascular outcomes (figure 1) (78). Results demonstrated a reduction in all-cause mortality (51.7 % vs. 73.2 %, p < 0.01), cardiovascular mortality (29.3 % vs. 67.9 %, p < 0.00001), and hospitalization (34.5 % vs. 58.9 %, p < 0.00001).
Figure 1.
Kaplan-Meyer curves for cardiovascular death (left) and for all-cause hospital admission (right) during 24-month follow-up cumulative survival rate according to use of carvedilol. Solid lines = carvedilol group; dashed lines = placebo group. Reprinted from J Am Coll Cardiol., Volume 41, Issue 9, Gennaro C, et al. “Carvedilol increases two-year survival in dialysis patients with dilated cardiomyopathy: A prospective, placebo-controlled trial,” pages 1438-1444, ©2003, with permission from Elsevier.
Beta-blockers possess renoprotective effects in hypertensive nephrosclerosis, similar to ACE-inhibitors but of less magnitude (79). In hemodialysis patients they have the most pronounced association with survival as compared to other anti-hypertensive drugs (80).
A systematic review revealed survival benefits with the use of beta-blockers in patients with CKD and chronic systolic heart failure (81). Nevertheless, these medications are underutilized in CKD with potential fears including metabolic disturbances, worsening renal function and hemodynamic abnormalities. The efficacy and safety profile of beta-blockers including metoprolol, atenolol, and carvedilol, have been well established in CKD, (82) although atenolol must be used with caution due to reduced renal clearance. Oxidative stress has been associated with microalbuminuria, (83) possibly accounting for some of the superior renoprotective properties of carvedilol (84).
Managing Volume Overload with Renal Replacement Therapy vs. Pharmacologic Therapy
Diuretics provide symptomatic benefit in those with CKD and heart failure. In addition they have an important role in interrupting the cardiorenal cascade. Decreased preload reduces right ventricular dilatation leading to improved left ventricular filling and contractility. This improvement is related to ventricular interdependence, referred to as the “reverse Bernheim phenomenon”. Furthermore, the central therapeutic role of reducing intra-abdominal pressure and central venous pressure in CRS has become evident over the years (85).
Although diuretics may result in a slight worsening of creatinine, when used judiciously they play a role in interrupting CRS and improving outcomes. Hemoconcentration as a marker of aggressive diuresis has a negative impact on renal function, but is associated with significantly improved survival in patients with congestive heart failure (86).
Newer hemodialysis strategies may provide physicians with superior means of managing volume status in RCS. Nocturnal hemodialysis allows for more frequent, longer duration dialysis sessions. This modality has shown regression of LVH (87) and improved LVEF (88) as compared to conventional hemodialysis. Peritoneal dialysis may have a theoretic advantage over conventional hemodialysis in patients with CRS due to the absence of major hemodynamic shifts. However, evidence suggests that sub-clinical volume expansion with resultant hypertension and LVH are more pronounced in CAPD than in hemodialysis (89) likely related to poor peritoneal dialysis prescription design rather than the therapy itself. Developments in the field of peritoneal dialysis have allowed an improvement in volume status and LVH with use of icodextrin and hypertonic dialysate solutions (90, 91). ACE-inhibitors e.g., ramipril and ARBs may play a role in preserving residual renal function, (92) an important marker of morbidity and mortality in patients receiving peritoneal dialysis (93).
Management of ESRD has typically focused on reducing extracellular volume overload through ultrafiltration, with a relative neglect of the pharmacologic measures that inhibit deleterious neurohormonal pathways. In one cohort, ACE-inhibitors and beta-blockers were used in less than 25 % of ESRD patients with a known history of heart failure (94). Balancing renal replacement therapy with pharmacologic management of CRS should be based on the current available evidence. The cardio-protective benefits of inhibiting SNS and RAAS that have been discussed in this review must be weighed against other factors. Activation of SNS and RAAS during dialysis helps prevent intra-dialytic hypotension. This compensatory mechanism is lost with beta-blockers and RAAS-inhibitors. In non-hypertensive patients, pharmacotherapy in renocardiac syndrome poses a dilemma due to the “U-shaped” BP curve in the ESRD population. Moreover, hypotension that occasionally results from these medications has the potential to limit the adequacy of dialysis. On the contrary, in an attempt to attain euvolemia, diuresis or dialysis may reduce effective circulating volume and cause hypotension limiting or prohibiting the use of neurohormonal antagonists. A narrow therapeutic window for managing volume in these patients exists, and is further confounded by the difficulty in differentiating between cardiac and renal causes of vascular congestion in those with concomitant heart failure and ESRD.
CONCLUSION AND FUTURE PERSPECTIVES
Despite a wealth of evidence suggesting the potential for a wide spectrum of pharmacologic therapy and interventions to manage heart failure, few studies have directly targeted RCS, partly because of the nebulous definitions and non-specific physiologic endpoints. Renal studies often lack precise cardiac measurements, and cardiac studies often exclude patients with significant renal impairment. To reinforce our limited pathophysiologic understanding of cardiorenal interactions, it is hoped that biomarkers that can provide mechanistic insights will allow the triage of patients for more targeted pharmacologic therapy. In the meantime, appropriate management must incorporate the multiple aspects of RCS including modifying conventional cardiovascular risk factors, reducing uremic complications through metabolic interventions, and balancing volume status to maximize appropriate therapeutic interventions. The latter requires a strategy to judiciously manage extracellular volume through diuretics and renal replacement therapy while simultaneously treating with medications that inhibit neurohormonal pathways. It is imperative that future studies be conducted to evaluate this fine balance.
Acknowledgements
W.H.W. Tang is supported by grants from the National Institutes of Health (NIH) and Abbott Laboratories.
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol. 2008 Nov 4;52(19):1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 2.Muntner P, He J, Hamm L, et al. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002 Mar;13(3):745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ. Cardiovascular mortality in end-stage renal disease. Am J Med Sci. 2003 Apr;325(4):163–167. doi: 10.1097/00000441-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004 Sep 23;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Levey AS. Cardiovascular disease and chronic renal disease: a new paradigm. Am J Kidney Dis. 2000 Apr;35(4 Suppl 1):S117–S131. doi: 10.1016/s0272-6386(00)70239-3. [DOI] [PubMed] [Google Scholar]
- 6.Muntner P, Hamm LL, Kusek JW, et al. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med. 2004 Jan 6;140(1):9–17. doi: 10.7326/0003-4819-140-1-200401060-00006. [DOI] [PubMed] [Google Scholar]
- •7. Shastri S, Sarnak MJ. Cardiovascular disease and CKD: core curriculum 2010. Am J Kidney Dis. 2010 Aug;56(2):399–417. doi: 10.1053/j.ajkd.2010.03.019. A comprehensive evidence-based review of cardiovascular disease in CKD including its epidemiology, pathophysiology, diagnosis, and treatment
- 8.FHN Trial Group. Chertow GM, Levin NW, Beck GJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010 Dec 9;363(24):2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wali RK, Wang GS, Gottlieb SS, et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol. 2005 Apr 5;45(7):1051–1060. doi: 10.1016/j.jacc.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 10.Julius S, Nesbitt S. Clinical consequences of the autonomic imbalance in hypertension and congestive heart failure. Scand Cardiovasc J Suppl. 1998;47:23–30. [PubMed] [Google Scholar]
- 11.Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol. 1931 Jun 6;72(1):49–61. doi: 10.1113/jphysiol.1931.sp002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol. 2008 Jan 22;51(3):300–306. doi: 10.1016/j.jacc.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 13.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004 Sep 23;351(13):1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 14.Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA. 2006 Sep 20;296(11):1377–1384. doi: 10.1001/jama.296.11.1377. [DOI] [PubMed] [Google Scholar]
- 15.Berger AK, Duval S, Krumholz HM. Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J Am Coll Cardiol. 2003 Jul 16;42(2):201–208. doi: 10.1016/s0735-1097(03)00572-2. [DOI] [PubMed] [Google Scholar]
- 16.Sohal AS, Gangji AS, Crowther MA, Treleaven D. Uremic bleeding: pathophysiology and clinical risk factors. Thromb Res. 2006;118(3):417–422. doi: 10.1016/j.thromres.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 17.Jardine MJ, Ninomiya T, Perkovic V, et al. Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol. 2010 Sep 14;56(12):956–965. doi: 10.1016/j.jacc.2010.02.068. [DOI] [PubMed] [Google Scholar]
- 18.Best PJ, Steinhubl SR, Berger PB, et al. The efficacy and safety of short- and long-term dual antiplatelet therapy in patients with mild or moderate chronic kidney disease: results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J. 2008 Apr;155(4):687–693. doi: 10.1016/j.ahj.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 19.Dasgupta A, Steinhubl SR, Bhatt DL, et al. Clinical outcomes of patients with diabetic nephropathy randomized to clopidogrel plus aspirin versus aspirin alone (a post hoc analysis of the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance [CHARISMA] trial) Am J Cardiol. 2009 May 15;103(10):1359–1363. doi: 10.1016/j.amjcard.2009.01.342. [DOI] [PubMed] [Google Scholar]
- 20.Turnbull F Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003 Nov 8;362(9395):1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 21.Perkovic V, Ninomiya T, Arima H, et al. Chronic kidney disease, cardiovascular events, and the effects of perindopril-based blood pressure lowering: data from the PROGRESS study. J Am Soc Nephrol. 2007 Oct;18(10):2766–2772. doi: 10.1681/ASN.2007020256. [DOI] [PubMed] [Google Scholar]
- 22.Mann JF, Gerstein HC, Pogue J, et al. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001 Apr 17;134(8):629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 23.Zager PG, Nikolic J, Brown RH, et al. "U" curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int. 1998 Aug;54(2):561–569. doi: 10.1046/j.1523-1755.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen CL, Huang SS, Huang JS. Cholesterol modulates cellular TGF-beta responsiveness by altering TGF-beta binding to TGF-beta receptors. J Cell Physiol. 2008 Apr;215(1):223–233. doi: 10.1002/jcp.21303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002 Oct;13(10):2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 26.Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol. 2006 Jul;17(7):2006–2016. doi: 10.1681/ASN.2006010012. [DOI] [PubMed] [Google Scholar]
- 27.Tonelli M, Moye L, Sacks FM, Kiberd B, Curhan G Cholesterol and Recurrent Events (CARE) Trial Investigators. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann Intern Med. 2003 Jan 21;138(2):98–104. doi: 10.7326/0003-4819-138-2-200301210-00010. [DOI] [PubMed] [Google Scholar]
- 28.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011 Jun 25;377(9784):2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Degoulet P, Legrain M, Reach I, et al. Mortality risk factors in patients treated by chronic hemodialysis. Report of the Diaphane collaborative study. Nephron. 1982;31(2):103–110. doi: 10.1159/000182627. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Coresh J, Eustace JA, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. 2004 Jan 28;291(4):451–459. doi: 10.1001/jama.291.4.451. [DOI] [PubMed] [Google Scholar]
- 31.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005 Jul 21;353(3):238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 32.Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009 Apr 2;360(14):1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 33.Marz W, Genser B, Drechsler C, et al. Atorvastatin and low-density lipoprotein cholesterol in type 2 diabetes mellitus patients on hemodialysis. Clin J Am Soc Nephrol. 2011 Jun;6(6):1316–1325. doi: 10.2215/CJN.09121010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerstein HC, Mann JF, Pogue J, et al. Prevalence and determinants of microalbuminuria in high-risk diabetic and nondiabetic patients in the Heart Outcomes Prevention Evaluation Study. The HOPE Study Investigators. Diabetes Care. 2000 Apr;23(Suppl 2):B35–B39. [PubMed] [Google Scholar]
- 35.Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients with left ventricular hypertrophy and diabetes. J Nephrol. 2008 Jul-Aug;21(4):566–569. [PubMed] [Google Scholar]
- 36.Araki S, Haneda M, Koya D, et al. Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes. 2007 Jun;56(6):1727–1730. doi: 10.2337/db06-1646. [DOI] [PubMed] [Google Scholar]
- 37.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837–853. [PubMed] [Google Scholar]
- 38.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004 Sep 21;141(6):421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 39.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 40.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009 Jan 8;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 41.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec 22;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metivier F, Marchais SJ, Guerin AP, et al. Pathophysiology of anaemia: focus on the heart and blood vessels. Nephrol Dial Transplant. 2000;15(Suppl 3):14–18. doi: 10.1093/oxfordjournals.ndt.a027970. [DOI] [PubMed] [Google Scholar]
- 43.Foley RN, Parfrey PS, Harnett JD, et al. The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am J Kidney Dis. 1996 Jul;28(1):53–61. doi: 10.1016/s0272-6386(96)90130-4. [DOI] [PubMed] [Google Scholar]
- 44.Silverberg DS, Wexler D, Blum M, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000 Jun;35(7):1737–1744. doi: 10.1016/s0735-1097(00)00613-6. [DOI] [PubMed] [Google Scholar]
- 45.Silverberg DS, Wexler D, Sheps D, et al. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol. 2001 Jun 1;37(7):1775–1780. doi: 10.1016/s0735-1097(01)01248-7. [DOI] [PubMed] [Google Scholar]
- 46.Besarab A, Bolton WK, Nissenson AR, et al. The Normal Haematocrit Trial in dialysis patients with cardiac disease. Nephrol Dial Transplant. 1999 Aug;14(8):2043–2044. doi: 10.1093/ndt/14.8.2043-a. [DOI] [PubMed] [Google Scholar]
- 47.Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007 Feb 3;369(9559):381–388. doi: 10.1016/S0140-6736(07)60194-9. [DOI] [PubMed] [Google Scholar]
- 48.Palmer SC, Navaneethan SD, Craig JC, et al. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med. 2010 Jul 6;153(1):23–33. doi: 10.7326/0003-4819-153-1-201007060-00252. [DOI] [PubMed] [Google Scholar]
- 49.Block G, Port FK. Calcium phosphate metabolism and cardiovascular disease in patients with chronic kidney disease. Semin Dial. 2003 Mar-Apr;16(2):140–147. doi: 10.1046/j.1525-139x.2003.160301.x. [DOI] [PubMed] [Google Scholar]
- 50.Okechukwu CN, Lopes AA, Stack AG, et al. Impact of years of dialysis therapy on mortality risk and the characteristics of longer term dialysis survivors. Am J Kidney Dis. 2002 Mar;39(3):533–538. doi: 10.1053/ajkd.2002.31403. [DOI] [PubMed] [Google Scholar]
- 51.Chertow GM, Burke SK, Raggi P Treat to Goal Working Group. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002 Jul;62(1):245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 52. [Accessed September 21st, 2012];Amgen. 2012 Available at: http://www.amgen.com/media/media_pr_detail.jsp?releaseID=1703773.
- 53.Navaneethan SD, Palmer SC, Vecchio M, et al. Phosphate binders for preventing and treating bone disease in chronic kidney disease patients. Cochrane Database Syst Rev. 2011 Feb 16;2(2):CD006023. doi: 10.1002/14651858.CD006023.pub2. [DOI] [PubMed] [Google Scholar]
- 54.Eikelboom JW, Lonn E, Genest J, Jr, et al. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Ann Intern Med. 1999 Sep 7;131(5):363–375. doi: 10.7326/0003-4819-131-5-199909070-00008. [DOI] [PubMed] [Google Scholar]
- 55.Wollesen F, Brattstrom L, Refsum H, et al. Plasma total homocysteine and cysteine in relation to glomerular filtration rate in diabetes mellitus. Kidney Int. 1999 Mar;55(3):1028–1035. doi: 10.1046/j.1523-1755.1999.0550031028.x. [DOI] [PubMed] [Google Scholar]
- 56.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002 Oct 23–30;288(16):2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 57.Bonaa KH, Njolstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006 Apr 13;354(15):1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 58.Wrone EM, Hornberger JM, Zehnder JL, et al. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol. 2004 Feb;15(2):420–426. doi: 10.1097/01.asn.0000110181.64655.6c. [DOI] [PubMed] [Google Scholar]
- 59.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998 Apr 9;338(15):1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 60.Kittleson M, Hurwitz S, Shah MR, et al. Development of circulatory-renal limitations to angiotensin-converting enzyme inhibitors identifies patients with severe heart failure and early mortality. J Am Coll Cardiol. 2003 Jun 4;41(11):2029–2035. doi: 10.1016/s0735-1097(03)00417-0. [DOI] [PubMed] [Google Scholar]
- 61.Ljungman S, Kjekshus J, Swedberg K. Renal function in severe congestive heart failure during treatment with enalapril (the Cooperative North Scandinavian Enalapril Survival Study [CONSENSUS] Trial) Am J Cardiol. 1992 Aug 15;70(4):479–487. doi: 10.1016/0002-9149(92)91194-9. [DOI] [PubMed] [Google Scholar]
- 62.Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail. 2011 Nov;4(6):685–691. doi: 10.1161/CIRCHEARTFAILURE.111.963256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006 Jan 12;354(2):131–140. doi: 10.1056/NEJMoa053107. [DOI] [PubMed] [Google Scholar]
- •64. Cice G, Di Benedetto A, D'Isa S, et al. Effects of telmisartan added to Angiotensin-converting enzyme inhibitors on mortality and morbidity in hemodialysis patients with chronic heart failure a double-blind, placebo-controlled trial. J Am Coll Cardiol. 2010 Nov 16;56(21):1701–1708. doi: 10.1016/j.jacc.2010.03.105. One of the few double-blinded, randomized controlled trials of cardiovascular medications in CKD
- 65.Takahashi A, Takase H, Toriyama T, et al. Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis--a randomized study. Nephrol Dial Transplant. 2006 Sep;21(9):2507–2512. doi: 10.1093/ndt/gfl293. [DOI] [PubMed] [Google Scholar]
- 66.Shibasaki Y, Masaki H, Nishiue T, et al. Angiotensin II type 1 receptor antagonist, losartan, causes regression of left ventricular hypertrophy in end-stage renal disease. Nephron. 2002 Mar;90(3):256–261. doi: 10.1159/000049060. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki H, Nakamoto H, Okada H, et al. A selective angiotensin receptor antagonist, Valsartan, produced regression of left ventricular hypertrophy associated with a reduction of arterial stiffness. Adv Perit Dial. 2003;19:59–66. [PubMed] [Google Scholar]
- 68.Edwards NC, Steeds RP, Stewart PM, et al. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009 Aug 4;54(6):505–512. doi: 10.1016/j.jacc.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 69.Edwards NC, Ferro CJ, Kirkwood H, et al. Effect of spironolactone on left ventricular systolic and diastolic function in patients with early stage chronic kidney disease. Am J Cardiol. 2010 Nov 15;106(10):1505–1511. doi: 10.1016/j.amjcard.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 70.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001 Sep 20;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 71.Bianchi S, Bigazzi R, Campese VM. Antagonists of aldosterone and proteinuria in patients with CKD: an uncontrolled pilot study. Am J Kidney Dis. 2005 Jul;46(1):45–51. doi: 10.1053/j.ajkd.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 72.Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009 Mar;4(3):542–551. doi: 10.2215/CJN.04750908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004 Aug 5;351(6):543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- •74. Shavit L, Lifschitz MD, Epstein M. Aldosterone blockade and the mineralocorticoid receptor in the management of chronic kidney disease: current concepts and emerging treatment paradigms. Kidney Int. 2012 May;81(10):955–968. doi: 10.1038/ki.2011.505. A comprehensive review of mineralocorticoid antagonism in the setting of CKD – renoprotective effects and safety.
- 75.Pitt B, Anker SD, Bushinsky DA, et al. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011 Apr;32(7):820–828. doi: 10.1093/eurheartj/ehq502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghali JK, Wikstrand J, Van Veldhuisen DJ, et al. The influence of renal function on clinical outcome and response to beta-blockade in systolic heart failure: insights from Metoprolol CR/XL Randomized Intervention Trial in Chronic HF (MERIT-HF) J Card Fail. 2009 May;15(4):310–318. doi: 10.1016/j.cardfail.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Cice G, Ferrara L, Di Benedetto A, et al. Dilated cardiomyopathy in dialysis patients--beneficial effects of carvedilol: a double-blind, placebo-controlled trial. J Am Coll Cardiol. 2001 Feb;37(2):407–411. doi: 10.1016/s0735-1097(00)01158-x. [DOI] [PubMed] [Google Scholar]
- 78.Cice G, Ferrara L, D'Andrea A, et al. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003 May 7;41(9):1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 79.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002 Nov 20;288(19):2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 80.Foley RN, Herzog CA, Collins AJ United States Renal Data System. Blood pressure and long-term mortality in United States hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney Int. 2002 Nov;62(5):1784–1790. doi: 10.1046/j.1523-1755.2002.00636.x. [DOI] [PubMed] [Google Scholar]
- 81.Badve SV, Roberts MA, Hawley CM, et al. Effects of beta-adrenergic antagonists in patients with chronic kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2011 Sep 6;58(11):1152–1161. doi: 10.1016/j.jacc.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 82.Bakris GL, Hart P, Ritz E. Beta blockers in the management of chronic kidney disease. Kidney Int. 2006 Dec;70(11):1905–1913. doi: 10.1038/sj.ki.5001835. [DOI] [PubMed] [Google Scholar]
- 83.Giner V, Tormos C, Chaves FJ, et al. Microalbuminuria and oxidative stress in essential hypertension. J Intern Med. 2004 May;255(5):588–594. doi: 10.1046/j.1365-2796.2003.01280.x. [DOI] [PubMed] [Google Scholar]
- 84.Marchi F, Ciriello G. Efficacy of carvedilol in mild to moderate essential hypertension and effects on microalbuminuria: a multicenter, randomized, open-label, controlled study versus atenolol. Adv Ther. 1995 Jul-Aug;12(4):212–221. [PubMed] [Google Scholar]
- 85.Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010 Jun 15;121(23):2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 86.Testani JM, Chen J, McCauley BD, et al. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010 Jul 20;122(3):265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chan CT, Floras JS, Miller JA, et al. Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int. 2002 Jun;61(6):2235–2239. doi: 10.1046/j.1523-1755.2002.00362.x. [DOI] [PubMed] [Google Scholar]
- 88.Chan C, Floras JS, Miller JA, Pierratos A. Improvement in ejection fraction by nocturnal haemodialysis in endstage renal failure patients with coexisting heart failure. Nephrol Dial Transplant. 2002 Aug;17(8):1518–1521. doi: 10.1093/ndt/17.8.1518. [DOI] [PubMed] [Google Scholar]
- 89.Enia G, Mallamaci F, Benedetto FA, et al. Long-term CAPD patients are volume expanded and display more severe left ventricular hypertrophy than haemodialysis patients. Nephrol Dial Transplant. 2001 Jul;16(7):1459–1464. doi: 10.1093/ndt/16.7.1459. [DOI] [PubMed] [Google Scholar]
- 90.Konings CJ, Kooman JP, Schonck M, et al. Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: a randomized study. Kidney Int. 2003 Apr;63(4):1556–1563. doi: 10.1046/j.1523-1755.2003.00887.x. [DOI] [PubMed] [Google Scholar]
- 91.Asci G, Ozkahya M, Duman S, et al. Volume control associated with better cardiac function in long-term peritoneal dialysis patients. Perit Dial Int. 2006 Jan-Feb;26(1):85–88. [PubMed] [Google Scholar]
- 92.Li PK, Chow KM, Wong TY, et al. Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis. A randomized, controlled study. Ann Intern Med. 2003 Jul 15;139(2):105–112. doi: 10.7326/0003-4819-139-2-200307150-00010. [DOI] [PubMed] [Google Scholar]
- 93.Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006 May;69(10):1726–1732. doi: 10.1038/sj.ki.5000382. [DOI] [PubMed] [Google Scholar]
- 94.Trespalacios FC, Taylor AJ, Agodoa LY, et al. Heart failure as a cause for hospitalization in chronic dialysis patients. Am J Kidney Dis. 2003 Jun;41(6):1267–1277. doi: 10.1016/s0272-6386(03)00359-7. [DOI] [PubMed] [Google Scholar]
- 95.Zannad F, Kessler M, Lehert P, et al. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 2006 Oct;70(7):1318–1324. doi: 10.1038/sj.ki.5001657. [DOI] [PubMed] [Google Scholar]