Abstract
As a member of the secreted extracellular matrix associated proteins of the CCN family, Wnt1 inducible signaling pathway protein 1 (WISP1/CCN4) is garnering increased attention not only as a potent proliferative entity, but also as a robust cytoprotective agent during toxic insults. Here we demonstrate that WISP1 prevents forkhead transcription factor FoxO3a mediated caspase 1 and caspase 3 apoptotic cell death in primary neurons during oxidant stress. Phosphoinositide 3-kinase (PI 3-K) and protein kinase B (Akt1) are necessary for WISP1 to foster post-translational phosphorylation of FoxO3a and sequester FoxO3a in the cytoplasm of neurons with protein 14-3-3. Through an autoregulatory loop, WISP1 also minimizes deacytelation of FoxO3a, prevents caspase 1 and 3 activation, and promotes an effective neuroprotective level of SIRT1 activity through SIRT1 nuclear trafficking and prevention of SIRT1 caspase degradation. Elucidation of the critical pathways of WISP1 that determine neuronal cell survival during oxidative stress may offer novel therapeutic avenues for neurodegenerative disorders.
Keywords: Akt1, apoptosis, caspase, CCN4, forkhead transcription factor, FoxO3a, histone deacetylase, neurons, oxidative stress, 14-3-3 protein, PI 3-K, sirtuin, SIRT1, WISP1
Introduction
As a cytoprotective agent, Wnt1 inducible signaling pathway protein 1 (WISP1) may offer a new therapeutic target for a number of disorders. WISP1 was initially identified as a component of the wingless Wnt1 signaling pathway and in the mouse mammary epithelial cell line C57MG transformed by Wnt1 (1). WISP1 (CCN4) is a member of six secreted extracellular matrix associated proteins of the CCN family that is characterized by the first three members of the family that include Cysteine-rich protein 61, Connective tissue growth factor, and Nephroblastoma over-expressed gene. WISP1 is expressed in several tissues including the epithelium, heart, kidney, lung, pancreas, placenta, ovaries, small intestine, spleen, and brain.
Early studies have demonstrated the ability of WISP1 to prevent p53 mediated apoptosis in kidney fibroblasts (2). Subsequent work has shown both a proliferative and protective role for WISP1 against apoptotic cell injury. WISP1 may promote cardiac remodeling after myocardial infarction (3), stimulate lung tissue repair (4), lead to cardiomyocyte proliferation (5), assist with vascular smooth muscle growth (6), block cell death during bone fractures (7, 8), and limit doxorubicin-induced cardiomyocyte death (9). In relation to neurodegenerative disease, WISP1 can avert microglial inflammatory cell death during β-amyloid (Aβ) toxicity (10) and prevent oxidative stress injury in primary neuronal cells (11, 12).
Although WISP1 is a component of the Wnt1 pathway, WISP1 utilizes protective pathways that include the traditional wingless canonical and non-canonical signaling of Wnt1 as well as pathways exclusive of this system. For example, WISP1 through canonical signaling controls the subcellular trafficking of β-catenin in neurons (12), osteoclasts (13), vascular cells (14), and cardiomyocytes (9). WISP1 can increase the nuclear expression of β-catenin and through a phosphoinositide 3-kinase (PI 3-K) mediated pathway can promote the nuclear translocation of β-catenin (12). Through pathways not involving canonical or non-canonical signaling, WISP1 relies upon PI 3-K and protein kinase B (Akt) to provide cellular protection in renal fibroblasts (2), cardiomyocytes (3, 6, 9), and neurons (11, 12).
Yet, the pathways that govern WISP1 cellular protection beyond the involvement of PI 3-K and Akt remain poorly defined. As a result, cellular signal transduction pathways that involve downstream pathways of PI 3-K and Akt, such as the forkhead transcription factor FoxO3a, are of considerable interest. PI 3-K through the activation of Akt can inhibit FoxO3a activity to block apoptotic cell death. Akt phosphorylates FoxO3a and sequesters FoxO3a in the cytoplasm through association with 14-3-3 protein (15-22). Activity of FoxO3a also is modulated by the sirtuin SIRT1, a mammalian homologues of Sir2 and a class III histone deacetylase (23-25). Dependent upon the post-translational changes on FoxO3a by SIRT1, SIRT1 can inhibit FoxO3a activity through Akt and post-translational phosphorylation of FoxO3a to promote cell survival (26-28). In contrast, SIRT1 also can increase the activity of FoxO3a through the deacetylation of FoxO3a (29-31). Increased FoxO3a activity can subsequently lead to caspase activity in the apoptotic cascade and be detrimental to cell survival (32, 33).
Given the intimate relationship WISP1 holds with PI 3-K and Akt, the signal transduction pathways of FoxO3a and SIRT1 may represent novel WISP1 targets that can determine neuronal cell survival. Here we show that WISP1 is neuroprotective against FoxO3a mediated caspase 1 and caspase 3 apoptotic cell death in primary neuronal cells during oxygen-glucose deprivation (OGD). WISP1 requires PI 3-K and Akt to promote inhibitory post-translational phosphorylation of FoxO3a and block nuclear translocation of FoxO3a through association with 14-3-3 protein. WISP1 effectively controls SIRT1 activity for neuronal survival, maintains nuclear expression of SIRT1, limits deacytelation of FoxO3a, and blocks caspase 1 and 3 activation during oxidative stress that can autoregulate SIRT1 expression and degradation.
Materials and Methods
Hippocampal neuronal cultures
Per our prior protocols (11, 12, 34, 35), hippocampi were obtained from E-19 Sprague-Dawley rat pups and incubated in Hanks’ balanced salt solution (HBBS) supplemented with 1 mM sodium pyruvate and 10 mM HEPES buffer solution (Invitrogen, Carlsbad, CA). The neurons were isolated by trituration for 10 times, centrifuged for 2 min at 200 g and then dissociated in growth medium (Leibovitz’s L-15 medium, Invitrogen, Carlsbad, CA) containing 6% sterile rat serum (ICN, Aurora, OH), 150 mM NaHCO3, 2.25 mg/ml of transferrin, 2.5 μg/ml of insulin, 10 nM progesterone, 90 μM putrescine, 15 nM selenium, 35 mM glucose, 1 mM L-glutamine, penicillin and streptomycin (50 μg/ml), and vitamins. Cells were then plated at a density of ~1.5 ×103 cells/mm2 in 35 mm polylysine/laminin-coated plates (Falcon Labware, Lincoln Park, NJ). Neurons were maintained in growth medium at 37 °C in a humidified atmosphere of 5% CO2 and 95% room air for 10-14 days.
Experimental treatments
Per our prior experimental protocols (11, 17, 36), oxygen-glucose deprivation (OGD) in primary neuronal cells was performed by replacing the media of the cultures in 35 mm2 dishes with cells of 60-70% confluence with glucose-free Hank’s balanced salt solution (HBSS) containing 116 mmol/l NaCl, 5.4 mmol/l KCl, 0.8 mmol/l MgSO4, 1 mmol/l NaH2PO4, 0.9 mmol/l CaCl2, and 10 mg/l phenol red (pH 7.4). Neuronal cultures were then placed into a Bactron II anaerobic glove box (Sheldon manufacturing, Inc, Cornelius, OR) and were maintained in an anoxic environment (95% N2 and 5% CO2) at 37 C for 3 hours. Following this period, the cultures were removed from the anoxic chamber and the glucose-free HBSS was replaced with media containing Dulbecco’s modified Eagle medium (DMEM) (Life Technologies Corp, Carlsbad, CA), supplemented with 10% heat-inactivated fetal bovine serum, 1 mM pyruvate, 1.5 g/L sodium bicarbonate, 100 IU/ml penicillin, and 100 μg/ml streptomycin and maintained at 37°C in 95%/5% (v/v) mixture of humidified atmospheric air and CO2.
For treatments applied prior to OGD, human recombinant WISP1 protein (R&D Systems, Minneapolis, MN) was continuous. The phosphatidylinositol-3-kinase (PI3-K) inhibitors wortmannin (0.5 μM, Calbiochem, La Jolla, CA) and LY294002 (10 μM, Sigma, St Louis, MO), the Akt1 inhibitor A6730 (2 μM, Sigma, St Louis, MO), the SIRT1 agonist SRT1720 [N-(2-(3-(piperazin-1-ylmethyl)imidazo[2,1-b]thiazol-6-yl)phenyl)quinoxaline-2-carboxamide hydrochloride] (Selleck, Munich, Germany), resveratrol (15 μM) [3,5,4′ -trihydroxy-trans-stilbene) (Tocris Bioscience, Ellisville, MO), the SIRT1 inhibitor 6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide (2 μM, EX527, Tocris Bioscience, Ellisville, MO), sirtinol (25 μM, Sigma, St Louis, MO), the caspase 1 inhibitor I (10 μM, American Peptide, Sunnyvale, CA), and the caspase 3 inhibitor I (5 μM, American Peptide, Sunnyvale, CA) were each administered directly to the cultures 1 hour prior to OGD and treatments were continuous.
Assessment of cell survival
Neuronal cell injury was determined by bright field microscopy using a 0.4% trypan blue dye exclusion method 24 hours following treatment with OGD per our previous protocols (35, 37). For each experimental condition, 8 × 35 mm2 dishes were used, and for each dish, the mean survival was determined by counting eight randomly selected non-overlapping fields with each containing approximately 20 cells (viable + non-viable). Each experiment was replicated 6 times with different cultures.
Assessment of DNA Fragmentation
Genomic DNA fragmentation was determined by the terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay (11, 38, 39). Briefly, neuronal cells were fixed in 4% paraformaldehyde/0.2% picric acid/0.05% glutaraldehyde and the 3′-hydroxy ends of cut DNA were labeled with biotinylated dUTP using the enzyme terminal deoxytransferase (Promega, Madison, WI) followed by streptavidin-peroxidase and visualized with 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA).
Akt kinase activity assessment
Per our prior work (40-42), Akt1 activity was determined by using a commercially available nonradioactive Akt1 kinase assay kit with a GSK-3β fusion protein. Cells were lysed in ice with 150 μl of lysis buffer containing 1% Triton X-100, 10% glycerol, 137 mM NaCl, 20 mM Tris-HCl (pH 7.5), 2 μg/ml aprotinin, 2 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, 20 mM NaF, 1 mM Na2PPi, and 1 mM Na3VO4. Equal amounts of lysates (200 μg) were pre-cleared by centrifugation and pre-absorbed with protein A-protein G (1:1) agarose slurry. Immunoprecipitation was carried out over night using the immobilized anti-Akt1G1 monoclonal antibody (Cell Signaling Technology, Beverly, MA) cross-linked to agarose. Immunoprecipitates were washed three times with lysis buffer and twice with Akt kinase buffer (20 mM HEPES, pH 7.4, 10 mM MgCl2, 10 mM MnCl2). Kinase assays were performed for 30 min at 30°C under continuous agitation in kinase buffer containing 200 μM ATP and 1 μg of GSK-3 fusion protein according to the manufacturer’s instructions (Cell Signaling Technology, Beverly, MA). Samples were analyzed by Western blot analysis using 12.5% SDS-polyacrylamide gel and rabbit antibody against p-GSK-3α/β (Cell Signaling Technology, Beverly, MA). Data for the kinase activity were expressed as percentage of control activity.
Expression of Akt1, FoxO3a, SIRT1, acetylated-lysine, caspase 1, and caspase 3 with relevant phosphorylated moieties
Cells were homogenized and following protein determination, each sample (25-50 μg/lane) was then subjected to 7.5% (p- = phosphorylated) (p-Akt1, Akt1, p-FoxO3a, FoxO3a, SIRT1, acetylated-lysine) or 12.5% (caspase 1 and caspase 3) SDS-polyacrylamide gel electrophoresis separation. After blocking for 1 hour at room temperature with 5% skim milk, the membranes were incubated overnight at 4 °C with a rabbit polyclonal antibody against p-Akt1 (Ser473, 1: 1000) (Cell Signaling, Beverly, MA) and total Akt1 (1:1000) (Cell Signaling, Beverly, MA), a rabbit monoclonal antibody against p-FoxO3a (1:1000) (Cell Signaling, Beverly, MA), a rabbit polyclonal antibody against total FoxO3a (1:1000) (Cell Signaling, Beverly, MA), a rabbit polyclonal antibody against SIRT1 (1:200) (Santa Cruz Biotechnologies, Santa Cruz, CA), a rabbit polyclonal antibody against acetylated-lysine (1:1000) (Cell Signaling, Beverly, MA), a rabbit polyclonal antibody against cleaved (active) caspase 1 (20 kDa) (1:1000) (Cell signaling, Beverly, MA), and a rabbit polyclonal antibody against cleaved (active) caspase 3 (17 kDa) (1:1000) (Cell signaling, Beverly, MA). Following incubation, the membranes were incubated with a horseradish peroxidase (HRP) conjugated secondary antibody goat anti-rabbit IgG (goat anti-rabbit IgG, 1:5000) (Pierce, Rockford, IL). The antibody-reactive bands were revealed by chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) and band density was performed using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
Western blot analysis for FoxO3a and SIRT1 in the cytoplasm and the nucleus
Cells were homogenized, the cytoplasmic and nuclear proteins were prepared by using NE-PER nuclear and cytoplasmic extraction reagents according to manufacture’s instructions (Pierce, Rockford, IL). The expression of FoxO3a and SIRT1 in nucleus and cytoplasm was determined by Western blot. Each sample (50 μg/lane) was subjected to 7.5% SDS-polyacrylamide gel electrophoresis and Western blot was performed as description as above.
SIRT1 histone deacetylase (HDAC) activity assay
Per our prior protocols (27), neurons were homogenized and following protein determination, each sample (30 μg/10 μl) was used for SIRT1 activity measurement. SIRT1 histone deacetylase (HDAC) activity was determined with the use of SIRT1 Fluorimetric Drug Discovery Kit (Biomol International, Plymouth Meeting, PA) and following the manufacturer’s protocol. Neuronal protein extracts were incubated in assay buffer with ß-nicotinamide adenine dinucleotide (NAD+) substrate at 37°C for 45 minutes. The fluorescence density was determined using a Multimode Detector (DTX880, Beckman Coulter, Brea, CA) and the relative activity of SIRT1 compared to untreated control neurons was assessed.
Immunocytochemistry for SIRT1 and FoxO3a
For immunocytochemical staining of SIRT1 andFoxO3a, neurons were fixed with 4% paraformaldehyde and permeabilized using 0.2% Triton X-100. Cells were then incubated with rabbit anti-SIRT1 (1:100, Santa Cruz Biotechnologies, Santa Cruz, CA) or anti-FoxO3a (1:100, Cell Signaling Technology, Beverly, MA) over night at 4 °C and then with biotinylated anti-rabbit IgG (1:50, Vector laboratories) for 2 hours followed by Texas Red streptavidin (1:50, Vector laboratories) for 1 hour. Cells were washed in PBS and then stained with DAPI (Sigma, St. Louis, MO) for nuclear identification. SIRT1, FoxO3a, and caspase 3 proteins were imaged with fluorescence at the wavelengths of 565 nm (red) and 400 nm (DAPI nuclear staining).
Transfection of FoxO3a cDNA, SIRT1 cDNA and SIRT1 shRNA constructs
To overexpress FoxO3a or SIRT1 in neurons, primary neurons were seeded into 35 mm dishes at a concentration of 1 × 106 cells/dish. Transfected adenoviral particles Ad-GFP-FoxO3a, Ad-SIRT1, and control vector Ad-Null construct (Vector Biolab, Philadelphia, PA) were administered to the neuronal cultures at a MOI of 500. Seventy-two hours later, reporter gene expression was examined using fluorescence microscopy or Western blot analysis.
To silence SIRT1 expression in neurons, transfection of short hairpin RNA (shRNA) was used against SIRT1 in neurons. Neurons were seeded into 35 mm dishes at a concentration of 1 × 106 cells/well. The media was subsequently removed from plate wells and replaced with 1 ml of Polybrene (5 μg/ml) to increase the infection efficiency. The lentiviral vector construct of shRNA pool for SIRT1 (Santa Cruz, Santa Cruz, CA) was administered into the cultures and incubated overnight. The culture medium with Polybene was replaced with 1 ml of complete medium (without Polybrene). Experimental assays were performed 72 hours post-transfection.
Immunoprecipitation of protein 14-3-3 and FoxO3a
Cell lysates of total protein (200 μg) were incubated with primary antibody against protein 14-3-3 (1:100, Santa Cruz Biotech, Santa Cruz, CA) or FoxO3a (1:100, Cell Signaling Technology, Beverly, MA) overnight at 4 °C. The complexes were collected with protein A/G-agarose beads, centrifuged, and then prepared for 14-3-3 and p-FoxO3a western analysis.
Acetylation of FoxO3a
To determine the expression of acetylated (Ac-) FoxO3a, cell lysates of total protein (200 μg) were incubated with an antibody against FoxO3a (1:100, Cell Signaling, Beverly, MA) overnight at 4°C. The complexes were collected with protein A/G-agarose beads, centrifuged and then prepared for Ac-FoxO3a Western analysis by using a rabbit polyclonal antibody against acetylated-lysine.
Statistical analysis
For each experiment, the mean and standard deviation (SD) was determined. Statistical differences among groups were assessed by means of analysis of variance (ANOVA) with the post-hoc Dunnett’s test. Statistical significance was considered at P<0.05.
Results
WISP1 prevents neuronal cell death during oxidant stress through PI 3-K and Akt1
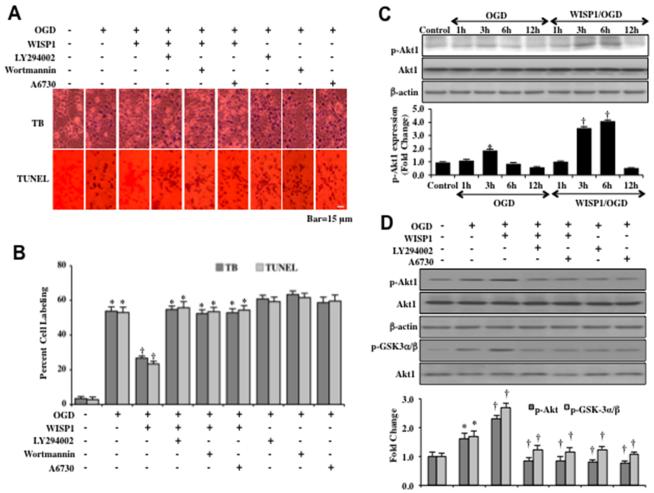
Recombinant human WISP1 protein (10 ng/ml), a concentration that has been demonstrated to offer neuronal protection during oxidative stress (11, 12), was administered to neuronal cultures 1 hour prior to a 3 hour period of OGD and cell injury was determined 24 hours later through trypan blue dye exclusion method and the TUNEL assay. In Figures 1A and 1B, OGD exposure resulted in significant trypan blue dye exclusion uptake and TUNEL staining in primary neurons. Application of WISP1 (10 ng/ml) significantly limited trypan blue uptake and TUNEL staining. However, application of the PI 3-K inhibitors wortmannin (0.5 μM) and LY294002 (20 μM) or the Akt1 direct inhibitor A6730 (2 μM) during treatment with WISP1 (10 ng/ml) prevented neuronal protection (Figures 1A and 1B). To inhibit PI 3-K, wortmannin (0.5 μM) forms a covalent link with the lysine residue of PI 3-K (43) and LY294002 (20 μM) reversibly competes for ATP binding (44).
Figure 1. WISP1 protects neurons against OGD through PI 3-K and Akt1.
(A) Representative images demonstrate that OGD leads to a significant increase in trypan blue staining and DNA fragmentation in neuronal cells 24 hours after a 3 hour period of OGD when compared to untreated control cultures. WISP1 (10 ng/ml) application 1 hour prior to OGD significantly decreases trypan blue and TUNEL staining. Yet, treatment with the specific PI 3-K inhibitors wortmannin (0.5 μM), LY294002 (10 μM), or the Akt1 inhibitor A6730 (2 μM) abrogates the ability of WISP1 to reduce trypan blue staining and DNA fragmentation. (B) Quantitative results illustrate that WISP1 (10 ng/ml) application significantly decreases percent trypan blue uptake and DNA fragmentation 24 hours following OGD exposure when compared to OGD exposure alone. Treatment with the specific PI 3-K inhibitors wortmannin, LY294002, or the Akt1 inhibitor A6730 significantly reduce the protective capacity of WISP1, resulting in an increase in percent trypan blue uptake and DNA fragmentation (*p <0.01 vs. untreated control; †p<0.01 vs. OGD). Each data point represents the mean and SD from 6 experiments. (C) Equal amounts of neuronal protein extracts (50 μg/lane) were immunoblotted at 1, 3, 6 and 12 hours following OGD exposure with the anti–phospho-Akt1 (p-Akt1, Ser473) antibody. The expression of p-Akt1 was mildly increased at 3 hours following OGD exposure and was significantly increased by WISP1 (10 ng/ml) administration 1 hour prior to OGD (*p<0.01 vs. Control; †p<0.01 vs. OGD of corresponding time point). Quantitative analysis of the Western blots from 3 experiments was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image/). (D) Representative images of western blot analysis illustrate that phospho-Akt1 (p-Akt1) expression was mildly increased 3 hours following OGD exposure but significantly increased by WISP1 (10 ng/ml) application. Assessment and quantitation of Akt1 kinase activity through the fusion protein GSK-3α/β and analysis of p-GSK-α/β expression also demonstrate that Akt1 kinase activity was mildly increased during OGD exposure when compared with control samples. Yet, the expression of p-Akt1 or Akt1 kinase activity during WISP1 (10 ng/ml) application was decreased by a 1-hour pretreatment with the Akt1 inhibitor A6730 (2 μM) or the PI 3-K inhibitor LY294002 (10 μM) (*p< 0.01 vs. control; †p<0.01 vs. OGD). Quantitative analysis of western blots from 3 experiments was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image/).
To investigate the role of WISP1 on the activation of Akt1 during progressive OGD exposure, western blot assay was performed for phosphorylated Akt1 (p-Akt1) (activated form of Akt1) and total Akt1 at 1, 3, 6, and 12 hours following a 3 hour period of OGD (Figure 1C). Administration of WISP1 (10 ng/ml) 1 hour prior to OGD significantly enhanced p-Akt1 expression in neurons. As shown in Figure 1C, the expression of p-Akt1 was significantly increased at 3 hours and 6 hours following OGD by WISP1. However, the increased expression of p-Akt1 by WISP1 was abrogated by the application of the PI 3-K inhibitor LY294002 (10 μM) and the Akt1 direct inhibitor A6730 (2 μM) (Figure 1D). In addition, we determined Akt1 activity through a GSK-3β fusion protein and assessed p-GSK-3α/β expression. As shown in Figure 1D, WISP1 (10 ng/ml) administration 1 hour prior to OGD significantly increased the activity of Akt1 (represented by p-GSK-3α/β expression) 3 hours following OGD. This increased Akt1 activity by WISP1 was abolished by administration of LY294002 (10 μM) or A6730 (2 μM) with or without the presence of WISP1 during OGD exposure (Figure 1D).
WISP1 fosters the post-translational phosphorylation of FoxO3a and the cytoplasmic retention of FoxO3a through PI 3-K and Akt1
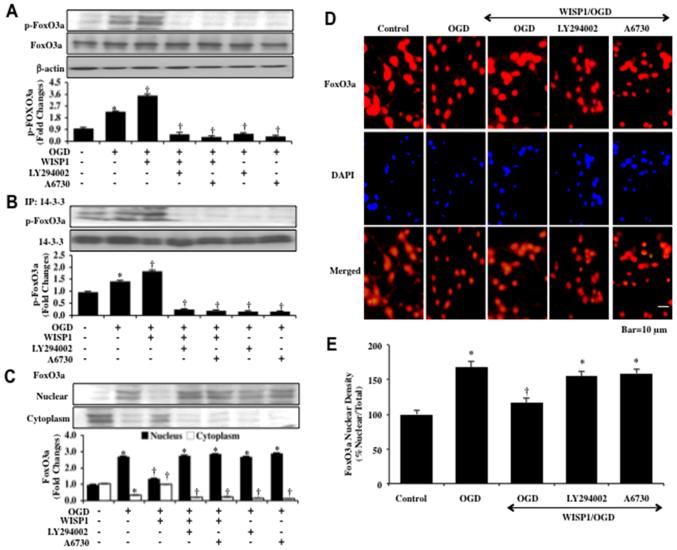
Western blot assay was performed for phosphorylated FoxO3a (p-FoxO3a) at Ser253, a preferential phosphorylation site for Akt1, as well as for the expression of total FoxO3a 3 hours following OGD exposure. OGD alone increased the expression of p-FoxO3a when compared to control cultures (Figure 2A). Yet, WISP1 (10 ng/ml) administered 1 hour prior to OGD significantly increased the expression of p-FoxO3a. The ability of WISP1 to phosphorylate FoxO3a was blocked during application of the PI 3-K inhibitor LY294002 (10 μM) or the Akt1 inhibitor A6730 (2 μM) (Figure 2A).
Figure 2. WISP1 phosphorylates FoxO3a and promotes its cytoplasmic retention during OGD through PI 3-K and Akt1.
(A) Equal amounts of neuronal protein extracts (50 μg/lane) were immunoblotted at 3 hours following OGD exposure with anti–phospho-FoxO3a (p-FoxO3a) antibody. The expression of p-FoxO3a was increased at 3 hours following OGD exposure but was significantly increased by WISP1 (10 ng/ml) administration 1 hour prior to OGD. Application of LY294002 (10 μM) or A6730 (2 μM) 1 hour prior to a 3 hour period of OGD blocked WISP1 expression of p-FoxO3a 3 hours following OGD (*p<0.01 vs. Control; †p<0.01 vs. OGD). Quantitative analysis of western analysis from 3 experiments was performed using the public domain NIH Image program (developed at the US National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). (B) Neuronal cell protein extracts were immunoprecipitated by using anti-14-3-3 antibody 3 hours following a 3 hour period of OGD and western blot analysis for p-FoxO3a and 14-3-3 precipitates was performed. OGD resulted in an increase in the expression of p-FoxO3a precipitated with 14-3-3. Application of WISP1 (10 ng/ml) 1 hour prior to OGD further increased the expression of p-FoxO3a precipitated with 14-3-3. Application of LY294002 (10 μM) or A6730 (2 μM) blocked the ability of WISP1 to promote the precipitation of p-FoxO3a precipitated with 14-3-3 (*p< 0.01 vs. untreated control; †p<0.01 vs. OGD). Quantification of western blot band intensity was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image/). Each data point represents the mean and SD from 3 experiments. (C) Equal amounts of cytoplasmic (cytoplasm) or nuclear (nucleus) protein extracts were immunoblotted with anti-FoxO3a antibody at 3 hours following a 3 hour period of OGD. WISP1 (10 ng/ml) significantly decreased the expression of FoxO3a in the nuclei of neurons following OGD. Application of the Akt1 inhibitor A6730 (2 μM) or the PI 3-K inhibitor LY294002 (10 μM) promoted the nuclear translocation of FoxO3a during WISP1 administration and OGD exposure (*p<0.01 vs. Control; †p< 0.01 vs. OGD). Quantitative analysis of the western blot data was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image/). (D) WISP1 (10 ng/ml) was administered 1 hour prior to a 3 hour period of OGD and immunofluorescence staining for FoxO3a (Texas-red) was performed 3 hours following OGD exposure. Nuclei of neurons were counterstained with DAPI. In merged images, cells with OGD alone show neuronal nuclei with significant FoxO3a staining (nuclei primarily red) and minimal FoxO3a staining (yellow) in the cytoplasm. In contrast, FoxO3a was predominantly in the cytoplasm of neurons (red) in neurons treated with WISP1 (10 ng/ml) during OGD exposure. Treatment of the Akt1 inhibitor A6730 (2 μM) or the PI 3-K inhibitor LY294002 (10 μM) with WISP1 resulted in predominantly nuclear staining of FoxO3a. (E) Intensity of FoxO3a nuclear staining was evaluated using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image/) (*p<0.01 vs. Control; †p< 0.01 vs. OGD) and control = untreated neurons.
Post-translational phosphorylation of FoxO3a leads to the association of FoxO3a with 14-3-3 protein and retention of FoxO3a in the cytoplasm (17, 45). We therefore examined whether WISP1 altered the binding of FoxO3a to protein 14-3-3 by immunoprecipitation (Figure 2B). WISP1 (10 ng/ml) during OGD exposure significantly increased the expression of p-FoxO3a in the lysate that was immunoprecipitated by antibody against 14-3-3 protein when compared to untreated neurons or neurons exposed to OGD alone. Treatment with WISP1 during OGD exposure or exposure to OGD alone with the PI 3-K inhibitor LY294002 (20 μM) or the Akt1 inhibitor A6730 (2 μM) significantly limited the expression of p-FoxO3a in the lysate that was immunoprecipitated by antibody against 14-3-3 protein, illustrating that WISP1 relies upon the PI 3-K and Akt1 pathways to promote FoxO3a binding to 14-3-3 (Figure 2B).
Since WISP1 can maintain post-translational phosphorylation of FoxO3a, we next examined whether WISP1 controls the subcellular trafficking of FoxO3a. In Figure 2C, WISP1 (10 ng/ml) was applied to neuronal cultures 1 hour prior to a 3 hour period of OGD and western blot analysis for FoxO3a in cell protein extracts of both nucleus and cytoplasm was performed. OGD exposure alone results in a significant expression of FoxO3a in the nuclei of neurons. In contrast, WISP1 (10 ng/ml) during OGD exposure maintains a high expression of FoxO3a in the cytoplasm of neurons similar to untreated control neurons. However, application of WISP1 during OGD exposure with the PI 3-K inhibitor LY294002 (20 μM) or the Akt1 inhibitor A6730 (2 μM) blocked the ability of WISP1 to maintain expression of FoxO3a in the cytoplasm of neurons (Figure 2C). We next performed immunofluorescent staining for FoxO3a and DAPI nuclear staining in primary neurons to follow the subcellular translocation of FoxO3a 3 hours following OGD exposure (Figures 2D and 2E). During OGD exposure, immunofluorescent staining for FoxO3a in the nucleus of neurons is markedly present. This is demonstrated by the inability to visualize a majority of DAPI nuclear staining (yellow in color) in cells during merged images since prominent FoxO3a staining is present in the nucleus (Figure 2D). Inhibition of PI 3-K with the inhibitor LY294002 (20 μM) or Akt1 with the inhibitor A6730 (2 μM) during OGD exposure also promotes the translocation of FoxO3a from the cell cytoplasm to the nucleus in neurons (Figures 2D and 2E). Application of WISP1 (10 ng/ml) during OGD exposure maintains FoxO3a in the cytoplasm of neurons similar to untreated control cells as shown by nuclear staining with DAPI (yellow nuclei in color) in the nuclei of neurons of merged images (Figures 2D and 2E).
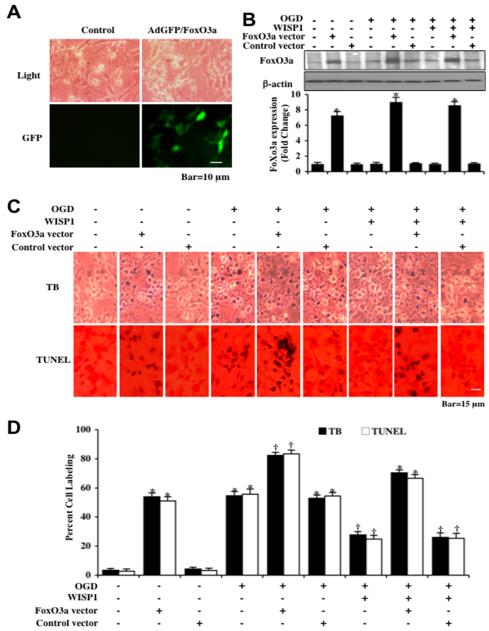
Over-expression of FoxO3a results in neuronal cell injury independently and during OGD exposure
Neurons were transfected with Ad-GFP-FoxO3a construct and the expression of FoxO3a was illustrated by imaging GFP in neurons with greater than 95% of neurons successfully transfected (Figure 3A). Expression of FoxO3a protein was assessed by immunocytochemistry and Western blot analysis without OGD and 3 hours following OGD (Figure 3B). Over-expression of FoxO3a in either untreated control neurons or in neurons exposed to OGD alone resulted in significant expression of FoxO3a protein (Figure 3B). As a control, empty vector transfection did not alter FoxO3a protein expression in untreated control cells or neurons exposed to OGD (Figure 3B). In Figure 3C, representative figures illustrate significant trypan blue and TUNEL staining in neurons 24 hours after OGD exposure alone or with vector transfection. In addition, more pronounced trypan blue and TUNEL staining is present in neurons following OGD with FoxO3a over-expression (Figures 3 C and 3D), demonstrating that over-expression of FoxO3a increases neuronal cell death during OGD exposure. Application of WISP1 (10 ng/ml) 1 hour prior to OGD significantly reduced trypan blue staining and DNA fragmentation in all scenarios, but the ability of WISP1 to reduce injury was attenuated by over-expression of FoxO3a in neurons.
Figure 3. Over-expression of FoxO3a increases neuronal injury during OGD.
(A) Representative images are illustrated following transfection of adenoviral construct of GFP-FoxO3a in neurons. Top panel illustrates neuron cells acquired through transmitted light and bottom panel represents FoxO3a overexpression tracked with GFP (green fluorescence protein). (B) Equal amounts of neuronal protein extracts (50 μg/lane) were immunoblotted with anti-FoxO3a antibody at 3 hours after OGD exposure. The expression of total FoxO3a was significantly increased by adenoviral FoxO3a cDNA construct transfection (*p<0.01 vs. Control). Quantitative analysis of the western blots from 3 experiments was performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image/). (C) Representative images demonstrate that FoxO3a over-expression or OGD led to a significant increase in trypan blue staining and DNA fragmentation in neuronal cells 24 hours after OGD compared to untreated control cultures. WISP1 (10 ng/ml) application 1 hour prior to OGD significantly decreased trypan blue and TUNEL staining. FoxO3a over-expression blocked WISP1 neuronal protection. (D) Quantitative results illustrate that WISP1 (10 ng/ml) application significantly decreased percent trypan blue uptake and DNA fragmentation 24 hours after OGD when compared to OGD treated alone, but FoxO3a over-expression prevented WISP1 neuronal protection. (*p <0.01 vs. untreated control; †p<0.01 vs. OGD). Each data point represents the mean and SD from 6 experiments.
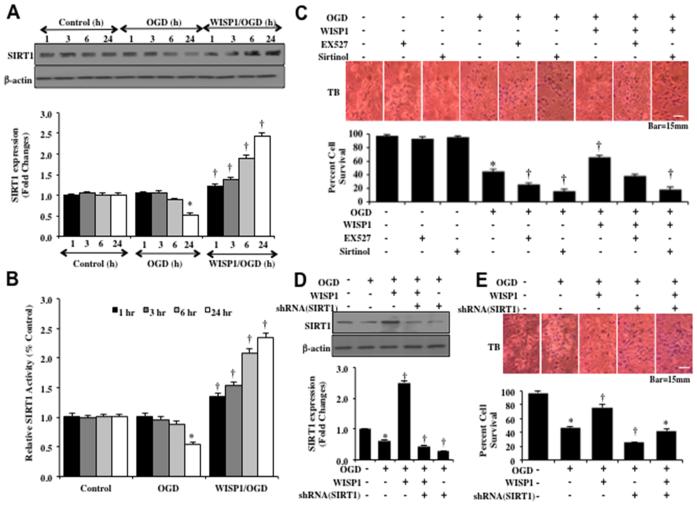
WISP1 increases endogenous SIRT1 activity and expression to promote neuronal cell protection
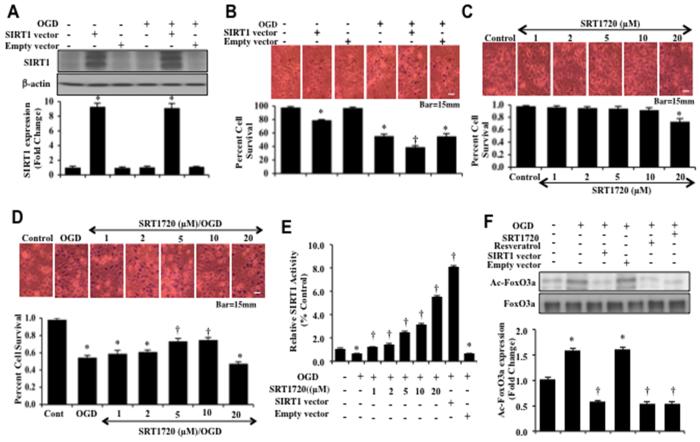
Western blot assay with hippocampal neurons protein extracts (50 μg/lane) were immunoblotted with anti-SIRT1 antibody at 1, 3, 6 and 24 hours following a 3 hour period of OGD. In Figure 4A, expression of endogenous SIRT1 was progressively decreased over 24 hours after OGD exposure. Application of WISP1 (10 ng/ml) 1 hour prior to OGD prevented the loss of SIRT1 expression and significantly increased the expression of SIRT1 in neurons at 1, 3, 6 and 24 hours to a greater degree than OGD alone (Figure 4A). We next examined whether WISP1 (10 ng/ml) could alter SIRT1 activity assessed by relative HDAC activity at 1, 3, 6 and 24 hours following OGD exposure (Figure 4B). Over a 24 hour course, OGD exposure led to a decrease in SIRT1 activity. However, Administration of WISP1 (10 ng/ml) 1 hour prior to OGD significantly increased HDAC activity of SIRT1 at 1, 3, 6 and 24 hours following OGD (Figure 4B).
Figure 4. WISP1 increases the endogenous expression and activity of SIRT1 that leads to neuronal protection during OGD exposure.
(A) Equal amounts of neuronal protein extracts (50 μg/lane) were immunoblotted at 1, 3, 6, and 24 hours after a 3 hour period of OGD with anti–SIRT1 antibody. The expression of SIRT1 was decreased at 6 and 24 hours following OGD exposure but was significantly increased by WISP1 (10 ng/ml) administration 1 hour prior to OGD at 3, 6 and 24 hours following OGD exposure (*p<0.01 vs. Control; †p<0.01 vs. OGD of corresponding time point). Quantitative analysis of the western blots from 3 experiments was performed using the public domain NIH image program (http://rsb.info.nih.gov/nih-image/). (B) WISP1 (10 ng/ml) was applied to neuronal cultures 1 hour prior to OGD and SIRT1 HDAC activity was determined at 1, 3, 6 and 24 hours following OGD. HDAC activity in neurons was significantly decreased at 24 hours after OGD (*P<0.05 vs. Control). WISP1 significantly increased HDAC in neurons (†p<0.05 vs. OGD). Each data point represents the mean and SD from 3 experiments. (C) Representative images and quantitative results of trypan blue staining demonstrate that OGD led to a significant increase in trypan blue staining in neurons at 24 hours after OGD compared to untreated control cultures. Trypan blue staining was significantly reduced by WISP1 (10 ng/ml) application. Yet, inhibition of SIRT1 activity with EX527 (2 μM) or sirtinol (25 μM) significantly increased apoptotic injury during OGD and attenuated the protective capacity of WISP1 (10 ng/ml) (*p<0.01 vs. untreated control; †p<0.05 vs. OGD). Inhibition of SIRT1 activity with EX527 (2 μM) or sirtinol (25 μM) significantly increased apoptotic injury to a greater level beyond OGD alone and significantly limited the protective capacity of WISP1 (10 ng/ml). (D) Equal amounts of neuronal protein extracts (50 μg/lane) were immunoblotted with anti–SIRT1 antibody at 24 hours after OGD. WISP1 (10 ng/ml) promotes the expression of SIRT1 24 hours following OGD. In contrast, cells with transfection of shRNA SIRT1 yield minimal SIRT1 expression. (*p<0.01 vs. Control; †p<0.01 vs. OGD). Quantitative analysis of the western blots from 3 experiments was performed using the public domain NIH image program (http://rsb.info.nih.gov/nih-image/). (E) Representative images demonstrate that OGD results in a significant increase in trypan blue staining in neurons at 24 hours after OGD compared to untreated control cultures. WISP1 (10 ng/ml) application significantly prevents trypan blue dye exclusion uptake. Transfection of shRNA SIRT1 significantly increased neuronal injury during OGD and significantly limited the ability of WISP1 (10 ng/ml) to prevent neuronal injury. Quantification of these results illustrates that WISP1 (10 ng/ml) application significant decreased percent trypan blue uptake 24 hours after OGD when compared to OGD treated alone. Transfection of shRNA SIRT1 significantly increased percent cell labeling during OGD treated alone and significantly attenuated the protective capacity of WISP1 (10 ng/ml) to reduce percent neuronal injury (*p<0.01 vs. untreated control; †p<0.05 vs. OGD). Each data point represents the mean and SD from 6 experiments.
To investigate whether SIRT1 activation is required for WISP1 neuronal protection, neuronal cell survival was assessed with trypan blue staining 24 hours following OGD. Representative images demonstrate that untreated control neurons had minimal trypan blue staining, but OGD led to a significant increase in trypan blue staining (Figure 4C). WISP1 (10 ng/ml) applied 1 hour prior to OGD significantly reduced trypan blue staining in neurons (Figure 4C). Yet, administration of the specific small-molecule inhibitor of SIRT1 catalytic activity EX527 (2 μM) (46) EX527 (2 μM) or the SIRT1 inhibitor sirtinol (25 μM) increased cell injury and blocked the ability of WISP1 to prevent neuronal cell injury during OGD exposure, suggesting that SIRT1 is necessary for WISP1 to protect neurons against OGD (Figure 4C). In addition, transfection of the lentiviral vector construct of shRNA against SIRT1 to knockdown SIRT1 expression in neurons significantly reduced SIRT1 expression in neurons (Figure 4D), increased neuronal cell injury in neurons exposed to OGD alone (Figure 4E), and limited the ability of WISP1 (10 μM) to protect neurons against OGD (Figure 4E), illustrating that SIRT1 provides both endogenous neuronal cell protection and is necessary, at least in part, for WISP1 neuronal protection. Transfection with empty vector did not alter cell injury (data not shown).
Limited SIRT1 activation prevents neuronal injury and leads to FoxO3a deacytelation
Neurons were transfected with Ad-SIRT1 cDNA construct and the expression of SIRT1 protein was assessed by Western blot analysis without OGD and 3 hours following OGD (Figure 5A). Over-expression of SIRT1 in either untreated control neurons or in neurons exposed to OGD alone resulted in significant expression of SIRT1 protein (Figure 5A). Empty vector transfection did not alter SIRT1 expression in untreated control cells or neurons exposed to OGD (Figure 5A). Representative figures illustrate significant trypan blue staining in neurons 24 hours after OGD exposure. SIRT1 vector transfection either alone or during OGD exposure significantly increased neuronal cell injury when compared to control or OGD exposed neurons.
Figure 5. Limited SIRT1 activation prevents neuronal injury and leads to FoxO3a deacytelation.
(A) Equal amounts of neuronal protein extracts (50 μg/lane) were immunoblotted with anti–SIRT1 antibody at 3 hours following OGD exposure. Total SIRT1 expression was significantly increased by SIRT1 adenoviral construct transfection without and with OGD exposure (*p<0.01 vs. Control). Quantitative analysis of the western blots from 3 experiments was performed using the public domain NIH image program (thttp://rsb.info.nih.gov/nih-image/). Control empty vector did not alter neuronal survival. (B) Representative images of primary neurons demonstrate that OGD exposure results in a significant increase in trypan blue staining in neurons at 24 hours after OGD when compared to untreated control cultures. Transfection of SIRT1 adenoviral construct significantly increased neuronal injury in untreated neurons and during OGD exposure. Quantification of these results illustrate that SIRT1 over-expression significantly increased trypan blue percent uptake 24 hours after OGD when compared to neurons exposed to OGD alone (*p<0.01 vs. untreated control; †p<0.05 vs. OGD). Control empty vector did not alter neuronal survival. (C) Representative images demonstrate that the SIRT1 agonist SRT1720 at the concentrations of 1, 2, 5, and 10 μM was not toxic to neurons, but SRT1720 at 20 μM results in significant neuronal injury. Quantification of these results illustrate that SRT1720 alone at the concentrations of 1, 2, 5, and 10 μM did not change percent trypan blue staining. SRT1720 at 20 μM significantly increases neuronal injury. (D) Increasing concentrations of SRT1720 (1, 2, 5, 10, and 20 μM) were administered to neuronal cultures 1 hour prior to a 3 hour period of OGD and cell injury was determined by trypan blue dye exclusion 24 hours after OGD. SRT1720 protected neurons against OGD in a concentration dependent manner, most prominently increasing neuronal survival at the concentrations of 5 μM and 10 μM. SRT1720 at 20 μM during OGD exposure decreased neuronal survival to a greater extent than OGD exposure alone (*p<0.01 vs. control; †p< 0.01 vs. OGD). (E) SIRT1 adenoviral construct was transfected into neurons or increasing concentrations of SRT1720 (1, 2, 5, 10, and 20 μM) was administered 1 hour prior to OGD and SIRT1 HDAC activity was assessed 24 hour following OGD exposure. HDAC activity in neurons significantly decreased at 24 hours after OGD (*P<0.05 vs. Control). SRT1720 and SIRT1 over-expression significantly increased HDAC activity in neurons (†p<0.05 vs. OGD). Each data point represents the mean and SD from 3 experiments. (F) Neuronal cell protein extracts were immunoprecipitated by using FoxO3a antibody 3 hours following a 3 hour period of OGD and western blot analysis for acetylated lysine (acetylation of FoxO3a, Ac-FoxO3a) was performed. OGD resulted in an increase in the acetylation of FoxO3a. SIRT1 over-expression and treatment with SRT1720 (10 μM) or resveratrol (15 μM) 1 hour prior to OGD decreased the acetylation of FoxO3a (*p < 0.01 vs. untreated control; †p <0.01 vs. OGD). Empty vector transfection did not alter FoxO3a acetylation. Quantification of western band intensity was performed using the public domain NIH image program (http://rsb.info.nih.gov/nih-image). Each data point represents the mean and SD from 3 experiments.
In Figure 5C, representative figures demonstrate that the SIRT1 agonist SRT1720 was not toxic to neurons at the concentrations of 1, 2, 5 and 10 μM. At the SRT1720 concentration of 20 μM, neuronal cell survival was decreased. During exposure to OGD, SRT1720 at the concentrations of 1, 2, 5 and 10 μM decreased trypan blue uptake and significantly increased neuronal survival (Figure 5D). The SRT1720 concentration of 20 μM was not neuroprotective and decreased neuronal survival to a greater degree than exposure to OGD alone (Figure 5D).
Given the neuronal toxicity observed with over-expression of SIRT1 and elevated SRT1720 concentration of 20 μM, we next assessed SIRT1 HDAC relative activity during SIRT1 over-expression and administration of SRT1720 (Figure 5E). SRT1720 at the concentrations of 1, 2, 5 and 10 μM increased HDAC relative activity between 2% to 4% over control that was similar to the degree WISP1 increases HDAC activity during neuroprotection against OGD (Figure 4B). In contrast, SIRT1 over-expression and the SRT1720 concentration of 20 μM, both toxic to neurons, markedly increased HDAC activity above control by 6% to 8% which may account for the toxicity observed with these agents (Figure 5E).
We subsequently investigated the ability of SIRT1 activation to alter the acetylation of FoxO3a during OGD. The SIRT1 agonist SRT1720 (10 μM) or the SIRT1 agonist resveratrol (15 μM) was administered 1 hour prior to a 3 hour period of OGD. During over-expression of SIRT1, neurons was transfected with Ad-SIRT1 cDNA construct prior to OGD. Neuronal protein extracts following OGD were then immunoprecipited using an antibody against FoxO3a and Western blot analysis for Ac-lysine was determined. As shown in Figure 5F, representative images for the acetylation of FoxO3a demonstrate that OGD alone resulted in a significant increase in the expression of acetylated FoxO3a when compared with untreated control cultures. However, application of the SIRT1 agonists SRT1720 or resveratrol, or over-expression of SIRT1 resulted in the deacetylation of FoxO3a (Figure 5F).
WISP1 promotes nuclear translocation of SIRT1 during OGD exposure
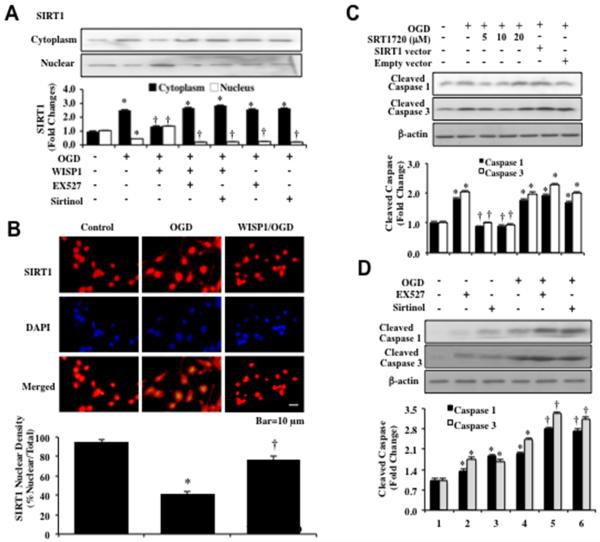
We assessed SIRT1 subcellular translocation from the cell cytoplasm to the nucleus through western analysis (Figure 6A). WISP1 (10 ng/ml) was applied to neuronal cultures 1 hour prior to a 3 hour period of OGD. Western blot analysis for SIRT1 was then assessed in the extracts from both the nucleus and cytoplasm of neurons. OGD resulted in an increase in cytoplasmic expression of SIRT1 and a decrease in nuclear expression of SIRT1. In contrast, WISP1 significantly increased the expression of SIRT1 in the nuclei of neurons. Inhibition of SIRT1 activity with EX527 (2 μM) or sirtinol (25 μM) during WISP1 application and OGD exposure prevented WISP1 from increasing the expression of SIRT1 in the nuclei of neurons (Figure 6A). We next performed immunofluorescent staining for SIRT1 and DAPI nuclear staining to follow the subcellular translocation of SIRT1 3 hours after OGD exposure (Figure 6B). In the presence of OGD, immunofluorescent staining for SIRT1 is primarily in the cytoplasm of neurons as shown by nuclear staining with DAPI (yellow nuclei in color) in the nuclei of neurons of merged images. WISP1 (10 ng/ml) promotes the nuclear translocation of SIRT1 as demonstrated by the inability to visualize a majority of DAPI nuclear staining (yellow in color) in cells during merged images (Figure 6B).
Figure 6. WISP1 promotes SIRT1 nuclear shuttling and proportionate SIRT1 activation blocks pro-caspase cleavage.
(A) Equal amounts of cytoplasmic or nuclear protein extracts (50 μg/lane) were immunoblotted with anti–SIRT1 antibody at 3 hours following a 3 hour period of OGD. SIRT1 expression is confined to the cytoplasm following OGD exposure, but WISP1 (10 ng/ml) applied 1 hour prior to OGD leads to the translocation of SIRT1 from the cytoplasm to the nucleus. SIRT1 inhibitors EX527 (2 μM) or sirtinol (25 μM) prevent the translocation of SIRT1 to the nucleus during OGD exposure (*p<0.01 vs. Control; †p<0.01 vs. OGD). Quantification of band density of SIRT1 was performed using the public domain NIH image program (http://rsb.info.nih.gov/nih-image). (B) WISP1 (10 ng/ml) was administered 1 hour prior to a 3 hour period of OGD exposure and immunofluorescence staining for SIRT1 (Texas-red streptavidin) was performed 3 hours after OGD exposure. Nuclei of neurons were counterstained with DAPI. In merged images, cells with OGD alone show neuronal nuclei with reduce SIRT1 staining (yellow) with significant SIRT1 staining (red) in the cytoplasm. In contrast, neurons treated with WISP1 (10 ng/ml) during OGD exposure have SIRT1 predominantly in the nuclei of neurons (nuclei primarily red). Quantification of the intensity of SIRT1 nuclear staining was performed using the public domain NIH image program (http://rsb.info.nih.gov/nih-image). Control = untreated neurons (*p<0.01 vs. Control; †p<0.01 vs. OGD). (C) and (D) Equal amounts of protein extracts (50 μg/lane) of neurons were immunoblotted with antibodies against cleaved caspase 1 and caspase 3 (active forms) at 3 hours following a 3 hour period of OGD exposure. Administration of the SIRT1 agonist SRT1720 at 5 μM or 10 μM 1 hour prior to OGD significantly reduced the expression of active caspase 1 and caspase 3. Yet, transfection of SIRT1 adenoviral construct or application of SRT1720 (20 μM) during OGD exposure increased caspase 1 and caspase 3 cleavage 3 hours following OGD exposure. Empty vector transfection did not alter caspase 1 or caspase 3 cleavage during OGD exposure (C). In (D), exposure to OGD alone as well as the administration of the SIRT1 inhibitors EX527 (2 μM) or sirtinol (25 μM) 1 hour prior to OGD significantly increased the expression of cleaved caspase 1 and caspase 3 (*p < 0.01 vs. Control; †p <0.01 vs. OGD). Quantification of western band intensity was performed using the public domain NIH image program (http://rsb.info.nih.gov/nih-image). Each data point represents the mean and SD from 3 experiments.
Proportionate SIRT1 activation prevents pro-caspase cleavage during OGD exposure
Since WISP1 relies upon SIRT1 activation to prevent apoptotic injury in primary neurons during OGD, we investigated whether SIRT1 alters caspase 1 and caspase 3 activity during OGD exposure (Figures 6C and 6D). In Figure 6C, the expression of cleaved (active) caspase 1 and caspase 3 on western analysis were assessed at 3 hours following OGD exposure that resulted in significant caspase 1 and caspase 3 cleavage. Administration of SRT1720 at the concentrations of 5 and 10 μM significantly prevented caspase 1 and caspase 3 cleavage during OGD exposure. Yet, administration of SRT1720 at the concentration of 20 μM or SIRT1 over-expression that significantly increase SIRT1 activity did not prevent caspase 1 and caspase 3 cleavage during OGD exposure. In addition, inhibition of SIRT1 activity with EX527 (2 μM) or sirtinol (25 μM) during OGD exposure resulted in greater caspase 1 and caspase 3 cleavage than during OGD exposure alone, suggesting that a minimal level of SIRT1 activity is required to block caspase 1 and caspase 3 activity (Figure 6D).
WISP1 preserves SIRT1 integrity through the modulation of FoxO3a generated caspase 1 and caspase 3 activity
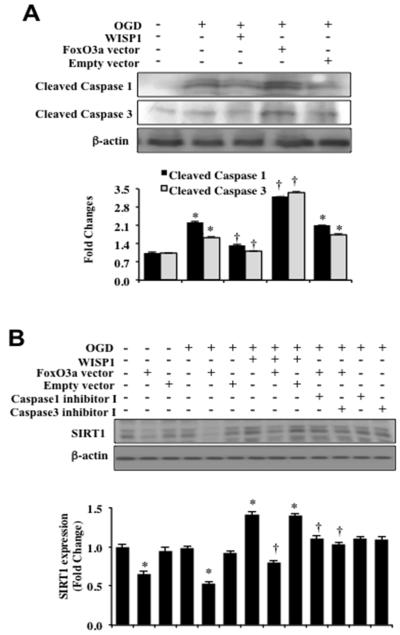
We next evaluated the ability of WISP1 to control caspase activity 3 hours following OGD exposure in neurons (Figure 7A). Expression of cleaved active caspase 1 and caspase 3 was significantly elevated over untreated control levels in neurons following OGD exposure, but WISP1 (10 ng/ml) significantly prevented cleaved active caspase 1 and caspase 3 activities. Neurons were also transfected with Ad-FoxO3a construct (Figure 7A). Over-expression of FoxO3a in neurons exposed to OGD resulted in significant caspase 1 and caspase 3 activities. Empty vector transfection did not caspase 1 and caspase 3 activities (Figure 7A).
Figure 7. WISP1 prevents the cleavage of SIRT1 by inhibiting FoxO3a mediated caspase activation during OGD.
(A) Equal amounts of protein extracts (50 μg/lane) of neurons were immunoblotted with antibodies against cleaved caspase 1 and caspase 3 (active forms) at 3 hours following a 3 hour period of OGD exposure. OGD alone significantly increased the expression of cleaved caspase 1 and caspase 3. In addition, transfection of adenoviral FoxO3a cDNA construct during OGD exposure significantly increased the expression of caspase 1 and caspase 3. Administration of WISP1 (10 ng/ml) 1 hour prior to OGD exposure prevented cleavage of caspase 1 and caspase 3 (*p < 0.01 vs. Control; †p <0.01 vs. OGD). Quantitative analysis of the western blots from 3 experiments was performed using the public domain NIH image program (http://rsb.info.nih.gov/nih-image/). (B) Equal amounts of neuronal protein extracts (50 μg/lane) were immunoblotted at 3 hours following a 3 hour period of OGD with anti-SIRT1 antibody. WISP1 (10 ng/ml) administration 1 hour prior to OGD significantly increased the expression of SIRT1. In contrast, FoxO3a over-expression significantly blocked the expression of SIRT1 without OGD exposure and during OGD exposure. FoxO3a over – expression also limited SIRT1 expression during administration of WISP1 (10 ng/ml) and OGD exposure. Application of the caspase1 inhibitor I or the caspase 3 inhibitor I maintained the expression of SIRT1 during FoxO3a over-expression and during OGD exposure (*p<0.01 vs. Control; †p<0.01 vs. FoxO3a/OGD). Empty vector transfection did not alter SIRT1 expression without OGD exposure or during OGD exposure with or without WISP1 (10 ng/ml) administration. Quantitative analysis of the western blots from 3 experiments was performed using the public domain NIH image program (http://rsb.info.nih.gov/nih-image/).
We also examined the effect of FoxO3a over-expression on SIRT1 expression. FoxO3a cDNA adenoviral construct was transfected into neuronal cultures and the expression of SIRT1 was determined 3 hours following OGD. As shown in Figure 7B, over-expression of FoxO3a significantly decreased the expression of SIRT1 with or without OGD exposure. WISP1 (10 ng/ml) administration 1 hour prior to OGD significantly increased the expression of SIRT1 during OGD exposure. However, the ability of WISP1 to maintain SIRT1 expression was compromised during over-expression of FoxO3a (Figure 7B). SIRT1 expression also was preserved during OGD exposure during application of inhibitors directed against caspase 1 or caspase 3, suggesting that caspase 1 and caspase 3 were responsible for the degradation of SIRT1.
Discussion
In multiple systems of the body, WISP1 can promote cellular development, survival, and if unchecked, tumorigenesis. During toxin exposure, WISP1 is up-regulated in primary kidney proximal tubule cells (47), cardiomyocytes (48), saphenous veins (49), inflammatory microglia (10), alveolar epithelial cells (50), and primary neurons (11, 12). WISP1 also appears to be vital for tissue development such as with pancreatic cells (51) and bone formation (7, 8, 52). The effects of WISP1 also extend to cellular hypertrophy (5) and cancer progression (53-55). In light of the proliferative cellular role for WISP1, these observations suggest a potential cytoprotective capacity for WISP1 under the appropriate conditions.
Consistent with prior studies, we show that WISP1 requires PI 3-K and Akt1 to protect primary neurons against OGD exposure. During pharmacological blockade of PI 3-K or Akt1, WISP1 cannot protect primary neurons from OGD. WISP1 also significantly increases and maintains the phosphorylation and activity of Akt1 in neurons during oxidative stress. PI 3-K and Akt have been shown to prevent cellular injury in multiple cell types (56) that can impact the nervous system such as endothelial cells (16, 28, 32, 37, 57, 58), inflammatory cells (36, 59-61), and neurons (40, 62-65). Outside of the nervous system, WISP1 has been shown to promote cellular protection through the PI 3-K and Akt systems (2, 3, 6, 9).
Although WISP1 has been shown in neurons to previously require PI 3-K and Akt1 to protect neurons against oxidative stress (11, 12), we now show that the activation of PI 3-K and Akt1 are vital for WISP1 to modulate the activity of the forkhead transcription factor FoxO3a that can lead to apoptotic cell injury. FoxO3a is closely involved with cellular survival, metabolism, and the toxic effects of oxidative stress (20, 31). Activation of Akt by PI 3-K limits apoptosis through the phosphorylation of FoxO3a (15-22). Akt phosphorylates serine253 of FoxO3a as well as other proteins that can potentiate cell death, such as the proline rich Akt substrate 40 kDa (62, 66). Once phosphorylated, FoxO3a is sequestered in the cytoplasm by association with 14-3-3 proteins and is prevented from initiating programs of apoptotic cell death (17). We show that WISP1 requires PI 3-K and Akt1 activation to phosphorylate FoxO3a and retain FoxO3a in the cytoplasm of neurons through its association with protein 14-4-3. The retention of FoxO3a in the cytoplasm of neurons by WISP1 prevents FoxO3a from reaching the nucleus to initiate apoptosis. Through studies with the over-expression of FoxO3a, we show that FoxO3a can significantly lead to neuronal cell death in untreated control cells and during OGD exposure. FoxO3a over-expression also can limit neuronal protection in the presence of WISP1, illustrating that post-translational phosphorylation of FoxO3a and the maintenance of FoxO3a in the cytoplasm of neurons by WISP1 may be critical for WISP1 to offer neuronal protection.
WISP1 in primary neurons controls the expression and activation of SIRT1 that also is important for WISP1 to provide cellular protection. During oxidative stress, SIRT1 activation can reduce cerebral infarction size (67), enhance neuronal survival (68, 69), and maintain cellular metabolism (70). SIRT1 may be important for maintaining normal learning, memory, and synaptic plasticity (71) as well as preventing chronic neurodegeneration that has been shown in models of Alzheimer’s disease (23, 24, 72). SIRT1 increases Akt activity that leads to cellular protection (27, 28, 73, 74). We demonstrate that WISP1 maintains SIRT1 expression and significantly increases SIRT1 activity during OGD exposure that would otherwise be lost during oxidative stress. In addition, WISP1 requires SIRT1 activity to foster neuronal survival during oxidative stress, since pharmacological inhibition of SIRT1 activity with EX527 or sirtinol as well as the knockdown of SIRT1 expression with shRNA against SIRT1 leads to increased neuronal injury.
However, our studies illustrate that a limited activation of SIRT1 is required by WISP1 to effectively yield neuronal protection. Over-expression of SIRT1 or elevated concentrations of the SIRT1 agonist SRT1720 of 20 μM resulted in increased neuronal injury in untreated cells and during OGD exposure in neurons. Relative SIRT1 activity is substantially increased during SIRT1 over-expression and administration of SRT1720 of 20 μM to a much greater degree than during application of lower concentrations of SRT1720 or WISP1, suggesting that a minimum level of SIRT1 activity that does not become excessive is necessary for effective neuronal protection during oxidative stress. The observed toxicity with SIRT1 over-expression and treatment with SRT1720 of 20 μM may be secondary to the activation of FoxO3a by SIRT1 through significant deacetylation of FoxO3a. Although SIRT1 can phosphorylate and inhibit the activity of FoxO3a through post-translation phosphorylation (26-28), activation of SIRT1 also can lead to the deacetylation of FoxO3a to increase the activity of FoxO3a and subsequent apoptotic cell death (29-31). Interestingly, the deacetylation of FoxOs by SIRT1 may ultimately lead to degradation of FoxO transcription factors (75). Our results are consistent with these prior studies and demonstrate that SIRT1 over-expression and treatment with SRT1720 of 20 μM result in the significant deacetylation of FoxO3a that subsequently can lead to apoptotic neuronal cell injury.
For SIRT1 to control gene transcription to prevent apoptotic cell death, SIRT1 under most scenarios must translocate to the cell nucleus. For example, in several cell systems but not all (76), nuclear translocation of SIRT1 is necessary for neuronal differentiation (77) as well as the protection of cells against apoptotic injury (27, 28, 78). We show that WISP1 not only increases the cellular expression and activity of SIRT1, but also promotes the subcellular trafficking of SIRT1 from the cytoplasm to the nucleus of neurons.
We also observed that during inhibition of SIRT1 activity with EX527 or sirtinol during OGD exposure alone or in the presence of WISP1 with OGD exposure, SIRT1 is retained in the cytoplasm and the expression of SIRT1 in the nucleus does not occur. Prior studies have shown that sirtinol can lead to caspase activation and the degradation of SIRT1 in the cell nucleus (79, 80). Furthermore, other work has suggested that SIRT1 degradation also may be mediated by other components of the apoptotic pathway such as p38 (81) and c-Jun N-terminal kinase 1 (82). We illustrate that during high SIRT1 activity in the presence of OGD with SIRT1 over-expression or with treatment of SRT1720 of 20 μM, a significant increase in caspase 1 and caspase 3 cleavage and activity occurs. Neuroprotective concentrations of SRT1720 of 5 μM and 10 μM block caspase 1 and caspase 3 cleavage during OGD exposure. In addition, inhibition of SIRT1 activity with EX527 and sirtinol results in elevated caspase 1 and caspase 3 cleavage during OGD exposure. These results suggest that either highly elevated SIRT1 activity or the loss of SIRT1 activity that is neuroprotective during oxidative stress can foster caspase 1 and caspase 3 activity that may play a role in the degradation of SIRT1 expression in the nucleus. Our next set of studies provide further support for this premise and illustrate a potential feedback mechanism for FoxO3a that can regulate SIRT1 expression and activation through caspase activity during oxidative stress. During acute nutrient withdrawal, FoxO3a has been shown to up-regulate the expression of SIRT1 through binding to two p53 binding sites within the SIRT1 promoter to induce SIRT1 transcription (83). We now show that over-expression of FoxO3a leads to significantly increased caspase 1 and caspase 3 cleavage during OGD exposure that also may account for the ability of FoxO3a to lead to apoptotic neuronal cell death similar to other cell systems (32, 84). WISP1 blocks caspase 1 and caspase 3 activation during OGD exposure. Yet, FoxO3a mediated caspase 1 and caspase 3 activation results in the significant degradative loss of SIRT1 expression that can be blocked by specific inhibition of caspase 1 and caspase 3 activity. As a result, SIRT1 expression is preserved during oxidative stress by WISP1 at two specific levels. First, WISP1 blocks FoxO3a activity through the inhibitory post-translational phosphorylation of FoxO3a and the sequestration of FoxO3a with 14-3-3 in the cytoplasm of neurons. Second, WISP1 prevents caspase 1 and caspase 3 activation during oxidative stress that would otherwise lead to the degradation of SIRT1.
WISP1 has a significant impact upon proliferative cellular growth and development but the pathways that determine cellular protection for WISP1 continue to unfold. Our studies highlight that both PI 3-K and Akt1 are necessary for WISP1 to provide neuronal protection against oxidative stress. We illustrate that the pathways of PI 3-K and Akt are critical for WISP1 to govern inhibitory post-translational phosphorylation of the forkhead transcription factor FoxO3a and sequester FoxO3a in the cytoplasm of neurons, since FoxO3a activation leads to caspase 1 and caspase 3 mediated apoptotic cell death. Furthermore, WISP1 also facilitates the nuclear subcellular trafficking of SIRT1 and requires a fine modulatory activation of SIRT1 that limits FoxO3a feedback activation through deacytelation and prevents caspase 1 and 3 activation that ultimately leads to the degradation of SIRT1. Identification of the specific cellular pathways that govern WISP1 cellular protection can discern new therapeutic targets and strategies for neurodegenerative and related disease entities.
Acknowledgments
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association (National), Bugher Foundation Award, LEARN Foundation Award, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
Footnotes
Conflicts of interest: The authors have no conflicts of interest.
References
- 1.Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert opinion on therapeutic targets. 2012 Aug 27; doi: 10.1517/14728222.2012.719499. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002 Jan 1;16(1):46–57. doi: 10.1101/gad.942902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colston JT, de la Rosa SD, Koehler M, Gonzales K, Mestril R, Freeman GL, et al. Wnt-induced secreted protein-1 is a prohypertrophic and profibrotic growth factor. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1839–46. doi: 10.1152/ajpheart.00428.2007. [DOI] [PubMed] [Google Scholar]
- 4.Heise RL, Stober V, Cheluvaraju C, Hollingsworth JW, Garantziotis S. Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J Biol Chem. 2011 May 20;286(20):17435–44. doi: 10.1074/jbc.M110.137273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanmugam P, Valente AJ, Prabhu SD, Venkatesan B, Yoshida T, Delafontaine P, et al. Angiotensin-II type 1 receptor and NOX2 mediate TCF/LEF and CREB dependent WISP1 induction and cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2011 Jun;50(6):928–38. doi: 10.1016/j.yjmcc.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy VS, Valente AJ, Delafontaine P, Chandrasekar B. Interleukin-18/WNT1-inducible signaling pathway protein-1 signaling mediates human saphenous vein smooth muscle cell proliferation. J Cell Physiol. 2011 Dec;226(12):3303–15. doi: 10.1002/jcp.22676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French DM, Kaul RJ, D’Souza AL, Crowley CW, Bao M, Frantz GD, et al. WISP-1 is an osteoblastic regulator expressed during skeletal development and fracture repair. Am J Pathol. 2004 Sep;165(3):855–67. doi: 10.1016/S0002-9440(10)63348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macsai CE, Georgiou KR, Foster BK, Zannettino AC, Xian CJ. Microarray expression analysis of genes and pathways involved in growth plate cartilage injury responses and bony repair. Bone. 2012 May;50(5):1081–91. doi: 10.1016/j.bone.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Venkatesan B, Prabhu SD, Venkatachalam K, Mummidi S, Valente AJ, Clark RA, et al. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal. 2010 May;22(5):809–20. doi: 10.1016/j.cellsig.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang YC, Chong ZZ, Wang S, Maiese K. WNT1 Inducible Signaling Pathway Protein 1 (WISP1) Targets PRAS40 to Govern beta-Amyloid Apoptotic Injury of Microglia. Curr Neurovasc Res. 2012 Aug 6;9(4):239–49. doi: 10.2174/156720212803530618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Chong ZZ, Shang YC, Maiese K. Wnt1 inducible signaling pathway protein 1 (WISP1) blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL. Curr Neurovasc Res. 2012 Feb;9(1):20–31. doi: 10.2174/156720212799297137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Chong ZZ, Shang YC, Maiese K. WISP1 (CCN4) autoregulates its expression and nuclear trafficking of beta-catenin during oxidant stress with limited effects upon neuronal autophagy. Curr Neurovasc Res. 2012 Apr 4;9(2):89–99. doi: 10.2174/156720212800410858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008 Oct 24;283(43):29196–205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchand A, Atassi F, Gaaya A, Leprince P, Le Feuvre C, Soubrier F, et al. The Wnt/beta-catenin pathway is activated during advanced arterial aging in humans. Aging Cell. 2011 Apr;10(2):220–32. doi: 10.1111/j.1474-9726.2010.00661.x. [DOI] [PubMed] [Google Scholar]
- 15.Bahia PK, Pugh V, Hoyland K, Hensley V, Rattray M, Williams RJ. Neuroprotective effects of phenolic antioxidant tBHQ associate with inhibition of FoxO3a nuclear translocation and activity. J Neurochem. 2012 Oct;123(1):182–91. doi: 10.1111/j.1471-4159.2012.07877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO Relies upon Novel Signaling of Wnt1 that Requires Akt1, FoxO3a, GSK-3beta, and beta-Catenin to Foster Vascular Integrity During Experimental Diabetes. Curr Neurovasc Res. 2011 May 1;8(2):103–20. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007 Apr;150(7):839–50. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai CS, Tsai ML, Badmaev V, Jimenez M, Ho CT, Pan MH. Xanthigen suppresses preadipocyte differentiation and adipogenesis through down-regulation of PPARgamma and C/EBPs and modulation of SIRT-1, AMPK, and FoxO pathways. Journal of agricultural and food chemistry. 2012 Feb 1;60(4):1094–101. doi: 10.1021/jf204862d. [DOI] [PubMed] [Google Scholar]
- 19.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008 May;14(5):219–27. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiese K, Chong ZZ, Shang YC, Hou J. A “FOXO” in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009 May;29(3):395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maiese K, Chong ZZ, Shang YC, Wang S. Erythropoietin: New Directions for the Nervous System. Int J Mol Sci. 2012;13:11102–29. doi: 10.3390/ijms130911102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Chen WR, Xing D. A pathway from JNK through decreased ERK and Akt activities for FOXO3a nuclear translocation in response to UV irradiation. J Cell Physiol. 2011 May 20; doi: 10.1002/jcp.22839. [DOI] [PubMed] [Google Scholar]
- 23.Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert opinion on therapeutic targets. 2012 Feb;16(2):167–78. doi: 10.1517/14728222.2012.648926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maiese K, Chong ZZ, Shang YC, Wang S. Translating cell survival and cell longevity into treatment strategies with SIRT1. Rom J Morphol Embryol. 2011;52(4):1173–85. [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Liang Y, Vanhoutte PM. SIRT1 and AMPK in regulating mammalian senescence: a critical review and a working model. FEBS Lett. 2011 Apr 6;585(7):986–94. doi: 10.1016/j.febslet.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 26.Frojdo S, Durand C, Molin L, Carey AL, El-Osta A, Kingwell BA, et al. Phosphoinositide 3-kinase as a novel functional target for the regulation of the insulin signaling pathway by SIRT1. Mol Cell Endocrinol. 2011 Mar 30;335(2):166–76. doi: 10.1016/j.mce.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010 May;7(2):95–112. doi: 10.2174/156720210791184899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou J, Wang S, Shang YC, Chong ZZ, Maiese K. Erythropoietin Employs Cell Longevity Pathways of SIRT1 to Foster Endothelial Vascular Integrity During Oxidant Stress. Curr Neurovasc Res. 2011 Aug 1;8(3):220–35. doi: 10.2174/156720211796558069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maiese K, Chong ZZ, Shang YC, Hou J. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond) 2009 Feb;116(3):191–203. doi: 10.1042/CS20080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiese K, Hou J, Chong ZZ, Shang YC. A fork in the path: Developing therapeutic inroads with FoxO proteins. Oxid Med Cell Longev. 2009 Jul;2(3):119–29. doi: 10.4161/oxim.2.3.8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storz P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal. 2011 Feb 15;14(4):593–605. doi: 10.1089/ars.2010.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou J, Chong ZZ, Shang YC, Maiese K. FoxO3a governs early and late apoptotic endothelial programs during elevated glucose through mitochondrial and caspase signaling. Mol Cell Endocrinol. 2010 Mar 4;321(2):194–206. doi: 10.1016/j.mce.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obexer P, Geiger K, Ambros PF, Meister B, Ausserlechner MJ. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 2007 Mar;14(3):534–47. doi: 10.1038/sj.cdd.4402017. [DOI] [PubMed] [Google Scholar]
- 34.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003 Mar;138(6):1107–18. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chong ZZ, Shang YC, Hou J, Maiese K. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid Med Cell Longev. 2010 Mar-Apr;3(2):153–65. doi: 10.4161/oxim.3.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shang YC, Chong ZZ, Hou J, Maiese K. FoxO3a governs early microglial proliferation and employs mitochondrial depolarization with caspase 3, 8, and 9 cleavage during oxidant induced apoptosis. Curr Neurovasc Res. 2009 Nov;6(4):223–38. doi: 10.2174/156720209789630302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002 Dec 3;106(23):2973–9. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 38.Chong ZZ, Lin SH, Kang JQ, Maiese K. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003 Mar 1;71(5):659–69. doi: 10.1002/jnr.10528. [DOI] [PubMed] [Google Scholar]
- 39.Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003 Sep;64(3):557–69. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- 40.Chong ZZ, Kang JQ, Maiese K. AKT1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-xL and caspase 1, 3, and 9. Exp Cell Res. 2004 Jun 10;296(2):196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 41.Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 Govern Pathways of mTOR, Apaf-1, and XIAP in Inflammatory Microglia. Curr Neurovasc Res. 2011 Oct 19;8(4):270–85. doi: 10.2174/156720211798120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY) 2012 Mar 3;4(3):187–201. doi: 10.18632/aging.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, et al. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996 Apr;16(4):1722–33. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994 Feb 18;269(7):5241–8. [PubMed] [Google Scholar]
- 45.Zhu W, Bijur GN, Styles NA, Li X. Regulation of FOXO3a by brain-derived neurotrophic factor in differentiated human SH-SY5Y neuroblastoma cells. Brain Res Mol Brain Res. 2004 Jul 5;126(1):45–56. doi: 10.1016/j.molbrainres.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 46.Solomon JM, Pasupuleti R, Xu L, McDonagh T, Curtis R, DiStefano PS, et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol. 2006 Jan;26(1):28–38. doi: 10.1128/MCB.26.1.28-38.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hennemeier I, Humpf HU, Gekle M, Schwerdt G. The food contaminant and nephrotoxin ochratoxin A enhances Wnt1 inducible signaling protein 1 and tumor necrosis factor-alpha expression in human primary proximal tubule cells. Molecular nutrition & food research. 2012 Sep;56(9):1375–84. doi: 10.1002/mnfr.201200164. [DOI] [PubMed] [Google Scholar]
- 48.Venkatachalam K, Venkatesan B, Valente AJ, Melby PC, Nandish S, Reusch JE, et al. WISP1, a pro-mitogenic, pro-survival factor, mediates tumor necrosis factor-alpha (TNF-alpha)-stimulated cardiac fibroblast proliferation but inhibits TNF-alpha-induced cardiomyocyte death. J Biol Chem. 2009 May 22;284(21):14414–27. doi: 10.1074/jbc.M809757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price RM, Tulsyan N, Dermody JJ, Schwalb M, Soteropoulos P, Castronuovo JJ., Jr Gene expression after crush injury of human saphenous vein: using microarrays to define the transcriptional profile. J Am Coll Surg. 2004 Sep;199(3):411–8. doi: 10.1016/j.jamcollsurg.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 50.Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009 Apr;119(4):772–87. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kapasa M, Serafimidis I, Gavalas A, Kossida S. Identification of phylogenetically conserved enhancer elements implicated in pancreas development in the WISP1 and CTGF orthologs. Genomics. 2008 Nov;92(5):301–8. doi: 10.1016/j.ygeno.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Kohara H, Tabata Y. Enhancement of ectopic osteoid formation following the dual release of bone morphogenetic protein 2 and Wnt1 inducible signaling pathway protein 1 from gelatin sponges. Biomaterials. 2011 Aug;32(24):5726–32. doi: 10.1016/j.biomaterials.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 53.Davies SR, Davies ML, Sanders A, Parr C, Torkington J, Jiang WG. Differential expression of the CCN family member WISP-1, WISP-2 and WISP-3 in human colorectal cancer and the prognostic implications. Int J Oncol. 2010 May;36(5):1129–36. doi: 10.3892/ijo_00000595. [DOI] [PubMed] [Google Scholar]
- 54.Nagai Y, Watanabe M, Ishikawa S, Karashima R, Kurashige J, Iwagami S, et al. Clinical significance of Wnt-induced secreted protein-1 (WISP-1/CCN4) in esophageal squamous cell carcinoma. Anticancer Res. 2011 Mar;31(3):991–7. [PubMed] [Google Scholar]
- 55.Wang Q, Liu H, Liu T, Shu S, Jiang H, Cheng S, et al. BRCA2 Dysfunction Promotes Malignant Transformation of Pancreatic Intraepithelial Neoplasia. Anticancer Agents Med Chem. 2012 Aug 24; doi: 10.2174/1871520611313020012. [DOI] [PubMed] [Google Scholar]
- 56.Maiese K, Chong ZZ, Wang S, Shang YC. Oxidant Stress and Signal Transduction in the Nervous System with the PI 3-K, Akt, and mTOR Cascade. Int J Mol Sci. 2012;13(11):13830–66. doi: 10.3390/ijms131113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mannell HK, Pircher J, Chaudhry DI, Alig SK, Koch EG, Mettler R, et al. ARNO regulates VEGF-dependent tissue responses by stabilizing endothelial VEGFR-2 surface expression. Cardiovasc Res. 2012 Jan 1;93(1):111–9. doi: 10.1093/cvr/cvr265. [DOI] [PubMed] [Google Scholar]
- 58.Su KH, Shyue SK, Kou YR, Ching LC, Chiang AN, Yu YB, et al. beta Common receptor integrates the erythropoietin signaling in activation of endothelial nitric oxide synthase. J Cell Physiol. 2011 Dec;226(12):3330–9. doi: 10.1002/jcp.22678. [DOI] [PubMed] [Google Scholar]
- 59.Pineda D, Ampurdanes C, Medina MG, Serratosa J, Tusell JM, Saura J, et al. Tissue plasminogen activator induces microglial inflammation via a noncatalytic molecular mechanism involving activation of mitogen-activated protein kinases and Akt signaling pathways and AnnexinA2 and Galectin-1 receptors. Glia. 2012 Apr;60(4):526–40. doi: 10.1002/glia.22284. [DOI] [PubMed] [Google Scholar]
- 60.Shang YC, Chong ZZ, Hou J, Maiese K. The forkhead transcription factor FoxO3a controls microglial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009 Feb;6(1):20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou X, Wang L, Wang M, Xu L, Yu L, Fang T, et al. Emodin-induced microglial apoptosis is associated with TRB3 induction. Immunopharmacol Immunotoxicol. 2011 Dec;33(4):594–602. doi: 10.3109/08923973.2010.549135. [DOI] [PubMed] [Google Scholar]
- 62.Chong ZZ, Shang YC, Wang S, Maiese K. PRAS40 Is an Integral Regulatory Component of Erythropoietin mTOR Signaling and Cytoprotection. PLoS ONE. 2012;7(9):e45456. doi: 10.1371/journal.pone.0045456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J Neurosci. 2010 Jan 20;30(3):1166–75. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen J, Wu Y, Xu JY, Zhang J, Sinclair SH, Yanoff M, et al. ERK- and Akt-dependent neuroprotection by erythropoietin (EPO) against glyoxal-AGEs via modulation of Bcl-xL, Bax, and BAD. Invest Ophthalmol Vis Sci. 2010 Jan;51(1):35–46. doi: 10.1167/iovs.09-3544. [DOI] [PubMed] [Google Scholar]
- 65.Zeng KW, Wang XM, Ko H, Kwon HC, Cha JW, Yang HO. Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid beta-protein via the PI3K/Akt/Bad/Bcl(XL)-regulated mitochondrial apoptotic pathway. Eur J Pharmacol. 2011 Dec 15;672(1-3):45–55. doi: 10.1016/j.ejphar.2011.09.177. [DOI] [PubMed] [Google Scholar]
- 66.Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol. 2012 Aug 15;99:128–48. doi: 10.1016/j.pneurobio.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simao F, Matte A, Matte C, Soares FM, Wyse AT, Netto CA, et al. Resveratrol prevents oxidative stress and inhibition of Na(+)K(+)-ATPase activity induced by transient global cerebral ischemia in rats. The Journal of nutritional biochemistry. 2011 Jan 3; doi: 10.1016/j.jnutbio.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 68.Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008 Oct 10;283(41):27810–9. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chong ZZ, Maiese K. Enhanced Tolerance against Early and Late Apoptotic Oxidative Stress in Mammalian Neurons through Nicotinamidase and Sirtuin Mediated Pathways. Curr Neurovasc Res. 2008 Aug;5(3):159–70. doi: 10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maiese K, Chong ZZ, Shang YC, Wang S. Novel directions for diabetes mellitus drug discovery. Expert opinion on drug discovery. 2012 Oct 24; doi: 10.1517/17460441.2013.736485. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Michan S, Li Y, Chou MM, Parrella E, Ge H, Long JM, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010 Jul 21;30(29):9695–707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010 Jul 23;142(2):320–32. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Sundaresan NR, Pillai VB, Wolfgeher D, Samant S, Vasudevan P, Parekh V, et al. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Science signaling. 2011;4(182):ra46. doi: 10.1126/scisignal.2001465. [DOI] [PubMed] [Google Scholar]
- 74.Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest. 2011 Nov;121(11):4477–90. doi: 10.1172/JCI46243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang F, Chan CH, Chen K, Guan X, Lin HK, Tong Q. Deacetylation of FOXO3 by SIRT1 or SIRT2 leads to Skp2-mediated FOXO3 ubiquitination and degradation. Oncogene. 2012 Mar 22;31(12):1546–57. doi: 10.1038/onc.2011.347. [DOI] [PubMed] [Google Scholar]
- 76.Oppenheimer H, Gabay O, Meir H, Haze A, Kandel L, Liebergall M, et al. 75-kd sirtuin 1 blocks tumor necrosis factor alpha-mediated apoptosis in human osteoarthritic chondrocytes. Arthritis Rheum. 2012 Mar;64(3):718–28. doi: 10.1002/art.33407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hisahara S, Chiba S, Matsumoto H, Tanno M, Yagi H, Shimohama S, et al. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci U S A. 2008 Oct 7;105(40):15599–604. doi: 10.1073/pnas.0800612105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, et al. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010 Mar 12;285(11):8375–82. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balaiya S, Ferguson LR, Chalam KV. Evaluation of sirtuin role in neuroprotection of retinal ganglion cells in hypoxia. Invest Ophthalmol Vis Sci. 2012;53(7):4315–22. doi: 10.1167/iovs.11-9259. [DOI] [PubMed] [Google Scholar]
- 80.Kozako T, Aikawa A, Shoji T, Fujimoto T, Yoshimitsu M, Shirasawa S, et al. High expression of the longevity gene product SIRT1 and apoptosis induction by sirtinol in adult T-cell leukemia cells. Int J Cancer. 2012 Nov 1;131(9):2044–55. doi: 10.1002/ijc.27481. [DOI] [PubMed] [Google Scholar]
- 81.Hong EH, Lee SJ, Kim JS, Lee KH, Um HD, Kim JH, et al. Ionizing radiation induces cellular senescence of articular chondrocytes via negative regulation of SIRT1 by p38 kinase. J Biol Chem. 2010 Jan 8;285(2):1283–95. doi: 10.1074/jbc.M109.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao Z, Zhang J, Kheterpal I, Kennedy N, Davis RJ, Ye J. Sirtuin 1 (SIRT1) protein degradation in response to persistent c-Jun N-terminal kinase 1 (JNK1) activation contributes to hepatic steatosis in obesity. J Biol Chem. 2011 Jun 24;286(25):22227–34. doi: 10.1074/jbc.M111.228874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004 Dec 17;306(5704):2105–8. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 84.Kelly KR, Nawrocki ST, Espitia CM, Zhang M, Yang JJ, Padmanabhan S, et al. Targeting Aurora A kinase activity with the investigational agent alisertib increases the efficacy of cytarabine through a FOXO-dependent mechanism. Int J Cancer. 2012 Dec 1;131(11):2693–703. doi: 10.1002/ijc.27579. [DOI] [PMC free article] [PubMed] [Google Scholar]