Abstract
The purpose of this study was to 1) compare trunk neuromuscular behavior between individuals with no history of low back pain (LBP) and individuals who experience exercise-induced LBP (eiLBP) when pain free, and 2) investigate changes in trunk neuromuscular behavior with eiLBP. Seventeen young adult males participated including eight reporting recurrent, acute eiLBP and nine control participants reporting no history of LBP. Intrinsic trunk stiffness and paraspinal muscle reflex delay were determined in both groups using sudden trunk flexion position perturbations 1-2 days following exercise when the eiLBP participants were experiencing an episode of LBP (termed post-exercise) and 4-5 days following exercise when eiLBP had subsided (termed post-recovery). Post-recovery, when the eiLBP group was experiencing minimal LBP, trunk stiffness was 26% higher in the eiLBP group compared to the control group (p=0.033) and reflex delay was not different (p=0.969) between groups. Trunk stiffness did not change (p=0.826) within the eiLBP group from post-exercise to post-recovery, but decreased 22% within the control group (p=0.002). Reflex delay decreased 11% within the eiLBP group from post-exercise to post-recovery (p=0.013), and increased 15% within the control group (p=0.006). Although the neuromuscular mechanisms associated with eiLBP and chronic LBP may differ, these results suggest that previously-reported differences in trunk neuromuscular behavior between individuals with chronic LBP and healthy controls reflect a combination of inherent differences in neuromuscular behavior between these individuals as well as changes in neuromuscular behavior elicited by pain.
Keywords: low back pain, exercise, trunk stiffness, reflex
Introduction
Low back pain (LBP) continues to be a significant health and economic problem in the United States (US) and around the world. An estimated 60-80% of all adults experience LBP at some point in their lifetime (van Tulder et al., 1995), and annual health care costs in the US associated with LBP exceed $90 billion (Luo et al., 2004). Numerous studies have reported differences in trunk neuromuscular behavior between individuals with and without LBP. For example, individuals with chronic LBP exhibit longer paraspinal reflex delays (Radebold et al., 2000; Radebold et al., 2001; Reeves et al., 2005), increased sway while sitting on an unstable seat (Radebold et al., 2001), and increased trunk muscle activity during slow trunk motions and isometric voluntary contractions while sitting (van Dieen et al., 2003a). While these descriptive studies suggest altered trunk neuromuscular behaviors that adversely affect spinal loads and stability (i.e., reduced stability and higher spinal loads), and which thereby may increase the risk of injury and pain, they are unable to discern whether these alterations were in response to LBP or whether they existed prior to the development of LBP.
To help understand the relationship between altered neuromuscular behavior and LBP, prior work has investigated changes in neuromuscular behavior after experimentally inducing LBP. Experimentally-induced LBP, caused by injecting a saline solution into the lumbar longissimus muscle, leads to decreased trunk motion during walking (Moe-Nilssen et al., 1999) and quiet standing (Smith et al., 2005), and altered trunk muscle activation (delayed onsets and decreased amplitudes) during a postural task involving rapid arm movements (Hodges et al., 2003). A recent study induced LBP using noxious heat applied on the skin, which decreased lumbar spine movement relative to the hip while performing trunk flexion (Dubois et al., 2011). The decreased voluntary trunk/spine motion in these studies is consistent with increased trunk stiffness and may thus represent a central nervous system response to LBP to help minimize pain and further injury (Dubois et al., 2011; Hodges and Moseley, 2003; Smith et al., 2005; van Dieen et al., 2003a).
An alternative approach, to further our understanding of the relationship between trunk neuromuscular behavior and LBP, is to study individuals who experience recurrent, acute, exercise-induced LBP (eiLBP). This approach provides the opportunity to determine whether neuromuscular behavior is altered among these individuals when they are not experiencing pain, which would suggest that altered neuromuscular behavior is an inherent characteristic of selected individuals that may contribute to the development of LBP. This approach can also determine if/how neuromuscular behavior changes with pain. Any such changes would suggest that altered neuromuscular behavior is a response to LBP. Therefore, the purpose of this study was to compare trunk neuromuscular behavior between individuals with no history of LBP and individuals who experience eiLBP when pain free, and to investigate changes in trunk neuromuscular behavior with eiLBP. Our general expectations were that neuromuscular behavior would differ between groups when the eiLBP group was not experiencing pain, and respond differently to exercise between groups. The following two specific hypotheses were tested: 1) neuromuscular behavior differs between an eiLBP group when not experiencing pain and a healthy control group, and 2) neuromuscular behavior responds differently to exercise in an eiLBP group compared to a healthy control group. Trunk neuromuscular behavior was quantified using measures of intrinsic trunk stiffness and paraspinal reflex delay, using sudden trunk flexion perturbations, and differences/changes in either measure were used to indicate differences/changes in underlying neuromuscular behavior.
Methods
Seventeen males, all members of the Virginia Tech Triathlon Club, completed the study. Only males were included to avoid the influence of gender differences in intrinsic trunk stiffness (Miller et al., 2012) and paraspinal muscle reflex delay (Miller et al., 2010). Eight of the participants reported experiencing recurrent, acute eiLBP [mean (SD) stature = 1.83 (0.05) m; mass = 72.9 (2.7) kg; and age = 20.7 (1.0) yr] and nine control participants reported no history of LBP [stature = 1.79 (0.03) m; mass =70.8 (7.3) kg; and age = 20.4 (1.6) yr]. None of the anthropometric or age differences between groups were significant (p > 0.05 from unpaired t-tests). Participants with eiLBP were required to pass a screening by a chiropractic physician that evaluated their ability to complete the experiment without further injury and ensured agreement with specific inclusion and exclusion criteria. Participants were included if they had recurrent acute eiLBP for at least six months, and excluded based on any neurological deficits, vestibular or visual disorders, major structural deformities of the spine, genetic spinal disorders, spinal surgery within the past five years, or spinal mechanical implants. The study protocols were approved by the Virginia Tech Institutional Review Board, and written consent was obtained from all participants prior to data collection.
Neuromuscular behavior was characterized in each participant during two experimental sessions. The first session (post-exercise) was 1-2 days following a triathlon race or simulated race, when the eiLBP participants were experiencing an episode of LBP. The second session (post-recovery) was 4-5 days following the triathlon activity, when eiLBP had subsided. The control group participated in experimental sessions on the same schedule (although they did not experience eiLBP). A visual analog scale (Scott and Huskisson, 1976) (VAS) was used for rating LBP during each session, and which quantified LBP on a numerical scale with text descriptors from 0 (none) to 10 (agonizing pain). During the first session, the eiLBP group provided a mean rating of 2.54 (0.91), which was between “annoying” (2) and “uncomfortable” (4), and is similar to that reported by chronic LBP individuals who exhibited increased sway measures while sitting compared to controls (Radebold et al., 2001). During the second session, each eiLBP participant provided lower ratings, and the mean (SD) decrease was 1.16 (0.74), which was significantly zero (paired t-test, p<0.001). All members of the control group provided ratings of 0 during both sessions.
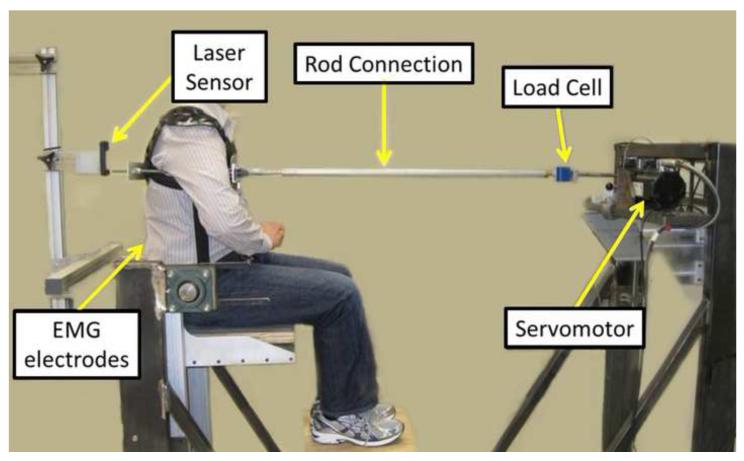
Intrinsic trunk stiffness and paraspinal muscle reflex delay were determined using trials of sudden trunk flexion position perturbations (Bazrgari et al., 2011a; Bazrgari et al., 2011b; Hendershot et al., 2011; Miller et al., 2012). Participants sat upright in a rigid metal frame (Figure 1), were strapped in at the pelvis, and were attached to a servomotor (Kollmorgen AKM53K, Radford, VA, USA) at the T8 level of the spine via a rigid harness/rod system. The motor height, foot height, and rod length were adjustable, and were configured so that the rod was horizontal, the trunk was upright, and the included hip angle was 90 degrees. During each 40-second perturbation trial, the motor applied a series of 12 anterior and 12 posterior position perturbations, each moving the rod 10 mm over ~40 ms (peak velocity of 360 mm/s), which is faster than typical erector spinae reflex delays (Granata et al., 2004; Hwang et al., 2008). Pseudorandom delays between each perturbation minimized participant anticipation, and participants were instructed to maintain an upright trunk and otherwise not attempt to resist or intervene with the perturbations. A real-time visual display of erector spinae (ES) and rectus abdominus (RA) electromyographic activity (EMG, see below) was used to minimize muscle activity during the perturbations. One practice trial was completed prior to a subsequent trial used for analysis.
Figure 1.
Experimental set up for measurements of trunk neuromuscular behavior in a seated posture.
During each perturbation trial, motor displacement was sampled at 1000 Hz using an encoder on the motor shaft, and trunk position was sampled at 1000 Hz with a CCD laser displacement sensor (Keyence LK-G 150, Osaka, Japan) focused on the midline of the dorsal aspect of the harness just above the rod (Figure 2). To account for the vertical offset between the laser sensor and the connecting rod, laser measurements were multiplied by the ratio of these heights (relative to L5S1). Forces in the connecting rod were sampled at 1000 Hz using an in-line load cell (Interface SM2000, Scottsdale, AZ, USA). Muscle activity was monitored using EMG electrodes (bipolar Ag/AgCl) placed bilaterally over the ES (~3 cm from the midline at the L1 level) and RA muscles. Raw EMG signals were amplified and bandpass filtered (20-500 Hz) in hardware (Measurement Systems Inc., Ann Arbor, MI, USA), sampled at 1000 Hz, then full-wave rectified and smoothed using a 25 Hz zero-phase-lag low-pass Butterworth filter. Displacement and force data were low-pass filtered at 10 Hz (7th-order, zero-phase-lag Butterworth filter).
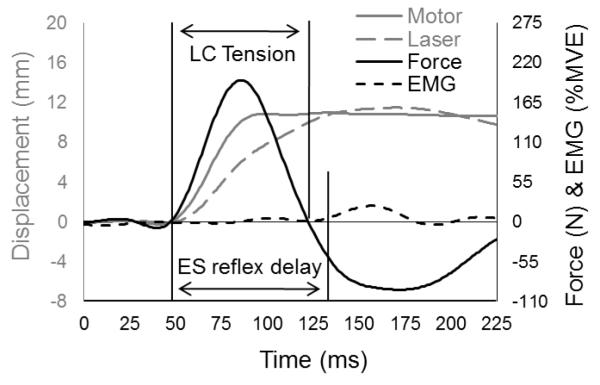
Figure 2.
Representative data illustrating motor displacement as measured by encoder, trunk displacement as measured by laser, perturbation force as measured by load cell (LC), and EMG from ES. Figure adapted from Bazrgari et al., 2011b.
Intrinsic trunk stiffness was estimated with a two degree-of-freedom model of the trunk and harness/rod connection as described elsewhere (Bazrgari et al., 2011a; Bazrgari et al., 2011b; Hendershot et al., 2011). Briefly, inputs to the model were the displacements collected from the motor encoder and laser sensor along with their numerically-calculated 1st and 2nd derivatives. The model output was an estimated force response. Model parameters of stiffness, damping, and effective mass (i.e. the driving point mass) were determined for each degree of freedom, using a curve fit routine in MATLAB (MathWorks, Natick, MA, USA) that minimized the sum of squared differences between estimated and measured force time series for each anterior perturbation. Initial efforts revealed an inability to consistently differentiate stiffness and damping, likely due to the short time interval over which the analysis was conducted. As such, trunk damping was set to zero, similar to previous studies (Bazrgari et al., 2011a; Gardner-Morse and Stokes, 2001; Hendershot et al., 2011). Therefore, changes in trunk mechanical behavior here were represented by changes in stiffness and effective mass. The model was fit to each of the 12 anterior trunk position perturbations within each trial using only data when the load cell measured a tensile force (i.e. when the trunk was being pulled forward by the motor). Only the value of trunk stiffness derived from the best model fit within each trial was used. The between-day reliability of stiffness estimates using this approach is very good, as demonstrated by an ICC = 0.82 (Hendershot et al., 2012). Paraspinal reflex delay was determined for the same perturbation, and was defined as the time between motor position onset and the onset of L1 level ES muscle activity (averaged across left and right sides). Motor position onset and ES muscle activity onset were determined as the instant that each signal exceeded its mean baseline (over 40 ms) plus two standard deviations, and all onsets were visually confirmed. Baseline paraspinal EMG amplitude was defined as the mean muscle activity over the 400 ms prior to the onset of the perturbation.
Separate two-way, mixed-factor analyses of variance were used to investigate the effects of group, session, and their interaction on intrinsic stiffness, reflex delay, effective mass, peak trunk velocity, and baseline paraspinal EMG amplitude. Significant interaction effects were explored using simple effects testing. All statistical tests were conducted using JMP 8 (SAS Software, Cary, NC, USA) with a significance level of p≤0.05.
Results
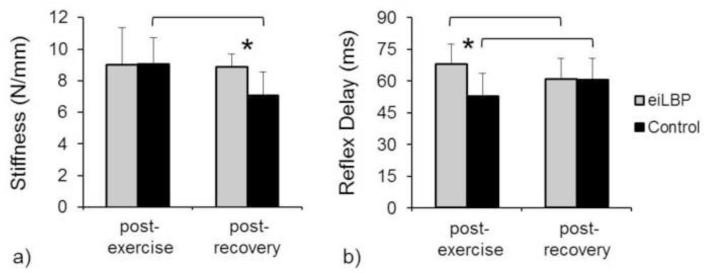
Intrinsic trunk stiffness exhibited a significant group × session interaction (p=0.032; Figure 3a). Trunk stiffness was not different (p=0.978) between eiLBP and control groups post-exercise, but was 26% higher in the eiLBP group compared to the control group post-recovery (p=0.033). In addition, trunk stiffness did not change (p=0.826) within the eiLBP group from post-exercise to post-recovery, but decreased 22% within the control group (p=0.002).
Figure 3.
Mean values of intrinsic trunk stiffness (a) and paraspinal reflex delay (b), by group and session. Asterisks indicate a significant group difference within session, and lines indicate a significant session difference within group. Error bars indicate standard deviation.
Paraspinal muscle reflex delay exhibited a significant group × session interaction (p=0.001; Figure 3b). Reflex delay was 29% longer in the eiLBP group compared to the control group post-exercise (p=0.006), but was not different (p=0.969) between the eiLBP and control group post-recovery. In addition, reflex delay decreased 11% within the eiLBP group from post-exercise to post-recovery (p=0.013), and increased 15% within the control group (p=0.006).
Effective mass decreased 14.5% from post-exercise to post-recovery (post-exercise: 15.9 ± 3.25 kg; post-recovery: 13.6 ± 3.98 kg; p=0.010), but there was not a main effect of group (eiLBP: 15.7 ± 3.70 kg; control: 13.9 ± 3.74 kg; p=0.268) or group × session interaction (p=0.415). Peak trunk velocity did not differ between groups (eiLBP: 276 ± 25 mm/s; control: 271 ± 13 mm/s; p=0.674), or between sessions (post-exercise: 275 ± 20 mm/s; post-recovery: 273 ± 20 mm/s; p=0.697), and there was no group × session interaction (p=0.808). Paraspinal EMG amplitude prior to perturbations did not differ between groups (eiLBP: 5.46 ± 2.88% MVC; control: 7.51 ± 3.75 % MVC; p=0.225), decreased 14.3% from post-exercise to post-recovery (post-exercise: 7.05 ± 3.66 %MVC; post-recovery: 6.04 ± 3.33 %MVC; p=0.027), and exhibited no group × session interaction (p=0.647).
Discussion
Participants in the current study were in a phase of their triathlon training during which they performed exercise sessions every weekend and used the weekdays to recover prior to the next exercise session the following weekend. Thus, our post-recovery measurements can also be considered pre-exercise measurements for the following weekend exercise, assuming there are no cumulative effects across subsequent weekend exercise sessions. Hence, the response of each group to exercise can be inferred by considering changes from post-recovery to post-exercise measurements. Our first hypothesis was that neuromuscular behavior would differ between the eiLBP group when not experiencing pain and the control group. This hypothesis was supported because the eiLBP group exhibited higher trunk stiffness (but not reflex delay) post-recovery. Our second hypothesis was that neuromuscular behavior would respond differently to exercise in the eiLBP group compared to the control group. This hypothesis was supported because exercise increased reflex delay (but had no effect on stiffness) in the eiLBP group, but increased stiffness and decreased reflex delay in the healthy control group. Overall, these results suggest inherent differences in neuromuscular behavior in individuals with eiLBP when not experiencing pain, and distinct changes in neuromuscular behavior with eiLBP.
Some important comments on the methods employed here are warranted. The trunk response to perturbations is non-linear and known to vary with the operating state of the joint (defined by the mean torque, perturbation magnitude, mean joint angle, and muscle activity) (Kearney and Hunter, 1990). Linear biomechanical models, as used here, provide valid estimates of trunk stiffness, but only over a relatively narrow range of operating states (Granata et al., 2002). Moreover, differences in experimental methods between studies can have a large influence on stiffness measurements (Bazrgari et al., 2011b). As such, it is difficult to compare stiffness values directly between studies. For example, intrinsic stiffness estimated here among individuals without eiLBP post-recovery (7.05 N/mm) was similar to those reported by Miller et al. (2012) among healthy individuals using similar experimental methods (6.29 N/mm), but 3-4 times higher than the effective stiffness reported by Hodges et al. (2009) using force perturbations among individuals with no history of LBP (1.64 N/mm). Despite the difficulty with estimating trunk stiffness, forces predicted by the model in the current study were highly correlated with those measured directly (mean r = 0.976), suggesting that the model provided a reasonable system representation. Reflex delay, estimated here without the model among individuals with eiLBP post-recovery (61 ms), was similar to those obtained from force perturbations (62-69 ms) (Radebold et al., 2000; Radebold et al., 2001; Reeves et al., 2005).
The percentage difference in stiffness found between groups post-recovery – presumably when neither group was affected by LBP or any exercise effects – was also consistent with previous studies. Here, intrinsic stiffness in the eiLBP group post-recovery (8.90 N/mm) was 26% higher than in the control group post-recovery (7.05 N/mm). This difference is comparable to a reported 22% higher effective trunk stiffness among individuals with a history of recurrent, acute LBP when not experiencing LBP (2.00 N/mm) compared to individuals with no history of LBP (1.64 N/mm) (Hodges et al., 2009). Researchers have suggested that the higher stiffness among individuals with LBP, even when not experiencing pain, may result from higher baseline EMG levels in the trunk musculature, and may reflect a compensatory technique to increase spinal stability (Lee et al., 2006; Radebold et al., 2000; van Dieen et al., 2003b; Wilder et al., 1996). Although we found no difference in reflex delay between groups post-recovery (again, presumably when neither group was affected by LBP or any post-exercise effects), alterations in this aspect of trunk neuromuscular behavior following exercise were quite opposite in the eiLBP vs. control groups. Such a difference is interesting given earlier findings of longer reflex delays in individuals with chronic LBP compared to healthy individuals (Radebold et al., 2000; Radebold et al., 2001; Reeves et al., 2005), although this finding is not without exception (Lariviere et al., 2010).
The control group responded to exercise with an increase in stiffness and a decrease in reflex delay while the eiLBP group responded to exercise/eiLBP with no change in stiffness and an increase in reflex delay. The increase in stiffness in the control group after exercise is consistent with other studies on eccentric exercise (Green et al., 2012; Hoang et al., 2007), although it is not clear how much eccentric activity of the trunk musculature occurred during triathlon exercise training. The eiLBP group exhibited higher stiffness than the control group prior to exercise (i.e. post-recovery). This higher stiffness has been associated with increased muscle activation (Gardner-Morse and Stokes, 2001; Lee et al., 2006), and may have limited this group’s ability to further increase stiffness through increased muscle activation after exercise. However, no group difference in baseline paraspinal muscle activity was detected in the present study. It is interesting to note that reflex delay in the control group decreased with exercise and increased with exercise/eiLBP in the eiLBP group. The decrease in reflex delay in the control group with exercise may be related to the increase in stiffness with exercise, in that higher stiffness would lead to a larger/faster change in muscle length of muscle that could lead to quicker reflex responses. The increased reflex delay after exercise may have resulted from proprioceptive deficits associated with the presence of pain (Radebold et al., 2001).
While the use of individuals with eiLBP is a convenient alternative to prospective studies for studying changes in neuromuscular behavior with LBP, there are two notable limitations. First, neuromuscular behavior among individuals with eiLBP may differ from individuals with chronic LBP or acute LBP with varying etiology. As such, caution should be exercised when generalizing these results to other populations with LBP. Second, the changes in neuromuscular behavior with eiLBP are due to the combined effects of pain and exercise. These changes may differ from the effects of LBP alone.
In conclusion, differences in neuromuscular behavior between individuals with no history of LBP and individuals with eiLBP when pain free suggest that altered neuromuscular behavior contributes to the development of eiLBP with exercise. Also, differences found among individuals with eiLBP after exercise suggest that altered neuromuscular behavior is also a response to eiLBP. Although the neuromuscular mechanisms associated with eiLBP and chronic LBP may differ, these results suggest that previously-reported differences in trunk neuromuscular behavior between individuals with chronic LBP and healthy controls reflect a combination of inherent differences in neuromuscular behavior between these individuals as well as changes in neuromuscular behavior elicited by pain.
Acknowledgements
This work is dedicated to the late Dr. Kevin P. Granata. This work was supported by awards R01AR046111 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH), and R01OH008504 from the National Institute for Occupational Safety and Health of the Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or CDC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
We have no conflict of interest to report.
References
- Bazrgari B, Hendershot B, Muslim K, Toosizadeh N, Nussbaum MA, Madigan ML. Disturbance and recovery of trunk mechanical and neuromuscular behaviours following prolonged trunk flexion: influences of duration and external load on creep-induced effects. Ergonomics. 2011a;54:1043–1052. doi: 10.1080/00140139.2011.614357. [DOI] [PubMed] [Google Scholar]
- Bazrgari B, Nussbaum MA, Madigan ML. Estimation of trunk mechanical properties using system identification: effects of experimental setup and modelling assumptions. Comput Methods Biomech Biomed Engin. 2011b;15:1001–1009. doi: 10.1080/10255842.2011.570340. [DOI] [PubMed] [Google Scholar]
- Dubois JD, Piche M, Cantin V, Descarreaux M. Effect of experimental low back pain on neuromuscular control of the trunk in healthy volunteers and patients with chronic low back pain. Journal of electromyography and kinesiology: official journal of the International Society of Electrophysiological Kinesiology. 2011;21:774–781. doi: 10.1016/j.jelekin.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Gardner-Morse MG, Stokes IA. Trunk stiffness increases with steady-state effort. Journal of biomechanics. 2001;34:457–463. doi: 10.1016/s0021-9290(00)00226-8. [DOI] [PubMed] [Google Scholar]
- Granata KP, Slota GP, Bennett BC. Paraspinal muscle reflex dynamics. Journal of biomechanics. 2004;37:241–247. doi: 10.1016/s0021-9290(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Granata KP, Wilson SE, Padua DA. Gender differences in active musculoskeletal stiffness. Part I. Quantification in controlled measurements of knee joint dynamics. Journal of electromyography and kinesiology: official journal of the International Society of Electrophysiological Kinesiology. 2002;12:119–126. doi: 10.1016/s1050-6411(02)00002-0. [DOI] [PubMed] [Google Scholar]
- Green MA, Sinkus R, Gandevia SC, Herbert RD, Bilston LE. Measuring changes in muscle stiffness after eccentric exercise using elastography. NMR Biomed. 2012;25:852–858. doi: 10.1002/nbm.1801. [DOI] [PubMed] [Google Scholar]
- Hendershot B, Bazrgari B, Muslim K, Toosizadeh N, Nussbaum MA, Madigan ML. Disturbance and recovery of trunk stiffness and reflexive muscle responses following prolonged trunk flexion: influences of flexion angle and duration. Clin Biomech (Bristol, Avon) 2011;26:250–256. doi: 10.1016/j.clinbiomech.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Hendershot B, Bazrgari B, Nussbaum MA, Madigan ML. Within- and between-day reliability of trunk mechanical behaviors estimated using position-controlled perturbations. Journal of Biomechanics. 2012;45:2019–2022. doi: 10.1016/j.jbiomech.2012.05.026. [DOI] [PubMed] [Google Scholar]
- Hoang PD, Herbert RD, Gandevia SC. Effects of eccentric exercise on passive mechanical properties of human gastrocnemius in vivo. Medicine and science in sports and exercise. 2007;39:849–857. doi: 10.1249/MSS.0b013e318033499b. [DOI] [PubMed] [Google Scholar]
- Hodges P, van den Hoorn W, Dawson A, Cholewicki J. Changes in the mechanical properties of the trunk in low back pain may be associated with recurrence. Journal of biomechanics. 2009;42:61–66. doi: 10.1016/j.jbiomech.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Moseley GL. Pain and motor control of the lumbopelvic region: effect and possible mechanisms. Journal of electromyography and kinesiology: official journal of the International Society of Electrophysiological Kinesiology. 2003;13:361–370. doi: 10.1016/s1050-6411(03)00042-7. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Moseley GL, Gabrielsson A, Gandevia SC. Experimental muscle pain changes feedforward postural responses of the trunk muscles. Exp Brain Res. 2003;151:262–271. doi: 10.1007/s00221-003-1457-x. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Lee YT, Park DS, Kwon TK. Age affects the latency of the erector spinae response to sudden loading. Clin Biomech (Bristol, Avon) 2008;23:23–29. doi: 10.1016/j.clinbiomech.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kearney RE, Hunter IW. System identification of human joint dynamics. Crit Rev Biomed Eng. 1990;18:55–87. [PubMed] [Google Scholar]
- Lariviere C, Forget R, Vadeboncoeur R, Bilodeau M, Mecheri H. The effect of sex and chronic low back pain on back muscle reflex responses. European journal of applied physiology. 2010;109:577–590. doi: 10.1007/s00421-010-1389-7. [DOI] [PubMed] [Google Scholar]
- Lee PJ, Rogers EL, Granata KP. Active trunk stiffness increases with co-contraction. Journal of electromyography and kinesiology: official journal of the International Society of Electrophysiological Kinesiology. 2006;16:51–57. doi: 10.1016/j.jelekin.2005.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Pietrobon R, Sun SX, Liu GG, Hey L. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine. 2004;29:79–86. doi: 10.1097/01.BRS.0000105527.13866.0F. [DOI] [PubMed] [Google Scholar]
- Miller EM, Bazrgari B, Nussbaum MA, Madigan ML. Effects of Gender, Preload, and Trunk Angle on Intrinsic Trunk Stiffness. Journal of Musculoskeletal Research. 2012;15:1250012. [Google Scholar]
- Miller EM, Slota GP, Agnew MJ, Madigan ML. Females exhibit shorter paraspinal reflex latencies than males in response to sudden trunk flexion perturbations. Clin Biomech (Bristol, Avon) 2010;25:541–545. doi: 10.1016/j.clinbiomech.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe-Nilssen R, Ljunggren AE, Torebjork E. Dynamic adjustments of walking behavior dependent on noxious input in experimental low back pain. Pain. 1999;83:477–485. doi: 10.1016/S0304-3959(99)00153-0. [DOI] [PubMed] [Google Scholar]
- Radebold A, Cholewicki J, Panjabi MM, Patel TC. Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine. 2000;25:947–954. doi: 10.1097/00007632-200004150-00009. [DOI] [PubMed] [Google Scholar]
- Radebold A, Cholewicki J, Polzhofer GK, Greene HS. Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine. 2001;26:724–730. doi: 10.1097/00007632-200104010-00004. [DOI] [PubMed] [Google Scholar]
- Reeves NP, Cholewicki J, Milner TE. Muscle reflex classification of low-back pain. Journal of electromyography and kinesiology: official journal of the International Society of Electrophysiological Kinesiology. 2005;15:53–60. doi: 10.1016/j.jelekin.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2:175–184. [PubMed] [Google Scholar]
- Smith M, Coppieters MW, Hodges PW. Effect of experimentally induced low back pain on postural sway with breathing. Exp Brain Res. 2005;166:109–117. doi: 10.1007/s00221-005-2352-4. [DOI] [PubMed] [Google Scholar]
- van Dieen JH, Cholewicki J, Radebold A. Trunk muscle recruitment patterns in patients with low back pain enhance the stability of the lumbar spine. Spine. 2003a;28:834–841. [PubMed] [Google Scholar]
- van Dieen JH, Selen LP, Cholewicki J. Trunk muscle activation in low-back pain patients, an analysis of the literature. Journal of electromyography and kinesiology: official journal of the International Society of Electrophysiological Kinesiology. 2003b;13:333–351. doi: 10.1016/s1050-6411(03)00041-5. [DOI] [PubMed] [Google Scholar]
- van Tulder MW, Koes BW, Bouter LM. A cost-of-illness study of back pain in The Netherlands. Pain. 1995;62:233–240. doi: 10.1016/0304-3959(94)00272-G. [DOI] [PubMed] [Google Scholar]
- Wilder DG, Aleksiev AR, Magnusson ML, Pope MH, Spratt KF, Goel VK. Muscular response to sudden load. A tool to evaluate fatigue and rehabilitation. Spine. 1996;21:2628–2639. doi: 10.1097/00007632-199611150-00013. [DOI] [PubMed] [Google Scholar]