Abstract
Polycystic ovary syndrome (PCOS) is a fertility disorder affecting 5–7% of reproductive-aged women. Women with PCOS manifest both reproductive and metabolic defects. Several animal models have evolved, which implicate excess steroid exposure during fetal life in the development of the PCOS phenotype. This review addresses the fetal and adult reproductive and metabolic consequences of prenatal steroid excess in sheep and the translational relevance of these findings to PCOS. By comparing findings in various breeds of sheep, the review targets the role of genetic susceptibility to fetal insults. Disruptions induced by prenatal testosterone excess are evident at both the reproductive and metabolic level with each influencing the other thus creating a self-perpetuating vicious cycle. The review highlights the need for identifying a common mediator of the dysfunctions at the reproductive and metabolic levels and developing prevention and treatment interventions targeting all sites of disruption in unison for achieving optimal success.
Keywords: Infertility, PCOS, fetal programming, androgens, oestrogens
1. Polycystic ovary syndrome and its origins
Since its first description by Stein and Leventhal (1935), the polycystic ovary syndrome has been recognized as a very complex disorder with not only reproductive but also metabolic complications. The diagnostic criteria for PCOS continue to be disputed. It was not until 1991 that the first PCOS diagnostic criteria were published as an initiative of NIH. Based on the NIH criteria, PCOS is diagnosed if both, chronic anovulation and clinical and/or biochemical signs of hyperandrogenism are present after exclusion of other etiologies (Zawadki and Dunaif, 1992). However, since 1991 diagnostic criteria for PCOS have been revised in two occasions. In 2003 the Rotterdam consensus meeting included the presence of polycystic ovaries as one of the diagnostic feature; PCOS diagnosis required the presence of two of the three following features; oligo- or anovulation, clinical and/or biochemical signs of hyperandrogenism, and polycystic ovaries after exclusion of other aetiologies (congenital adrenal hyperplasia, androgen-secreting tumours, Cushing’s syndrome) (The Rotterdam ESHRE-ASRM PCOS consensus, 2004). In 2006, the AE-PCOSS established a different set of criteria for PCOS diagnosis; presence of clinical/biochemical hyperandrogenism and oligomenorrhea or polycystic ovaries (Azziz et al., 2006). Given the different diagnostic criteria used to define PCOS development of animal models to understand the aetiology of PCOS that meets the different diagnostic criteria poses challenges. In addition to the reproductive deficits, about 70% of PCOS women also manifest insulin resistance. Insulin-lowering drugs reduce hyperandrogenism implicating a metabolic component in the etiology of PCOS. In addition, women with PCOS are at increased risk of cardiovascular disease, dyslipidemia, hypertension, diabetes mellitus, and endometrial cancer. Only recently, the AE-PCOSS has reviewed the cardiovascular risk aspects of PCOS (Wild et al., 2010). The metabolic disruptions seen in PCOS women emphasize the need to not only address the issues of infertility but also the long-term goals of preventing debilitating diseases.
Despite the multitude of studies addressing PCOS very little is known about its aetiology. A number of gene-association studies undertaken to pin-point a genetic basis for the syndrome have yielded differing outcomes due to the variability of the syndrome, the different diagnostic criteria used, and the different populations studied. A genetic basis in the aetiology of PCOS arises from familial clustering in first-degree relatives of PCOS women (Yildiz et al., 2003) and higher prevalence of PCOS characteristics in monozygotic than dizygotic twins (Vink et al., 2006). Some candidate gene or chromosome regions found to be associated with PCOS include chromosome 19p13.2 (Urbanek et al., 2005), fibrillin 3 (although reports are contradictory) [Prodoehl et al., 2009; Ewens et al., 2010]), and SNP rs2414096 in the aromatase gene (Jin et al., 2009). However, the phenotypic heterogeneity in PCOS siblings points to contribution from environmental factors (Diamanti-Kandarakis et al., 2006). This is consistent with the recent endocrine society scientific statement pointing to a role of endocrine disruptors in the etiology of complex diseases such as obesity, diabetes mellitus, and cardiovascular disease (Diamanti-Kandarakis et al., 2009). Interestingly, women with PCOS have been found to have higher levels of the endocrine disruptor, bisphenol A, (Takeuchi et al., 2004; Kandaraki et al., 2011), possibly the result of decreased bisphenol A clearance in these women (Takeuchi et al., 2006).
Others believe that androgen excess early in life may lead to manifestation of PCOS in adulthood (Abbott et al., 2002, Barnes et al., 1994, Dumesic et al., 2007; Davies and Norman, 2002). In support, the PCOS phenotype is associated with conditions such as classical 21-hydroxylase deficiency in which the fetus has been exposed to high concentrations of sex steroids before birth (Barnes et al., 1994). A recent study found androgen levels to be elevated in female offspring of PCOS at birth (Barry et al., 2010), while another study reported reduced androstendione and oestradiol levels at term in umbilical cord blood of female offspring of PCOS women (Anderson et al., 2010).
A caveat of using term cord blood measures is that cord blood is of mixed origin and the time point well past the critical period of brain and ovarian differentiation in precocial species (Padmanabhan et al., 2007). Another study also failed to find a correlation between androgens in maternal blood at 18 weeks of gestation and the development of PCOS traits (Rotterdam criteria) at age 14–17 (Hickey et al., 2009). Considering that gestational levels of T in males start increasing at ~6 weeks of age and then begin to decline at 16 weeks of age remaining low thereafter until birth (Rouiller-Fabre et al., 2008), the timing of this study is at the tail end or beyond the period of masculinization. A recent study also indicates that androgen programming of masculinization in males occurs before 11–13 weeks of gestation in humans as evidenced by significantly longer anogenital distance in male fetuses by that stage (Fowler et al., 2011). One possible explanation for phenotypic variability is an interaction between genetic susceptibility and developmental insults.
2. Animal models to study PCOS
Several animal models have evolved focusing on the impact of perinatal exposure to steroids on the development of adult reproductive and metabolic pathologies (Abbott et al., 2006). These models stem from treatment of non-human primate, rat, mouse, and sheep with T or DHT. Comparative aspects of the various animal models and the quality of the steroid responsible for inducing reproductive and metabolic deficits paralleling that seen in women with PCOS have been reviewed previously (Abbott et al., 2002, 2006; Padmanabhan et al., 2010a; Padmanabhan and Veiga-Lopez, 2011). For translating the findings from these animal models to humans, it is important to interpret findings from the various models in relation to the developmental trajectory of the organ system being studied. The focus of this review is restricted to models that use sheep in their investigations. Readers are referred to other reviews in this issue for information relative to other animal models.
3. Benefits of developing a sheep as a model for developmental programming
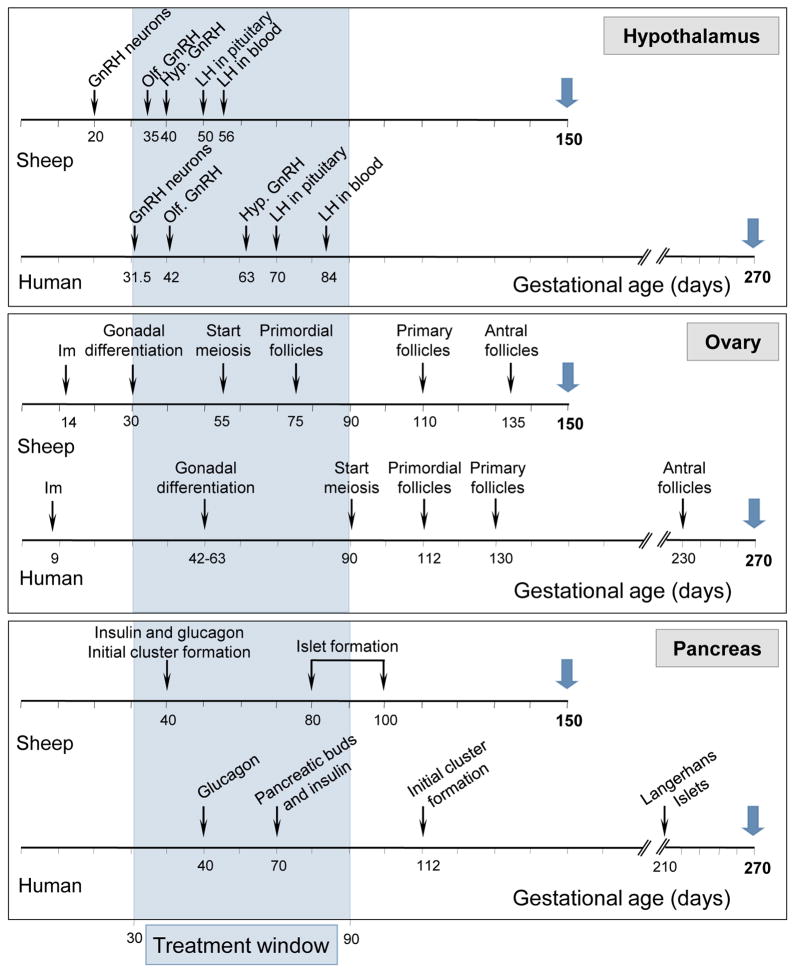
There are several benefits relative to the use of sheep as an animal model for endocrine research. Sheep are amenable for many interventions. Their large size compared to rodent models permits performance of detailed and repetitive hormonal profiling, non-invasive sequential monitoring of ovarian follicular dynamics via ultrasound, and multiple neurotransmitter measures. Because they are a domesticated species, animals can be kept in a natural setting free from stress associated with caging. Sheep has been an invaluable model for understanding fetal physiology (Harding and Bloomfield, 2004) and consequently studies focusing on developmental programming of reproductive dysfunction. Being precocial, their reproductive developmental trajectory follows a similar time line as in humans (Padmanabhan et al., 2007, see Fig 1). For instance, gonadal differentiation, start of meiosis, and appearance of primordial follicles occur at D35, D55, and D75 in sheep, respectively. In humans these events occur at D50, D90, and D112, respectively. In relation to gestational length (human: 280; sheep: 147 days), these events occur at similar time points during gestation in both species; 18, 32, and 40% in humans and 20, 37, and 51% in sheep of gestation. More importantly, full follicular differentiation in sheep, as in monkeys and humans, occurs before birth, unlike rodents where it occurs postnatally (Padmanabhan et al., 2007). The ontogeny of other organs such as the hypothalamus and the pancreas is also very similar to that described in humans (Fig 1). There is considerable parallel between sheep and human in mediators of cyclic ovarian function. For example, from a neuroendocrine perspective, progesterone blocks generation of LH surge in sheep as in humans, in contrast to progesterone being a facilitator in rodents (Kasa-Vubu et al., 1992; Levine, 1997; Gemzell-Danielsson and Marions, 2004). The perivoulatory events in sheep are similar to humans, albeit on a compressed time frame (length of follicular phase is 3 days in sheep vs. 14 days in human). Importantly, like humans, sheep are not litter-bearing. Finally, compared to non-human primates, sheep models are very cost effective.
Figure 1.
Developmental ontogeny of the GnRH neural circuitry (top panel), ovary (middle panel), and pancreas (bottom panel) in sheep and humans. The schema is developed based on information from following publications: 1) human hypothalamus (Kaplan, 1976; Seminara et al., 1998; Schwanzel-Fukuda, 1999), 2) ovine hypothalamus (Polkowska et al., 1987; Matwijiw et al., 1989; Brooks and McNeilly, 1992; Caldani et al. 1995), 3) human ovary (Francavilla et al., 1990; Bukovsky et al., 2005; Makabe et al., 2006), 4) ovine ovary (McNatty et al. 2000; Rhind et al., 2001; Sawyer et al. 2002), 5) human pancreas (Fowden, 1985; Bouwens et al., 1997; Fowden and Hill, 2001), and 6) ovine pancreas (Reddy et al., 1988; Fowden and Hill, 2001).
Abbreviations used: Hypothalamus: GnRH neurons: appearance of first GnRH immunoreactive neurons, Olf. GnRH: GnRH neurons visible in olfactory bulb, and Hyp. GnRH: appearance of GnRH neurons in the hypothalamus. Ovary: Im: implantation.
4. Early studies providing the basis for development of a sheep model of PCOS phenotype
The basis for developing sheep as a model to understand the impact of excess prenatal T exposure stems from the very early observations made by Short and Clarke in the 70’s in Finish-Landrace x Dorset Horn sheep. Using s.c. T implants (1 g) they tested various fetal exposure windows (D30-80, D50-100, D70-120, and D90-140) and established that the critical period for masculanization of the external genitalia is between D40 and D50 (Clarke et al., 1976a). This group had also reported earlier that prenatal T treatment disrupts reproductive cyclicity leading to oligo- and anovulation (Short, 1974) with severity of disruption dependent on the treatment window (Clarke et al., 1977). In addition, sheep treated with T from D30-D80 or D50-D100, but not D70-120 or D90-140 (later initiation of treatment; Clarke et al., 1976b), displayed male-typical behavior including increased aggressive behavior. The impact of T treatment beginning early during gestation (different window than used by Clarke) on behavioral and genital masculinization were confirmed in another breed of sheep, the New Zealand Romney sheep (treated on D20, D27, and D40; 200 mg/dose), by Wilson and Tarttelin (1978a).
A large body of information exists using prenatal T-treated sheep for studies centering on the sexual differentiation of the brain. These studies focused on organizational effects of prenatal T on oestradiol negative feedback, oestradiol positive feedback, progesterone negative feedback, and onset of neuroendocrine puberty. To remove influence from other ovarian factors, in these studies, animals were ovariectomized neonatally and clamped with an oestradiol implant (ovx+E model). Findings from prenatal T-treated Suffolk lambs that were ovariectomized at 3–5 weeks of age and inserted with s.c. implants of oestradiol (produces 3–5 pg/ml) found that prenatal T treatment (T cypionate [Wood et al., 1991], T propionate [Herbosa et al., 1995]) advanced neuroendocrine puberty, suggestive of an earlier escape from oestradiol negative feedback. The finding in the the ovx+E model demonstrating that both an early (D30-51) and late (D65-81) T treatment advances neuroendocrine puberty suggests that the critical period for programming the escape from oestradiol negative feedback spans between days 30–81(Wood et al., 1995). Subsequent studies with the ovx+E model found that prenatal DHT treatment from D30-90 (200, 400, and 800mg/twice a week) also advanced neuroendocrine puberty to 7–11 weeks of age (Masek et al., 1999) and co-treatment with flutamide, an antiandrogen, prevented this advancement (Jackson et al., 2008) suggestive of androgenic mediation of oestradiol negative feedback disruption. Further studies demonstrated that early prenatal T treatment (D30-76) diminishes the inhibition of long constant photoperiod to neuroendocrine puberty, while a later window (D89-135) was much less effective (Herbosa et al., 1996).
Studies with ovx+E Suffolk sheep also documented the disruptive effects of excess prenatal T on oestradiol positive feedback (Wood et al., 1991; Kosut et al., 1997; Herbosa et al., 1996), a finding first reported in ovary-intact Finish-Landrace x Dorset Horn sheep (Clarke and Scaramuzzi, 1978). These studies demonstrated that there is a dose threshold for programming oestradiol positive feedback disruptions; while 80 and 200 mg of T/week lead to complete blockade of the oestradiol positive feedback response, 32 mg/week only partially masculinized this response (Kosut et al., 1997). Studies with 3 doses of DHT (200, 400, and 800 mg/twice a week), a potent androgen (Handa et al., 2008), found lack of positive feedback disruptions with all 3 doses suggesting that organizational effects on oestradiol positive feedback disruptions are likely programmed via oestrogenic programming due to aromatization of T to oestrogen. Clearly, in these studies DHT treatment was effective in phenotypically masculinizing the external genitalia (Masek et al., 1999). Studies with the ovx+E Poll Dorset sheep (100 mg of T propionate/twice a week) demonstrated that prenatal treatment with T also reduces sensitivity to the progesterone negative feedback (Robinson et al., 1999). Both the D30-90 and D60-90 window of treatment produced this defect, suggesting that the critical period for this organization may reside between days 60–90 of gestation.
The initial observations in ovary-intact sheep (Short, 1974; Clarke et al., 1976a) and subsequent studies of sexual differentiation of brain using the ovx+E model established critical periods to target cycle disruptions and provide proof of principle for developing an ovary-intact animal model for understanding infertility disorders.
5. Adult reproductive phenotype of ovary-intact gestational T-treated sheep
Several sheep breeds have been used to study the effects of prenatal T on reproductive dysfunctions (see Table 1 for details). To date, the impact of prenatal T excess on the adult phenotype of three breeds, Finish-Landrace x Dorset Horn, Poll Dorset, and Suffolk breeds has been studied. Recent studies of prenatal T treatment with Scottish Greyface sheep have focused only on the fetal impact but not on the adult phenotypic outcome.
Table 1.
Top panel: Comparison of attributes of prenatal T-treated sheep (Finish-Landrace x Dorset Horn, Poll Dorset, Suffolk, and Scottish-Greyface) with that of women with PCOS. Bottom panel: Comparison of attributes of the above groups with the diagnostic criteria for PCOS. Numbers in superscript refer to citations from which information was derived.
| Sheep Breed (gestational days of treatment) | ||||||||
|---|---|---|---|---|---|---|---|---|
| PCOS women | Finish-Dorset (see text for days) | New Zealand Romney (D20, 27, 40) | Poll Dorset (D30-90) | Suffolk (D30-90) | Suffolk (D60-90) | Scottish Greyface (D60-90) | ||
| Reproduction | Functional hyperandrogenism | yes1,2 | - | - | - | yes24 | - | - |
| LH excess | yes3 | - | no19 | - | yes25 | yes36 | - | |
| Oligo- anovulation | yes1,2,4 | yes17 | - | yes21,22 | yes26 | no29, 36 | - | |
| Infertility | yes1, 2, 5 | - | - | - | - | yes37 | - | |
| PCO morphology | yes6 | - | - | - | yes27 | nounpublished | - | |
| Increased ovary weight/vol | yes6 | - | - | - | yes28 | nounpublished | - | |
| Follicular persistence | ? | - | - | - | yes26 | - | - | |
| Enhanced follicular recruitment | yes7 | - | - | yes23 | yes28 | - | - | |
| Disrupted E2 positive feedback | ? | yes18 | - | yes21, 22 | yes29 | yes29 | - | |
| Reduced E2 negative feedback | ? | - | - | - | yes30 | - | - | |
| Reduced P4 negative feedback | yes8 | - | - | - | yes31 | - | - | |
| Increased GnRH sensitivity | yes9 | - | no20 | - | yes25 | - | - | |
| Metabolism | Reduced insulin sensitivity | yes10, 11 | - | - | - | yes32 | yes32 | - |
| Increased total free fatty acids | yes12 | - | - | - | yes33 | - | - | |
| Hypertension | at risk13, 14 | - | - | - | yes34 | - | - | |
| Altered visceral adiposity | yes15, 16 | - | - | - | yes35 | - | - | |
| PCOS criteria | NIH criteria (chronic anovulation and biochemical/clinical signs of hyperandrogenism) | - | incomplete information | incomplete information | incomplete information | yes | incomplete information | adult phenotype not studied |
| Rotterdam criteria (2 of 3; oligo- or anovulation, clinical and/or biochemical signs of hyperandrogenism, polycystic ovaries) | - | incomplete information | incomplete information | yes | yes | incomplete information | adult phenotype not studied | |
| AE-PCOSS (clinical/biochemical hyperandrogenism and oligomenorrhea or polycystic ovaries) | - | incomplete information | incomplete information | incomplete information | yes | incomplete information | adult phenotype not studied | |
Abbreviations: AE-PCOSS: androgen excess-polycystic ovary syndrome society, D: gestational day, E2: oestradiol, GnRH: gonadotropin releasing hormone, LH: luteinizing hormone, NIH: National Institutes of Health, and P4: progesterone.
5.1. Reproductive cyclicity
Studies with Finish-Landrace x Dorset Horn sheep (Short, 1974) were the first to report perturbed reproductive cyclicity in prenatal T-treated females (D20-birth and D60-birth). Subsequent studies carried out with the Poll Dorset breed (D30-90) found that 70% of the prenatally T-treated females had regular cycles during the first breeding season with 100% of prenatal T-treated females becoming anovulatory in the second breeding season (Birch et al., 2003). Follow-up studies using the same breed of sheep and the same prenatal T treatment paradigm found 64% of prenatal T-treated females to manifest irregular cycles with more severe perturbations in the second than the first breeding season (Unsworth et al., 2005). Studies with the Suffolk breed using similar treatment paradigm as the Poll Dorset also found progressive deterioration of the reproductive axis with 64.3% of prenatal T-treated females having disrupted cycles in the first breeding season and 75% either not cycling or with severely disrupted reproductive cycles in the second breeding season (Manikkam et al., 2006). Postnatal overfeeding was found to amplify the severity of phenotype with majority being anovulatory in the first breeding season (Steckler et al., 2009).
Poll Dorset and Suffolk females treated during a shorter but later treatment window (D60-90) showed a less severe phenotype (Birch et al., 2003; Unsworth et al., 2005; Sharma et al., 2002). Although treatment dose used with Poll Dorset sheep was slightly lower than the Suffolk breed (80 vs. 100 mg/dose) 14% and 30% of Poll Dorset females failed to cycle during the first and second breeding season, respectively (Birch et al., 2003; Unsworth et al., 2005). In Suffolk sheep, all females displayed cycles during the first breeding season with only one out of 8 females studied during the second breeding season showing disrupted progestogenic cycles (Savabiesfahani et al., 2005). Finish-Landrace x Dorset Horn sheep treated from D80-birth showed no cycle disruptions (Short, 1974). The differences in severity of defects between Poll Dorset and Suffolk involving both early-long and late-short treatment models suggest that the Poll Dorset may be more susceptible than the Suffolk breed to prenatal T excess. The difference between the two Poll Dorset studies, 100% becoming anovulatory in the first study but not the second, suggests potential influence from maternal body condition, nutrition, or environment. Together, these studies emphasize the importance of the interaction between genetic background and environmental factors in an individual’s response to prenatal insults. An aspect to note is that the later window treatment does not lead to virilization of females as the early window.
5.2. Perivoulatory changes
Studies characterizing the effects of prenatal T on the periovulatory hormonal events are only available with the Suffolk breed (D30-90) and come from prostaglandin F2α-synchronized cycles (Veiga-Lopez et al., 2008). There was a gradation of response of prenatal T females to this synchronization protocol ranging from normal to anovulatory. Disruptions observed during the follicular phase of those that cycled include an elevated basal and delayed preovulatory oestradiol rise, a delayed and severely reduced primary gonadotropin surge, a trend for an amplified secondary FSH surge, and a shift in the relative balance of FSH regulatory proteins manifested as an increase in the ratio of activin A:follistatin+inhibin B during the preovulatory phase in prenatal T compared to control females. Differential degree of disruptions at various levels in the periovulatory hormonal sequence, stemming possibly from differences in individual genetic susceptibility, likely contribute to variety of ovulatory outcomes ranging from normal, reduced, or absent progesterone response. Subsequent studies comparing prenatal T and DHT-treated females demonstrated that follicular phase defects in oestradiol and LH surge dynamics were not evident in prenatal DHT-treated females, suggesting that the periovulatory defects are likely programmed via oestrogenic actions of T (Veiga-Lopez et al., 2009).
5.3. Neuroendocrine defects
Neuroendocrine feedback defects in ovary-intact females have been investigated in the Finish-Landrace x Dorset Horn, Poll Dorset, and Suffolk sheep breeds. These studies confirm predictions from the ovx+E model (Wood and Foster, 1998) and show that prenatal T treatment (D30-90) compromises all 3 neuroendocrine feedback mechanisms controlling cyclic changes in gonadotropin releasing hormone (GnRH)/LH secretion, namely oestradiol negative, oestradiol positive, and progesterone negative feedback (Padmanabhan et al., 2010a). Focused testing of oestradiol negative feedback in ovary-intact females has been carried out only in the Suffolk breed (Sarma et al., 2005). These studies document reduced sensitivity to the oestradiol negative feedback, a finding predicted by the advancement of neuroendocrine puberty in the prenatal T-treated ovx+E clamped model (Wood et al., 1991; Herbosa et al., 1995). That the programming of reduced oestradiol negative feedback appears to be androgen mediated is supported by similar outcomes in the prenatal DHT-treated ovary-intact Suffolk females (Veiga-Lopez et al., 2009).
Prenatal T excess also disrupts the oestradiol positive feedback in all 3 breeds studied. Studies using Finish-Landrace x Dorset Horn breed found only 2 of 6 of D30-80 T-treated females responded to oestradiol feedback challenge as opposed to 1 of the seven D50-80 treated females (Clarke et al., 1976b). Comparative breed-specific effects could be addressed between Poll Dorset vs. Suffolk sheep as they used similar prenatal T treatment window. A more severe phenotype, namely complete absence of positive feedback response, was reported in the Poll Dorset breed (no LH response after an oestradiol challenge) (Unsworth et al., 2005) compared to the Suffolk breed, where many showed a delayed and lower amplitude LH response (Sharma et al., 2002; Veiga-Lopez et al., 2009). In contrast to these findings in ovary-intact Suffolk sheep, oestradiol positive feedback response was completely absent when tests were conducted with the ovx+E Suffolk model (Wood et al., 1991; Herbosa et al., 1996; Kosut et al., 1997), indicative of protection from ovarian factors or cyclic oestradiol changes in the ovary-intact model. Oestradiol positive feedback response was not disrupted in prenatal DHT-treated ovary-intact (Veiga-Lopez et al., 2009) or ovx+E models (Masek et al., 1999) suggestive of programming via oestrogenic rather than androgenic actions of T. Compared to the early-long treatment (D30-90) the late-short T treatment (D60-90) has a lesser impact on the oestradiol positive feedback response. In the ovary-intact Suffolk sheep a small delay in response was observed with no effect on amplitude (Sharma et al., 2002), a finding in keeping with that seen also in the ovx+E model (Wood et al., 1995). Studies with Poll Dorset sheep (ovx+E model; D60-90) showed not only a delay but also a blunted LH response (Birch et al., 2003; Unsworth et al., 2005).
Studies characterizing LH pulsatility during the luteal phase in ovary-intact prenatal T-treated Suffolk sheep also provide evidence in support of reduced progesterone negative feedback in prenatal T-treated females (Veiga-Lopez et al., 2009). In these studies luteal frequency of LH pulses was found to be increased in prenatal T-treated females compared to controls, in the face of similar concentrations of progesterone. These findings substantiate earlier documentation of reduced progesterone negative feedback in the ovx+E Poll Dorset sheep model (Robinson et al., 1999). Recent neuroanatomical studies have found that prenatal T treatment decreases the number of dynorphin positive cells (inhibitory input to GnRH neurons) but not the number of kisspeptin positive cells (stimulatory input to GnRH neurons) in the kisspeptin/neurokinin B/dynorphin neurons of arcuate nucleus (Cheng et al., 2010). Since these neurons signal directly to GnRH neurons, these neuropeptide changes have been proposed as the underlying mechanism for a decreased sensitivity to the progesterone negative feedback in the prenatal T-treated animals.
Effects of prenatal T excess on pituitary sensitivity have been tested in Suffolk and in New Zealand Romney sheep with different outcomes. Studies with prenatal T-treated (D20, D27, and D40) Romney sheep (Wilson and Tarttelin, 1978b) found no disruption in the pituitary sensitivity. In contrast, studies with prenatal T- and DHT-treated Suffolk sheep (D30-90) demonstrated an increase in pituitary sensitivity to GnRH, suggestive of mediation via androgenic actions of T (Manikkam et al., 2008). The differences in outcomes between the 2 breeds may relate to lack of ablation of endogenous GnRH input prior to testing in the New Zealand Romney sheep.
5.4. Ovarian defects
Ovarian perturbations induced by prenatal T excess have been studied for the most part in the Suffolk (D30-90) breed with some information coming from the Poll Dorset breed (D30-90). Studies carried out with the Suffolk breed have provided evidence in support of a multifollicular ovarian phenotype (West et al., 2001). Studies carried out in Poll Dorset sheep using ovarian cortical biopsies taken at 8 months of age found a lower percentage of primordial follicles and higher percentage of primary follicles (Forsdike et al., 2007) suggestive of increased follicular recruitment. More detailed morphometric studies involving whole ovaries in Suffolk sheep found the presence of increased number of small and large antral follicles (Smith et al., 2009) in 10-month-old ovaries consistent with observations of multifollicular ovarian morphology (West et al., 2001) and a nearly 50% reduction in number of primordial follicles supportive of not only enhanced recruitment but also of follicular depletion (Smith et al., 2009). Ultrasonographic studies carried out only with the Suffolk breed spanning 25 days found large antral follicles to survive longer, thereby providing evidence in support of follicular persistence (Manikkam et al., 2006). The multifollicular phenotype and follicular persistence seen in prenatal T-treated sheep was not evident in prenatal DHT-treated sheep, indicative of oestrogenic mediation of this phenotype (Steckler et al., 2007a; West et al., 2001). Follicular persistence is a likely contributor to the ovulatory dysfunction evident in prenatal T-treated females. Eliminating persistent follicles by blocking gonadotropin input with a GnRH antagonist and subsequent stimulation with exogenous gonadotropins in a manner mimicking that seen during natural follicular phase was found to restore ovulatory and luteal response in prenatal T-treated Suffolk females (Steckler et al., 2008).
Intrafollicular changes in expression of key regulators of follicular development in adults have been undertaken mainly in the Suffolk breed. These studies found that prenatal T excess 1) increases expression of AR in granulosa cells of large preantral and antral follicles (Ortega et al., 2009) supportive of functional ovarian hyperandrogenism, 2) induces an imbalance in the expression of the two ESRs manifested as an increase in ESR1 and decrease in ESR2 in granulosa cells of antral follicles of 10- and 21-month-old females (puberty occurs at ~7 months of age, 3) disrupts the balance in ovarian paracrine factors; the percentage of follicles expressing follistatin mRNA was found to be increased while those that expressed activin B subunit tended to be lower in prenatal T females, suggestive of compromised intra-follicular activin availability (West et al., 2001), and 4) reduces expression of AMH in large preantral follicles but increases its expression in large antral follicles (Veiga-Lopez and Padmanabhan, 2010). Since AMH is known to reduce follicular sensitivity to FSH, the increased AMH expression in these late-staged follicles, may contribute to the follicular arrest and persistence seen in prenatal T-treated females. In Poll Dorset sheep, prenatal T excess was found to reduce the proportion of AMH positive non-growing and early-transitional follicles in adult ovaries (Bull et al., 2004). Studies addressing changes in insulin receptor signaling cascade and metabolic mediators in the Suffolk breed found a reduction in granulosa cell expression of adiponectin (Ortega et al., 2010), a protein hormone implicated in remodeling of the periovulatory follicle (Ledoux et al., 2006). Recent studies also suggest an imbalance in proliferative vs. apoptotic markers in granulosa and theca cells of antral follicles manifested as an increase in proliferating cell nuclear antigen and a decrease in B-cell lymphoma 2 protein in granulosa and theca cells of antral follicles of 10 and 21-month old prenatal T-treated females (Ortega et al., 2011). Together, these intrafollicular changes likely contribute to the disrupted fate of antral follicles leading to follicular arrest and persistence (Manikkam et al., 2006; Steckler et al., 2007a).
5.5. Fertility
Fertility tests have been conducted only with the Suffolk breed. Because early prenatal T treatment leads to virilization of the external genitalia (Wood and Foster, 1998, Manikkam et al., 2004), it is not possible to assess fertility potential through natural mating. The later and shorter window of T treatment (D60-90), which does not lead to virilization, provides a valuable resource for such investigations. Fertility tests carried out with the prenatal D60-90 T-treated females found that males chose controls and avoided prenatal T-treated females, when both groups were housed together. However, 100% mating success was achieved with the prenatal T-treated females, when mating tests were carried out in the absence co-housed controls. Only 40% of mated T females gave birth to live offspring, as opposed to 90% of the control breeding herd (Steckler et al., 2007b), providing evidence in support of reduced fecundity of prenatal T-treated females.
6. Adult metabolic phenotype of ovary-intact gestational T-treated sheep
Prenatal T-treated sheep also induces insulin resistance evident as early as 5 weeks of age (Recabarren et al., 2005) and during adult life (Padmanabhan et al., 2010b). Comparative studies with D30-90 T and DHT-treated females suggest that this reprogramming occurs via androgenic actions of T (Padmanabhan et al., 2010b). Peripheral insulin resistance was supported also by the findings that prenatal T excess modulates insulin sensitivity in a tissue-specific manner with liver and muscle being insulin resistant and adipose tissue insulin sensitive (Nada et al., 2010). These findings parallel what has been reported for women with PCOS in muscle (Dunaif et al., 2001; Corbould et al., 2005; Glintborg et al., 2008; Hojlund et al., 2008) and adipose tissue (Corbould and Dunaif, 2007; Corton et al., 2007; Seow et al., 2007). Improving insulin sensitivity with an insulin sensitizer (rosiglitazone) improved reproductive function in prenatal T-treated females by preventing progressive deterioration of the reproductive axis (Veiga-Lopez et al., 2010). Neuroanatomical studies found that prenatal T excess increases the number of immunopositive agouti-related peptide neurons in the arcuate nucleus of the hypothalamus (Sheppard et al., 2011) implicating metabolic neural circuitry in the development of insulin resistance. Our recent studies also indicate that prenatal T excess increases total as well as saturated FFA such as palmitic [16:0] (Veiga-Lopez et al., 2011a); FFA imbalance has been suggested as a possible cause in the development of hyperandrogenemia in PCOS women (Mai et al., 2008). Ongoing studies assessing adipose tissue distribution using computerized tomography at 16 months of age found prenatal T-treated females (D30-90) to have less visceral fat compared to controls (Padmanabhan et al., 2011). These findings parallel observations made in lean PCOS women (Dolfing et al., 2011), the reproductive and metabolic phenotype of whom prenatal T-treated females recapitulates (Padmanabhan et al., 2010a; Padmanabhan and Veiga-Lopez, 2011). Telemetry studies found prenatal T excess (D30-90) to increase arterial and diastolic blood pressure (King et al., 2007), suggestive of cardiovascular compromise.
7. Fetal phenotype of gestational T-treated sheep
7.1. Fetal phenotype following maternal administration of T
To understand how early insults program subsequent adult pathology, it is essential to focus on early changes in critical mediators. Majority of studies addressing fetal phenotype used the Suffolk breed with two studies in the Poll Dorset and one in the Scottish Greyface breed. While studies with the Suffolk breed have addressed the impact of prenatal T excess at the pituitary, ovary, and metabolic levels, studies with Poll Dorset and Scottish-Greyface breeds have thus far addressed only ovarian aspects.
7.1.1. Pituitary
So far, only one study involving Suffolk sheep has addressed the effects of prenatal T excess (D30-90) at the pituitary level during fetal life. These studies show that prenatal T excess induces developmental changes in gene expression of pituitary ESRs (decrease in ESR1) consistent with programming of reduced oestradiol negative feedback and increased LH release (Manikkam et al., 2008). Furthermore, prenatal T excess also induced changes in paracrine mediators of FSH regulation during fetal life. The composite changes in expression pattern of pituitary genes induced by prenatal T excess were in general consistent with changes seen in differential expression of LH and FSH subunit mRNAs seen during fetal life.
7.1.2. Ovary
Detailed morphometric studies have been carried out during 3 time points in fetal life (D65, D90, and D140) corresponding to middle, end and 50 days post T treatment only with Suffolk sheep treated during days 30 to 90 of gestation (Steckler et al., 2005; Smith et al., 2009). These studies provide evidence in support of programming of increased follicular recruitment by prenatal T excess as early as D140 of gestation, a feature seen also in adult life. The increase in number of activated follicles was evident not only in prenatal T but also DHT-treated fetuses suggestive of androgenic mediation of this programming. Prenatal T excess also induced an increase in the oocyte diameter of both the primordial and primary follicles, evident in D140 fetuses (Steckler et al., 2005), consistent with altered ovarian developmental trajectory. This impact of prenatal T excess was however not evident at fetal day 65 or 90 (Smith et al., 2009). Studies with the Scottish-Greyface breed (D60-90) also failed to find any disruption in ovarian morphology at fetal day 70 or 90 (Hogg et al., 2011). Later time points, when effects were seen in Suffolk breed (Steckler et al., 2005; Smith et al., 2009), have not been studied with the Scottish-Greyface breed.
Studies addressing changes in key ovarian regulatory proteins during fetal life has been carried out in Suffolk (D30-90) and Scottish-Greyface (D60-90) breed. It needs to be recognized that while findings during fetal life can be related to adult pathology in Suffolk breed, this is not possible with the Scottish-Greyface, due to lack of information on adult phenotype. Immunocytochemical studies with fetal ovaries involving Suffolk breed of sheep found prenatal T excess (D30-90) increases AR immunostaining in granulosa and stromal cells at fetal days 90 and140 (Ortega et al., 2009), but has no effect on ESR1, ESR2 or progesterone receptor protein expression. In contrast, the mRNA expression of steroid receptors (AR, ESR1, ESR2, and progesterone receptor) did not change in Suffolk female fetuses (D30-90) at days 65 or 90 of gestation (Luense et al., 2010a) or in Scottish-Greyface maternally T treated female fetuses (D60-90) at days 70 or 90 of gestation (Hogg et al., 2011). Since androgens are implicated in early follicular differentiation (Hillier and Tetsuka, 1997; Vendola et al.,1998; McGee and Hsueh, 2000; Walters et al., 2008) the upregulation of AR protein during early fetal life may be a contributing factor in the increased follicular recruitment (Steckler et al., 2005; Smith et al., 2009) evident in prenatal T-treated females.
Changes in key steroidogenic mediators have also been studied with Suffolk and Scottish-Greyface breed. Prenatal T treatment of Suffolk (D30-90) increased expression of aromatase, and 5α-reductase (Luense et al., 2010a) involved in the conversion of T to oestradiol and DHT, respectively in fetal day 65 ovaries. Prenatal T also decreased mRNA expression in Cyp11A1, which is the first step in conversion of cholesterol to pregnenolone. In Scottish-Greyface sheep prenatal T treatment (D60-90) reduced Cyp11A1, steroidogenic acute regulatory protein, 17α-hydroxylase, and LH receptor expression at fetal day 90 (Hogg et al., 2011). The impact of prenatal T excess on key paracrine regulators of ovarian differentiation has been studied in Poll Dorset and Suffolk breeds. Studies of prenatal T excess in Poll Dorset sheep (D30-90) found that very few follicles in the fetal ovaries from control animals stain positive for AMH (age not reported) with a lower percentage being stained in the T group (6.5 vs. 4.7%) (Bull et al., 2004). In the Suffolk model (D30-90) expression of growth differentiation factor 9 was found to be not altered (Veiga-Lopez and Padmanabhan, 2010).
In the context of insulin acting as co-gonadotropin, prenatal T excess (D30-90) in Suffolk breed did not alter immunostaining of members of insulin signaling cascade in fetal days 90 and 140 ovaries although an increase in peroxisome proliferator-activated receptor gamma protein expression in granulosa cells of fetal day 140 preantral follicles and a decrease in stromal cells during fetal days 90 and 140 were evident (Ortega et al., 2010). Relative to mediators of follicular survival/demise, prenatal T excess induced a selective decrease in expression of the pro-apoptotic mediator BAX in granulosa cells of fetal day 90 primordial and primary follicles (Ortega et al., 2011). The decreased expression of BAX may be the result of androgenic programming stemming from increased AR expression (Ortega et al., 2009), since prenatal DHT treatment also induced similar decrease in BAX. In concert, the changes in ovarian steroidogenic enzymes, steroid receptors, paracrine, and metabolic mediators as well as pro- and anti-apoptotic mediators may be responsible for the altered ovarian developmental trajectory.
There are various mechanisms by which prenatal T excess may program the changes in gene/protein expression of regulators of ovarian differentiation. These may involve epigenetic modifications including changes at the miRNA level (Narayanan et al., 2010; Delic et al., 2010). The versatility of functions attributed for miRNAs makes them a likely target for early programming by T. Our recent investigation using the Suffolk sheep (D30-90) found that prenatal T excess upregulates miRNAs (miR-497 and -15b) that have been implicated in the insulin signaling pathway (Luense et al., 2010b) in fetal D65 ovaries. The functional significance of these findings remains to be determined.
7.1.3. Metabolism
At the metabolic level studies are very limited and are only beginning to emerge. Preliminary studies carried out in the Suffolk sheep (D30-90) addressing the effects of prenatal T excess on insulin resistance at the tissue level have demonstrated that there is a tissue-specific programming of members of insulin signaling cascade (Nada et al., 2009).
7.2. Fetal phenotype following direct administration of T
A new model with Scottish Greyface sheep, which involve direct fetal administration of T has been developed (Hogg et al., 2011). In this model, a single injection of T (20 mg) is administered to the fetus through the flank at day 60 of gestation. This intervention was found to reduce mRNA expression of ESR1, ESR2, Cyp11A1, as well as roundabout homolog 1 and increase aromatase at fetal day 70 (only time point studied). The changes in ESR mRNA expression induced by T administration in this fetal T-administered model differs from lack of changes seen in the maternal T-administered Scottish-Greyface (D60-90) (Hogg et al., 2011) as well as Suffolk (D30-90) (Luense et al., 2010a) models. The impact of fetal T administration has not been studied at the pituitary or metabolic level.
8. Relating fetal steroid levels achieved with gestational T administration to human pathology
To relate findings from the sheep model to humans, it is important to address fetal levels of T and oestradiol (since T can be aromatized to oestradiol) achieved following gestational administration of T and compare them to physiological levels seen in control males. Fetal concentrations of T achieved during the treatment window (D30-90) have been addressed only in two studies. Our recent study with Suffolk sheep found that maternal administration of T propionate 100 mg/twice a week beginning day 30 of gestation resulted in 0.59±0.17 ng/ml T in umbilical arterial samples of D65 female fetuses, comparable to physiologic levels seen in control male fetuses of same age (0.45±0.16 ng/ml; Veiga-Lopez et al., 2011b). To place this in context, concentrations of T in male fetuses are elevated from D35 to D70 of gestation, declining between D70 and D90 (Attal et al., 1969), and remaining low until term (Pomerantz and Nalvandov, 1975). In a recent study with Scottish Greyface, maternal administration of 100 mg T propionate (D60-90) resulted in 0.30±0.14 ng/ml T on D70 and 0.15±0.02 ng/ml on D90 (Hogg et al., 2011). Corresponding levels in control males were 0.17±0.05 at D70 and 0.14±0.03 ng/ml at D90. The differing T levels reported with the Suffolk and Scottish Greyface breeds may relate to differences in pharmacokinetics, approaches used to measure T (LCMS with Suffolk [Veiga-Lopez et al., 2011b] vs. in house radioimmunoassay with Scottish-Greyface [Hogg et al., 2011]) or timing of measurement relative to the injection. On the other hand, a single direct intraperitoneal administration of 20 mg T to fetuses on D60 of gestation resulted in high fetal T levels of 4.53±2.20 ng/ml 10 days later (measured on D70 after D60 administration [Hogg et al., 2011]). The lack of information on the adult phenotype and the >20-fold higher levels of T levels achieved in the female fetus following direct fetal T administration relative to the male physiologic range limits the translational significance of this animal model.
Fetal levels of oestradiol achieved after administration maternal T has been studied in maternally treated Suffolk and the Scottish-Greyface breeds. Both breeds show elevated exposure of fetuses to oestradiol following maternal administration of T. Levels achieved in gestational T-treated Suffolk female fetuses were 2–3 fold higher than controls at D65 (C: 4.0±0.01, T: 14.4±2.7 pg/ml) or D90 (C: 4.0±0.05; T: 9.3±1.9 pg/ml) (Veiga-Lopez et al., 2011b). Levels achieved in Scottish-Greyface breed at D70 were of comparable magnitude (C: 4.6±1; T: 14.0±3.5 pg/ml) (Hogg et al., 2011) to that seen with the Suffolk breed.
9. Translational relevance to PCOS
Amongst all the prenatal T-treated sheep models discussed in this review, the D30-90 treated Suffolk sheep model is the most well-characterized. The adult attributes of this model meets the diagnostic criteria of human PCOS, whether it involves NIH, Rotterdam, or AE-PCOSS criteria (see Table 1 for details). The assumption that the D30-90 treated Suffolk sheep meets the NIH criteria is based on the increased androgen receptor expression found at the hypothalamic (Cernea et al., 2011), pituitary (Nada and Padmanabhan unpublished) and ovarian (Ortega et al., 2009) levels and associated functional outcomes such as ovarian follicular persistence (Manikkam et al., 2006) and LH excess (Manikkam et al., 2008) and not based on elevated systemic androgen levels (circulating levels of androgens in sheep are too low to get reliable estimates). The D30-90 T-treated Poll Dorset breed manifests oligo-anovulation and mutifollicular phenotype (surmised from enhanced follicular activation), hence meets the Rotterdam criteria. It is important to note that several features of women with PCOS such as LH excess, insulin resistance, and reduced progesterone negative feedback, although not part of diagnostic criteria, are also seen in the gestational D30-90 Suffolk breed (Manikkam et al., 2008; Padmanabhan et al., 2010b; Veiga Lopez et al., 2009). The Poll Dorset breed treated during similar intervals (D30-90) also shows reduced progesterone negative feedback (Robinson et al., 2009). Conclusions regarding the D60-90 Suffolk and Poll Dorset models cannot be made due to lack of information on ovarian phenotype and functional androgenism. Adult phenotype of the Scottish Greyface breed is yet to be characterized.
It is important to recognize that while the gestational D30-90 sheep model recapitulates all features of PCOS, and there are several conditions (eg. 21-hydroxylase deficiency) where the human fetus is exposed to high sex steroids levels and show PCOS features (Barnes et al., 1994), this does not establish that prenatal T excess is the cause of PCOS. Heritability studies point to clear genetic contribution (Yildiz et al., 2003; Vink et al., 2006). A logical explanation is that prenatal T excess acts as a decoder or amplifier of PCOS attributes dictated by genetics. What evidence exists in support of excess fetal T exposure in humans thus facilitating androgenic and oestrogenic decoding or amplification of genetic traits? Our studies in the sheep model indicate that some aspects of PCOS phenotype are programmed by androgenic while others by oestrogenic actions of T (Padmanabhan and Veiga-Lopez, 2011). Studies with various breeds of sheep also show the severity of phenotype varies with breed (discussed above). Relative to excess steroid exposure in human, exposure of female fetuses to excess T can occur during diabetic pregnancies (Driscoll et al., 1960) and clinical conditions such as congenital adrenal hyperplasia and adrenal virilizing tumors (Barnes et al., 1994; Yildiz and Azziz, 2007). Human fetuses can also be exposed to excess androgens or oestrogens from inadvertent exposure to contraceptive pills, use of anabolic steroids (Li et al., 1995; Kallen et al., 1991; Hartgens and Kuipers, 2004), or environmental steroid mimics (Hileman, 1994). A human cordocentesis study found 40% of female fetuses are exposed to T in levels reported in male fetuses (Beck-Peccoz et al., 1991). The fact that 40% of females do not manifest PCOS argues against prenatal T excess being the cause of human PCOS but rather to be an amplifier of genetic traits. A recent study reported high levels of T in female offspring born to PCOS women (Barry et al., 2010), although other studies failed to do so (Anderson et al., 2010; Hickey et al., 2009). These differences may relate to the timing when T was measured. To place this in context, human male fetuses are exposed to increased levels of T from ~6 to 16 weeks of gestation (Rouiller-Fabre et al., 2008). Changes in maternal and fetal T levels from lean, obese, diabetic, and PCOS pregnancies across gestational time points are required to resolve this issue, which indeed is a formidable task.
While the focus of this review has been on phenotype of prenatal T / DHT treated female offspring and their translational relevance to women with PCOS, it needs to be recognized that prenatal T excess also has an impact on the male phenotype. Prenatal T excess reduces sperm count and motility (Recabarren et al., 2008), increases number of Sertoli cells and reduces germ cells per seminiferous tubule (Rojas-García et al., 2010), and occludes tubule lumen (Bormann et al., 2011) in the Suffolk sheep (other breeds have not been studied). While the male PCOS phenotype remains to be investigated in depth relative to reproductive consequences, hyperandrogenemia and insulin resistance are features of male first-degree relatives of women with PCOS (Sam et al., 2008).
9. Future Directions
While not directly addressing the causality of human PCOS, the sheep model provides a unique resource for addressing underlying mechanisms and developing prevention and treatment options that are likely to be of human translational value. From a mechanistic perspective, future studies should focus on 1) distinguishing the relative contribution of androgens and oestrogens in the development of adult PCOS phenotype, 2) narrowing the developmental window of susceptibility taking into consideration the parallel with human developmental trajectory (sheep are precocial like humans while rodents are not [Padmanabhan et al., 2007]) (Fig. 1), 3) the role of environmental chemical exposures during pregnancy and early development in the development of PCOS phenotype, 4) the neural, ovarian, and metabolic mediaries of this programming, 5) early embryonic developmental defects, and 6) the epigenetic mechanisms involved. From a disease development perspective, the less severe phenotype of D60-90 sheep model (Birch et al., 2003; Sabaviesfahani et al., 2005; Steckler et al., 2007b) provides a valuable resource for addressing amplification of programmed traits by the postnatal metabolic environment as well as transgenerational transfer of traits programmed by prenatal T excess.
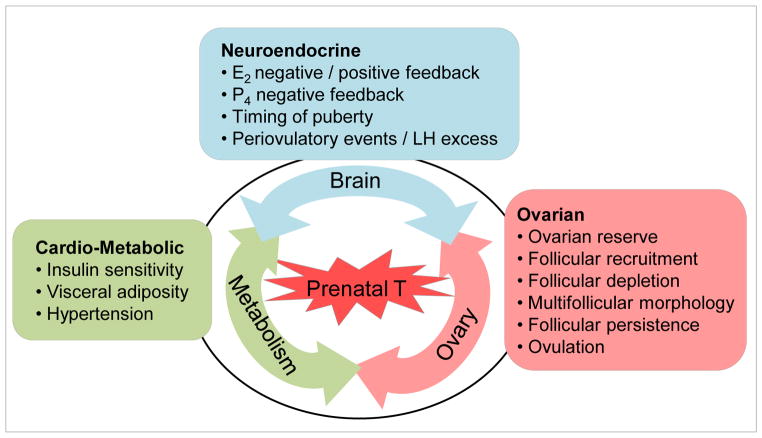
Even more importantly, from a translational stand point, the model provides a means to develop prevention and treatment strategies. Considering the impact of prenatal T is evident at the fetal level, interventions should be targeted during pregnancy to prevent development of disease phenotype. If epigenetic mechanisms are found to be the primary means by which reprogramming occurs, dietary changes involving methyl donors can be imposed to overcome development of such defects. From a treatment perspective, once pathology is developed, approaches can be targeted to prevent manifestation of masked phenotype or lessen the severity of phenotype. Exacerbation of severity of established pathologies, such as PCOS, with obesity suggests that such treatment strategies should encompass lifestyle modifications. Because prenatal T excess impacts reproductive and metabolic systems and sets in motion a self perpetuating cycle with defect at one level amplifying the other (Fig 2), treatments targeted to one site may not yield optimal success. An integrative systems approach with interventions targeted at all affected sites in unison would be required to overcome such developmentally programmed pathologies. Ideally, a common mediary responsible for inducing defects at the various sites needs to be identified. At the same time, studies are needed in humans for mapping accurately the developmental changes in fetal steroidal and metabolic environment. Measures obtained from cord samples taken at birth are not optimal for addressing insults occurring early during gestation. All in all, a bench to bedside and back to bench studies and partnering of clinicians and basic scientists would be required to develop appropriate prevention and treatment strategies aimed towards overcoming infertility disorders such as seen in women with PCOS.
Figure 2.
Self-perpetuating vicious cycle involving the three systems (neuroendocrine, ovarian, and metabolic) impacted by prenatal T excess. Abbreviations: E2: oestradiol, LH: luteinizing hormone, P4: progesterone.
Highlights.
Prenatal T excess programs PCOS phenotype in sheep.
Outcomes in different breeds support genetic environmental interactions.
Prenatal T excess impacts reproductive and metabolic systems and sets an interactive self-perpetuating vicious cycle.
Intervention at all sites in unison is needed to achieve optimal success.
A common mediator of prenatal T effects at reproductive and metabolic levels needs to be identified.
Abbreviations
- AE-PCOSS
Androgen Excess and PCOS Society
- AMH
antimullerian hormone
- AR
androgen receptor
- BAX
Bcl-2-associated X protein
- Cyp11A1
cholesterol side-chain cleavage enzyme
- DHT
dihydrotestosterone
- D
gestational day
- ESHRE-ASRM
European Society of Human Reproduction and Embryology-American Society for Reproductive Medicine
- ESHRE-ASRM
European Society of Human Reproduction and Embryology-American Society for Reproductive Medicine
- ESR
oestradiol receptor
- FFA
free fatty acids
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin releasing hormone
- LH
luteinizing hormone
- miRNA
microRNAs
- ovx+E
ovariectomized neonatally and clamped with an oestradiol implant
- NIH
National Institutes of Health
- PCOS
polycystic ovary syndrome
- T
testosterone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vasantha Padmanabhan, Email: vasantha@umich.edu, Professor, Departments of Pediatrics, Obstetrics and Gynecology, and Molecular and Integrative Physiology, The University of Michigan, Ann Arbor, MI, 300 North Ingalls, Room 1138, Phone: 734.647.0276, FAX: 734.615.5441.
Almudena Veiga-Lopez, Email: aveiga@umich.edu, Research Investigator, Department of Pediatrics, The University of Michigan, Ann Arbor, MI, 300 North Ingalls, Room 1135, Phone: 734.615.8607, FAX: 734.615.5441.
References
- Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome - a hypothesis. J Endocrinol. 2002;174:1–5. doi: 10.1677/joe.0.1740001. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Dumesic DA, Levine JE, Dunaif A, Padmanabhan V. Animal models and fetal programming of PCOS. In: Azziz JE, Nestler JE, Dewailly D, editors. Contemporary endocrinology: androgen excess disorders in women: polycystic ovary syndrome and other disorders. Humana Press Inc; Totowa, NJ: 2006. pp. 259–272. [Google Scholar]
- Anderson H, Fogel N, Grebe SK, Singh RJ, Taylor RL, Dunaif A. Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J Clin Endocrinol Metab. 2010;95:2180–2186. doi: 10.1210/jc.2009-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attal JA. Levels of testosterone, androstenedione, estrone and estradiol-17 beta in the testes of fetal sheep. Endocrinology. 1969;85:280–289. doi: 10.1210/endo-85-2-280. [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- Barber TM, McCarthy MI, Wass JA, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf) 2008;65:137–145. doi: 10.1111/j.1365-2265.2006.02587.x. [DOI] [PubMed] [Google Scholar]
- Barnes RB, Rosenfield RL, Ehrmann DA, Cara JF, Cuttler L, Levitsky LL, Rosenthal IM. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79:1328–1333. doi: 10.1210/jcem.79.5.7962325. [DOI] [PubMed] [Google Scholar]
- Barry JA, Kay AR, Navaratnarajah R, Iqbal S, Bamfo JE, David AL, Hines M, Hardiman PJ. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Obstet Gynaecol. 2010;30:444–446. doi: 10.3109/01443615.2010.485254. [DOI] [PubMed] [Google Scholar]
- Beck-Peccoz P, Padmanabhan V, Baggiani AM, Cortelazzi D, Buscaglia M, Medri G, Marconi AM, Pardi G, Beitins IZ. Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: circulating levels of gonadotropins, their common alpha-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-stimulating hormone. J Clin Endocrinol Metab. 1991;73:525–532. doi: 10.1210/jcem-73-3-525. [DOI] [PubMed] [Google Scholar]
- Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144:1426–1434. doi: 10.1210/en.2002-220965. [DOI] [PubMed] [Google Scholar]
- Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update. 2006;12:351–361. doi: 10.1093/humupd/dml017. [DOI] [PubMed] [Google Scholar]
- Bormann CL, Smith GD, Padmanabhan V, Lee TM. Prenatal testosterone and dihydrotestosterone exposure disrupts ovine testicular development. Reproduction. 2011;142:167–173. doi: 10.1530/REP-10-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AN, McNeilly AS. Inhibitory effects of a luteinizing-hormone-releasing hormone agonist implant on ovine fetal gonadotrophin secretion and pituitary sensitivity to luteinizing-hormone-releasing hormone. J Reprod Fertil. 1992;96:785–792. doi: 10.1530/jrf.0.0960785. [DOI] [PubMed] [Google Scholar]
- Bouwens L, Lu WG, De Krijger R. Proliferation and differentiation in the human fetal endocrine pancreas. Diabetologia. 1997;40:398–404. doi: 10.1007/s001250050693. [DOI] [PubMed] [Google Scholar]
- Bull L, Stubbs S, Birch R, Robinson J, Themmen AP, Visser JA, Groome NP, Franks S. Reduced expression of anti-Mullerian hormone (AMH) protein in the androgenised sheep ovary. Endocrine Abstracts. 2004;8:OC13. [Google Scholar]
- Bukovsky A, Caudle MR, Svetlikova M, Wimalasena J, Ayala ME, Dominguez R. Oogenesis in adult mammals, including humans: a review. Endocrine. 2005;26:301–316. doi: 10.1385/ENDO:26:3:301. [DOI] [PubMed] [Google Scholar]
- Caldani M, Antoine M, Batailler M, Duittoz A. Ontogeny of GnRH systems. J Reprod Fertil Suppl. 1995;49:147–162. [PubMed] [Google Scholar]
- Cernea M, Lee T, Cheng G, Padmanabhan V, Lehman M, Coolen L. Excess prenatal testosterone increases androgen receptor expression in the female sheep brain. Program of the Annual Meeting of the Society for Neuroscience; Washington DC, USA. 2011. p. 499.20. [Google Scholar]
- Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151:301–311. doi: 10.1210/en.2009-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke IJ, Scaramuzzi RJ, Short RV. Effects of testosterone implants in pregnant ewes on their female offspring. J Embryol Exp Morphol. 1976a;36:87–99. [PubMed] [Google Scholar]
- Clarke IJ, Scaramuzzi RJ, Short RV. Sexual differentiation of the brain: endocrine and behavioural responses of androgenized ewes to oestrogen. J Endocrinol. 1976b;71:175–176. doi: 10.1677/joe.0.0710175. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Scaramuzzi RJ, Short RV. Ovulation in prenatally androgenized ewes. J Endocrinol. 1977;73:385–389. doi: 10.1677/joe.0.0730385. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Scaramuzzi RJ. Release of luteinizing hormone in androgenized ewes after prostaglandin-induced luteolysis or luteinizing hormone releasing hormone. J Endocrinol. 1978;77:261–262. doi: 10.1677/joe.0.0770261. [DOI] [PubMed] [Google Scholar]
- Corbould A, Kim YB, Youngren JF, Pender C, Kahn BB, Lee A, Dunaif A. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab. 2005;288:E1047–1054. doi: 10.1152/ajpendo.00361.2004. [DOI] [PubMed] [Google Scholar]
- Corbould A, Dunaif A. The adipose cell lineage is not intrinsically insulin resistant in polycystic ovary syndrome. Metabolism. 2007;56:716–722. doi: 10.1016/j.metabol.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corton M, Botella-Carretero JI, Benguria A, Villuendas G, Zaballos A, San Millan JL, Escobar-Morreale HF, Peral B. Differential gene expression profile in omental adipose tissue in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:328–337. doi: 10.1210/jc.2006-1665. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Norman RJ. Programming and reproductive functioning. Trends Endocrinol Metab. 2002;13:386–392. doi: 10.1016/s1043-2760(02)00691-4. [DOI] [PubMed] [Google Scholar]
- Delic D, Grosser C, Dkhil M, Al-Quraishy S, Wunderlich F. Testosterone-induced upregulation of miRNAs in the female mouse liver. Steroids. 2010;75:998–1004. doi: 10.1016/j.steroids.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Kandarakis H, Legro RS. The role of genes and environment in the etiology of PCOS. Endocrine. 2006;30:19–26. doi: 10.1385/ENDO:30:1:19. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E. Role of obesity and adiposity in polycystic ovary syndrome. Int J Obes (Lond) 2007;31(Suppl 2):S8–13. doi: 10.1038/sj.ijo.0803730. discussion, S31–32. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolfing JG, Stassen CM, van Haard PM, Wolffenbuttel BH, Schweitzer DH. Comparison of MRI-assessed body fat content between lean women with polycystic ovary syndrome (PCOS) and matched controls: less visceral fat with PCOS. Hum Reprod. 2011;26:1495–1500. doi: 10.1093/humrep/der070. [DOI] [PubMed] [Google Scholar]
- Driscoll SG, Benirschke K, Curtis GW. Neonatal deaths among infants of diabetic mothers: postmortem findings in ninety-five infants. Am J Dis Child. 1960;100:818–835. doi: 10.1001/archpedi.1960.04020040820004. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8:127–141. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Wu X, Lee A, Diamanti-Kandarakis E. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS) Am J Physiol Endocrinol Metab. 2001;281:E392–399. doi: 10.1152/ajpendo.2001.281.2.E392. [DOI] [PubMed] [Google Scholar]
- Ehrmann DA. β-cell dysfunction, glucose intolerance, and diabetes in the polycystic syndrome. In: Azziz R, Nestler JE, Dewailly D, editors. Androgen excess disorders in women. Humana Press Inc; Totowa, NJ: 2007. pp. 319–324. [Google Scholar]
- Ewens KG, Stewart DR, Ankener W, Urbanek M, McAllister JM, Chen C, Baig KM, Parker SC, Margulies EH, Legro RS, Dunaif A, Strauss JF, 3rd, Spielman RS. Family-based analysis of candidate genes for polycystic ovary syndrome. J Clin Endocrinol Metab. 2010;95:2306–2315. doi: 10.1210/jc.2009-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsdike RA, Hardy K, Bull L, Stark J, Webber LJ, Stubbs S, Robinson JE, Franks S. Disordered follicle development in ovaries of prenatally androgenized ewes. J Endocrinol. 2007;192:421–428. doi: 10.1677/joe.1.07097. [DOI] [PubMed] [Google Scholar]
- Fowden AL. Pancreatic endocrine function and carbohydrate metabolism in the fetus. In: Abrecht E, Pepe GJ, editors. Research in Perinatal Medicine IV. Perinatology Press; Ithaca, NY: 1985. pp. 71–90. [Google Scholar]
- Fowden AL, Hill DJ. Intra-uterine programming of the endocrine pancreas. Br Med Bull. 2001;60:123–142. doi: 10.1093/bmb/60.1.123. [DOI] [PubMed] [Google Scholar]
- Fowler PA, Bhattacharya S, Flannigan S, Drake AJ, O’Shaughnessy PJ. Maternal cigarette smoking and effects on androgen action in male offspring: unexpected effects on second-trimester anogenital distance. J Clin Endocrin Metab. 2011 doi: 10.1210/jc.2011-1100. First published ahead of print July 13, 2011. [DOI] [PubMed] [Google Scholar]
- Francavilla S, Cordeschi G, Properzi G, Concordia N, Cappa F, Pozzi V. Ultrastructure of fetal human gonad before sexual differentiation and during early testicular and ovarian development. J Submicrosc Cytol Pathol. 1990;22:389–400. [PubMed] [Google Scholar]
- Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- Franks S, Roberts R, Hardy K. Gonadotrophin regimens and oocyte quality in women with polycystic ovaries. Reprod Biomed Online. 2003;6:181–184. doi: 10.1016/s1472-6483(10)61708-7. [DOI] [PubMed] [Google Scholar]
- Gemzell-Danielsson K, Marions L. Mechanisms of action of mifepristone and levonorgestrel when used for emergency contraception. Hum Reprod Update. 2004;10:341–348. doi: 10.1093/humupd/dmh027. [DOI] [PubMed] [Google Scholar]
- Glintborg D, Hojlund K, Andersen NR, Hansen BF, Beck-Nielsen H, Wojtaszewski JF. Impaired insulin activation and dephosphorylation of glycogen synthase in skeletal muscle of women with polycystic ovary syndrome is reversed by pioglitazone treatment. J Clin Endocrinol Metab. 2008;93:3618–3626. doi: 10.1210/jc.2008-0760. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding JE, Bloomfield FH. Prenatal treatment of intrauterine growth restriction: lessons from the sheep model. Pediatr Endocrinol Rev. 2004;2:182–192. [PubMed] [Google Scholar]
- Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34:513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- Herbosa CG, Wood RI, Foster DL. Prenatal androgens modify the reproductive response to photoperiod in the developing sheep. Biol Reprod. 1995;52:163–169. doi: 10.1095/biolreprod52.1.163. [DOI] [PubMed] [Google Scholar]
- Herbosa CG, Foster DL. Defeminization of the reproductive response to photoperiod occurs early in prenatal development in the sheep. Biol Reprod. 1996;54:420–428. doi: 10.1095/biolreprod54.2.420. [DOI] [PubMed] [Google Scholar]
- Hickey M, Sloboda DM, Atkinson HC, Doherty DA, Franks S, Norman RJ, Newnham JP, Hart R. The relationship between maternal and umbilical cord androgen levels and polycystic ovary syndrome in adolescence: a prospective cohort study. J Clin Endocrinol Metab. 2009;94:3714–3720. doi: 10.1210/jc.2009-0544. [DOI] [PubMed] [Google Scholar]
- Hileman B. Environmental estrogens linked to reproductive abnormalities, cancer. Chem Eng News. 1994;72:19–23. [Google Scholar]
- Hillier SG, Tetsuka M. Role of androgens in follicle maturation and atresia. Baillieres Clin Obstet Gynaecol. 1997;11:249–260. doi: 10.1016/s0950-3552(97)80036-3. [DOI] [PubMed] [Google Scholar]
- Hogg K, McNeilly AS, Duncan WC. Prenatal androgen exposure leads to alterations in gene and protein expression in the ovine fetal ovary. Endocrinology. 2011;152:2048–2059. doi: 10.1210/en.2010-1219. [DOI] [PubMed] [Google Scholar]
- Hoffman LK, Ehrmann DA. Cardiometabolic features of polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2008;4:215–222. doi: 10.1038/ncpendmet0755. [DOI] [PubMed] [Google Scholar]
- Hojlund K, Glintborg D, Andersen NR, Birk JB, Treebak JT, Frosig C, Beck-Nielsen H, Wojtaszewski JF. Impaired insulin-stimulated phosphorylation of Akt and AS160 in skeletal muscle of women with polycystic ovary syndrome is reversed by pioglitazone treatment. Diabetes. 2008;57:357–366. doi: 10.2337/db07-0706. [DOI] [PubMed] [Google Scholar]
- Holte J, Bergh T, Berne C, Lithell H. Serum lipoprotein lipid profile in women with the polycystic ovary syndrome: relation to anthropometric, endocrine and metabolic variables. Clin Endocrinol (Oxf) 1994;41:463–471. doi: 10.1111/j.1365-2265.1994.tb02577.x. [DOI] [PubMed] [Google Scholar]
- Homburg R. Polycystic ovary syndrome: induction of ovulation. Baillieres Clin Endocrinol Metab. 1996;10:281–292. doi: 10.1016/s0950-351x(96)80127-3. [DOI] [PubMed] [Google Scholar]
- Jackson LM, Timmer KM, Foster DL. Sexual differentiation of the external genitalia and the timing of puberty in the presence of an antiandrogen in sheep. Endocrinology. 2008;149:4200–4208. doi: 10.1210/en.2007-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JL, Sun J, Ge HJ, Cao YX, Wu XK, Liang FJ, Sun HX, Ke L, Yi L, Wu ZW, Wang Y. Association between CYP19 gene SNP rs2414096 polymorphism and polycystic ovary syndrome in Chinese women. BMC Med Genet. 2009;10:139. doi: 10.1186/1471-2350-10-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen B, Mastroiacovo P, Lancaster PA, Mutchinick O, Kringelbach M, Martinez-Frias ML, Robert E, Castilla EE. Oral contraceptives in the etiology of isolated hypospadias. Contraception. 1991;44:173–182. doi: 10.1016/0010-7824(91)90117-x. [DOI] [PubMed] [Google Scholar]
- Kaplan SL, Grumbach MM, Aubert ML. The ontogenesis of pituitary hormones and hypothalamic factors in the human fetus: maturation of central nervous system regulation of anterior pituitary function. Recent Prog Horm Res. 1976;32:161–243. doi: 10.1016/b978-0-12-571132-6.50015-4. [DOI] [PubMed] [Google Scholar]
- Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, Palimeri S, Panidis D, Diamanti-Kandarakis E. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab. 2011;96:E480–484. doi: 10.1210/jc.2010-1658. [DOI] [PubMed] [Google Scholar]
- Kasa-Vubu JZ, Dahl GE, Evans NP, Thrun LA, Moenter SM, Padmanabhan V, Karsch FJ. Progesterone blocks the estradiol-induced gonadotropin discharge in the ewe by inhibiting the surge of gonadotropin-releasing hormone. Endocrinology. 1992;131:208–212. doi: 10.1210/endo.131.1.1611998. [DOI] [PubMed] [Google Scholar]
- King AJ, Olivier NB, Mohankumar PS, Lee JS, Padmanabhan V, Fink GD. Hypertension caused by prenatal testosterone excess in female sheep. Am J Physiol Endocrinol Metab. 2007;292:E1837–1841. doi: 10.1152/ajpendo.00668.2006. [DOI] [PubMed] [Google Scholar]
- Kosut SS, Wood RI, Herbosa-Encarnacion C, Foster DL. Prenatal androgens time neuroendocrine puberty in the sheep: effect of testosterone dose. Endocrinology. 1997;138:1072–1077. doi: 10.1210/endo.138.3.4993. [DOI] [PubMed] [Google Scholar]
- Ledoux S, Campos DB, Lopes FL, Dobias-Goff M, Palin MF, Murphy BD. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology. 2006;147:5178–5186. doi: 10.1210/en.2006-0679. [DOI] [PubMed] [Google Scholar]
- Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod. 1997;56:293–302. doi: 10.1095/biolreprod56.2.293. [DOI] [PubMed] [Google Scholar]
- Li DK, Daling JR, Mueller BA, Hickok DE, Fantel AG, Weiss NS. Oral contraceptive use after conception in relation to the risk of congenital urinary tract anomalies. Teratology. 1995;51:30–36. doi: 10.1002/tera.1420510105. [DOI] [PubMed] [Google Scholar]
- Luense LJ, Christenson LK, Padmanabhan V. Developmental programming: Gestational testosterone treatment alters fetal ovarian steroidogenic gene expression. Biol Reprod; 43rd Annual Meeting of the Society for Study of Reproduction; Milwaukee, Wisconsin. 2010a. p. 183. [Google Scholar]
- Luense LJ, Padmanabhan V, Christenson LK. Developmental Programming: Maternal testosterone excess fetal ovarian mRNA and microRNA gene expression. 17th Ovarian Workshop; Milwaukee, MD. 2010b. p. 44. [Google Scholar]
- Mai K, Bobbert T, Reinecke F, Andres J, Maser-Gluth C, Wudy SA, Mohlig M, Weickert MO, Hartmann MF, Schulte HM, Diederich S, Pfeiffer AF, Spranger J. Intravenous lipid and heparin infusion-induced elevation in free fatty acids and triglycerides modifies circulating androgen levels in women: a randomized, controlled trial. J Clin Endocrinol Metab. 2008;93:3900–3906. doi: 10.1210/jc.2008-0714. [DOI] [PubMed] [Google Scholar]
- Makabe S, Naguro T, Stallone T. Oocyte-follicle cell interactions during ovarian follicle development, as seen by high resolution scanning and transmission electron microscopy in humans. Microsc Res Tech. 2006;69:436–449. doi: 10.1002/jemt.20303. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147:1997–2007. doi: 10.1210/en.2005-1338. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Thompson RC, Herkimer C, Welch KB, Flak J, Karsch FJ, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on pre- and postnatal gonadotropin regulation in sheep. Biol Reprod. 2008;78:648–660. doi: 10.1095/biolreprod.107.063347. [DOI] [PubMed] [Google Scholar]
- Martínez-Bermejo E, Luque-Ramírez M, Escobar-Morreale HF. Obesity and the polycystic ovary syndrome. Minerva Endocrinol. 2007;32:129–140. [PubMed] [Google Scholar]
- Masek KS, Wood RI, Foster DL. Prenatal dihydrotestosterone differentially masculinizes tonic and surge modes of luteinizing hormone secretion in sheep. Endocrinology. 1999;140:3459–3466. doi: 10.1210/endo.140.8.6913. [DOI] [PubMed] [Google Scholar]
- Matwijiw I, Thliveris JA, Faiman C. Hypothalamo-pituitary portal development in the ovine fetus. Biol Reprod. 1989;40:1127–1130. doi: 10.1095/biolreprod40.5.1127. [DOI] [PubMed] [Google Scholar]
- McCartney CR, Eagleson CA, Marshall JC. Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin Reprod Med. 2002;20:317–326. doi: 10.1055/s-2002-36706. [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Fidler AE, Juengel JL, Quirke LD, Smith PR, Heath DA, Lundy T, O’Connell A, Tisdall DJ. Growth and paracrine factors regulating follicular formation and cellular function. Mol Cell Endocrinol. 2000;163:11–20. doi: 10.1016/s0303-7207(99)00235-x. [DOI] [PubMed] [Google Scholar]
- Nada S, Thompson RC, Padmanabhan V. Developmental programming: prenatal testosterone excess has differential effects on the developmental trajectory of members of the insulin signaling cascade in liver and skeletal muscle. Biol Reprod. 2009;81(Suppl):342. [Google Scholar]
- Nada SE, Thompson RC, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone excess on insulin target tissues. Endocrinology. 2010;151:5165–5173. doi: 10.1210/en.2010-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Jiang J, Gusev Y, Jones A, Kearbey JD, Miller DD, Schmittgen TD, Dalton JT. MicroRNAs are mediators of androgen action in prostate and muscle. PLoS One. 2010;5:e13637. doi: 10.1371/journal.pone.0013637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega HH, Salvetti NR, Padmanabhan V. Developmental programming: prenatal androgen excess disrupts ovarian steroid receptor balance. Reproduction. 2009;137:865–877. doi: 10.1530/REP-08-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega HH, Rey F, Velazquez MM, Padmanabhan V. Developmental programming: effect of prenatal steroid excess on intraovarian components of insulin signaling pathway and related proteins in sheep. Biol Reprod. 2010;82:1065–1075. doi: 10.1095/biolreprod.109.082719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega H, Salvetti N, Veiga-Lopez A, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on ovarian cell proliferation and survival factors. 44th Annual Meeting of the Society for the Study of Reproduction; Portland, Oregon (USA). 31 July–4 August, 2011. [Google Scholar]
- Padmanabhan V, Veiga-Lopez A, Abbott DH, Dumesic DA. Research Signpost. Developmental programming of ovarian dysfunction. In: Gonzalez-Bulnes A, editor. Novel concepts in ovarian endocrinology. Kerala, India: 2007. pp. 329–352. [Google Scholar]
- Padmanabhan V, Sarma HN, Savabieasfahani M, Steckler TL, Veiga-Lopez A. Developmental reprogramming of reproductive and metabolic dysfunction in sheep: native steroids vs. environmental steroid receptor modulators. Int J Androl. 2010a;33:394–404. doi: 10.1111/j.1365-2605.2009.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A, Abbott DH, Recabarren SE, Herkimer C. Developmental programming: impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology. 2010b;151:595–605. doi: 10.1210/en.2009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Developmental origin of reproductive and metabolic dysfunctions: androgenic versus estrogenic reprogramming. Semin Reprod Med. 2011;29:173–186. doi: 10.1055/s-0031-1275519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Patel D, Veiga-Lopez A, Pease A, Kinns J. Developmental Programming: adipose tissue distribution in prenatal testosterone-treated sheep. 93rd Annual Meeting of the Endocrine Society; Boston, USA. 2011. p. 443. [Google Scholar]
- Polkowska J, Dubois MP, Jutisz M. Maturation of luteinizing hormone-releasing hormone (LHRH) and somatostatin (SRIF) neuronal systems in the hypothalamus of growing ewe lambs. Reprod Nutr Dev. 1987;27:627–639. doi: 10.1051/rnd:19870502. [DOI] [PubMed] [Google Scholar]
- Pomerantz DK, Nalbandov AV. Androgen level in the sheep fetus during gestation. Proc Soc Exp Biol Med. 1975;149:413–416. doi: 10.3181/00379727-149-38818. [DOI] [PubMed] [Google Scholar]
- Prodoehl MJ, Hatzirodos N, Irving-Rodgers HF, Zhao ZZ, Painter JN, Hickey TE, Gibson MA, Rainey WE, Carr BR, Mason HD, Norman RJ, Montgomery GW, Rodgers RJ. Genetic and gene expression analyses of the polycystic ovary syndrome candidate gene fibrillin-3 and other fibrillin family members in human ovaries. Mol Hum Reprod. 2009;15:829–841. doi: 10.1093/molehr/gap072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebar R, Judd HL, Yen SS, Rakoff J, Vandenberg G, Naftolin F. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest. 1976;57:1320–1329. doi: 10.1172/JCI108400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recabarren SE, Padmanabhan V, Codner E, Lobos A, Duran C, Vidal M, Foster DL, Sir-Petermann T. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol Endocrinol Metab. 2005;289:E801–806. doi: 10.1152/ajpendo.00107.2005. [DOI] [PubMed] [Google Scholar]
- Recabarren SE, Rojas-García PP, Recabarren MP, Alfaro VH, Smith R, Padmanabhan V, Sir-Petermann T. Prenatal testosterone excess reduces sperm count and motility. Endocrinology. 2008;149:6444–6448. doi: 10.1210/en.2008-0785. [DOI] [PubMed] [Google Scholar]
- Reddy S, Bibby NJ, Elliott RB. An immunofluorescent study of insulin-, glucagon-, pancreatic polypeptide- and somatostatin-containing cells in the early ovine fetal pancreas. Q J Exp Physiol. 1988;73:225–232. doi: 10.1113/expphysiol.1988.sp003135. [DOI] [PubMed] [Google Scholar]
- Rhind SM, Rae MT, Brooks AN. Effects of nutrition and environmental factors on the fetal programming of the reproductive axis. Reproduction. 2001;122:205–214. doi: 10.1530/rep.0.1220205. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Forsdike RA, Taylor JA. In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology. 1999;140:5797–5805. doi: 10.1210/endo.140.12.7205. [DOI] [PubMed] [Google Scholar]
- Rojas-García PP, Recabarren MP, Sarabia L, Schön J, Gabler C, Einspanier R, Maliqueo M, Sir-Petermann T, Rey R, Recabarren SE. Prenatal testosterone excess alters Sertoli and germ cell number and testicular FSH receptor expression in rams. Am J Physiol Endocrinol Metab. 2010;299:E998–E1005. doi: 10.1152/ajpendo.00032.2010. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL. Current concepts of polycystic ovary syndrome. Baillieres Clin Obstet Gynaecol. 1997;11:307–333. doi: 10.1016/s0950-3552(97)80039-9. [DOI] [PubMed] [Google Scholar]
- Rouiller-Fabre V, Lambrot R, Muczynski V, Coffigny H, Lecureuil C, Pairault C, Bakalska M, Courtot AM, Frydman R, Habert R. Development and regulations of testicular functions in the human foetus. Gynecol Obstet Fertil. 2008;36:898–907. doi: 10.1016/j.gyobfe.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL. Current concepts of polycystic ovary syndrome. Baillieres Clin Obstet Gynaecol. 1997;11:307–333. doi: 10.1016/s0950-3552(97)80039-9. [DOI] [PubMed] [Google Scholar]
- Sam S, Coviello AD, Sung YA, Legro RS, Dunaif A. Metabolic phenotype in the brothers of women with polycystic ovary syndrome. Diabetes Care. 2008;31:1237–1241. doi: 10.2337/dc07-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma HN, Manikkam M, Herkimer C, Dell’Orco J, Welch KB, Foster DL, Padmanabhan V. Fetal programming: excess prenatal testosterone reduces postnatal luteinizing hormone, but not follicle-stimulating hormone responsiveness, to estradiol negative feedback in the female. Endocrinology. 2005;146:4281–4291. doi: 10.1210/en.2005-0322. [DOI] [PubMed] [Google Scholar]
- Savabieasfahani M, Lee JS, Herkimer C, Sharma TP, Foster DL, Padmanabhan V. Fetal programming: testosterone exposure of the female sheep during midgestation disrupts the dynamics of its adult gonadotropin secretion during the periovulatory period. Biol Reprod. 2005;72:221–229. doi: 10.1095/biolreprod.104.031070. [DOI] [PubMed] [Google Scholar]
- Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP. Formation of ovarian follicles during fetal development in sheep. Biol Reprod. 2002;66:1134–1150. doi: 10.1095/biolreprod66.4.1134. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M. Origin and migration of luteinizing hormone-releasing hormone neurons in mammals. Microsc Res Tech. 1999;44:2–10. doi: 10.1002/(SICI)1097-0029(19990101)44:1<2::AID-JEMT2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Hayes FJ, Crowley WF., Jr Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann’s syndrome): pathophysiological and genetic considerations. Endocr Rev. 1998;19:521–539. doi: 10.1210/edrv.19.5.0344. [DOI] [PubMed] [Google Scholar]
- Seow KM, Juan CC, Hsu YP, Hwang JL, Huang LW, Ho LT. Amelioration of insulin resistance in women with PCOS via reduced insulin receptor substrate-1 Ser312 phosphorylation following laparoscopic ovarian electrocautery. Hum Reprod. 2007;22:1003–1010. doi: 10.1093/humrep/del466. [DOI] [PubMed] [Google Scholar]
- Sharma TP, Herkimer C, West C, Ye W, Birch R, Robinson JE, Foster DL, Padmanabhan V. Fetal programming: prenatal androgen disrupts positive feedback actions of estradiol but does not affect timing of puberty in female sheep. Biol Reprod. 2002;66:924–933. doi: 10.1095/biolreprod66.4.924. [DOI] [PubMed] [Google Scholar]
- Sheppard KM, Padmanabhan V, Coolen LM, Lehman MN. Prenatal programming by testosterone of hypothalamic metabolic control neurones in the ewe. J Neuroendocrinol. 2011;23:401–411. doi: 10.1111/j.1365-2826.2011.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short RV. Sexual differentiation of the sheep brain. Sexual Endocrinology Perinatal Period. 1974;73:121–142. [Google Scholar]
- Smith P, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology in sheep. Biol Reprod. 2009;80:726–736. doi: 10.1095/biolreprod.108.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–3193. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology. 2007a;148:3532–3540. doi: 10.1210/en.2007-0339. [DOI] [PubMed] [Google Scholar]
- Steckler TL, Roberts EK, Doop DD, Lee TM, Padmanabhan V. Developmental programming in sheep: administration of testosterone during 60–90 days of pregnancy reduces breeding success and pregnancy outcome. Theriogenology. 2007b;67:459–467. doi: 10.1016/j.theriogenology.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Steckler TL, Lee JS, Ye W, Inskeep EK, Padmanabhan V. Developmental programming: exogenous gonadotropin treatment rescues ovulatory function but does not completely normalize ovarian function in sheep treated prenatally with testosterone. Biol Reprod. 2008;79:686–695. doi: 10.1095/biolreprod.108.068643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler TL, Herkimer C, Dumesic DA, Padmanabhan V. Developmental programming: excess weight gain amplifies the effects of prenatal testosterone excess on reproductive cyclicity -implication for polycystic ovary syndrome. Endocrinology. 2009;150:1456–1465. doi: 10.1210/en.2008-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–191. [Google Scholar]
- Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr J. 2004;51:165–169. doi: 10.1507/endocrj.51.165. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Tsutsumi O, Ikezuki Y, Kamei Y, Osuga Y, Fujiwara T, Takai Y, Momoeda M, Yano T, Taketani Y. Elevated serum bisphenol A levels under hyperandrogenic conditions may be caused by decreased UDP-glucuronosyltransferase activity. Endocr J. 2006;53:485–491. doi: 10.1507/endocrj.k06-032. [DOI] [PubMed] [Google Scholar]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- Unsworth WP, Taylor JA, Robinson JE. Prenatal programming of reproductive neuroendocrine function: the effect of prenatal androgens on the development of estrogen positive feedback and ovarian cycles in the ewe. Biol Reprod. 2005;72:619–627. doi: 10.1095/biolreprod.104.035691. [DOI] [PubMed] [Google Scholar]
- Urbanek M, Woodroffe A, Ewens KG, Diamanti-Kandarakis E, Legro RS, Strauss JF, 3rd, Dunaif A, Spielman RS. Candidate gene region for polycystic ovary syndrome on chromosome 19p13.2. J Clin Endocrinol Metab. 2005;90:6623–6629. doi: 10.1210/jc.2005-0622. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Ye W, Phillips DJ, Herkimer C, Knight PG, Padmanabhan V. Developmental programming: deficits in reproductive hormone dynamics and ovulatory outcomes in prenatal, testosterone-treated sheep. Biol Reprod. 2008;78:636–647. doi: 10.1095/biolreprod.107.065904. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Astapova OI, Aizenberg EF, Lee JS, Padmanabhan V. Developmental programming: contribution of prenatal androgen and estrogen to estradiol feedback systems and periovulatory hormonal dynamics in sheep. Biol Reprod. 2009;80:718–725. doi: 10.1095/biolreprod.108.074781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Lee JS, Padmanabhan V. Developmental programming: insulin sensitizer treatment improves reproductive function in prenatal testosterone-treated female sheep. Endocrinology. 2010;151:4007–4017. doi: 10.1210/en.2010-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Padmanabhan V. Developmental programming: prenatal testosterone excess disrupts expression of oocyte and granulosa cell growth factors in sheep. Biol Reprod; 43rd Annual Meeting of the Society for the Study of Reproduction; Milwaukee, Wisconsin, USA. 2010. p. 79. [Google Scholar]
- Veiga-Lopez A, Burant C, Padmanabhan V. Developmental Programming: prenatal testosterone and postnatal obesity induce free fatty acid imbalance in sheep. 93rd Annual Meeting of the Endocrine Society; Boston, USA. 2011a. p. 55. [Google Scholar]
- Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, Refsal K, Padmanabhan V. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011b;84:87–96. doi: 10.1095/biolreprod.110.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91:2100–2104. doi: 10.1210/jc.2005-1494. [DOI] [PubMed] [Google Scholar]
- Walters KA, Allan CM, Handelsman DJ. Androgen actions and the ovary. Biol Reprod. 2008;78:380–389. doi: 10.1095/biolreprod.107.064089. [DOI] [PubMed] [Google Scholar]
- Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–1021. doi: 10.1016/s0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- West C, Foster DL, Evans NP, Robinson J, Padmanabhan V. Intra-follicular activin availability is altered in prenatally-androgenized lambs. Mol Cell Endocrinol. 2001;185:51–59. doi: 10.1016/s0303-7207(01)00632-3. [DOI] [PubMed] [Google Scholar]
- Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95:2038–2049. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- Wilson PR, Tarttelin MF. Studies on sexual differentiation of sheep. I Foetal and maternal modifications and post-natal plasma LH and testosterone content following androgenisation early in gestation. Acta Endocrinol (Copenh) 1978a;89:182–189. [PubMed] [Google Scholar]
- Wilson PR, Tarttelin MF. Studies of sexual differentiation of sheep. II Evidence for post-natal hypothalamic hypophysiotrophic depression of basal LH secretion following pre-natal androgenisation. Acta Endocrinol (Copenh) 1978b;89:190–195. [PubMed] [Google Scholar]
- Wood RI, Ebling FJ, I’Anson H, Bucholtz DC, Yellon SM, Foster DL. Prenatal androgens time neuroendocrine sexual maturation. Endocrinology. 1991;128:2457–2468. doi: 10.1210/endo-128-5-2457. [DOI] [PubMed] [Google Scholar]
- Wood RI, Mehta V, Herbosa CG, Foster DL. Prenatal testosterone differentially masculinizes tonic and surge modes of luteinizing hormone secretion in the developing sheep. NeuroEndocrinology. 1995;62:238–247. doi: 10.1159/000127010. [DOI] [PubMed] [Google Scholar]
- Wood RI, Foster DL. Sexual differentiation of reproductive neuroendocrine function in sheep. Rev Reprod. 1998;3:130–140. doi: 10.1530/ror.0.0030130. [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Yarali H, Oguz H, Bayraktar M. Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2031–2036. doi: 10.1210/jc.2002-021499. [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Azziz R. The adrenal and polycystic ovary syndrome. Rev Endocr Metab Disord. 2007;8:331–342. doi: 10.1007/s11154-007-9054-0. [DOI] [PubMed] [Google Scholar]
- Zawadki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic ovary syndrome. Blackwell Scientific Publications; Boston: 1992. pp. 377–384. [Google Scholar]