Abstract
Mesothelin, a glycosylphosphatidylinositol (GPI) anchored cell surface protein, is a potential target for antibody-based cancer therapy due to its high expression in mesothelioma, ovarian cancer, pancreatic cancer, cholangiocarcinoma and other cancers. The SS1P immunotoxin and MORAb-009 (amatuximab), a chimeric monoclonal antibody, are currently being evaluated in clinical trials. In this review, we discuss the role of mesothelin in cancer progression and provide new insights into mesothelin-targeted cancer therapy. Recent studies highlight three mechanisms by which mesothelin plays a role in cancer progression. First, mesothelin may aid in the peritoneal implantation and metastasis of tumors through its interaction with mucin MUC16 (also known as CA125). Second, mesothelin may promote cancer cell survival and proliferation via the NF-κB signaling pathway. Finally, mesothelin expression promotes resistance to certain chemotherapy drugs such as TNF-α, paclitaxel, and a combination of platinum and cyclophosphamide. However, its cancer-specific expression makes mesothelin a potential target for monoclonal antibody therapy. New human monoclonal antibodies targeting mesothelin have been isolated by phage display technology and may provide opportunities for novel cancer therapy.
Keywords: antibody dependent cell mediated cytotoxicity/ADCC, apoptosis, cell surface proteins, cell survival/proliferation, complement dependent cytotoxicity/CDC, human monoclonal antibodies, immunotoxin, mesothelin, MORAb-009/amatuximab, MUC16/CA125, NF-κB, PI3K/Akt, SS1P
Introduction
Mesothelin (MSLN) is highly expressed in mesothelioma, ovarian cancer and pancreatic adenocarcinomas [1, 2, 3, 4, 5, 6, 7], and recent studies show that it is also expressed in lung adenocarcinomas [8], uterine serous carcinoma [9], acute myeloid leukemia [10] and cholangiocarcinoma [11, 12]. A full understanding of the biological functions of mesothelin is lacking given that mesothelin knockout mice do not show any developmental phenotype [13]. Interestingly, recent reports indicate that mesothelin may play an important role in cell adherence [14], cell survival/proliferation, tumor progression [15, 16, 17, 18] and chemoresistance [18, 19, 20, 21].
Structure of mesothelin
The mesothelin cDNA and protein were identified using the K1 monoclonal antibody (mAb) generated by the immunization of mice with human ovarian carcinoma (OVCAR)-3 cells [1, 2]. The human MSLN gene encodes a ~71kDa precursor protein of 622 amino acids (Fig. 1). The N-terminal signal peptide (residues 1–33) and the C-terminal glycosylphosphatidylinositol (GPI) anchor addition signal (at predicted cleavage site: Ser598) are removed and the latter replaced with a GPI anchor. The MSLN precursor is cleaved at Arg295 into two products, a ~31kDa mature megakaryocyte potentiating factor (MPF residues Ser34 – Arg286) [22] and a ~40kDa GPI-anchored membrane-bound mature mesothelin starting from Glu296 [2, 6, 7]. The C-terminus is displayed on the surface of cancer cells via a GPI anchor. Mesothelin has four N-linked glycans (Asn57, Asn388, Asn488 and Asn515). While the three dimensional structure of mesothelin has not been solved, a recent study predicted both the secondary and three dimensional structures of both mesothelin precursor and mature mesothelin [23]. Nine secondary structure prediction programs predict that both the mesothelin precursor and mature mesothelin are predominantly of helical structure, which is composed of small helical segments separated by short non-helical regions. Based on this secondary structure prediction, four three-dimensional structure prediction programs (INHUB, 3D-PSSM, BasD, and I-TASSER) provide the same type of structure for the mesothelin precursor: a superhelical structure with ARM-type repeats. The model also predicts that the conformation of mature mesothelin may not change after cleavage into its mature form. Interestingly, the structure model for mesothelin is made of tandem repeats of approximately 50 residue-long helix-turn-helix motifs [23]. However, the structure of mesothelin remains unknown.
Figure 1.
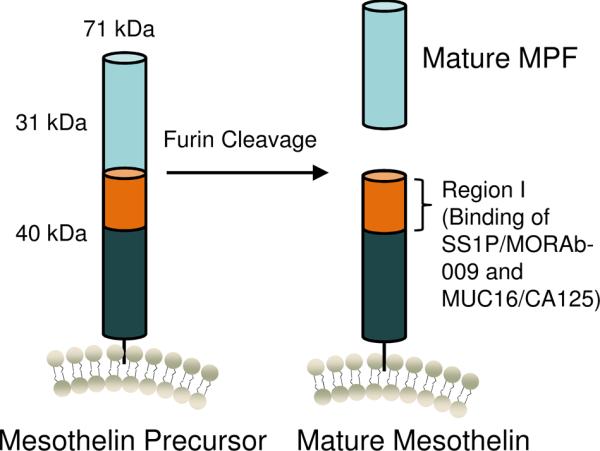
Structure of mesothelin. The mesothelin precursor protein (71 kDa) is cleaved by furin to release its 31 kDa N-terminal megakaryocyte potentiating factor (MPF) and is displayed as mature mesothelin on the cell surface. Region I (residues 296–390) of mature mesothelin contains the binding site for SS1P/MORAb-009 and MUC16/CA125.
Biological functions of mesothelin
The biological functions of mesothelin remain largely unknown as mesothelin knockout mice do not show a detectable phenotype [13]. It has been suggested that mesothelin plays a role in tumor adhesion and metastasis based on evidence that it can bind to MUC16 (also known as CA125), which is highly glycosylated, with both O-linked and N-linked oligosaccharides, to mediate heterotypic cell adhesion [14]. For example, OVCAR-3 cells, which express MUC16, can specifically attach to mesothelin positive LO cells and an anti-mesothelin antibody blocks this interaction [14]. It has been suggested that N-linked glycans on MUC16 are essential for mediating mesothelin-MUC16 binding [24]. Mesothelin can specifically bind to the MUC16-expressing ovarian cancer cell line OVCAR-3 with a KD of approximately 5nM, whereas it does not bind to OVCAR-3 derived sublines that lack MUC16 expression. The N-linked oligosaccharides of MUC16 are required for mesothelin binding as treatment of MUC16 with peptide-N-glycosidase (PNGaseF), which removes the N-linked oligosaccharides, inhibits MUC16-mesothelin binding [24]. Our group experimentally established that the N-terminal domain (named IAB) of cell-surface mature mesothelin is the minimal recognition sequence for MUC16 binding by using truncated mutagenesis and alanine replacement techniques [25]. We generated truncated alanine mesothelin mutants and characterized them using western blot, enzyme-linked immunosorbent assay and flow cytometry. We identified an N terminal region (residues 296–359) at the extracellular domain of cell surface mesothelin that is sufficient and necessary for binding to cell surface-associated MUC16 [25]. In addition, we showed that the IAB-Fc fusion protein can block the mesothelin-MUC16 interaction on cancer cells, indicating that the IAB domain could be a new therapeutic target in preventing or treating peritoneal malignant tumors.
According to these studies, we further developed the novel human immunoadhesin HN125 against tumor-associated MUC16 [26]. HN125 consists of IAB and the Fc portion of human IgG1, and it is able to specifically bind to the MUC16-expressing cell line OVCAR-3 with a KD value of 13nM [26]. HN125 can significantly inhibit the MUC16-mesothelin interaction on cancer cells. For example, at 100μg/ml, HN125 reduced the adherence of mesothelin-positive A431/H9 cells [26] onto the MUC16-positive OVCAR3 monolayer by approximately 80%. Furthermore, HN125 can exert significant antibody dependent cell mediated cytotoxicity (ADCC) by killing about 35% of OVCAR3 cells while there was no ADCC activity on MUC16-negative cells, which indicates that HN125 has anti-tumor activity [26]. Together, these studies provide evidence that inhibiting the mesothelin-MUC16 interaction could be a new therapeutic strategy for ovarian cancer and other cancers.
Mesothelin is also involved in tumor progression and confers resistance to chemotherapy [15, 16, 17, 18, 19, 20, 21], Fig. (2). The proliferative effect of mesothelin was recently examined in pancreatic cancer [15, 16, 17, 18]. MIA PaCa-2 cells stably overexpressing mesothelin significantly increased cell proliferation by 90% and cell migration by 300% in vitro. These cells also formed large tumors in nude mice xenograft models [15]. In contrast, silencing mesothelin in BxPC-3 cells reduced cell proliferation and cell cycle progression by slowing cell entry into S phase [15, 16]. In addition, mesothelin could inhibit TNF-α-induced apoptosis [18].
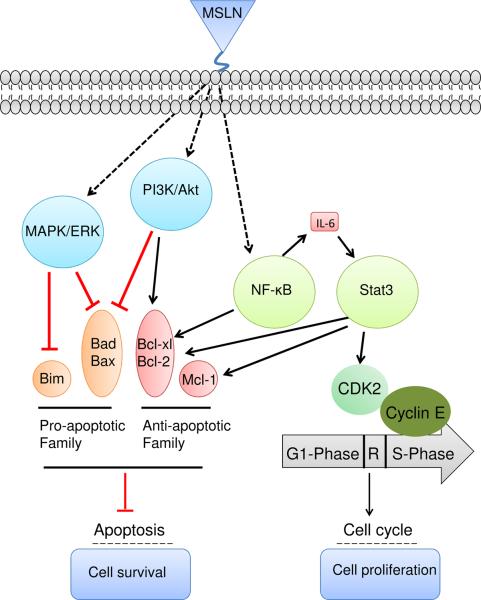
Figure 2.
Role of mesothelin in cancer progression
Overexpression of mesothelin leads to higher IL-6 production by constitutively activating NF-κB. High IL-6 can trigger the transcription protein 3 (Stat3), resulting in higher expression levels of the cyclin E/cyclin-dependent kinase (CDK2) complex, as well as speeding the G1-S transition. In addition, activation of NF-κB and Stat3 may induce expression of Bcl-xl and Bcl-2 and inhibit apoptosis signaling. Mesothelin can protect cancer cells from drug-induced apoptosis by stimulating Akt phosphorylation under PI3K activation or the MAPK/ERK signaling pathway to promote the expression of anti-apoptotic genes such as Bcl-2 and Mcl-1 or inhibit the expression of pro-apoptotic factors such as Bad and Bax.
IL-6 is related to cancer cell survival/proliferation and tumor progression. Overexpression of mesothelin in pancreatic cancer cells led to higher IL-6 production by constitutively activating NF-κB. Conversely, silencing mesothelin reduced levels of IL-6. In pancreatic cells with mesothelin overexpression, high IL-6 may be responsible for triggering the transcription protein 3 (Stat3), resulting in higher expression levels of the cyclin E/cyclin-dependent kinase (CDK2) complex, as well as speeding the G1-S transition [16]. High levels of IL-6 production could up-regulate the soluble IL-6 receptor to stimulate cell proliferation under serum-reduced conditions via an IL-6/sIL-6R (soluble IL-6 receptor) trans-signaling pathway. Even in serum-free medium, cancer cells with forced mesothelin expression grow faster than control cells by producing higher quantities of IL-6. In this case, IL-6 may act as a growth factor to support cancer cell survival [17]. In addition, activation of NF-κB and Stat3 induces expression of Bcl-xl and Bcl-2 and inhibits apoptosis signaling [18, 28, 29].
Another mechanism may involve the PI3K/Akt pathway to protect cancer cells from drug-induced apoptosis [18, 19]. Mesothelin protects cancer cells from TNF-α induced apoptosis by rapidly stimulating Akt phosphorylation under PI3K activation, inhibiting the expression of pro-apoptotic factors, such as Bad and Bax, and promoting the expression of anti-apoptotic genes, such as Bcl-2 and Mcl-1. Cyclin A was also increased in mesothelin overexpressing MIA PaCa-2 cells by TNF-α treatment [18].
Several other groups found that mesothelin could confer resistance to cytotoxic drug-induced apoptosis. In a human breast cancer model, mesothelin induces anchorage-independent growth and down-regulates the pro-apoptotic protein Bim to confer cancer cell resistance to anoikis-induced apoptosis via stimulation of the ERK signaling pathway [21]. Among the ovarian epithelial carcinoma patients treated with platinum plus cyclophosphamide, chemoresistant patients showed significantly higher mesothelin expression than chemosensitive patients. In addition, overexpressing mesothelin in C57BL/6 murine peritoneal cells could protect cells from paclitaxel-induced apoptosis through both the PI3/Akt and MAPK/ERK pathways [19].
In an early study, Prieve and Moon demonstrated that stably expressing Wnt-1 in the mouse mammary epithelial cell line C57 or co-culturing C57mg cells with Wnt-1 secreting cells could up-regulate mesothelin co-culturing C57mg cells [30]. Also, the expression of mesothelin could be stimulated by Li+, an inhibitor of GSK-3β that mimics Wnt-1. In contrast, mesothelin expression was down-regulated by Wnt-5a, which may be due to the antagonism of endogenous Wnt/β-catenin signaling. These results suggest that mesothelin expression can be regulated by different Wnt proteins. However, further studies are necessary to evaluate whether mesothelin is a direct target of Wnt-1 and Wnt-5a.
Collectively, these studies signify the potential role of mesothelin in cancer cell proliferation and indicate that a drug neutralizing the functionality of mesothelin may be useful for novel cancer therapy. Further studies will reveal the role of mesothelin in tumor progression.
Gene regulation of mesothelin in cancer cells
Recently a promoter of mesothelin called Canscript, located −65 to −46 bp 5' of the transcription start site in mesothelin, was identified in highly expressing cancer cells, which may contribute to the highly cancer-specific overexpression of mesothelin [31, 32]. The activity of Canscript was increased over 100-fold in cancer cells. The Canscript promoter consists of two motifs: a conventional MCAT motif and a SP1-like motif. Single nucleotide transitional substitution survey confirmed that all eight nucleotides in MCAT are essential for its function. However, the SP1-like element has two point mutations compared to the conserved SP1 motif and binding of an unknown transcription factor to the SP1-like motif may be responsible for cancer-specific expression of mesothelin. Several transcriptional factors such as KLF6 and YAP1 have been investigated. The expression patterns of these transcription factors are consistent with the mesothelin expression pattern in various cell lines but are not sufficient for mesothelin overexpression [32]. The key transcriptional factor which regulates cancer-specific overexpression of mesothelin has not been found.
Mesothelin-based antibody therapy for human malignancies
Mesothelin has been suggested as an attractive target for immunotherapy. Several therapeutic agents that target cell surface mesothelin have been developed and some are being evaluated in preclinical and clinical studies. Recombinant immunotoxin SS1P is composed of a variable fragment (Fv) of SS1 and a truncated form of Pseudomonas exotoxin A (PE) [33, 34]. Two phase I clinical trials of SS1P were completed at the U.S. National Cancer Institute (NCI) [35, 36]. Based on phase I clinical studies showing the safety of SS1P and its anti-tumor activity, a clinical trial of SS1P in combination with chemotherapy is currently ongoing. MORAb-009 (amatuximab), a chimeric (mouse/human) antibody containing murine SS1 Fv and human IgGγ1 and k constant regions, was developed [37]. A phase I clinical trial of MORAb-009 for mesothelioma, pancreatic cancer and ovarian cancer patients was recently completed [38]. A total of 24 subjects were treated, including 13 mesothelioma, 7 pancreatic cancer, and 4 ovarian cancer patients. Eleven subjects had stable disease. Phase II studies of MORAb-009 in different mesothelin-expressing cancers are ongoing.
With two antibodies currently undergoing clinical trials, new antibodies are being investigated as potential therapeutic agents. A human mAb, m912, was isolated from a human Fab library. M912 in Fab, single-chain variable fragment (scFv), and IgG formats can specifically bind to cell surface associated mesothelin and induce ADCC [39]. Our group generated a high-affinity human mAb (named HN1) based on a scFv isolated by phage display technology [40]. The HN1 human antibody can specifically bind to cell surface mesothelin with high affinity (KD = 3nM) and kill mesothelin-expressing cancer cells with strong ADCC. A recombinant immuntoxin by fusing the HN1 scFv to a truncated PE kills cancer cells with high cytotoxic activity. We believe that the new HN1 antibody and immunotoxin have significant potential for mesothelin-expressing cancer treatment.
Furthermore, based on the MUC16 functional binding domain to mesothelin, we developed a novel human immunoadhesion, HN125, against tumor-associated MUC16. HN125 can significantly inhibit the interaction between mesothelin-MUC16 as well as killing ovarian tumor cells via ADCC, indicating its potential anti-tumor activity in treating ovarian cancer and other MUC16-expressing tumors [26]. Despite the number of mesothelin mAbs available, none have been able to inhibit cancer cell proliferation. This is most likely because the majority of these antibodies (including SS1 and MORAb-009) target N terminal Region I rather than a key signaling domain in mesothelin. Our work and that of other groups indicate that Region I is highly immunogenic; therefore, it is difficult to obtain mAbs targeting domains outside of this region. Our unpublished data show that mAbs targeting Region I do not inhibit mesothelin-expressing cancer cell proliferation. We believe that the potential for mesothelin-targeted therapy will not be fully exhausted until mAbs against all functional domains of mesothelin are generated and evaluated for anti-tumor activity. Desirable epitopes may be related to the functions of mesothelin in cancer progression.
Conclusions
Although mesothelin is an attractive therapeutic target and antibody drugs targeting mesothelin are currently being evaluated in clinical trials, the role of mesothelin in cancer progression remains poorly understood. Recent studies have revealed oncogenic functions of mesothelin in cancer survival/proliferation and drug resistance through Wnt/NF-κB/PI3K/Akt signaling pathways. Activity at the Canscript promoter may play a role in the cancer-specific expression of mesothelin. More studies are needed to validate and further investigate the potential role of mesothelin in tumor metastasis, cancer cell survival and proliferation, and drug resistance. The mechanistic studies on mesothelin biology may provide important insights and opportunities for more effective antibody therapy targeting mesothelin in solid tumors.
Acknowledgements
This research is supported by the Intramural Research Program of the National Institutes of Health (NIH), NCI, Center for Cancer Research. Dr. Mitchell Ho is also supported by the NCI Director's Intramural Innovation Award for Principal Investigators, an Ovarian Cancer Research Fund Individual Investigator Award, and a Mesothelioma Applied Research Foundation Craig Kozicki Memorial Grant. Dr. Ho is a Zi-jiang Lecture Professor of East China Normal University. Zhewei Tang is in the Graduate Partnerships Program of the NIH. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. The authors have no conflict of interest directly relevant to the content of this review. We thank the NIH Fellows Editorial Board and Yen Phung for editorial assistance.
References
- [1].Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int. J. Cancer. 1992;50:373–381. doi: 10.1002/ijc.2910500308. [DOI] [PubMed] [Google Scholar]
- [2].Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc. Natl. Acad. Sci. USA. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin. Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- [4].Ordonez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod. Pathol. 2003;16:192–197. doi: 10.1097/01.MP.0000056981.16578.C3. [DOI] [PubMed] [Google Scholar]
- [5].Hassan R, Kreitman RJ, Pastan I, Willingham MC. Localization of mesothelin in epithelial ovarian cancer. Appl. Immunohistochem. Mol. Morphol. 2005;13:243–247. doi: 10.1097/01.pai.00000141545.36485.d6. [DOI] [PubMed] [Google Scholar]
- [6].Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin. Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- [7].Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur. J. Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ho M, Bera TK, Willingham MC, Onda M, Hassan R, FitzGerald D, Pastan I. Mesothelin expression in human lung cancer. Clin. Cancer Res. 2007;13:1571–1575. doi: 10.1158/1078-0432.CCR-06-2161. [DOI] [PubMed] [Google Scholar]
- [9].Dainty LA, Risinger JI, Morrison C, Chandramouli GV, Bidus MA, Zahn C, Rose GS, Fowler J, Berchuck A, Maxwell GL. Overexpression of folate binding protein and mesothelin are associated with uterine serous carcinoma. Gynecol. Oncol. 2007;105:563–570. doi: 10.1016/j.ygyno.2006.10.063. [DOI] [PubMed] [Google Scholar]
- [10].Steinbach D, Onda M, Voigt A, Dawczynski K, Wittig S, Hassan R, Gruhn B, Pastan I. Mesothelin, a possible target for immunotherapy, is expressed in primary AML cells. Eur. J. Haematol. 2007;79:281–286. doi: 10.1111/j.1600-0609.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- [11].Ordonez NG. Application of mesothelin immunostaining in tumor diagnosis. Am. J. Surg. Pathol. 2003;27:1418–1428. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- [12].Yu L, Feng M, Kim H, Phung Y, Kleiner DE, Gores GJ, Qian M, Wang XW, Ho M. Mesothelin as a potential therapeutic target in human cholangiocarcinoma. J. Cancer. 2010;1:141–149. doi: 10.7150/jca.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol. Cell Biol. 2000;20:2902–1906. doi: 10.1128/mcb.20.8.2902-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, Miyajima A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 2004;279:9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- [15].Li M, Bharadwaj U, Zhang R, Zhang S, Mu H, Fisher WE, Brunicardi FC, Chen C, Yao Q. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol. Cancer Ther. 2008;7:286–296. doi: 10.1158/1535-7163.MCT-07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bharadwaj U, Li M, Chen C, Yao Q. Mesothelin-induced pancreatic cancer cell proliferation involves alteration of cyclin E via activation of signal transducer and activator of transcription protein 3. Mol. Cancer Res. 2008;6:1755–1765. doi: 10.1158/1541-7786.MCR-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bharadwaj U, Marin-Muller C, Li M, Chen C, Yao Q. Mesothelin overexpression promotes autocrine IL-6/sIL-6R trans-signaling to stimulate pancreatic cancer cell proliferation. Carcinogenesis. 2011;32:1013–1024. doi: 10.1093/carcin/bgr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bharadwaj U, Marin-Muller C, Li M, Chen C, Yao Q. Mesothelin confers pancreatic cancer cell resistance to TNF-alpha-induced apoptosis through Akt/PI3K/NF-kappaB activation and IL-6/Mcl-1 overexpression. Mol. Cancer. 2011;10:106. doi: 10.1186/1476-4598-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chang MC, Chen CA, Hsieh CY, Lee CN, Su YN, Hu YH, Cheng WF. Mesothelin inhibits paclitaxel-induced apoptosis through the PI3K pathway. Biochem. J. 2009;424:449–458. doi: 10.1042/BJ20082196. [DOI] [PubMed] [Google Scholar]
- [20].Cheng WF, Huang CY, Chang MC, Hu YH, Chiang YC, Chen YL, Hsieh CY, Chen CA. High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma. Br. J. Cancer. 2009;100:1144–1153. doi: 10.1038/sj.bjc.6604964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Uehara N, Matsuoka Y, Tsubura A. Mesothelin promotes anchorage-independent growth and prevents anoikis via extracellular signal-regulated kinase signaling pathway in human breast cancer cells. Mol. Cancer Res. 2008;6:186–193. doi: 10.1158/1541-7786.MCR-07-0254. [DOI] [PubMed] [Google Scholar]
- [22].Yamaguchi N, Hattori K, Oh-eda M, Kojima T, Imai N, Ochi N. A novel cytokine exhibiting megakaryocyte potentiating activity from a human pancreatic tumor cell line HPC-Y5. J. Biol. Chem. 1994;269:805–808. [PubMed] [Google Scholar]
- [23].Sathyanarayana BK, Hahn Y, Patankar MS, Pastan I, Lee B. Mesothelin, Stereocilin, and Otoancorin are predicted to have superhelical structures with ARM-type repeats. BMC. Struct. Biol. 2009;9:1. doi: 10.1186/1472-6807-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gubbels JA, Belisle J, Onda M, Rancourt C, Migneault M, Ho M, Bera TK, Connor J, Sathyanarayana BK, Lee B, Pastan I, Patankar MS. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kaneko O, Gong L, Zhang J, Hansen JK, Hassan R, Lee B, Ho M. A binding domain on mesothelin for CA125/MUC16. J. Biol. Chem. 2009;284:3739–3749. doi: 10.1074/jbc.M806776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xiang X, Feng M, Felder M, Connor JP, Man YG, Patankar MS, Ho M. HN125: A novel immunoadhesin targeting MUC16 with potential for cancer therapy. J. Cancer. 2011;2:280–291. doi: 10.7150/jca.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ho M, Hassan R, Zhang J, Wang QC, Onda M, Bera T, Pastan I. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin. Cancer Res. 2005;11:3814–3820. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- [28].Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem. Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- [29].Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- [30].Prieve MG, Moon RT. Stromelysin-1 and mesothelin are differentially regulated by Wnt-5a and Wnt-1 in C57mg mouse mammary epithelial cells. BMC. Dev. Biol. 2003;3:2. doi: 10.1186/1471-213X-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hucl T, Brody JR, Gallmeier E, Iacobuzio-Donahue CA, Farrance IK, Kern SE. High cancer-specific expression of mesothelin (MSLN) is attributable to an upstream enhancer containing a transcription enhancer factor dependent MCAT motif. Cancer Res. 2007;67:9055–9065. doi: 10.1158/0008-5472.CAN-07-0474. [DOI] [PubMed] [Google Scholar]
- [32].Ren YR, Patel K, Paun BC, Kern SE. Structural analysis of the cancer-specific promoter in mesothelin and in other genes overexpressed in cancers. J. Biol. Chem. 2011;286:11960–11969. doi: 10.1074/jbc.M110.193458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chowdhury PS, Viner JL, Beers R, Pastan I. Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc. Natl. Acad. Sci. USA. 1998;95:669–674. doi: 10.1073/pnas.95.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chowdhury PS, Pastan I. Improving antibody affinity by mimicking somatic hypermutation in vitro. Nat. Biotechnol. 1999;17:568–572. doi: 10.1038/9872. [DOI] [PubMed] [Google Scholar]
- [35].Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, Pastan I. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin. Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- [36].Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin. Cancer Res. 2009;15:5274–5279. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hassan R, Ebel W, Routhier EL, Patel R, Kline JB, Zhang J, Chao Q, Jacob S, Turchin H, Gibbs L, Phillips MD, Mudali S, Iacobuzio-Donahue C, Jaffee EM, Moreno M, Pastan I, Sass PM, Nicolaides NC, Grasso L. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20. [PMC free article] [PubMed] [Google Scholar]
- [38].Hassan R, Cohen SJ, Phillips M, Pastan I, Sharon E, Kelly RJ, Schweizer C, Weil S, Laheru D. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin. Cancer Res. 2010;16:6132–6138. doi: 10.1158/1078-0432.CCR-10-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Feng Y, Xiao X, Zhu Z, Streaker E, Ho M, Pastan I, Dimitrov DS. A novel human monoclonal antibody that binds with high affinity to mesothelin-expressing cells and kills them by antibody-dependent cell-mediated cytotoxicity. Mol. Cancer Ther. 2009;8:1113–1118. doi: 10.1158/1535-7163.MCT-08-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ho M, Feng M, Fisher RJ, Rader C, Pastan I. A novel high-affinity human monoclonal antibody to mesothelin. Int. J. Cancer. 2011;128:2020–2030. doi: 10.1002/ijc.25557. [DOI] [PMC free article] [PubMed] [Google Scholar]