Abstract
Interleukin (IL)-17 is a pro-inflammatory cytokine that plays critical roles in host defense against extracellular bacteria and fungi and also in the pathogenesis of autoimmune diseases. While CD4+ TCRαβ+ T helper (Th) 17 cells are the best-described cellular source of IL-17, many innate-like T cells are in fact potent producers of IL-17. Given the increasing interest in therapeutic modulation of the IL-17 axis, it is crucial to better understand the cellular origins of IL-17 in various infection and diseases settings. While the diverse population of IL-17-producing T cells share many common characteristics, notable differences also exist. In this review, we discuss the heterogeneity of IL-17-producing T cell types focusing on their development, regulation, and function.
Keywords: Cytokine, Th17 cells, nTh17 cells, γδ T cells, iNKT cells, Host defense, Autoimmunity
Introduction
CD4+ TCRαβ+ T helper (Th) cells play a central role in orchestrating immune response by producing a distinct array of cytokines depending on each subset. The Th1/Th2 paradigm of CD4+ T cell differentiation, first proposed by Mosmann and Coffman 25 years ago [1], helped clarify many phenomena of the adaptive immune system, albeit with some unexplained enigmas. While an imbalance in Th1 cell function was thought to result in autoimmunity, subsequent studies demonstrated that mice lacking interferon (IFN)-γ, a Th1 cytokine, as well as mice deficient of molecules required for Th1 cell differentiation developed more severe experimental autoimmune encephalomyelitis (EAE) [2–4], a mouse model of multiple sclerosis (MS). This paradox was solved when interleukin (IL)-23 was found to be crucial for the induction of EAE [5] and by the following discovery that IL-23 expands a population of IL-17-producing CD4+ T cells that are capable of inducing EAE [6]. Closely following this observation, multiple studies established this novel population as a distinct T helper cell subset, Th17 cells, and the immunology community welcomed a new and important member to the CD4+ T cell family.
Discovery of Th17 cells generated new interest and excitement for the cytokine IL-17. Murine IL-17 was first identified in 1993 [7] (human IL-17 was cloned in 1996 [8]) but had remained underexplored. IL-17 is a pro-inflammatory cytokine that induces production of other pro-inflammatory cytokines and chemokines from target cells; the IL-17 receptor is ubiquitously expressed on hematopoietic and non-hematopoietic cells throughout the body. Since the identification of the Th17 lineage in 2005, IL-17 has gained much attention due to its critical role in host defense against extracellular bacteria and fungi, especially at mucosal and barrier sites. In addition to EAE, Th17 cells and IL-17 have been shown to be crucial in the pathogenesis of other autoimmune diseases including arthritis, psoriasis, and inflammatory bowel diseases [9]. These findings have been translated into therapeutic advances that include the use of anti-IL-17 and anti-IL-17 receptor antibodies to treat a number of autoimmune syndromes.
With the growing interest in clinical modulation and targeting of IL-17, it is important to better understand the cellular sources of IL-17 at distinct physiological sites and in specific disease settings. IL-17 is produced by a number of adaptive and innate immune cells [10]. Among T lymphocytes, the best-characterized source of IL-17 is Th17 cells; however, CD8+ T cells produce IL-17 as do “innate-like” T cell lineages including natural Th17 cells, γδ T cells, and natural killer T (NKT) cells (Table 1). Within the innate arm of the immune system, activated neutrophils, mast cells, alveolar macrophages from asthmatic lungs, natural killer (NK) cells, and lymphoid tissue-inducer cells have also been shown to be potent sources of IL-17. The development and function of many of these IL-17-producing innate lymphoid cells have been the subject of recent reviews [10, 11]. In this review, we will focus on the diversity of IL-17-producing T cells and the differences and/or similarities in their development, regulation, and function.
Table 1.
Summary of IL-17-producing T cell types
| T cell type | Effector cytokines | Cytokine requirements | Transcription factors |
|---|---|---|---|
| CD4+ αβTCR+ Th17 cell | IL-17, IL-17F, IL-22 | IL-6, TGFβ, IL-1β, IL-23 | RORγt, RORα, AHR, c-Rel, IκBζ [168], BATF [169], RUNX1 [170], IRF4 [171], HIF1α [172, 173] |
| CD4+ αβTCR+ nTh17 cell | IL-17, IL-17F, IL-22 | IL-6, TGFβ | RORγt, RelA, RelB |
|
CD1d-tetramer+ NK1.1− CD4− IL-17+ iNKT cell |
IL-17 | TGFβ | RORγt |
|
CD27− γδTCR+ IL-17+ γδ T cell |
IL-17 | TGFβ | RORγt, RelB, RUNX1 [29], AHR, Hes1 [174] |
Th T helper, n natural, iNKT invariant natural killer T, IL interleukin, TGF transforming growth factor, ROR retinoid orphan receptor, AHR aryl hydrocarbon receptor, BATF basic leucine zipper transcription factor ATF-like, RUNX1 runt-related transcription factor 1, IRF4 interferon-regulatory factor 4, HIF hypoxia inducible factor
Classification of IL-17-producing T cells
Conventional Th17 cells
Following the identification of IL-17-producing CD4+ T cells critical for the induction of EAE [6], two independent groups showed that these cells constitute a distinct subset of CD4+ T helper cells, Th17 cells, that develop from naive CD4+ T cells independently from Th1 or Th2 cells [12, 13]. Soon after, Littman and colleagues identified the master regulator for the Th17 subset, retinoic orphan receptor (ROR)γt, a transcription factor both necessary and sufficient for Th17 cell development [14]. Further characterization revealed that in addition to IL-17 (also known as IL-17A), Th17 cells produce high levels of IL-17F, another member of the IL-17 family, and IL-22 and express IL-23 receptor and CCR6 [15, 16]. Human Th17 cells have also been identified and characterized [17–19]. Initial studies highlighted several discrepancies between mice and human Th17 cells, such as the requirement of TGFβ for differentiation (reviewed below), however, additional studies reveal that they are more similar than originally considered.
One intriguing aspect of both murine and human Th17 cells is their considerable heterogeneity. Co-production of IFNγ and IL-17 by CD4+ T cells has been readily observed under inflammatory conditions [14], and a recent study using IL-17 reporter mice demonstrated that these “double-producers” originate from Th17 cells [20]. In addition, the presence of Th17 cells expressing Foxp3, the transcription factor specific for CD4+ regulatory T (Treg) cells, has been reported both in mice [21] and human [22] although the differentiation pathway of these cells is unknown.
Natural Th17 cells
While it was initially put forth that all Th17 cells differentiate from mature naive CD4+ T cells at peripheral effector sites, recent work has identified another developmental pathway for IL-17-producing CD4+ T cells. Studies from the Craft laboratory and our group have independently identified a population of such cells that acquire effector function in the thymus during development prior to antigen exposure in the periphery [23, 24]. Using multiple experimental approaches including recombinase-activating gene-green fluorescence protein (Rag-GFP) reporter mice [23] and fetal thymic organ culture (FTOC) [24], these thymic Th17 cells have been demonstrated to be of bona fide thymic origin rather than re-circulating cells generated in the periphery. Based on their site of origin, this population has been termed natural Th17 (nTh17) cells. Furthermore, these nTh17 cells have been shown to be a population distinct from conventional Th17 cells with distinct TCR gene usage, thymic selection, and TCR signaling requirements [24].
γδ T cells
γδ T cells are a potent source of innate IL-17 [25], IL-17-producing γδ T cells share characteristics of Th17 cells, including expression of CCR6, IL-23R, and RORγt. Unlike conventional Th17 cells, they also express Toll-like receptor 1 (TLR1), TLR2, and Dectin-1, however, it is unclear whether γδ T cells directly respond to TLR or Dectin-1 ligand to expand and secrete IL-17 [26], or whether activation in the presence of innate ligands is due to stimulation by IL-1 and IL-23 produced by myeloid cells in a TLR-induced manner [27, 28]. These IL-17-producing γδ T cells constitute one of two distinct functional subsets of γδ T cells, the other being IFNγ-producers. Data suggest that these effector fates are determined during thymic selection. Consistent with this hypothesis, fetal thymocytes can be distinguished as either IL-17- or IFNγ-producing γδ T cell precursors by CD27 expression with IL-17-producing γδ T cells being CD27− [29]. Human IL-17-producing γδ T cells have also been characterized, and these cells are present at an increased frequency during some bacterial infections [30].
Invariant natural killer T (iNKT) cells
iNKT cells are characterized by the expression of a highly restricted TCR that recognizes glycolipid antigens presented by the non-polymorphic major histocompatibility complex (MHC) class I-like molecule CD1d [31]. In addition to iNKT subsets producing Th1 or Th2-associated cytokines, an IL-17-producing iNKT cell subset has been described [32]. These IL-17-producing CD44+ NK1.1− CD4− iNKT cells develop in the thymus and readily produce IL-17 in response to α-galactoceramide (α-GalCer) stimulation. A more recent study identified another marker for IL-17-producing iNKT cells, IL-17RB, and demonstrated a role for these cells in the pathogenesis of a virus-induced airway hyperreactivity disease model [33].
Tc17 cells
IL-17-producing CD8+ cells, termed Tc17 cells, have been described. Tc17 cells share developmental requirements similar to those of Th17 cells [34, 35]. Transcription factors that promote conventional IFNγ-producing cytotoxic T lymphocyte (CTL) development, such as T-bet and Eomesodermin, inhibit Tc17 development [36]. The physiological role of Tc17 cells is yet unclear.
In vivo ontogeny of IL-17+ T cells
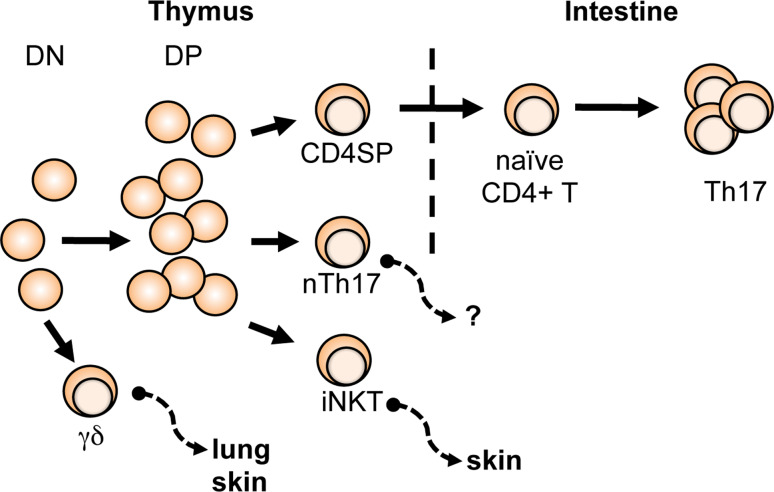
At steady state in vivo, Th17 cells are enriched in the lamina propria (LP) of the small intestine; 80–90 % of IL-17+ cells in the small intestinal LP are CD4+ TCRαβ+ cells [37]. However, these Th17 cells are not found in the intestine of neonatal mice until approximately the 25th day of life, which coincides with the timing of weaning and subsequent colonization of the intestine with normal commensal bacteria. Consistent with these observations, studies using germ-free mice and mice administered a broad antibiotic cocktail are also devoid of LP Th17 cells [37, 38]. Taken together, these findings demonstrate that Th17 cells differentiate from mature naive CD4+ T cells at the intestinal sites in vivo (Fig. 1). It remains to be determined whether CD4+ TCRαβ+ Th17 cells found at other mucosal or barrier sites, such as the lung or skin, are also induced from naive CD4+ T cells at those sites or are originated from the thymus-derived nTh17 cells. Of note, IL-17+ CD4+ TCRαβ+ are present in the peripheral lymphoid organs of germ-free mice, raising the possibility that these sites may be seeded by nTh17 cells (J.S.K. and M.S.J., unpublished observation). Interestingly, intestinal IL-17+ γδ T cells are not significantly affected by commensal colonization as they constitute roughly 1–2 % of CD3+ LP lymphocytes throughout the neonatal period without alteration (day 8 to day 33 of age) [37] and are only slightly reduced in germ-free mice (~7 % of γδ T cells are IL-17+) compared to conventional mice (~10 % IL-17+ γδ T cells) [37].
Fig. 1.
In vivo sites of origin and distribution of IL-17+ T cells. Conventional Th17 cells differentiate from naive CD4+ T cells at intestinal sites. The innate IL-17+ T cells—nTh17, γδ T, and iNKT cells—acquire effector function during development within in the thymus and subsequently emigrate to effector site
For the innate IL-17+ T cells, the thymus is the site of development and commitment as an IL-17+ cell (Fig. 1). Studies using FTOC have clearly demonstrated that the IL-17-producing effector function is programmed and acquired in the thymus during development in nTh17 [24], γδ T [29], and iNKT cells [39]. Since these innate IL-17+ T cells are present in many peripheral tissues, an intriguing question is whether the effector site to which the different thymus-generated IL-17+ T cells emigrate is determined/imprinted during development.
Developmental requirements of IL-17+ T cells
Cytokines
Th17 cells: the “IL-6 plus TGFβ” recipe challenged
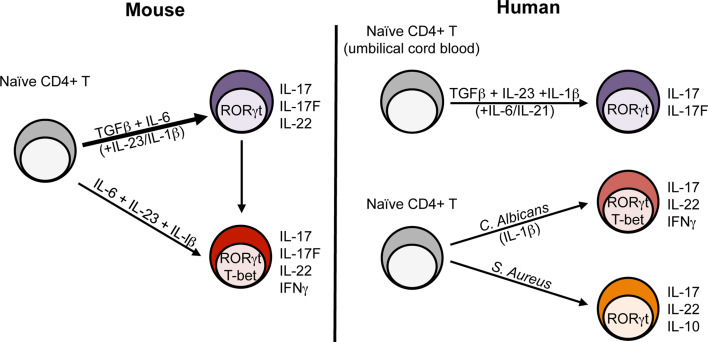
While the identification of Th17 cells was driven by IL-23, it initially was unclear how Th17 cells are derived from naive CD4+ T cells, as they do not express IL-23 receptor (IL-23R). This mystery was solved through parallel studies examining the requirements for differentiation of naive CD4+ cells into Th17 cells and for expression of the IL-23R. Key to this work was three independent studies demonstrating that the combination of transforming growth factor (TGF)β and IL-6 is required to efficiently induce Th17 cells from naive CD4+ T cells in vitro (Fig. 2) [15, 40, 41].
Fig. 2.
Heterogeneity of mouse and human Th17 cells. Differentiation of murine Th17 cells from naive CD4+ T cells (CD62Lhi CD44lo Foxp3-) includes TGFβ and IL-6 for initial differentiation and IL-23 and IL-β for stabilization and commitment (thick arrow). Recent studies suggest a pathogenic subset of Th17 cells (IFNγ+ T-bet+) can be generated in a TGFβ-independent manner from naive CD4+ T cells (thin line). In vivo, IFNγ+ IL-17+ double-producers are generated and are presumed to be converted from conventional Th17 cells. The cytokine requirements for human Th17 cell differentiation is similar to that of mouse Th17 cells (contrary to some initial discrepancies—see text for details) yet the role of each cytokine is still unclear. Heterogeneity within human CD4+ Th17 cells can be appreciated following stimulation of naïve CD4+ T cells with C. albicans or S. aureus, in which IFNγ and IL-10, respectively, have been found to be co-expressed with classic Th17 associated cytokines
IL-6 is a pro-inflammatory cytokine produced by many cell types including innate immune cells [42]. IL-6-deficient mice have drastically reduced numbers of Th17 cells in the intestinal LP [14], and in vitro differentiation of Th17 cells can be completely abolished by adding a blocking antibody against IL-6 [40]. IL-6 leads to expression of IL-23R and strong activation of signal transducer and activator of transcription 3 (STAT3), which is necessary to induce RORγt. However, the IL-6-dependent STAT3 activation is not sufficient for RORγt expression; full induction of RORγt requires the additional presence of TGFβ [43, 44]. Notably, IL-6 (via a STAT3-dependent, RORγt-independent mechanism) also increases the production IL-21, a cytokine capable of upregulating IL-23R expression. Moreover, IL-21 in concert with TGFβ can lead to robust RORγt expression and support Th17 cell differentiation [45–47].
TGFβ is an immunoregulatory cytokine with pleiotropic functions in T cell development and homeostasis [48]. The importance of TGFβ in Th17 cell development was initially established by a number of groups. Mice defective in TGFβ signaling (CD4dnTGFβRII) [49] or deficient in TGFβ1 expression [15] show impaired Th17 cell differentiation in vitro and in vivo [as measured by the paucity of Th17 cells in intestinal LP and mesenteric lymph nodes (MLN)] and are protected from EAE. In contrast, transgenic overexpression of TGFβ in T cells resulted in more severe EAE with increased Th17 cell generation [41]. Taken together, these studies supported the role of TGFβ as an essential initiating factor for Th17 cell fate commitment. This finding was intriguing, as TGFβ was known to be a cytokine crucial for the generation of Treg cells and thereby provided the first indication that these two Th cell subsets, with opposing roles in the immune system, were developmentally linked. Additional studies support the notion that Th17 and Treg cells have a reciprocal developmental relationship and that IL-6 plays a pivotal role in determining the balance between the two [41]. In support of the in vitro studies, IL-6-deficient mice show increased numbers of Treg cells in the periphery [41, 46]. Moreover, RORγt and Foxp3, the lineage-specific transcription factors for Th17 and Treg cells, respectively, physically interact and antagonize each other’s functions [21], providing a molecular mechanism for the reciprocal relationship between the two CD4+ subsets.
The role of TGFβ in Th17 cell generation, however, has recently been challenged. O’Shea and colleagues demonstrated that IL-17+ T cells can be generated from naive CD4+ T cells with the combination of IL-6, IL-1β, and IL-23, though significantly fewer IL-17+ cells are generated in this setting compared to the conventional IL-6 plus TGFβ condition [50]. Moreover, these TGFβ-independent Th17 cells, “Th17(23) cells”, show a different transcriptional profile compared to TGFβ-dependent Th17 cells “Th17(β) cells”. Th17(23) cells have more a “Th1-like” profile characterized by IFNγ and T-bet expression and demonstrate more pathogenicity in a transfer model of EAE compared to Th17(β) cells. How can this be reconciled with previous studies highlighting the indispensable role of TGFβ in Th17 cell differentiation? Since TGFβ is ubiquitously expressed, it is challenging to create an in vivo condition where Th17 cells are generated in the complete absence of TGFβ. In vitro, TGFβ is produced by activated T cells, and Th17 cells themselves appear to provide the TGFβ required for their own generation [51]. Therefore, instead of a strict TGFβ-dependent versus -independent mechanism, perhaps it is the amount of TGFβ signaling received by naive CD4+ T cells that shapes the heterogeneity within the Th17 cell population. In fact, it has been shown that low concentrations of TGFβ promote the Th17 cell program, while high concentrations of TGFβ inhibit IL-23R expression and RORγt activity through induction of Foxp3 [21]. While understanding the definitive role of TGFβ requires further studies, it is clear that there is considerable heterogeneity within the Th17 cell lineage and that TGFβ likely serves as an important contributing factor.
IL-1β is a proinflammatory cytokine that belongs to the IL-1 superfamily. While initial in vitro studies suggested an accessory role for IL-1β in Th17 cell generation [40], mice lacking IL-1R1 were shown to be resistant to EAE, with severe defects in IL-17+ T cell generation in vivo [52]. Later it was demonstrated that the IL-1R1 is highly expressed on Th17 cells, and IL-1 signaling in T cells, in fact, is required for Th17 cell development in EAE [53]. The addition of IL-1 (both IL-1β and IL-1α) to the classical IL-6 plus TGFβ combination significantly enhances Th17 cell generation in vitro, and IL-1 regulates the expression of RORγt and IFN regulatory factor 4 (IRF4)—an additional regulator of IL-17 gene transcription—during this process [53]. A more recent study further emphasized the role of IL-1β in intestinal Th17 cell development at steady state. IL-1R1-deficient mice have greatly reduced numbers of Th17 cells in the intestinal LP, and in vivo administration of IL-1β induces the generation of intestinal LP Th17 cells in germ-free mice, which are normally devoid of Th17 cells at this site [54]. Collectively, these studies highlight the less appreciated role of IL-1β in murine Th17 cells.
While IL-23 is not required for Th17 cell development at the initial stages, it is essential for the full and sustained differentiation of the lineage. Specifically, developing Th17 cells from IL-23R-deficient mice fail to undergo normal effector cell differentiation in vivo, as assessed by their altered CD27 and IL-7Rα expression, and they have defective cell expansion [55]. Importantly, IFNγ responses are not inhibited in the absence of IL-23 signaling. With the emerging concept of heterogeneity within the Th17 lineage, such as Th17(23) versus Th17(β) cells discussed above, IL-23 might be a crucial factor promoting the more “pathogenic” subpopulations of the Th17 cells.
Cytokine requirements for Th17 cell differentiation in humans
The cytokine requirements for human Th17 cell development have been a point of controversy. A number of studies initially claimed that human Th17 cells are induced efficiently by the combination of IL-23, IL-1β, and IL-6, and do not require TGFβ [18, 19, 56, 57]. These findings suggested an intriguing discrepancy between mice and human Th17 cell biology. However, based on the method by which naive cells were purified and the conditions under which these cells were cultured, cellular and serum sources of TGFβ could not be ruled out. When these studies were revisited in 2008, three independent groups demonstrated that TGFβ is necessary for human Th17 cell differentiation [58–60]. Using stringent purification methods for the isolation of naive CD4+ T cells from umbilical cord blood [59] and serum-free media [58] or carefully selected serum lacking TGFβ [59], these studies showed that TGFβ is in fact required for human Th17 cell differentiation in combination with other inflammatory cytokines (Fig. 2). Controversy over how human Th17 cells develop is not completely settled, though, as disagreement surrounding the relative roles of IL-6, IL-1β, and IL-23 remains, and future studies are required to understand the root of this discrepancy. Nonetheless, emerging data suggest that mouse and human Th17 cells are more alike than previously thought and that studies in one system will aid the other to deepen our understanding of the Th17 cell lineage. As with murine Th17 cells, heterogeneity exists within human Th17 cells. Recently, in an in vitro naive T cell priming system using intact microbes and monocytes as antigen presenting cells (APCs), Candida albicans-specific Th17 cells were found to co-produce IL-17 and IFNγ and express T-bet and RORγt. In contrast, Staphylococcus aureus-specific Th17 cells did not produce IFNγ nor express T-bet but were capable of producing IL-10 upon restimulation (Fig. 2) [61].
Cytokine requirements for innate IL-17-producing T cells
The importance of TGFβ and IL-6 in nTh17 cell development has been demonstrated [23]. In this aspect, nTh17 cells appear to share similar developmental requirements as conventional Th17 cells although the cellular source of those cytokines within the thymic environment has yet to be determined. However, the role of IL-1β and IL-23 in nTh17 cell generation has not been studied. Interestingly, requirements for IL-17+ iNKT and γδ T cell development appear quite distinct. Examination of IL-6-deficient mice revealed that these two IL-17-producing T cell populations develop independently of IL-6 and do not require IL-6 stimulation to produce IL-17 [62–64]. This finding is in contrast to the two TCRαβ+ Th17 cell types and suggests that iNKT and γδ T cells might serve as an alternative source of IL-17 when IL-6 is not present in the milieu.
Although IL-6 is dispensable, TGFβ plays an essential role in the development of IL-17+ iNKT and γδ T cells. In TGFβ-deficient mice, IL-17+ γδ T cells are greatly reduced in the thymus and completely absent in the periphery, while the overall γδ T cell development remains intact [65]. This suggests that TGFβ is crucial for the development and maintenance of IL-17+ γδ T cells. A recent study using mice either deficient for TGFβ or expressing a constitutively active form of TGFβR on T cells also demonstrated the need for TGFβ/Smad4 signaling in IL-17+ iNKT cell development and IL-17 production from this subset [66]. Since both IL-17+ iNKT and γδ T cells develop independently of IL-6, the question arises whether TGFβ alone is sufficient to induce RORγt expression in these cells and, if so, what the mechanism may be.
Alternatively, a common characteristic of IL-17+ iNKT and γδ T cells is the constitutive expression of IL-23R and IL-1R1 [26, 63, 64]. Thus, constitutive IL-23R/IL-1R1 expression on innate-like T cells may contribute to the IL-6-independent nature of IL-17 production observed by these cell types. In vitro stimulation of γδ T cells with IL-23 and IL-1β, in the absence of antigen, induces rapid IL-17 production [26, 27], and only 4 h after injection of IL-23 and IL-1β into the foot pad of mice, γδ T cells are stimulated to produce IL-17 [27]. iNKT cells also produce IL-17 after ex vivo stimulation with IL-23 alone [63]. In terms of development, studies using IL-23p19-deficient mice demonstrated that IL-23 is dispensable for the development of IL-17+ γδ T cells [65]. However, whether IL-23 and/or IL-1β are truly required for the development of IL-17+ iNKT cells (and IL-1β for γδ T cells) has not been determined.
T cell receptor (TCR) signal
The strength of TCR signaling, determined by the avidity between TCRs and peptide:MHC complexes on APCs, is an important determinant in the development of various T cell subsets. Indeed, within the CD4+ Th subsets, TCR signal strength is known to be an important factor in Th1 versus Th2 cell differentiation [67]; yet, how TCR signals control Th17 cell differentiation is incompletely understood. Several reports show that CD4+ T cells from mutant mice with dampened TCR signaling exhibit defective Th17 cell differentiation. SH2 domain-containing leukocyte protein of 76 kDa (SLP-76) is a key adaptor protein in the TCR signaling pathway [68]. SLP-76 Y145F mice, where the N-terminal tyrosine 145 residue is mutated to phenylalanine thereby creating a hypomorphic TCR signaling mutant, have defective Th17 cell differentiation both in vitro and in vivo in the intestinal LP [69]. In addition, mice lacking inducible T cell kinase (Itk), a Tec family tyrosine kinase required for TCR-induced PLCγ1 activation and a binding partner of SLP-76 at the Y145 reside, also exhibit decreased IL-17 production [70]. Altering lipid rafts in CD4+ T cells via deleting Raftlin [71] or lowering glycosphingolipid levels [72] attenuates TCR signaling and results in defective Th17 cell differentiation and reduced severity of EAE. Moreover, IL-17 can be induced in vitro under Treg skewing conditions (TGFβ and IL-2) if in the presence of high TCR stimuli [73]. Together, these studies suggest that weak TCR signal strength is insufficient for Th17 cell generation. However, weak TCR stimulation was shown to favor Th17 cell differentiation of human CD4+ T cells stimulated with low versus high numbers of anti-CD3/anti-CD28 coated beads or antigen-pulsed dendritic cells (DCs) [74].
The role of TCR signal strength in nTh17 cell development is also unclear. nTh17 cells are greatly enriched in double transgenic mice where T cells bearing a transgenic TCR develop in the presence of their ubiquitously expressed cognate self-antigen [23]. However, SLP-76 Y145F mice, with attenuated TCR signaling in thymocytes, also show enhanced nTh17 cell development [24]. These studies seem to conflict with each other. However, the apparent differences may be explained by alterations in the sensitivity of Y145F thymocytes to both positive and negative selection. More definitive studies are required to determine the relative TCR signal strength for optimal nTh17 cell development. To this end, it will be interesting to dissect the peptide requirement of nTh17 cells compared to that of nTreg cells, as their peripheral counterparts share an antagonistic developmental relationship. While strong TCR signals are often thought to drive γδ T cell development versus αβ T cell commitment, how signals through the TCR influence the generation of different γδ T cell subsets is not fully known due in part to our incomplete knowledge surrounding the ligand requirements for γδ T cell development. Despite this limitation, recent studies demonstrated that IL-17+ γδ T cells develop in the absence of antigen encounter during their development in the thymus [75]. These data suggest that a lack of TCR engagement supports the IL-17+ subset. However, selection of these cells may be due to ligand-independent signaling, for example through TCR oligomerization or TCRγ/TCRδ pairing, for which “signal strength” has not been fully defined [75–77]. The strength of TCR signal for IL-17+ iNKT, in comparison to their non-IL-17-producing subsets, has not been studied. Clearly, further work is needed to understand how TCR signaling influences IL-17+ T cell subset development and discoveries in this area will provide valuable insights into how lineage choice of these IL-17+ T cells is controlled during development.
Transcription factors
Retinoid orphan receptors (RORs)
RORs are orphan nuclear receptors that belong to the retinoid receptor family. There are three members of the family, RORα, RORβ, and RORγ, each encoded by a different gene; a splice variant of RORγ is expressed exclusively in lymphoid cells and termed RORγt. Every Th cell subset has a key transcription factor that specifies most of the phenotypic and genotypic characteristics of the subset and is usually referred to as a “master regulator”. RORγt serves as the master regulator for Th17 cells and is selectively expressed in Th17 cells generated in vitro or in vivo in the intestinal LP, a physiological site enriched with this Th subset. RORγt is both necessary and sufficient for Th17 cell development, as RORγt-deficient CD4+ T cells show impaired Th17 cell differentiation in vitro and in vivo and retroviral transduction of RORγt into naive CD4+ T cells induces IL-17 production [14]. Furthermore, chromatin immunoprecipitation (ChIP) analysis revealed that RORγt drives IL-17A transcription by directly binding to the IL-17A promoter [78]. However, RORγt-deficient mice are not completely devoid of Th17 cells. Dong and colleagues demonstrated that RORα is also preferentially expressed in Th17 cells and is required for optimal IL-17 production in these cells. While RORα deficiency results in a relatively mild defect in Th17 cell development compared to RORγt deficiency, CD4+ T cells from mice deficient of both RORα and RORγt show complete abrogation of Th17 cell polarization in vitro, and RAG1−/− chimeric mice reconstituted with RORα−/−RORγt−/− double-deficient stem cells have no Th17 cells in the intestinal LP and are completely protected from EAE [44]. As in mice, RORγt is both necessary and sufficient to induce human Th17 cells in experiments using human umbilical cord blood. In addition, RORα also promotes IL-17 production in human Th17 cells [58].
nTh17 cells also have selectively high expression levels of RORγt compared to their CD4 single-positive (SP) thymocyte counterparts [24]. During thymocyte development, RORγt serves as a survival factor for CD4+ CD8+ double-positive (DP) thymocytes [79]. As cells undergo selection and mature into either CD8 or CD4 SP thymocytes, RORγt expression is repressed. Further studies are required to understand how the expression of RORγt is maintained (or upregulated) specifically in nTh17 cells compared to other maturing CD4SP thymocytes. The dependency of nTh17 cells on IL-6 and TGFβ suggests that mechanisms regulating RORγt expression in nTh17 cells may at least partially overlap with those regulating conventional Th17 cells.
Both mouse and human IL-17+ γδ T cells show high expression of RORγt [29, 30]. RORγt appears to be required for their development, as RORγt-deficient mice lack IL-17+ γδ T cells [14]. IL-17+ iNKT cells constitutively express RORγt (both mouse [63, 80] and human [64]) and require RORγt for their development as RORγt− iNKT cells cannot be induced to produce IL-17 following a FTOC-like culture with Jα18−/− thymocytes, as feeder cells, in the presence of IL-7 [39]. While IL-6-dependent STAT3 activation is considered to be crucial for RORγt expression in Th17 cells, both IL-17+ γδ T and iNKT cells develop independently of IL-6 (reviewed above), thereby distinguishing them from conventional Th17 and nTh17 cells. What drives RORγt expression in these cells is currently unknown.
Aryl hydrocarbon receptor (AHR)
AHR is a ligand-activated transcription factor belonging to the basic helix-loop-helix (bHLH)-Per-Arnt-Sim homology domain (PAS) family of transcription factors. The AHR pathway is evolutionarily conserved and is activated following detection of naturally occurring or environmental ligands including the well-known toxin, dioxin [81]. Interest in the role of AHR in the immune system was heightened recently, as three groups independently showed that AHR is selectively expressed on Th17 cells (both mouse and human) [82–84]. Activation of AHR by the endogenous ligand b-formylindolo[3,2-b]carbazole (FICZ), a tryptophan-derived photoproduct, promotes IL-17, IL-17F, and IL-22 expression in Th17 cells in vitro and increased the severity of EAE in vivo [82, 83]. Moreover, the differential presence of natural AHR agonists between commercial culture media was shown to contribute to the variability of in vitro Th17 cell polarization efficiencies observed among independent laboratories [85]. However, AHR is not necessary or sufficient for Th17 cell differentiation, since AHR-deficient CD4+ T cells cultured under in vitro Th17-promoting conditions show intact expression of RORγt, IL-17A, IL-17F, and retroviral transduction of AHR into CD4+ T cells under neutral, Th1, Th2, or Treg polarizing conditions does not induce IL-17 [82]. However, AHR-deficient Th17 cells do not produce IL-22, indicating a specific requirement of AHR in the induction of IL-22 [82]. Interestingly, not all AHR ligands promote Th17 cell-mediated immune responses; in contrast to FICZ, AHR activation by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces Treg cell differentiation, resulting in protection against EAE in mice [83]. This ligand-specific regulation of Th17 versus Treg cell differentiation makes AHR a potentially attractive therapeutic target.
IL-17+ γδ T cells also express AHR and respond to FICZ-mediated AHR activation [26]. Mice immunized with heat-killed Mycobacterium tuberculosis (Mtb) and FICZ show increased numbers of IL-17+ γδ T cells with even greater enhancement in the number of IL-17+ IL-22+ double-producing γδ T cells compared to Mtb alone [26]. Similar to Th17 cells, AHR-deficient mice have intact numbers of IL-17+ γδ T cells, but those cells fail to produce IL-22. It is currently unknown whether nTh17 cells express AHR. One report showed that human iNKT cells (either from peripheral blood or cord blood) expanded with α-GalCer and IL-2 in the presence of TGFβ, IL-1β, and IL-23 upregulate AHR expression. Interestingly, FICZ-induced AHR activation in these iNKT cells suppresses IL-17 while increasing IL-22 production [80], indicating that the same AHR ligand can have opposing effects on Th17-associated cytokine production. Whether AHR plays a role in murine IL-17+ NKT cells is unknown.
It remains unclear how AHR promotes Th17 cell responses and IL-22 induction in Th17 cells. AHR was shown to co-immunoprecipitate with STAT1 and STAT5, and this interaction has been suggested to suppress STAT1 and STAT5 signaling to negatively regulate Th17 cell development [84]. The expression of AHR by most IL-17-producing T cells might be an evolutionarily conserved phenomenon linked to their prominent roles in host defense at barrier sites with proximity to the environment.
Nuclear factor-κB (NF-κB)
NF-κB is an inducible transcription factor playing an essential role in controlling both innate and adaptive immunity. The mammalian NF-κB family consists of five members: RelA (p65), RelB, c-Rel, NK-κB1 (p50:p105), and NK-κB2 (p52:p100) [86]. Several NF-κB family members play critical roles in Th17 cell differentiation, including c-Rel. Mice lacking c-Rel show defective Th17 cell development in vitro and in vivo and are resistant to EAE [87]. ChIP analysis revealed that c-Rel binds to the RORγt promoter region in Th17 cells, thereby directly inducing the Th17 cell program [87, 88]. While data regarding the dispensable role of RelB in Th17 cells are consistent, the role of RelA is controversial. While one study showed defective in vitro Th17 cell differentiation of RelA-deficient CD4+ T cells from chimeric mice generated with RelA−/− fetal liver cells [88], another study demonstrated intact Th17 cell polarization of RelA-deficient CD4+ T cells from Lck-Cre RelA fl/fl mice [89]. This discrepancy is potentially due to the difference in timing of RelA deletion during development, and further work is required to determine the role of RelA in Th17 cells.
In contrast to conventional Th17 cells, mice deficient of RelA or RelB have drastically reduced numbers of nTh17 cells, indicating a role for these two NF-κB family members in nTh17 cell development [89]. These mice also show defective development of IL-17+ γδ T cells. Experiments using mice which lack RelA or RelB in both γδ and DP thymocytes (Lck-Cre) or only in DP thymocytes (CD4-Cre) alone revealed that RelA controls IL-17+ γδ T cell development via a cell-extrinsic mechanism by regulating LTβR ligand expression on accessory thymocytes, while RelB has an intrinsic role in the development of IL-17+ γδ T cells regulating RORγt and RORα expression [89]. Global disruption of the NF-κB pathway results in defective iNKT cell development, and c-Rel, RelA, and NF-κB1 each have differential roles in distinct states of iNKT cell development [90]. However, mice with individual deletion of c-Rel, RelA, or NF-κB1 all have intact IL-17+ iNKT cell development [91]. It is possible that these factors play redundant roles in the development of IL-17+ iNKT cells, and further comprehensive studies are needed to reveal the role of NK-κB in these cells.
Given the central role of NF-κB in the immune system, especially in inflammation, it is not surprising that this transcription factor regulates all IL-17+ T cell types. Individual NF-κB family members appear to play differential roles in distinct IL-17+ T cell populations. Future studies revealing mechanistic details on how each NF-κB transcription factor mediates this function and interaction with other pathways will provide a global picture of how NF-κB fine-tunes the IL-17 axis of the immune system.
In vivo function of IL-17+ T cells
IL-17+ T cells in infection and host defense
Early studies demonstrated that IL-17 is a potent inducer of inflammatory cytokines [IL-1, IL-6, IL-8, tumor necrosis factor α (TNFα), granulocyte colony-stimulating factor (G-CSF), granulocyte–macrophage (GM)-CSF)], chemokines (CXCL1, CXCL5, CXCL8, CXCL10, CCL2, CCL7), matrix metalloproteinases (MMP1, MMP3, MMP13), and recruits neutrophils and monocytes to the site of inflammation [92, 93]. Thus, as would be predicted, IL-17-deficient mice are highly susceptible to bacterial and fungal infections (Table 2). This is true not only in animal model studies as humans with mutations leading to defects in the IL-17-axis are impaired in their ability to mount effective immune responses. Job’s syndrome (or hyper-IgE syndrome) is a rare immune disorder characterized by recurrent pulmonary infections, pneumatoceles, staphylococcal abscesses, mucocutaneous candidiasis, eczema, and abnormalities of the bone [94]. Dominant-negative mutations in STAT3 have been characterized as the underlying cause of this disease [95], and in accordance with the role of STAT3 in IL-17 induction, these patients lack IL-17-producing T cells. Furthermore, naïve T cells from these patients fail to differentiate into Th17 cells in vitro [96]. More recently, genetic deficiencies in IL-17F or the IL-17 receptor A have been found in patients with chronic mucocutaneous candidiasis disease (CMCD), a disorder characterized by recurrent and/or persistent C. albicans infection in the skin and mucosal areas [97]. In addition, thymoma or autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) patients with CMCD were found to have neutralizing autoantibodies against IL-17 that correlated with the infections [98]. While these reports demonstrate the importance of IL-17 in bacterial and fungal infections, the precise cellular source of IL-17 in each infection is unclear. This section reviews the specific roles of IL-17-producing T cells (based on studies using mouse models) in host defense at various mucosal and barrier sites (Table 2).
Table 2.
Role of IL-17-producing T cell populations in host defense
| Site | T cell type | Cytokine | Experimental system | References |
|---|---|---|---|---|
| Intestine | CD4+ αβTCR+ | IL-17, IL-17F | IL-17−/−, IL-17F−/−, and IL-17−/− IL-17F−/− mice are less efficient in clearing Citrobacter rodentium infection | [104] |
| CD4+ αβTCR+ | IL-17 | Nod1- and Nod2-dependent “innate” Th17 cells contribute to the early phase (4–7 days post infection) of Citrobacter rodentium infection | [110] | |
| γδ, CD4+ αβTCR+ | IL-17 | γδ T cells are the major source of IL-17 in Salmonella Typhimutium infection; IL-23p19−/− mice have reduced level of IL-17 and neutrophil recruitment into the cecal mucosa during Salmonella Typhimutium infection | [110, 175] | |
| CD4+ αβTCR+ | IL-17 | Administration of anti-IL-17 antibody results in impaired vaccine-induced clearance of Helicobacter pylori infection | [105] | |
| Skin | γδ | IL-17 | Mice deficient in γδ T cells show defects in IL-17 production and impaired clearance in Staphylococcus aureus infection | [112] |
| ? | IL-17 | IL-17R−/− mice are more susceptible to Candida albicans infection | [176] | |
| CD4+ αβTCR+, γδ | IL-17 | Both CD4+ and γδ T cells produce IL-17 during Candida albicans infection | [20] | |
| Lung | ? | IL-17 | IL-17−/− and IL-23p19−/− mice are greatly susceptible to K. pneumoniae; recombinant IL-17 enhances bacterial clearance in K. pneumoniae infection | [99, 101, 177] |
| CD4+ αβTCR+ | IL-17 | IL-17 and IL-17F from CD4+ T cells contribute to bacterial clearance in Mycoplasma pneumoniae infection | [178] | |
| CD4+ αβTCR+ |
IL-17, IL-17F |
Ag-specific IL-17+ CD4+ T cells rapidly respond to infection after Mycobacterium tuberculosis vaccination | [103] | |
| γδ | IL-17 | γδ T cells are the major source of IL-17 in Mycobacterium tuberculosis infection | [102] | |
| ? | IL-17 | Ab-mediate IL-17 depletion or IL-17R-deficiency abrogates vaccine-induced protection against Pseudomonas aeruginosa infection | [179] | |
| CD4+ αβTCR+, γδ | IL-17 | γδ T cells are the major source of IL-17 in Mycobacterium bovis bacille Calmette-Guérin (BCG) infection | [180] |
Lung
In the lung, IL-17 is induced in a time- and dose-dependent manner in a number of infections, including Klebsiella pneumoniae [99]. Administration of IL-17 to mice results in more neutrophils in the bronchoalveolar lavage fluid (BALF) and enhances the clearance of bacteria following intranasal K. pneumoniae challenge [99]. Conversely, IL-17R-deficient mice show increased bacteremia and mortality following K. pneumoniae infection [100, 101]. Similarly, mice deficient in IL-23p19 showed reduced survival after infection with this bacteria species that was associated with decreased levels of IL-17 and IL-17-induced cytokines and chemokines [101]. Administration of recombinant IL-17 to IL-23p19-deficient mice rescues these defects [101]. While the exact cellular origin of IL-17 in K. pneumoniae infection is unclear, given that IL-17 is detectable in BALF as early as 12 h following infection [99], it is likely to be produced by innate cells. In M. tuberculosis infection, γδ T cells have been shown as a major source of IL-17 in the lung [102]. However, CD4+ Th17 cells and other non-γδ T cells also produce IL-17 during M. tuberculosis infection, and the functional contribution of each subset has not been dissected fully. In an M. tuberculosis vaccination model, upon vaccination, antigen-specific IL-17+ CD4+ T cells populated the lung and rapidly responded to subsequent infections [103].
Intestine
In the gastrointestinal tract, IL-17 confers protection against Citrobacter rodentium, Helicobacter pylori, and Salmonella enterica serovar Typhimurium [104–106]. The C. rodentium model has been valuable for investigating how various IL-17-producing cell types contribute to protection against intestinal infection. Mice deficient in both IL-17 and IL-17F (IL-17−/− IL-17F−/−) or either cytokine (IL-17−/− or IL-17F−/−) show increased bacterial burdens and disrupted intestinal pathology following C. rodentium infection [92, 104]. In addition, mice lacking IL-6 or IL-23 fail to control the infection and show enhanced mortality [12, 15, 107]. C. rodentium induces an early innate IL-17 response (in the colon and cecum) at 4–7 days post-infection, followed by a robust adaptive IL-17 response at 10–14 days post-infection [108]. Th17 cells are the major source of IL-17 in the later adaptive phase [104], while lymphoid tissue-inducer (LTi)-like cells have been shown to control the innate response following the infection [109]. Recently, an “innate” Th17 cell population, which is dependent on nucleotide oligomerization domain (Nod)-like receptors Nod1 and Nod2, in the colon has been characterized to contribute to the early phase of C. rodentium infection (4 days post-infection) [110]. Whether and how these Th17 cells are different from the conventional Th17 cells in the gut, which control the adaptive response to C. rodentium infection, are unclear. While an IL-17+ γδ T cell response is not observed during the course of C. rodentium infection, in S. Typhimurium infection, strong induction of IL-17 expression in γδ T cells is seen in the acute phase of the infection (1 day post-infection). This observation demonstrates that although Th17 cells are the dominant IL-17-producing cell type in the gut, innate IL-17+ T cells are likely to play important roles in more acute and earlier phases of host defense against enteric pathogens.
Skin
IL-17+ γδ T and iNKT cells are readily observed in the skin of naive (uninfected) mice [20, 64, 111]. Following cutaneous S. aureus infection, mice deficient in γδ T but not αβ T cells show defects in IL-17 production after intra-dermal challenge with S. aureus, substantially larger skin lesions with higher bacterial burden, and impaired neutrophil recruitment [112]. Administration of recombinant IL-17 to γδ T cell-deficient mice rescued the impaired immune response [112], demonstrating that IL-17+ γδ T cells play a critical role in host defense against S. aureus. While there are no studies yet demonstrating iNKT cells participating in antimicrobial responses in the skin, the skin-resident IL-17+ iNKT cells expand and produce IL-17 rapidly in a mitogen-induced injury model [64], suggesting that these cells might contribute to innate responses in cutaneous infection. In C. albicans infection, both Th17 and γδ T cells upregulate IL-17 expression, where γδ T cells mediate the early response (day 3 post-infection) followed by progressive involvement of Th17 cells (day 5 post-infection) [20]. It is still not known whether γδ T cells are the functionally dominant IL-17 producers critical for clearance of C. albicans infection, as in S. aureus. Further studies are required to better dissect the cellular origin of IL-17 in host defense against various infections in the skin as evidence from humans clearly demonstrate the crucial role of IL-17 in cutaneous infections and the severe clinical burden of the patients, especially in CMCD.
IL-17+ T cells in autoimmunity and inflammatory disorders
Conventional Th7 cells
IL-17 has garnered interest in part due to its association with many autoimmune and inflammatory diseases and the hope that, because of its presumed causal role in disease, it may serve as an effective therapeutic target. As mentioned above, intense experimental interest in IL-17 and Th17 cells quickly followed the discovery that IL-23, at the time a newly described cytokine, was important for the pathogenesis of EAE [5] and could promote Th17 responses [6]. IL-12 is composed of the cytokine subunits p35 and p40, and the protection of p40−/− mice from EAE, for many years, was attributed to defective IL-12 production and Th1 differentiation. The realization that p40 also pairs with p19 to form IL-23 led to a reevaluation of the role of IL-12 and IFNγ in EAE, and studies revealed that lack of IL-12 (through p35 deficiency) does not protect against EAE [113, 114]; rather, it is the lack of IL-23 (revealed through utilization of p19−/− mice) that provides protection against EAE [5]. In line with the role of other cytokines in the differentiation and/or expansion of Th17 cells, IL-1βR1−/− mice [52] and mice deficient for TGFβ1 [51] in activated T cells also have ameliorated EAE.
Despite these data, the role of IL-17 secreting CD4+ T cells in EAE is not without debate. Several investigators have approached identifying the role of Th17 cells in EAE through induction of disease in mice lacking IL-17 and by adoptive transfer of Th17 versus Th1 cells; these studies provide examples of the complex nature of cytokine involvement in EAE. While transfer of myelin oligodendrocyte glycoprotein (MOG)-primed wild-type CD4+ T cells into mice induces EAE, transfer of similar cells from IL-17−/− mice does not [115]. In contrast, mice deficient in IL-17A or IL-17F are still susceptible to EAE, and this susceptibility cannot be explained by redundancy within the IL-17 family, as administration of anti-IL-17A blocking antibodies in the context of IL-17F deficiency does not significantly alter the disease course [115, 116]. However, close evaluation of the disease kinetics in IL-17-deficient mice revealed that in the absence of IL-17, disease onset is delayed, and prolonged evaluation revealed that IL-17A−/− mice display early amelioration of disease [115]. These data indicate that IL-17 and Th17 cells are indeed pathogenic in EAE but are not required for disease induction. The precise role of IL-17 and Th17 cells in the course of EAE is still under investigation. Several studies indicate that Th17 cells promote atypical EAE [117, 118] characterized by high levels of IL-17 in the brain that triggers inflammation and cellular infiltration at this site [117]. This disease course is in contrast to mice receiving in vitro generated Th1 cells that induce classic EAE characterized by spinal cord inflammation [117]. A combined pathogenic effect of Th17- and Th1-cytokines has also been suggested, and several studies show that dual IL-17- and IFNγ-producing CD4+ T cells are associated with severe EAE [20, 50].
Initial queries into a potential role for IL-17 in MS revealed elevated IL-17 message in mononuclear cells from the blood and cerebrospinal fluid of MS patients compared to controls, and these differences were augmented during periods of active disease compared to remission [119]. Additional studies have corroborated and extended these findings to implicate CD4+ as well as CD8+ T cells as sources of IL-17 in active MS lesions [120]. As in EAE, there is also a role for IFNγ-producing CD4+ T cells in MS [121], and their presence positively correlates with disease severity. Moreover, treatment of patients with IFNγ exacerbates disease [122] and anti-IFNγ administration delays disease progression [123]. Similar to studies in mice, whether the co-production of IL-17 and IFNγ leads to severe disease in MS patients is also being investigated. To this end, IL-17/IFNγ double-producing CD4+ cells can be readily seen in active lesions of MS patients [124].
The finding that IL-23, not IL-12, was the major inducer of EAE prompted reevaluation of the role of IL-12 and Th1 cells in other autoimmune diseases. In mouse models of arthritis, IL-23 deficiency protects from organ-specific inflammation and this protection correlates with decreased IL-17 production from CD4+ T cells [125]. Additionally, blocking IL-17 alleviates disease in some murine models of arthritis [126–129]. In the case of arthritis, the pathogenic nature of IL-17 may be two-fold. In addition to promoting infiltration of inflammatory cells, IL-17 stimulates differentiation and activation of osteoclasts, which directly mediate bone erosion [130, 131]. Consistent with elevated levels of IL-17 in the synovium of rheumatoid arthritis patients, trials with anti-IL-17 antibodies are being met with success [132, 133].
As described above, IL-17 functions at barrier sites to protect the host against infection. However, if not properly regulated, IL-17 can instead play a pathological role promoting autoimmunity and autoinflammation at these sites. Psoriasis, a chronic skin disorder characterized by inflammation and keratinocyte hyperproliferation, is thought to be a consequence of dysregulated T cell responses. The effective use of blocking antibodies targeting Th17 cells in active psoriasis has rapidly focused the field’s attention on the IL-23/IL-17 axis and its role in this disease. A monoclonal antibody specific for IL-12/IL-23p40 (ustekinumab) was approved for the treatment of psoriasis in 2009. More recent studies have focused on specific targeting of IL-17 and monoclonal antibodies against IL-17 (ixekizumab) [134] or the IL-17 receptor (brodalumab) [135] have demonstrated efficacy and safety for the treatment of psoriasis in phase 2 trials.
Crohn’s disease and ulcerative colitis (UC) are the two major types of inflammatory bowel disease (IBD). While the etiology of IBD is unknown, a number of studies show that the inflamed intestine of patients with Crohn’s disease or UC contains increased Th17 cells (and increased IL-17 RNA expression) compared to normal colonic mucosa [136, 137]. Also, genome-wide association studies have identified multiple single-nucleotide polymorphisms (SNPs) in the IL-23R gene region with both Crohn’s disease and UC. In mouse studies, the role of Th17 cells in intestinal inflammation has been demonstrated in a number of different models. Administration of IL-17-neutralizing antibodies results in attenuated intestinal inflammation in a T cell transfer model of colitis [138], suggesting that IL-17+ T cells are necessary for colitis. A more recent study has demonstrated that Bacteroides fragilis, a human colonic bacterium, can colonize mice and trigger colitis that is dependent on Th17 cells in the colon [139].
In asthma, a classic Th2-mediated disease, a role for IL-17 is emerging. In humans, several reports link elevated IL-17 levels in serum and sputum with increased asthma severity. Additionally, an IL-17F polymorphism resulting in antagonism of IL-17 function appears to be protective against asthma [140]. In murine models, IL-17 deficiency renders mice resistant to allergic asthma as determined by decreased granulocytic lung infiltration, Th2-cytokine production, and IgE production [141]. Consistent with these findings, adoptive transfer of allergen-primed Th17 cells followed by nasal allergen challenge results in lung neutrophilia, mucus secretion, and airway hyperreactivity [142, 143]. When administered with Th2 cells, Th17 cells augment Th2 cell-induced eosinophilia in addition to eliciting neutrophil infiltration, suggesting that IL-17 can exacerbate the Th2 response [144]. Importantly, in this adoptive transfer model, the Th17 cell-mediated arm of asthma is not quelled by steroids, leading to the notion that IL-17 may contribute to steroid-resistant asthma [142]. In contrast to these findings, however, provision of exogenous IL-17 during established asthma can lessen disease symptoms via a mechanism leading to decreased Th2 cytokine and chemokine expression [145, 146]. Infection with viruses or challenge with bacterial products that evoke a Th17 response, either concurrently or directly following asthma induction, can also alter the course of this disease. In such a scenario, IL-17 drives development of neutrophilic asthma and suppresses eosinophilic asthma [147]. These data suggest administration of IL-17 may be therapeutic for established asthma but that its presence during asthma induction or sensitization augments the disease.
Innate IL-17+ T cells in autoimmunity
A growing number of reports illustrate the contribution of IL-17 from innate-like T cells in Th17-mediated autoimmune and inflammatory diseases. These studies have largely focused on γδ T cells with the exception of one report implicating nTh17 cells as an early source of IL-17 in asthma [148]. IL-17 from γδ T cells have been implicated in murine models of psoriasis, arthritis, EAE, and colitis. Although IL-17+ γδ T cells are not required for arthritis induction in mice, deletion of these cells alleviates disease severity and incidence [149]. Among γδ subtypes, Vγ4/Vδ4 cells have been specifically implicated in collagen-induced arthritis, EAE, and psoriasis, where deletion of γδ T cells is associated with decreased IL-17 production and delayed and diminished disease [27, 150]. While the precise role of γδ T cells in these autoimmune diseases is not completely understood, their localization within the target tissues and effect on disease course suggests that IL-17 from these cells may serve to amplify further IL-17 production [131, 151]. This hypothesis is consistent with recent data demonstrating that IL-17 from γδ T cells facilitates CD4+ Th17 differentiation in an adoptive transfer model of colitis [152].
IL-17+ T cells in cancer
The accumulating number of studies investigating the role of IL-17 and Th17 cells in malignancy reflects both the growing interest in IL-17 during immunosurveillance and the controversy over its pro- or anti-tumor effects. Inflammation is known to promote tumorigenesis, tumor growth, and metastasis [153], and early studies showed that IL-17 produced by CD4+ T cells induces angiogenesis and tumor size [154]. The pro-tumor effects of Th17 cells heavily rely on induction of angiogenesis, recruitment of other inflammatory cells, and activating oncogenic transcription factors. Recent studies demonstrated that IL-17 produced by Th17 cells promotes tumor growth in melanoma and bladder carcinoma models in a STAT3-dependent manner [155] as well as tumorigenesis in enterotoxigenic Bacteroides fragilis-induced inflammatory colon cancer [139]. In contrast, other studies suggest that Th17 cells mediate anti-tumor effects. In the setting of established murine B16 tumors, injection of in vitro–generated tumor-specific Th17 cells resulted in tumor regression and increased survival compared to IFNγ-producing Th1 cells [156]. Interestingly, protection in this model was dependent upon IFNγ, but not IL-17, from the Th17 cells and was associated with increased persistence of Th17 over Th1 cells within the tumors. Additional studies have manipulated the tumor microenvironment to favor the generation of Th17 versus Treg cells [157–159]. In these studies, slowed tumor growth was associated with increased IL-17+ cells, decreased Treg cell numbers and in some cases, increased numbers of tumor infiltrating CD8+ T cells. While these data point to a positive correlation of Th17 cells with anti-tumor immunity, the mechanism of protection in these models remains unclear. The role of Th17 cells in the tumor microenvironment may depend more on their requirements for survival, cytokine profile, plasticity and/or their developmental relationship with other T cell populations than their ability to secrete IL-17.
The role of IL-17+ γδ T cells in tumor biology is also highly context-dependent. Following chemotherapy, IL-17-producing γδ T cells have been implicated in directing the accumulation of cytotoxic CD8+ T cells at tumor sites and in mediating the ensuing anti-tumor immune response [160]. IL-17+ γδ T cells have also been shown to be important in Mycobacterium bovis BCG adjuvant therapy used for the treatment of bladder cancers. In a murine model for this treatment, it was suggested that γδ T cells were the IL-17 source required for neutrophil recruitment to the bladder, an important parameter for successful BCG treatment [161]. Conversely, IL-17 from γδ T cells, as well as other T cell sources have been suggested to drive angiogenesis and thus support tumor growth [162]. It should be noted that not all sources of IL-17 and/or its isoforms regulate angiogenesis in the same way as illustrated by the findings that IL-17F from non-T cell sources appears to inhibit this process [163, 164].
Human studies correlating the presence of IL-17-producing cells with disease outcomes have also given conflicting conclusions. Some studies have shown that IL-17+ T cells correlate with slower tumor growth suggesting they play a protective role while other studies find the reverse relationship or no association at all [165]. It is likely that the complexity reflected in these studies lies in the tumor type, location, and underlying inflammatory state of the tumor microenvironment. These issues have been more fully addressed in recent reviews [165, 166].
Concluding remarks
The IL-17 field has experienced a rapid expansion over the past 7 years. In addition to advancing our understanding on the cytokine itself, the findings have inspired insights to the broader concepts in the field of immunology, such as lineage identity and commitment. While terminally differentiated effector CD4+ T cells (Th1 and Th2 cells) were thought to represent a stable and irreversible stage of lineage commitment, Th17 cells do not seem to obey that paradigm. In vitro generated Th17 cells can become IFNγ+ Th1-like or IL-4+ Th2-like cells when further polarized with IL-12 or IL-4, respectively [167]. This plasticity can also be seen in vivo using IL-17 fate-mapping reporter mice where Th17 cells became IFNγ+ T-bet+ “ex-Th17” cells during chronic inflammation [20]. Do innate IL-17+ T cells also possess a certain degree of plasticity, or are they at a more stable stage of terminal differentiation? What is the developmental and/or functional relationship between adaptive and innate IL-17+ T cells? With these remaining questions and increasing interest in IL-17 in clinical settings, future studies investigating the differentiation, activation, and maintenance of the diverse population of IL-17+ T cells will not only provide better understanding of the immune system but also improve the ongoing therapeutic targeting of the IL-17 axis in immune-mediated diseases.
Acknowledgments
We thank Dr. Gary Koretzky for helpful discussions and critical reading of the manuscript, and Justina Stadanlick for editorial assistance. The authors declare no competing financial interests.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26(7):1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 3.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169(12):7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 4.Zhang GX, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol. 2003;170(4):2153–2160. doi: 10.4049/jimmunol.170.4.2153. [DOI] [PubMed] [Google Scholar]
- 5.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 6.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150(12):5445–5456. [PubMed] [Google Scholar]
- 8.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman L. Mixed results with modulation of TH-17 cells in human autoimmune diseases. Nat Immunol. 2010;11(1):41–44. doi: 10.1038/ni.1803. [DOI] [PubMed] [Google Scholar]
- 10.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10(7):479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 11.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12(1):21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 12.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 13.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, Craig S, Watowich SS, Jetten AM, Tian Q, Dong C. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181(12):8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, Annunziato F. Human interleukin 17-producing cells originate from a CD161+ CD4+ T cell precursor. J Exp Med. 2008;205(8):1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8(6):639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 19.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8(9):950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 20.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12(3):255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voo KS, Wang YH, Santori FR, Boggiano C, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, Zheng B, Littman DR, Liu YJ. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106(12):4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks BR, Nowyhed HN, Choi JY, Poholek AC, Odegard JM, Flavell RA, Craft J. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol. 2009;10(10):1125–1132. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JS, Smith-Garvin JE, Koretzky GA, Jordan MS (2011) The requirements for natural Th17 cell development are distinct from those of conventional Th17 cells. J Exp Med. doi:10.1084/jem.20110680 [DOI] [PMC free article] [PubMed] [Retracted]
- 25.Roark CL, Simonian PL, Fontenot AP, Born WK, O’Brien RL. gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20(3):353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31(2):321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31(2):331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Ribot JC, Chaves-Ferreira M, d’Orey F, Wencker M, Goncalves-Sousa N, Decalf J, Simas JP, Hayday AC, Silva-Santos B. Cutting edge: adaptive versus innate receptor signals selectively control the pool sizes of murine IFN-gamma- or IL-17-producing gammadelta T cells upon infection. J Immunol. 2010;185(11):6421–6425. doi: 10.4049/jimmunol.1002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10(4):427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caccamo N, La Mendola C, Orlando V, Meraviglia S, Todaro M, Stassi G, Sireci G, Fournie JJ, Dieli F. Differentiation, phenotype, and function of interleukin-17-producing human Vgamma9Vdelta2 T cells. Blood. 2011;118(1):129–138. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- 31.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2(8):557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 32.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204(5):995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, Yoshida H, Kubo M, Kawamoto H, Koseki H, Taniguchi M. Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol. 2012;10(2):e1001255. doi: 10.1371/journal.pbio.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber M, Heink S, Grothe H, Guralnik A, Reinhard K, Elflein K, Hunig T, Mittrucker HW, Brustle A, Kamradt T, Lohoff M. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol. 2009;39(7):1716–1725. doi: 10.1002/eji.200939412. [DOI] [PubMed] [Google Scholar]
- 35.Ciric B, El-behi M, Cabrera R, Zhang GX, Rostami A. IL-23 drives pathogenic IL-17-producing CD8+ T cells. J Immunol. 2009;182(9):5296–5305. doi: 10.4049/jimmunol.0900036. [DOI] [PubMed] [Google Scholar]
- 36.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321(5887):408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michel ML, Mendes-da-Cruz D, Keller AC, Lochner M, Schneider E, Dy M, Eberl G, Leite-de-Moraes MC. Critical role of ROR-gammat in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc Natl Acad Sci U S A. 2008;105(50):19845–19850. doi: 10.1073/pnas.0806472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 42.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 43.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282(13):9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 44.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 46.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 48.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 49.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7(11):1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 50.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467(7318):967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gutcher I, Donkor MK, Ma Q, Rudensky AY, Flavell RA, Li MO. Autocrine transforming growth factor-beta1 promotes in vivo Th17 cell differentiation. Immunity. 2011;34(3):396–408. doi: 10.1016/j.immuni.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203(7):1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30(4):576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw MH, Kamada N, Kim YG, Nunez G. Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med. 2012;209(2):251–258. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10(3):314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Z, Tato CM, Muul L, Laurence A, O’Shea JJ. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56(9):2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci U S A. 2007;104(43):17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9(6):641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9(6):650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 60.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454(7202):350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F (2012) Pathogen-induced human T(H)17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. doi:10.1038/nature10957 [DOI] [PubMed]
- 62.Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008;205(6):1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180(8):5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doisne JM, Becourt C, Amniai L, Duarte N, Le Luduec JB, Eberl G, Benlagha K. Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor (gamma)t+ and respond preferentially under inflammatory conditions. J Immunol. 2009;183(3):2142–2149. doi: 10.4049/jimmunol.0901059. [DOI] [PubMed] [Google Scholar]
- 65.Do JS, Fink PJ, Li L, Spolski R, Robinson J, Leonard WJ, Letterio JJ, Min B. Cutting edge: spontaneous development of IL-17-producing gamma delta T cells in the thymus occurs via a TGF-beta 1-dependent mechanism. J Immunol. 2010;184(4):1675–1679. doi: 10.4049/jimmunol.0903539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Havenar-Daughton C, Li S, Benlagha K, Marie JC (2012) Development and function of murine RORgammat+ iNKT cells are under TGF-beta control. Blood. doi:10.1182/blood-2012-01-401604 [DOI] [PubMed]
- 67.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gordon SM, Carty SA, Kim JS, Zou T, Smith-Garvin JE, Alonzo ES, Haimm E, Sant’Angelo DB, Koretzky GA, Reiner SL, Jordan MS. Requirements for Eomesodermin and PLZF in the development of innate-like CD8+ T cells. J Immunol. 2011;186(8):4573–4578. doi: 10.4049/jimmunol.1100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gomez-Rodriguez J, Sahu N, Handon R, Davidson TS, Anderson SM, Kirby MR, August A, Schwartzberg PL. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 2009;31(4):587–597. doi: 10.1016/j.immuni.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saeki K, Fukuyama S, Ayada T, Nakaya M, Aki D, Takaesu G, Hanada T, Matsumura Y, Kobayashi T, Nakagawa R, Yoshimura A. A major lipid raft protein raftlin modulates T cell receptor signaling and enhances th17-mediated autoimmune responses. J Immunol. 2009;182(10):5929–5937. doi: 10.4049/jimmunol.0802672. [DOI] [PubMed] [Google Scholar]
- 72.Zhu Y, Gumlaw N, Karman J, Zhao H, Zhang J, Jiang JL, Maniatis P, Edling A, Chuang WL, Siegel C, Shayman JA, Kaplan J, Jiang C, Cheng SH. Lowering glycosphingolipid levels in CD4+ T cells attenuates T cell receptor signaling, cytokine production, and differentiation to the Th17 lineage. J Biol Chem. 2011;286(17):14787–14794. doi: 10.1074/jbc.M111.218610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Molinero LL, Miller ML, Evaristo C, Alegre M-L. High TCR stimuli prevent induced regulatory T cell differentiation in a NF-κB-dependent manner. J Immunol. 2011;186(8):4609–4617. doi: 10.4049/jimmunol.1002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Purvis HA, Stoop JN, Mann J, Woods S, Kozijn AE, Hambleton S, Robinson JH, Isaacs JD, Anderson AE, Hilkens CM. Low-strength T-cell activation promotes Th17 responses. Blood. 2010;116(23):4829–4837. doi: 10.1182/blood-2010-03-272153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien YH. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29(1):90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turchinovich G, Pennington DJ. T cell receptor signalling in gammadelta cell development: strength isn’t everything. Trends Immunol. 2011;32(12):567–573. doi: 10.1016/j.it.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 77.Mahtani-Patching J, Neves JF, Pang DJ, Stoenchev KV, Aguirre-Blanco AM, Silva-Santos B, Pennington DJ. PreTCR and TCRgammadelta signal initiation in thymocyte progenitors does not require domains implicated in receptor oligomerization. Sci Signal. 2011;4(182):ra47. doi: 10.1126/scisignal.2001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, Takaesu G, Hori S, Yoshimura A, Kobayashi T. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem. 2008;283(25):17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 79.He YW, Deftos ML, Ojala EW, Bevan MJ. RORgamma t, a novel isoform of an orphan receptor, negatively regulates Fas ligand expression and IL-2 production in T cells. Immunity. 1998;9(6):797–806. doi: 10.1016/S1074-7613(00)80645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moreira-Teixeira L, Resende M, Coffre M, Devergne O, Herbeuval JP, Hermine O, Schneider E, Rogge L, Ruemmele FM, Dy M, Cordeiro-da-Silva A, Leite-de-Moraes MC. Proinflammatory environment dictates the IL-17-producing capacity of human invariant NKT cells. J Immunol. 2011;186(10):5758–5765. doi: 10.4049/jimmunol.1003043. [DOI] [PubMed] [Google Scholar]
- 81.Barouki R, Coumoul X, Fernandez-Salguero PM. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007;581(19):3608–3615. doi: 10.1016/j.febslet.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 82.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 83.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 84.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci U S A. 2008;105(28):9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Veldhoen M, Hirota K, Christensen J, O’Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206(1):43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 87.Chen G, Hardy K, Pagler E, Ma L, Lee S, Gerondakis S, Daley S, Shannon MF. The NF-kappaB transcription factor c-Rel is required for Th17 effector cell development in experimental autoimmune encephalomyelitis. J Immunol. 2011;187(9):4483–4491. doi: 10.4049/jimmunol.1101757. [DOI] [PubMed] [Google Scholar]
- 88.Ruan Q, Kameswaran V, Zhang Y, Zheng S, Sun J, Wang J, DeVirgiliis J, Liou HC, Beg AA, Chen YH. The Th17 immune response is controlled by the Rel-RORgamma-RORgamma T transcriptional axis. J Exp Med. 2011;208(11):2321–2333. doi: 10.1084/jem.20110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Powolny-Budnicka I, Riemann M, Tanzer S, Schmid RM, Hehlgans T, Weih F. RelA and RelB transcription factors in distinct thymocyte populations control lymphotoxin-dependent interleukin-17 production in gammadelta T cells. Immunity. 2011;34(3):364–374. doi: 10.1016/j.immuni.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 90.Gerondakis S, Banerjee A, Grigoriadis G, Vasanthakumar A, Gugasyan R, Sidwell T, Grumont RJ. NF-kappaB subunit specificity in hemopoiesis. Immunol Rev. 2012;246(1):272–285. doi: 10.1111/j.1600-065X.2011.01090.x. [DOI] [PubMed] [Google Scholar]
- 91.Stankovic S, Gugasyan R, Kyparissoudis K, Grumont R, Banerjee A, Tsichlis P, Gerondakis S, Godfrey DI. Distinct roles in NKT cell maturation and function for the different transcription factors in the classical NF-kappaB pathway. Immunol Cell Biol. 2011;89(2):294–303. doi: 10.1038/icb.2010.93. [DOI] [PubMed] [Google Scholar]
- 92.Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;226:57–79. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 93.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 94.Freeman AF, Holland SM. The hyper-IgE syndromes. Immunol Allergy Clin North Am. 2008;28(2):277–291. doi: 10.1016/j.iac.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, Anderson VL, Darnell DN, Welch PA, Kuhns DB, Frucht DM, Malech HL, Gallin JI, Kobayashi SD, Whitney AR, Voyich JM, Musser JM, Woellner C, Schaffer AA, Puck JM, Grimbacher B. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357(16):1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 96.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O’Shea J, Holland SM, Paul WE, Douek DC. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452(7188):773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, Gumbleton M, Toulon A, Bodemer C, El-Baghdadi J, Whitters M, Paradis T, Brooks J, Collins M, Wolfman NM, Al-Muhsen S, Galicchio M, Abel L, Picard C, Casanova JL. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, Meloni A, Cetani F, Perniola R, Ergun-Longmire B, Maclaren N, Krohn KJ, Pura M, Schalke B, Strobel P, Leite MI, Battelino T, Husebye ES, Peterson P, Willcox N, Meager A. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207(2):299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25(3):335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 100.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194(4):519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202(6):761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177(7):4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 103.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 104.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30(1):108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 105.Velin D, Favre L, Bernasconi E, Bachmann D, Pythoud C, Saiji E, Bouzourene H, Michetti P. Interleukin-17 is a critical mediator of vaccine-induced reduction of Helicobacter infection in the mouse model. Gastroenterology. 2009;136(7):2237–2246. doi: 10.1053/j.gastro.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 106.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14(4):421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dann SM, Spehlmann ME, Hammond DC, Iimura M, Hase K, Choi LJ, Hanson E, Eckmann L. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J Immunol. 2008;180(10):6816–6826. doi: 10.4049/jimmunol.180.10.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14(3):282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 109.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34(1):122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Geddes K, Rubino SJ, Magalhaes JG, Streutker C, Le Bourhis L, Cho JH, Robertson SJ, Kim CJ, Kaul R, Philpott DJ, Girardin SE. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat Med. 2011;17(7):837–844. doi: 10.1038/nm.2391. [DOI] [PubMed] [Google Scholar]
- 111.Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186(11):6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120(5):1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110(4):493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gran B, Zhang G-X, Yu S, Li J, Chen X-H, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169(12):7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 115.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177(1):566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 116.Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B, Waisman A. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest. 2009;119(1):61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14(3):337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS ONE. 2010;5(11):e15531. doi: 10.1371/journal.pone.0015531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Matusevicius D, Kivisäkk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler (Houndmills, Basingstoke, England) 1999;5(2):101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 120.Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. The American journal of pathology. 2008;172(1):146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pelfrey CM, Rudick RA, Cotleur AC, Lee JC, Tary-Lehmann M, Lehmann PV. Quantification of self-recognition in multiple sclerosis by single-cell analysis of cytokine production. J Immunol. 2000;165(3):1641–1651. doi: 10.4049/jimmunol.165.3.1641. [DOI] [PubMed] [Google Scholar]
- 122.Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1(8538):893–895. doi: 10.1016/S0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 123.Skurkovich S, Boiko A, Beliaeva I, Buglak A, Alekseeva T, Smirnova N, Kulakova O, Tchechonin V, Gurova O, Deomina T, Favorova OO, Skurkovic B, Gusev E. Randomized study of antibodies to IFN-gamma and TNF-alpha in secondary progressive multiple sclerosis. Mult Scler (Houndmills, Basingstoke, England) 2001;7(5):277–284. doi: 10.1177/135245850100700502. [DOI] [PubMed] [Google Scholar]
- 124.Kebir H, Ifergan I, Alvarez JI, Bernard M, Poirier J, Arbour N, Duquette P, Prat A. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Annals of neurology. 2009;66(3):390–402. doi: 10.1002/ana.21748. [DOI] [PubMed] [Google Scholar]
- 125.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198(12):1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lubberts E, Joosten LA, Oppers B, van den Bersselaar L, Coenen-de Roo CJ, Kolls JK, Schwarzenberger P, van de Loo FA, van den Berg WB (2001) IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. Journal of immunology (Baltimore, MD: 1950) 167(2):1004–1013 [DOI] [PubMed]
- 127.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171(11):6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 128.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100(10):5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Irmler IM, Gajda M, Bräuer R (2007) Exacerbation of antigen-induced arthritis in IFN-gamma-deficient mice as a result of unrestricted IL-17 response. J Immunol (Baltimore, MD: 1950) 179(9):6228–6236 [DOI] [PubMed]
- 130.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203(12):2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pöllinger B, Junt T, Metzler B, Walker UA, Tyndall A, Allard C, Bay S, Keller R, Raulf F, Di Padova F, O’Reilly T, Horwood NJ, Patel DD, Littlewood-Evans A. Th17 cells, not IL-17+ γδ T cells, drive arthritic bone destruction in mice and humans. J Immunol. 2011;186(4):2602–2612. doi: 10.4049/jimmunol.1003370. [DOI] [PubMed] [Google Scholar]
- 132.Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, Sloan-Lancaster J. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62(4):929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- 133.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, Group PS, Durez P, Tak PP, Gomez-Reino JJ, Group RAS, Foster CS, Kim RY, Samson CM, Falk NS, Chu DS, Callanan D, Nguyen QD, Group US, Rose K, Haider A, Di Padova F (2010) Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med 2(52):52ra72. doi:10.1126/scitranslmed.3001107 [DOI] [PubMed]
- 134.Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, Braun D, Banerjee S. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366(13):1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 135.Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, Aras G, Li J, Russell CB, Thompson EH, Baumgartner S. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 136.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52(1):65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Seiderer J, Elben I, Diegelmann J, Glas J, Stallhofer J, Tillack C, Pfennig S, Jurgens M, Schmechel S, Konrad A, Goke B, Ochsenkuhn T, Muller-Myhsok B, Lohse P, Brand S. Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): upregulated colonic IL-17F expression in active Crohn’s disease and analysis of the IL17F p.His161Arg polymorphism in IBD. Inflamm Bowel Dis. 2008;14(4):437–445. doi: 10.1002/ibd.20339. [DOI] [PubMed] [Google Scholar]
- 138.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116(5):1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15(9):1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kawaguchi M, Takahashi D, Hizawa N, Suzuki S, Matsukura S, Kokubu F, Maeda Y, Fukui Y, Konno S, Huang SK, Nishimura M, Adachi M. IL-17F sequence variant (His161Arg) is associated with protection against asthma and antagonizes wild-type IL-17F activity. J Allergy Clin Immunol. 2006;117(4):795–801. doi: 10.1016/j.jaci.2005.12.1346. [DOI] [PubMed] [Google Scholar]
- 141.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17(3):375–387. doi: 10.1016/S1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 142.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181(6):4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams CM, Wright JF, Fouser LA. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179(11):7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 144.Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y, Puccetti P, Iwamoto I, Nakajima H. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008;178(10):1023–1032. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- 145.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203(12):2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barlow JL, Flynn RJ, Ballantyne SJ, McKenzie AN. Reciprocal expression of IL-25 and IL-17A is important for allergic airways hyperreactivity. Clin Exp Allergy. 2011;41(10):1447–1455. doi: 10.1111/j.1365-2222.2011.03806.x. [DOI] [PubMed] [Google Scholar]
- 147.Essilfie AT, Simpson JL, Horvat JC, Preston JA, Dunkley ML, Foster PS, Gibson PG, Hansbro PM. Haemophilus influenzae infection drives IL-17-mediated neutrophilic allergic airways disease. PLoS Pathog. 2011;7(10):e1002244. doi: 10.1371/journal.ppat.1002244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tanaka S, Yoshimoto T, Naka T, Nakae S, Iwakura Y, Cua D, Kubo M. Natural occurring IL-17-producing T cells regulate the initial phase of neutrophil-mediated airway responses. J Immunol. 2009;183(11):7523–7530. doi: 10.4049/jimmunol.0803828. [DOI] [PubMed] [Google Scholar]
- 149.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL (2007) Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J Immunol (Baltimore, MD: 1950) 179(8):5576–5583 [DOI] [PMC free article] [PubMed]
- 150.Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, Becher B. Rorgammat + innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J Clin Invest. 2012;122(6):2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, Prinz I, Hemmer B, Kuchroo VK, Oukka M, Korn T. gammadelta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33(3):351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Do J-s, Visperas A, Dong C, Baldwin WM, Min B. Cutting edge: Generation of colitogenic Th17 CD4 T cells is enhanced by IL-17+ γδ T cells. J Immunol. 2011;186(8):4546–4550. doi: 10.4049/jimmunol.1004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 154.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101(7):2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 155.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206(7):1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112(2):362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178(11):6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 158.Gnerlich JL, Mitchem JB, Weir JS, Sankpal NV, Kashiwagi H, Belt BA, Porembka MR, Herndon JM, Eberlein TJ, Goedegebuure P, Linehan DC. Induction of Th17 cells in the tumor microenvironment improves survival in a murine model of pancreatic cancer. J Immunol. 2010;185(7):4063–4071. doi: 10.4049/jimmunol.0902609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, Mellor AL, He Y, Munn DH. Indoleamine 2, 3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113(24):6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N, Lasarte JJ, Matsuzaki G, Ikuta K, Ryffel B, Benlagha K, Tesniere A, Ibrahim N, Dechanet-Merville J, Chaput N, Smyth MJ, Kroemer G, Zitvogel L. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208(3):491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Takeuchi A, Dejima T, Yamada H, Shibata K, Nakamura R, Eto M, Nakatani T, Naito S, Yoshikai Y. IL-17 production by gammadelta T cells is important for the antitumor effect of Mycobacterium bovis bacillus Calmette-Guerin treatment against bladder cancer. Eur J Immunol. 2011;41(1):246–251. doi: 10.1002/eji.201040773. [DOI] [PubMed] [Google Scholar]
- 162.Wakita D, Sumida K, Iwakura Y, Nishikawa H, Ohkuri T, Chamoto K, Kitamura H, Nishimura T. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol. 2010;40(7):1927–1937. doi: 10.1002/eji.200940157. [DOI] [PubMed] [Google Scholar]
- 163.Tong Z, Yang XO, Yan H, Liu W, Niu X, Shi Y, Fang W, Xiong B, Wan Y, Dong C. A protective role by interleukin-17F in colon tumorigenesis. PLoS ONE. 2012;7(4):e34959. doi: 10.1371/journal.pone.0034959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xie Y, Sheng W, Xiang J, Ye Z, Yang J. Interleukin-17F suppresses hepatocarcinoma cell growth via inhibition of tumor angiogenesis. Cancer Invest. 2010;28(6):598–607. doi: 10.3109/07357900903287030. [DOI] [PubMed] [Google Scholar]
- 165.Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, Zou W. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32(5):643–649. doi: 10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10(4):248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30(1):92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Okamoto K, Iwai Y, Oh-Hora M, Yamamoto M, Morio T, Aoki K, Ohya K, Jetten AM, Akira S, Muta T, Takayanagi H. IkappaBzeta regulates T(H)17 development by cooperating with ROR nuclear receptors. Nature. 2010;464(7293):1381–1385. doi: 10.1038/nature08922. [DOI] [PubMed] [Google Scholar]
- 169.Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, Hatton RD, Stormo GD, Weaver CT, Russell JH, Murphy TL, Murphy KM. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460(7253):405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, Oukka M, Kuchroo VK, Glimcher LH. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol. 2011;12(1):96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8(9):958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 172.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208(7):1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shibata K, Yamada H, Sato T, Dejima T, Nakamura M, Ikawa T, Hara H, Yamasaki S, Kageyama R, Iwakura Y, Kawamoto H, Toh H, Yoshikai Y. Notch-Hes1 pathway is required for the development of IL-17-producing gammadelta T cells. Blood. 2011;118(3):586–593. doi: 10.1182/blood-2011-02-334995. [DOI] [PubMed] [Google Scholar]
- 175.Godinez I, Raffatellu M, Chu H, Paixao TA, Haneda T, Santos RL, Bevins CL, Tsolis RM, Baumler AJ. Interleukin-23 orchestrates mucosal responses to Salmonella enterica serotype Typhimurium in the intestine. Infect Immun. 2009;77(1):387–398. doi: 10.1128/IAI.00933-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190(3):624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 177.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14(3):275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9(1):78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Priebe GP, Walsh RL, Cederroth TA, Kamei A, Coutinho-Sledge YS, Goldberg JB, Pier GB. IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharide-heterologous strains of Pseudomonas aeruginosa. J Immunol. 2008;181(7):4965–4975. doi: 10.4049/jimmunol.181.7.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178(6):3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]